Abstract
Objective
To evaluate the long-term safety and efficacy of intranasal esketamine in patients with treatment-resistant depression from the Asian subgroup of the SUSTAIN-2 study.
Methods
SUSTAIN-2 was a phase 3, open-label, single-arm, multicenter study comprising a 4-week screening, 4-week induction, 48-week optimization/maintenance, and 4-week follow-up (upon esketamine discontinuation) phase. Patients with treatment-resistant depression received esketamine plus an oral antidepressant during the treatment period.
Results
The incidence of ≥ 1 serious treatment-emergent adverse event (TEAE) among the 78 subjects from the Asian subgroup (Taiwan 33, Korea 26, Malaysia 19) was 11.5% (n = 9); with no fatal TEAE. 13 Asian patients (16.7%) discontinued esketamine due to TEAEs. The most common TEAEs were dizziness (37.2%), nausea (29.5%), dissociation (28.2%), and headache (21.8%). Most TEAEs were mild to moderate in severity, transient and resolved on the same day. Upon discontinuation of esketamine, no trend in withdrawal symptoms was observed to associate long-term use of esketamine with withdrawal syndrome. There were no reports of drug seeking, abuse, or overdose. Improvements in symptoms, functioning and quality of life, occurred during in the induction phase and were generally maintained through the optimization/maintenance phases of the study.
Conclusion
The safety and efficacy of esketamine in the Asian subgroup was generally consistent with the total SUSTAIN-2 population. There was no new safety signal and no indication of a high potential for abuse with the long-term (up to one year) use of esketamine in the Asian subgroup. Most of the benefits of esketamine occurred early during the induction phase.
Keywords: Administration, intranasal; Esketamine; Asia; Depressive disorder, treatment-resistant; Antidepressive agents;
INTRODUCTION
Major depressive disorder (MDD) is a serious psychiatric illness associated with significant disease burden, impact on quality of life, functional and cognitive impairment, and excess morbidity and mortality [1,2]. Around one-third of individuals with MDD fail to achieve remission despite multiple courses of antidepressant treatment of adequate dose and duration, and are considered to have treatment-resistant depression (TRD) [3,4]. In Asia, the prevalence of MDD is estimated to be lower compared with the United States (US) and Europe although under-reporting is suspected [5,6]. Based on epidemiologic data, the percentage of pharmaceutically- treated depression (PTD) patients in Taiwan who developed TRD was around 7 to 12% [7-9]. In Korea, the estimated proportion of PTD patients who developed TRD was 4.2% [10].
Patients with TRD have more co-morbidities, a higher psychiatric hospitalization rate, longer length of hospital stay and more emergency room visits than patients who are responsive to treatment [4,11-13]. MDD is the most common mental health disorder associated with suicide-related behaviors [13-15]. A recent study using Swedish national registers showed markedly higher mortality from external causes (including suicides and accidents) among TRD patients compared with non-TRD patients (hazard ratio [HR] 1.97 [95% confidence interval, 95% CI: 1.69−2.29]) [16]. TRD is often associated with greater functional impairment and poorer quality of life compared with treatment-responsive depression; with high unemployment rates [12], loss of productivity [17], and increased healthcare resource utilization [18]. Hence, the lack of effective options for treating TRD represents a large unmet need for patients with TRD.
Esketamine has been approved for use, in conjunction with an oral antidepressant, in the US [19] and Europe [20] as a nasal spray formulation for the treatment of adult TRD. Esketamine is the S-enantiomer of ketamine racemate and has a higher affinity for the N-methyl-D-aspartate receptor than the R-enantiomer [21]. Phase 3 randomized studies assessing the efficacy and safety of esketamine as a nasal spray administered with a newly initiated oral antidepressant in TRD have been completed. These included short-term (4-week) studies with patients aged 18 to 64 [22,23] and elderly patients ≥ 65 years [24], and a long-term maintenance of effect study [25].
The US Food and Drug Administration (FDA) approved the use of esketamine in adult TRD based on “substantial evidence of effectiveness” from one of the short-term and the long-term maintenance studies [19,26]. In the short-term (4-week) study involving adult TRD patients, flexibly-dosed esketamine plus a newly initiated oral antidepressant was statistically superior (mean difference −4.0 [95% CI: −7.3 to −0.6]) in the change in Montgomery- Åsberg Depression Rating Scale (MADRS) total score at Week 4 versus baseline compared with placebo plus a newly initiated oral antidepressant. This difference between the esketamine and placebo treatment arms was observed as early as 24 hours and generally remained through Day 28 of the study [19,23]. In the long-term maintenance study, continued use of esketamine in adult TRD patients resulted in a statistically significant delay in time to relapse compared with placebo among stable remitters (HR 0.49 [95% CI: 0.29−0.84]) and stable responders (HR 0.30 [95% CI: 0.16−0.55]) [19,25].
There is a concern regarding the potential for abuse with esketamine due to its similar pharmacological profile to ketamine, a known controlled substance [27]. Ketamine abuse is relatively common in some parts of Asia, including Hong Kong, Taiwan, and mainland China [28,29]. In the US, esketamine is only available through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) [19]. The boxed warning of esketamine’s US prescribing information further states “because of the risks of sedation and dissociation, patients must be monitored for at least 2 hours at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting” [19].
Separate from the long-term maintenance (SUSTAIN-1) study mentioned above, SUSTAIN-2 was another phase 3 study designed primarily to assess the long-term (up to one year) safety of esketamine nasal spray plus an oral antidepressant in patients with TRD. Efficacy was assessed as a secondary objective [30]. Currently there is no published safety and efficacy data on the long-term use of intranasal esketamine in the Asian population. Hence, we conducted an Asian subgroup analysis involving Korean, Malaysian, and Taiwanese subjects from the SUSTAIN-2 study. To inform on the potential for esketamine abuse, our safety analysis also included assessments on withdrawal and rebound symptoms after cessation of esketamine treatment.
METHODS
This post-hoc analysis of SUSTAIN-2 evaluated the safety and efficacy of intranasal esketamine in the Asian subgroup, including subjects from Korea, Malaysia, and Taiwan, and compared it with the overall study population.
The SUSTAIN-2 study design and its primary results have been described in detail elsewhere [30]. Briefly, SUSTAIN-2 was a phase 3, open-label, single-arm, multicenter study conducted across 21 countries/regions (Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Finland, France, Germany, Italy, Korea, Malaysia, Mexico, Poland, South Africa, Spain, Sweden, Taiwan, Turkey, United Kingdom, and the US) between October 2015 and October 2017. All applicable Institutional Review Boards (IRBs) or Independent Ethics Committees (IECs) approved the study protocol and amendments (the IRB/IEC approval numbers relevant to this sub-analysis are listed in Supplementary Material 1; available online). The study was conducted in accordance with ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. All individuals provided written informed consent before participating in the study. The SUSTAIN-2 study is registered at clinicaltrials. gov, identifier: NCT02497287.
Patients
Eligibility criteria of SUSTAIN-2 patients are detailed at https:// clinicaltrials.gov/ct2/show/NCT02497287. Eligible patients were ≥ 18 years of age with a diagnosis of MDD (Diagnostic and Statistical Manual of Mental Disorders-fifth edition) [31], and must have had non-response to ≥ 2 oral antidepressant treatments in the current depressive episode. Eligible patients entered the study directly, or were transferred after completing a separate short-term (4-week) double-blind efficacy study comparing the use of flexibly-dosed esketamine vs. placebo in elderly subjects (≥ 65 years) with TRD [24]. At study entry, patients had a MADRS [32] total score ≥ 22. Key exclusion criteria were suicidal ideation with an intent to act within the prior 6 months or suicidal behavior within the prior year; diagnosis of psychotic disorder, bipolar or related disorders; recent history (within prior 6 months) of moderate or severe substance use disorder; and, positive test result(s) for specified drugs of abuse.
Study Design
The study consisted of a screening period of up to 4 weeks, a 4 week induction phase followed by a 48 week optimization/maintenance phase, and a 4-week follow-up phase after the discontinuation of esketamine. Direct-entry patients were treated with flexible-dose intranasal esketamine and a newly-assigned oral antidepressant (duloxetine, escitalopram, sertraline, or venlafaxine extended release) during the 4-week induction phase. Transferred-entry non-responder patients entered the study at the induction phase of SUSTAIN-2 while transferred-entry responder patients joined the study at the optimization/maintenance phase. These transferred-entry patients received open-label flexible-dose intranasal esketamine and continued with the same oral antidepressant initiated from the earlier short-term (4-week) efficacy study.
During the induction phase, direct-entry and transferred-entry non-responder patients were started on esketamine 28 mg (≥ 65-years) or 56 mg (< 65-years); and were flexibly-dosed (≥ 65 years: 28 mg, 56 mg, or 84 mg; < 65 years: 56 mg or 84 mg), based on efficacy and tolerability, twice a week from Day 4 till the end of the 4-week induction phase.
Following the induction phase, patients who met the response criteria (≥ 50% reduction in MADRS total score from baseline) continued with the optimization/maintenance phase and were administered esketamine once weekly for the first 4 weeks (week 5 to 8) at the same dose as the induction phase. Transferred-entry responders joining the optimization/maintenance phase directly were started on esketamine 28 mg for the first week (week 5) and were subsequently flexibly-dosed (28 mg, 56 mg, or 84 mg) with esketamine weekly (week 6 to 8) based on efficacy and tolerability. Beyond week 8, no further dose increase was permitted but the frequency of esketamine administration could still be adjusted based on the subject’s MADRS total score: treatment frequency was either weekly (MADRS total score > 12) or every-other-week (MADRS total score ≤ 12).
In the follow-up phase, there was no administration of intranasal esketamine, and patients were encouraged to continue treatment with their oral antidepressants at the discretion of the investigator. The discontinuation of esketamine during the follow-up phase facilitated the assessment for potential withdrawal symptoms after the long-term use (up to one year) of esketamine.
Safety Assessments
The key safety assessments for SUSTAIN-2 included treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), and “TEAEs of special interest” related to suicidality (assessed by the Columbia Suicide Severity Rating Scale) [33], dissociative symptoms (assessed by the Clinician Administered Dissociative States Scale, CADSS) [34], psychotic and affective symptoms (assessed by the Brief Psychiatric Rating Scale) [35], sedation (assessed by the Modified Observer’s Assessment of Alertness/ Sedation scale, MOAA/S) [36], and cognitive impairment (assessed by the Cogstate Computerized Test Battery [30], computerized cognitive battery and Hopkins Verbal Learning Test-Revised) [37].
The Physician Withdrawal Checklist (PWC-20) [38] was administered upon discontinuing esketamine during the induction or optimization/maintenance phases, to assess potential withdrawal symptoms following the long term (up to one year) use of intranasal esketamine. PWC-20 assessments were conducted at the treatment endpoint (i.e., last dose of esketamine) and at weeks 1, 2, and 4 of the follow-up phase. PWC-20 included symptoms associated with depression, posing a challenge of attributing these symptoms to withdrawal or worsening of the depressive condition after discontinuing esketamine. Hence, PWC-20 symptoms overlapping with depressive symptoms or deemed as comorbidities of depression were grouped together as the PWC-Depression Symptoms (PWC-DS) subscale. The remaining PWC-20 symptoms not associated with depression were grouped together as the PWC-Withdrawal Symptoms (PWC-WS) subscale that was considered more specific to potential signs of withdrawal in this study. Study subjects being encouraged to continue their oral antidepressants during the follow-up phase could also help minimize this confounder.
Bladder symptoms were monitored using Bladder Pain/Interstitial Cystitis Symptom Score (BPIC-SS) [39]. Clinical laboratory tests, vital signs assessments, electrocardiograms, nasal examinations, and nasal symptom questionnaires were also performed at prespecified timepoints throughout the study.
Efficacy Assessments
The SUSTAIN-2 efficacy endpoints included change in MADRS total score, response rate (proportion of subjects achieving ≥ 50% reduction in MADRS total score) and remission rate (proportion of subjects achieving MADRS total score ≤ 12) [40,41], that were assessed through the induction and optimization/maintenance phases. Other efficacy assessments included Patient Health Questionnaire 9-item Depression module (PHQ-9) [42], Generalized Anxiety Disorder 7-item scale (GAD 7) [43], Clinical Global Impression-Severity of Illness Scale (CGI-S) [44], Sheehan Disability Scale (SDS) [45] and EuroQol-5 dimension-5 level (EQ-5D-5L) [46].
Statistical Analyses
There was no formal sample size calculation for this open-label, single-arm safety study. In general, the safety and efficacy outcomes were summarized descriptively based on the full analysis set across the induction or optimization/maintenance phases of SUSTAIN-2. The full analysis set included all patients who received at least one dose of intranasal esketamine or one dose of oral antidepressant in the respective study phases. Selected safety analyses were performed for the entire treatment period based on the all-enrolled analysis set; that included all patients who were not screen failures and entered the study receiving at least one dose of intranasal esketamine or one dose of oral antidepressant. PWC-20-related analyses were summarized based on the follow-up analysis set that included all patients who entered the follow-up phase upon discontinuing esketamine. Efficacy outcomes were analyzed and summarized descriptively using the last observation carried forward data and observed data.
Analyses were conducted for following subgroups: (i) Korean subgroup; (ii) Malaysian subgroup; (iii) Taiwanese subgroup; (iv) Asian subgroup (all patients from Korea, Malaysia and Taiwan); (v) Non-Asian sub-group (all patients other than Asian subgroup); and (vi) Total group (all patients). Analyses were performed using Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Demographics and Baseline Characteristics
A total of 802 patients were enrolled in SUSTAIN-2, of whom 78 (9.7%) were enrolled from Asia with 33 patients from Taiwan, 26 patients from Korea and 19 from Malaysia (Table 1). All patients in the Asian subgroup were direct-entry patients. Fifty-three of the patients from Asia (67.9%) completed the induction phase (compared with 74.5% for the total SUSTAIN-2 population) and 12 patients (22.6% of the 53 Asians completing the induction phase) completed the optimization/maintenance phase (compared with 24.9% for the total population). Follow- up data were obtained for 42 patients (53.8%) from Asia compared with 357 patients (44.5%) from the total study population.
Table 1.
Demographics and baseline characteristics (all enrolled analysis set)
| Characteristic | Korea (n = 26) | Malaysia (n = 19) | Taiwan (n = 33) | Asian (n = 78) | Non-Asian (n = 724) | Total (n = 802) |
|---|---|---|---|---|---|---|
| Sex, male | 13 (50.0) | 11 (57.9) | 7 (21.2) | 31 (39.7) | 269 (37.2) | 300 (37.4) |
| Mean age (yr) | 49.0 ± 13.10 | 48.3 ± 10.50 | 43.8 ± 11.24 | 46.6 ± 11.84 | 52.8 ± 13.75 | 52.2 ± 13.69 |
| Age ≥ 65 years | 3 (11.5) | 0 | 1 (3.0) | 4 (5.1) | 174 (24.0) | 178 (22.2) |
| Mean age of MDD diagnosis (yr) | 36.2 ± 14.64 | 40.3 ± 12.68 | 34.3 ± 10.56 | 36.4 ± 12.62 | 35.6 ± 13.87 | 35.7 ± 13.75 |
| No. of previous MDD episodesa | ||||||
| 1 | 1 (3.8) | 8 (42.1) | 1 (3.0) | 10 (12.8) | 101 (14.0) | 111 (13.9) |
| 2−5 | 22 (84.6) | 9 (47.4) | 24 (72.7) | 55 (70.5) | 479 (66.3) | 534 (66.7) |
| 6−10 | 3 (11.5) | 2 (10.5) | 4 (12.1) | 9 (11.5) | 112 (15.5) | 121 (15.1) |
| > 10 | 0 | 0 | 4 (12.1) | 4 (5.1) | 31 (4.3) | 35 (4.4) |
| No. of previous AD medications | ||||||
| 1 | 0 | 0 | 0 | 0 | 17 (2.3) | 17 (2.1) |
| 2 | 14 (53.8) | 12 (63.2) | 25 (75.8) | 51 (65.4) | 414 (57.2) | 465 (58.0) |
| 3 | 9 (34.6) | 4 (21.1) | 4 (12.1) | 17 (21.8) | 170 (23.5) | 187 (23.3) |
| 4 | 3 (11.5) | 2 (10.5) | 2 (6.1) | 7 (9.0) | 77 (10.6) | 84 (10.5) |
| 5 | 0 | 0 | 2 (6.1) | 2 (2.6) | 21 (2.9) | 23 (2.9) |
| 6 | 0 | 1 (5.3) | 0 | 1 (1.3) | 16 (2.2) | 17 (2.1) |
| 7 | 0 | 0 | 0 | 0 | 4 (0.6) | 4 (0.5) |
| 8 | 0 | 0 | 0 | 0 | 5 (0.7) | 5 (0.6) |
| Mean MADRS total score | 33.8 ± 5.74 | 33.6 ± 5.49 | 28.6 ± 4.06 | 31.6 ± 5.58 | 31.4 ± 5.38 | 31.4 ± 5.39 |
| Mean CGI-S score | 5.3 ± 0.80 | 5.1 ± 0.71 | 5.1 ± 0.74 | 5.2 ± 0.75 | 4.8 ± 0.77 | 4.8 ± 0.77 |
| CGI-S category | ||||||
| Normal, not at all ill | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Borderline mentally ill | 0 | 0 | 0 | 0 | 3 (0.4) | 3 (0.4) |
| Mildly ill | 0 | 0 | 0 | 0 | 18 (2.5) | 18 (2.2) |
| Moderately ill | 3 (11.5) | 4 (21.1) | 6 (18.2) | 13 (16.7) | 222 (30.7) | 235 (29.3) |
| Markedly ill | 13 (50.0) | 10 (52.6) | 18 (54.5) | 41 (52.6) | 368 (50.8) | 409 (51.0) |
| Severely ill | 8 (30.8) | 5 (26.3) | 8 (24.2) | 21 (26.9) | 109 (15.1) | 130 (16.2) |
| Extremely ill patients | 2 (7.7) | 0 | 1 (3.0) | 3 (3.8) | 3 (0.4) | 6 (0.7) |
| Mean PHQ-9 total score | 17.3 ± 4.88 | 18.7 ± 4.81 | 16.9 ± 5.54 | 17.5 ± 5.14 | 17.3 ± 5.00 | 17.3 ± 5.01 |
| Screening C-SSRS lifetimeb | ||||||
| No event | 14 (53.8) | 12 (63.2) | 10 (30.3) | 36 (46.2) | 438 (60.7) | 474 (59.3) |
| Suicidal ideation | 6 (23.1) | 4 (21.1) | 12 (36.4) | 22 (28.2) | 181 (25.1) | 203 (25.4) |
| Suicidal behavior | 6 (23.1) | 3 (15.8) | 11 (33.3) | 20 (25.6) | 103 (14.3) | 123 (15.4) |
| Screening C-SSRS past 6 or 12 monthsb | ||||||
| No event | 17 (65.4) | 14 (73.7) | 22 (66.7) | 53 (67.9) | 530 (73.4) | 583 (72.9) |
| Suicidal ideation (past 6 months) | 9 (34.6) | 5 (26.3) | 11 (33.3) | 25 (32.1) | 190 (26.3) | 215 (26.9) |
| Suicidal behavior (past 12 months) | 0 | 0 | 0 | 0 | 2 (0.3) | 2 (0.3) |
| Class of newly assigned oral AD | ||||||
| SNRI | 15 (57.7) | 12 (63.2) | 9 (27.3) | 36 (46.2) | 371 (51.3) | 407 (50.8) |
| SSRI | 11 (42.3) | 7 (36.8) | 24 (72.7) | 42 (53.8) | 352 (48.7) | 394 (49.2) |
| Employment status | ||||||
| Any employment | 15 (57.7) | 11 (57.9) | 16 (48.5) | 42 (53.8) | 408 (56.4) | 450 (56.1) |
| Any unemployment | 10 (38.5) | 6 (31.6) | 14 (42.4) | 30 (38.5) | 145 (20.0) | 175 (21.8) |
| Other | 1 (3.8) | 2 (10.5) | 3 (9.1) | 6 (7.7) | 171 (23.6) | 177 (22.1) |
Values are presented as number (%) or mean ± standard deviation.
MDD, major depressive disorder; AD, antidepressant; MADRS, Montgomery-Åsberg Depression Rating Scale; CGI-S, Clinical Global Impression-Severity of Illness Scale; PHQ-9, Patient Health Questionnaire 9-item Depression module; C-SSRS, Columbia Suicide Severity Rating Scale; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
aSample size of n = 723 and n = 801 for non-Asian subgroup and total group respectively. bSample size of n = 722 and n = 800 for non-Asian subgroup and total group respectively.
The median final daily dose of esketamine (defined as the last non-zero dose) during the induction phase was 56 mg in both the Asian subgroup and the Total population. At the end of the induction phase, 41.0% of subjects in the Asian subgroup were on the 84 mg dose and 50.0% were on 56 mg. During the optimization/maintenance phase, the median final daily dose of esketamine was 56 mg in the Asian subgroup and 84 mg in the Total population. 49.1% of Asian subjects were on the 84 mg dose and 45.3% were on 56 mg at the end of the optimization/ maintenance phase.
The mean age and the proportion of patients ≥ 65 years were lower among Asian patients (46.6 years; 5%) compared with the total study population (52.2 years; 22%) (Table 1). The mean CGI-S scores were 5.2 and 4.8 for Asian subjects and the total population, respectively. The proportion of severely and extremely ill patients was higher among Asians, especially the Korean subgroup, compared with the total population. Asians, especially the Taiwanese subgroup, had a higher lifetime suicide risk compared with the total population. The proportion of subjects with suicidal ideation in the past 6 months was also higher among the Korean and Taiwanese subgroups compared with the total study population. There was slightly more use of selective serotonin reuptake inhibitor (SSRI) vs. serotonin-norepinephrine reuptake inhibitor (SNRI) in Asian patients compared with the total population. The proportion of Asian subjects (53.8%) who were employed (including any category containing “employed”; sheltered work; housewife or dependent husband; and student) was similar to the total population (56.1%); but unemployment (including any category containing “unemployed”) was higher among Asians (38.5%) compared with the total population (21.8%). Otherwise, the baseline characteristics, including mean MADRS and PHQ-9 total scores, of patients from Asia were generally comparable with the total population (Table 1).
Safety Assessments
A summary of TEAEs that occurred over the treatment period with intranasal esketamine plus a newly assigned oral antidepressant is provided in Table 2. The overall incidence of TEAEs was 92.3% in the Asian subgroup and 90.1% in the total population. There were 2 deaths reported during the SUSTAIN-2 study; but neither occurred in the Asian subgroup. The incidence of ≥ 1 serious TEAE was 11.5% (9 of 78) in the Asian subgroup compared with 6.9% (55 of 802) in the total population. The incidence of TEAEs assessed as possibly related to intranasal esketamine by the investigator was 73.1% in the Asian subgroup and 78.9% in the total population. TEAEs leading to esketamine discontinuation occurred in 16.7% (13 of 78) of Asian subjects compared with 9.5% (76 of 802) for the total population (Table 2).
Table 2.
TEAEs over induction and optimization/maintenance phases (all enrolled analysis set)
| TEAE | Korea (n = 26) | Malaysia (n = 19) | Taiwan (n = 33) | Asian (n = 78) | Non-Asian (n = 724) | Total (n = 802) |
|---|---|---|---|---|---|---|
| Any TEAE | 24 (92.3) | 16 (84.2) | 32 (97.0) | 72 (92.3) | 651 (89.9) | 723 (90.1) |
| TEAE possibly related to intranasal esketamine | 18 (69.2) | 13 (68.4) | 26 (78.8) | 57 (73.1) | 576 (79.6) | 633 (78.9) |
| TEAE possibly related to oral AD | 7 (26.9) | 9 (47.4) | 5 (15.2) | 21 (26.9) | 220 (30.4) | 241 (30.0) |
| TEAE leading to death | 0 | 0 | 0 | 0 | 2 (0.3) | 2 (0.2) |
| ≥ 1 serious TEAE | 4 (15.4) | 2 (10.5) | 3 (9.1) | 9 (11.5) | 46 (6.4) | 55 (6.9) |
| TEAE leading to intranasal esketamine discontinuation | 5 (19.2) | 3 (15.8) | 5 (15.2) | 13 (16.7) | 63 (8.7) | 76 (9.5) |
| TEAE leading to oral AD withdrawn | 4 (15.4) | 2 (10.5) | 1 (3.0) | 7 (9.0) | 26 (3.6) | 33 (4.1) |
Values are presented as number (%).
TEAE, treatment-emergent adverse event; AD, antidepressant.
A total of 21 (26.9%) subjects in the Asian subgroup and 118 (14.7%) subjects in the total population reported ≥ 1 treatment-emergent severe adverse event. The most common treatment-emergent severe adverse events in the Asian subgroup included dissociation (3.8%, n = 3), suicidal ideation (3.8%, n = 3) and dizziness (3.8%, n = 3) compared with 1.9% (n = 15), 0.6% (n = 5) and 1.6% (n = 13) in the total study population, respectively.
Common TEAEs (≥ 5% of patients in any group) reported during the treatment period with intranasal esketamine plus a newly assigned oral antidepressant are shown in Table 3. The most common TEAEs reported in both the Asian subgroup and total population group were dizziness (37.2% and 32.9%, respectively), nausea (29.5% and 25.1%, respectively), dissociation (28.2% and 27.6%, respectively), and headache (21.8% and 24.9%, respectively). Most TEAEs reported during the treatment period were mild to moderate in severity, reported on the day of esketamine dosing, transient, and resolved on the same day. Reporting of these TEAEs in the Asian subgroup was generally consistent with the total population.
Table 3.
Common TEAEs (≥ 5% in any group) over induction and optimization/maintenance phases (all enrolled analysis set)
| Common TEAE | Korea (n = 26) | Malaysia (n = 19) | Taiwan (n = 33) | Asian (n = 78) | Non-Asian (n = 724) | Total (n = 802) |
|---|---|---|---|---|---|---|
| Nervous system disorders | 14 (53.8) | 13 (68.4) | 26 (78.8) | 53 (67.9) | 475 (65.6) | 528 (65.8) |
| Dizziness | 6 (23.1) | 7 (36.8) | 16 (48.5) | 29 (37.2) | 235 (32.5) | 264 (32.9) |
| Headache | 3 (11.5) | 4 (21.1) | 10 (30.3) | 17 (21.8) | 183 (25.3) | 200 (24.9) |
| Somnolence | 1 (3.8) | 6 (31.6) | 5 (15.2) | 12 (15.4) | 122 (16.9) | 134 (16.7) |
| Dysgeusia | 0 | 6 (31.6) | 1 (3.0) | 7 (9.0) | 88 (12.2) | 95 (11.8) |
| Hypoesthesia | 0 | 2 (10.5) | 7 (21.2) | 9 (11.5) | 86 (11.9) | 95 (11.8) |
| Sedation | 1 (3.8) | 6 (31.6) | 2 (6.1) | 9 (11.5) | 62 (8.6) | 71 (8.9) |
| Dizziness postural | 7 (26.9) | 0 | 2 (6.1) | 9 (11.5) | 58 (8.0) | 67 (8.4) |
| Paraesthesia | 0 | 0 | 0 | 0 | 58 (8.0) | 58 (7.2) |
| Psychiatric disorders | 14 (53.8) | 10 (52.6) | 21 (63.6) | 45 (57.7) | 339 (46.8) | 384 (47.9) |
| Dissociation | 8 (30.8) | 3 (15.8) | 11 (33.3) | 22 (28.2) | 199 (27.5) | 221 (27.6) |
| Anxiety | 0 | 1 (5.3) | 2 (6.1) | 3 (3.8) | 69 (9.5) | 72 (9.0) |
| Insomnia | 2 (7.7) | 1 (5.3) | 9 (27.3) | 12 (15.4) | 51 (7.0) | 63 (7.9) |
| Gastrointestinal disorders | 15 (57.7) | 7 (36.8) | 17 (51.5) | 39 (50.0) | 334 (46.1) | 373 (46.5) |
| Nausea | 10 (38.5) | 5 (26.3) | 8 (24.2) | 23 (29.5) | 178 (24.6) | 201 (25.1) |
| Vomiting | 2 (7.7) | 2 (10.5) | 5 (15.2) | 9 (11.5) | 78 (10.8) | 87 (10.8) |
| Hypoesthesia oral | 0 | 0 | 1 (3.0) | 1 (1.3) | 72 (9.9) | 73 (9.1) |
| Diarrhoea | 3 (11.5) | 0 | 9 (27.3) | 12 (15.4) | 48 (6.6) | 60 (7.5) |
| Infections and infestations | 8 (30.8) | 5 (26.3) | 16 (48.5) | 29 (37.2) | 250 (34.5) | 279 (34.8) |
| Viral upper respiratory tract infection | 6 (23.1) | 0 | 10 (30.3) | 16 (20.5) | 66 (9.1) | 82 (10.2) |
| Urinary tract infection | 0 | 1 (5.3) | 6 (18.2) | 7 (9.0) | 58 (8.0) | 65 (8.1) |
| Influenza | 0 | 0 | 0 | 0 | 43 (5.9) | 43 (5.4) |
| General disorders and administration site conditions | 9 (34.6) | 2 (10.5) | 8 (24.2) | 19 (24.4) | 168 (23.2) | 187 (23.3) |
| Fatigue | 1 (3.8) | 0 | 3 (9.1) | 4 (5.1) | 59 (8.1) | 63 (7.9) |
| Musculoskeletal and connective tissue disorders | 8 (30.8) | 3 (15.8) | 11 (33.3) | 22 (28.2) | 132 (18.2) | 154 (19.2) |
| Back pain | 2 (7.7) | 0 | 2 (6.1) | 4 (5.1) | 37 (5.1) | 41 (5.1) |
| Investigations | 3 (11.5) | 1 (5.3) | 9 (27.3) | 13 (16.7) | 130 (18.0) | 143 (17.8) |
| Blood pressure increased | 0 | 1 (5.3) | 2 (6.1) | 3 (3.8) | 72 (9.9) | 75 (9.4) |
| Ear and labyrinth disorders | 1 (3.8) | 3 (15.8) | 6 (18.2) | 10 (12.8) | 116 (16.0) | 126 (15.7) |
| Vertigo | 0 | 1 (5.3) | 6 (18.2) | 7 (9.0) | 81 (11.2) | 88 (11.0) |
| Eye disorders | 4 (15.4) | 3 (15.8) | 5 (15.2) | 12 (15.4) | 93 (12.8) | 105 (13.1) |
| Vision blurred | 1 (3.8) | 0 | 2 (6.1) | 3 (3.8) | 57 (7.9) | 60 (7.5) |
Values are presented as number (%).
TEAE, treatment-emergent adverse event.
TEAEs of Special Interest
Suicidality
Suicidality-related TEAEs were reported in 10.3% (n = 8) of patients in the Asian subgroup vs. 5.2% (n = 42) in the total population (Table 4). The most common TEAEs related to suicide was suicidal ideation at 7.7% (n = 6) in the Asian subgroup and 3.2% (n = 26) in the total population. One Asian patient (from Korea) attempted suicide; but none completed suicide in the Asian subgroup over the treatment period with intranasal esketamine plus a newly assigned oral antidepressant (Table 4).
Table 4.
TEAEs related to suicide over induction and optimization/maintenance phases (all enrolled analysis set)
| TEAEs related to suicide | Korea (n = 26) | Malaysia (n = 19) | Taiwan (n = 33) | Asian (n = 78) | Non-Asian (n = 724) | Total (n = 802) |
|---|---|---|---|---|---|---|
| TEAEs related to suicide | 2 (7.7) | 1 (5.3) | 5 (15.2) | 8 (10.3) | 34 (4.7) | 42 (5.2) |
| Suicidal ideation | 2 (7.7) | 1 (5.3) | 3 (9.1) | 6 (7.7) | 20 (2.8) | 26 (3.2) |
| Intentional self-injury | 0 | 0 | 2 (6.1) | 2 (2.6) | 5 (0.7) | 7 (0.9) |
| Suicide attempt | 1 (3.8) | 0 | 0 | 1 (1.3) | 6 (0.8) | 7 (0.9) |
| Suicidal behavior | 0 | 0 | 0 | 0 | 3 (0.4) | 3 (0.4) |
| Completed suicide | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
| Depression suicidal | 0 | 0 | 0 | 0 | 1 (0.1) | 1 (0.1) |
Values are presented as number (%).
TEAE, treatment-emergent adverse event.
Dissociation
For the total population, nearly all TEAEs related to dissociation, assessed by CADSS, were transient and resolved spontaneously without the need for concomitant medication. No subjects in the Asian subgroup had a treatment-emergent dissociation that required concomitant medication or resulted in discontinuation of intranasal esketamine.
Sedation
MOAA/S was used to measure treatment-emergent sedation. Nine (11.5%) Asian subjects, mainly from the Malaysian subgroup (6 out of 19 subjects), experienced treatment-emergent sedation compared with 8.9% in the total population. Similar to the total population, only one patient discontinued intranasal esketamine because of treatment-emergent sedation.
Cognitive impairment
No TEAE in the category of cognitive disorder was reported during the esketamine treatment period. The mean group performance on tests of attention/processing speed and higher-level cognitive domains, assessed by the standardized Cogstate computerized cognitive battery, either remained stable or showed slight improvement in both the Asian and total study populations.
Cystitis
There was no report of cystitis, including interstitial/ulcerative cystitis, among Asian subjects during the study. Overall, BPIC-SS total score remained low during the esketamine treatment period, suggesting no/minimal bladder symptoms.
Blood pressure and heart rate
The number of subjects with ≥ 1 TEAE related to increased blood pressure over the induction and optimization/maintenance phases was 5 (6.4%) in the Asian subgroup and 103 (12.8%) in the total population. The proportion of subjects who met the study criteria for acute hypertension (i.e., systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg) was similar between the Asian subgroup (n = 2, 2.6%) and the total population (n = 18, 2.3%) during the induction phase. There was no report of acute hypertension among Asian subjects during the optimization/maintenance phase. No Asian patients discontinued intranasal esketamine because of TEAEs related to increased blood pressure. TEAEs related to increased heart rate was low at 2.6% (n = 2) in the Asian subgroup vs. 1.7% (n = 14) in the total population.
Withdrawal Symptoms Following Discontinuation of Esketamine
The mean PWC-20 total scores and the mean scores for the PWC-DS and PWC-WS subscales during the follow-up phase are shown in Table 5. The PWC-20, PWC-DS and PWC-WS scores were generally higher in the Asian subgroup compared with the total population and non-Asians from the end of treatment through week 4 of the follow-up phase. Unlike the relatively stable scores for the total population and non-Asian subgroup, the scores for Asian subjects fluctuated randomly throughout the follow-up phase; with the PWC-WS scores appearing to be relatively more stable compared with its corresponding PWC-20 and PWC-DS scores. The scores for the Taiwanese subgroup also appeared less fluctuant than the Korean and Malaysian subgroups.
Table 5.
Physician withdrawal checklist (PWC-20, PWC-DS, and PWC-WS) scores over the follow-up phase (follow-up analysis set)
| PWC scores | Korea | Malaysia | Taiwan | Asian | Non-Asian | Total |
|---|---|---|---|---|---|---|
| End of treatment | n = 16 | n = 4 | n = 9 | n = 29 | n = 273 | n = 302 |
| Mean PWC-20 total score | 13.25 | 6.25 | 7.56 | 10.52 | 6.84 | 7.19 |
| Mean PWC-DS score | 11.31 | 5.25 | 6.78 | 9.07 | 6.01 | 6.30 |
| Mean PWC-WS score | 1.94 | 1.00 | 0.78 | 1.45 | 0.83 | 0.89 |
| Week 1 (F/U) | n = 6 | n = 1 | n = 8 | n = 15 | n = 118 | n = 133 |
| Mean PWC-20 total score | 6.83 | 16.00 | 7.75 | 7.93 | 7.44 | 7.50 |
| Mean PWC-DS score | 6.17 | 12.00 | 6.88 | 6.93 | 6.48 | 6.53 |
| Mean PWC-WS score | 0.67 | 4.00 | 0.88 | 1.00 | 0.96 | 0.96 |
| Week 2 (F/U) | n = 13 | n = 2 | n = 9 | n = 24 | n = 207 | n = 231 |
| Mean PWC-20 total score | 14.85 | 19.50 | 7.22 | 12.38 | 6.87 | 7.45 |
| Mean PWC-DS score | 11.77 | 16.50 | 6.11 | 10.04 | 6.05 | 6.47 |
| Mean PWC-WS score | 3.08 | 3.00 | 1.11 | 2.33 | 0.82 | 0.98 |
| Week 4 (F/U) | n = 13 | n = 1 | n = 8 | n = 22 | n = 189 | n = 211 |
| Mean PWC-20 total score | 11.62 | 24.00 | 9.00 | 11.23 | 6.73 | 7.20 |
| Mean PWC-DS score | 9.15 | 22.00 | 7.75 | 9.23 | 5.98 | 6.32 |
| Mean PWC-WS score | 2.46 | 2.00 | 1.25 | 2.00 | 0.75 | 0.88 |
The PWC-Depression Symptoms (PWC-DS) subscale consisted of the following 9 items overlapping with depressive symptoms: anxiety-nervousness; restlessness-agitation; irritability; difficulty concentrating/remembering; dysphoric mood-depression; fatigue-lethargy-lack of energy; insomnia; depersonalization-derealization; and loss of appetite, and 3 items deemed as comorbidities of depression: headaches; muscle aches or stiffness; and weakness. The PWC-Withdrawal Symptoms (PWC-WS) subscale consisted of the following 8 items: diaphoresis; diarrhea; dizziness-lightheadedness; increased acuity for sound, smell, touch and pain; nausea-vomiting; paresthesias; poor coordination; and tremors-tremulousness. Each PWC symptom was rated using a 0–3-point scale (not present = 0; mild = 1; moderate = 2; severe = 3) with a maximum score of 60, 36 and 24 for PWC-20, PWC-DS and PWC-WS, respectively.
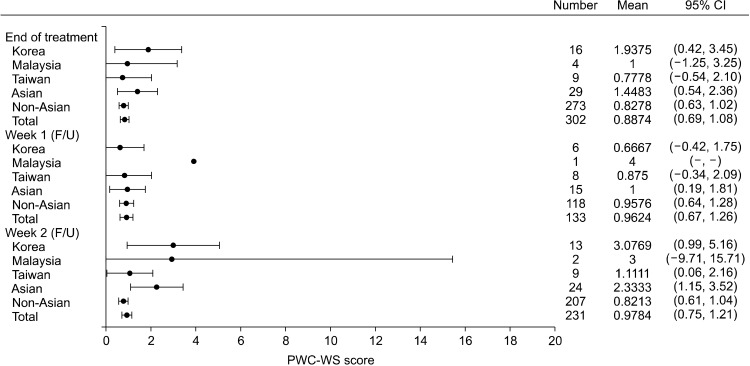
Figure 1 shows the mean PWC-WS subscale scores for the different patient groups during end of treatment, weeks 1 and 2 of the follow-up phase. The mean PWC-WS scores for the Asian subgroup and total population were closer (i.e., more similar) at end of treatment and week 1 compared with week 2; with overlapping 95% CIs at all 3 timepoints. In the Asian subgroup, a smaller change in mean PWC-WS score was observed between end of treatment vs. week 1 compared with week 1 vs. 2. Random fluctuations of mean PWC-WS scores occurred among Asian subjects (range 0.67 to 3.08), especially for the Korean and Malaysia subgroups with wide 95% CI.
Fig. 1.
Physician withdrawal checklist-withdrawal symptoms (PWC-WS) subscale scores over the follow-up phase (follow-up analysis set). CI, confidence interval; F/U, follow-up.
A total of 43 (55.1%) subjects in the Asian subgroup, and 429 (53.5%) in the total population had a TEAE suggestive of abuse potential during esketamine treatment. In the Asian subgroup and total population, the most common TEAEs suggestive of abuse potential were dizziness (37.2% and 32.9%, respectively) and dissociation (28.2% and 27.6%, respectively). These and other TEAEs observed after esketamine administration were considered related to the mechanism of action of the compound and not indicative of abuse [47].
There was no report of overdose, drug seeking or drug abuse of esketamine during the SUSTAIN-2 study. There was no request from study subjects to increase the dose (> 84 mg) or dosing frequency of esketamine beyond what was specified in the protocol.
Efficacy Assessments
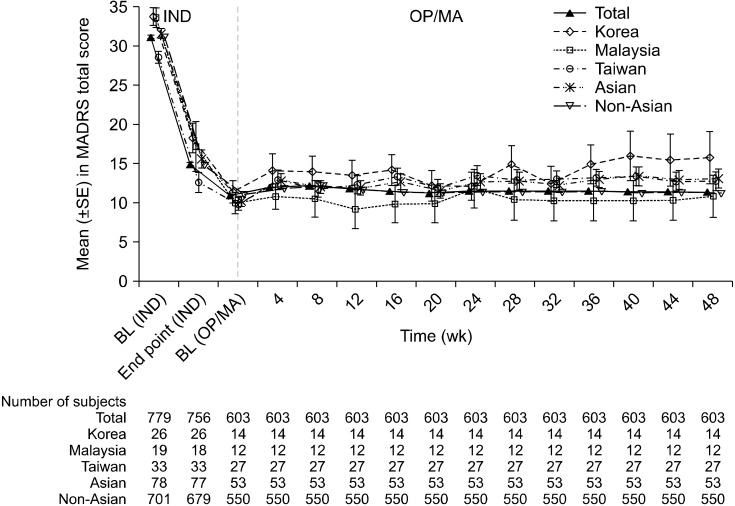
The mean MADRS total score for the Asian subgroup and the total population improved over time during the 4-week induction period and was generally maintained during the 48-week optimization/maintenance phase. The mean MADRS total score for the Asian subgroup was generally overlapping with the total study population through the induction and optimization/maintenance phases (Fig. 2). The mean change from baseline in MADRS total score at the end of the induction phase was −15.8 (standard deviation [SD] 10.00), and 2.9 (SD 7.55) from the start to the end of the optimization/maintenance phase in the Asian population; compared with −16.4 (SD 8.76) and 0.3 (SD 8.12) in the total population, respectively.
Fig. 2.
MADRS total score over induction and OP/MA phases, LOCF (all enrolled analysis set). MADRS, Montgomery-Åsberg De-pression Rating Scale; LOCF, last observation carried forward; SE, standard error; IND, induction phase; OP, optimization; MA, maintenance.
The proportion of Asian patients who met the response criteria (≥ 50% improvement from baseline in MADRS total score) at the end of the induction phase and optimization/maintenance phases were 72.7% and 62.3%, respectively, compared with 78.4% and 76.5% in the total population, respectively. The proportion of Asian subjects who achieved remission (MADRS total score ≤ 12) at the end of the induction and optimization/maintenance phases were 46.8% and 45.3%, respectively; compared with 47.2% and 58.2% in the total population, respectively.
Similarly, improvements from baseline in PHQ-9 total score, GAD-7 total score, and CGI-S score were observed during the 4-week induction phase and were maintained till the end of the optimization/maintenance phase for both the Asian subgroup and total population. The mean change from baseline to the end of the induction phase in PHQ-9 total score was −7.9 (SD 7.31), and 0.9 (SD 6.16) from the start to the end of the optimization/maintenance phase in the Asian population; compared with −8.9 (SD 6.67) and −0.2 (SD 5.65) in the total population, respectively. The mean change from baseline to the end of the induction phase in GAD-7 total score was −6.3 (SD 6.57) in the Asian population, and −5.9 (SD 5.85) in the total population. The corresponding change from the start to the end of the optimization/maintenance phase was 1.0 (SD 5.24) in the Asian population, and 0.2 (SD 4.23) in the total population. The median change from baseline to the end of the induction phase in CGI-S score was −1.0 (range −5 to 1), and 0.0 (range −3 to 2) from the start to the end of optimization/maintenance in the Asian population; compared with −2.0 (range −6 to 2) and 0.0 (range −3 to 4) in the total population, respectively.
Functioning and Quality of Life
In the Asian subgroup, the mean changes in SDS total score were −7.7 and 2.4 at the end of the induction and optimization/maintenance phases, respectively compared with −9.3 and −1.6 in the total population, respectively (Table 6).
Table 6.
Sheehan disability scale total scores over induction and optimization/maintenance phases
| SDS total scores | Induction phase | Optimization/maintenance phase | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Asian | Non-Asian | Total | Asian | Non-Asian | Total | ||
| Baseline | |||||||
| Number | 76 | 633 | 709 | 53 | 511 | 564 | |
| Mean ± SD | 21.2 ± 7.21 | 22.3 ± 5.19 | 22.2 ± 5.45 | 10.6 ± 8.19 | 11.4 ± 7.17 | 11.3 ± 7.27 | |
| End of phase | |||||||
| Number | 73 | 575 | 648 | 50 | 507 | 557 | |
| Mean ± SD | 13.1 ± 8.98 | 12.8 ± 7.75 | 12.8 ± 7.89 | 12.5 ± 8.98 | 9.2 ± 7.72 | 9.5 ± 7.89 | |
| Change | |||||||
| Number | 71 | 555 | 626 | 50 | 491 | 541 | |
| Mean ± SD | −7.7 ± 8.57 | −9.6 ± 7.75 | −9.3 ± 7.86 | 2.4 ± 7.54 | −2.0 ± 8.22 | −1.6 ± 8.25 | |
SD, standard deviation; SDS, sheehan disability scale.
EQ-5D-5L health status index, EQ visual analogue scale (VAS) and sum scores improved over the treatment period, with most of the changes occurring during the 4-week induction phase vs. the optimization/maintenance phase, for both the Asian subgroup and total population (Table 7). The mean changes in EQ-5D-5L health status index, EQ VAS and Sum scores from baseline at the end of the induction phase in the Asian subgroup (0.166, 12.3, and −13.0, respectively) were comparable with the total population (0.190, 17.0, and −15.3, respectively). These improvements were generally maintained through the optimization/maintenance phase.
Table 7.
EQ-5D-5L health status index, EQ VAS and sum score over induction and optimization/maintenance phases
| EQ-5D-5L results | Induction phase | Optimization/maintenance phase | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Asian | Non-Asian | Total | Asian | Non-Asian | Total | ||
| Baseline | n = 78 | n = 701 | n = 779 | n = 53 | n = 550 | n = 603 | |
| Mean health status index | 0.612 ± 0.2390 | 0.599 ± 0.2017 | 0.601 ± 0.2056 | 0.838 ± 0.1104 | 0.838 ± 0.1193 | 0.838 ± 0.1185 | |
| Mean EQ VAS | 48.9 ± 22.65 | 44.3 ± 20.20 | 44.7 ± 20.49 | 67.2 ± 15.68 | 67.6 ± 17.10 | 67.6 ± 16.97 | |
| Mean sum score | 31.9 ± 17.66 | 33.9 ± 15.34 | 33.7 ± 15.58 | 14.6 ± 12.32 | 14.5 ± 11.90 | 14.5 ± 11.92 | |
| End of phase | n = 76 | n = 669 | n = 745 | n = 53 | n = 550 | n = 603 | |
| Mean health status index | 0.784 ± 0.1693 | 0.793 ± 0.1729 | 0.792 ± 0.1725 | 0.784 ± 0.1861 | 0.833 ± 0.1474 | 0.829 ± 0.1517 | |
| Mean EQ VAS | 61.6 ± 19.99 | 62.2 ± 20.66a | 62.2 ± 20.58b | 64.0 ± 22.97 | 69.7 ± 19.38 | 69.2 ± 19.76 | |
| Mean sum score | 18.6 ± 14.85 | 18.3 ± 15.08 | 18.3 ± 15.05 | 18.5 ± 16.97 | 13.4 ± 13.77 | 13.8 ± 14.14 | |
| Change | n = 76 | n = 669 | n = 745 | n = 53 | n = 550 | n = 603 | |
| Mean health status index | 0.166 ± 0.2451 | 0.193 ± 0.2100 | 0.190 ± 0.2138 | −0.054 ± 0.1314 | −0.005 ± 0.1413 | −0.009 ± 0.1411 | |
| Mean EQ VAS | 12.3 ± 24.23 | 17.6 ± 21.34a | 17.0 ± 21.69b | −3.2 ± 17.53 | 2.0 ± 18.55 | 1.6 ± 18.51 | |
| Mean sum score | −13.0 ± 18.89 | −15.5 ± 15.93 | −15.3 ± 16.26 | 3.9 ± 12.58 | −1.1 ± 13.18 | −0.7 ± 13.19 | |
Values are presented as mean ± standard deviation.
EQ-5D-5L, EuroQol-5 dimension-5 level; VAS, visual analogue scale.
an = 670; bn = 746.
DISCUSSION
The results of this Asian subgroup analysis, based on subjects from Korea, Malaysia and Taiwan, participating in the SUSTAIN-2 study, provide important insights on the safety and efficacy of long-term (of up to one year) use of esketamine in the Asian population.
Although there were no TEAE leading to death in the Asian subgroup, and the proportion Asian patients with any TEAE (92.3%) or TEAE possibly related to esketamine (73.1%) appears comparable to the total population at 90.1% and 78.9%, respectively; the percentage of Asian patients with ≥ 1 serious TEAE (11.5%, n = 9) or TEAE leading to esketamine discontinuation (16.7%, n = 13) was higher than the total population at 6.9% (n = 55) and 9.5% (n = 76), respectively. Moreover, 26.9% (n = 21) of Asian subjects reported ≥ 1 treatment-emergent severe adverse event compared with 14.7% (n = 118) in the total population. The most common treatment-emergent severe adverse events in the Asian subgroup included dissociation (3.8%, n = 3) and dizziness (3.8%, n = 3) compared with 1.9% (n = 15) and 1.6% (n = 13) in the total study population, respectively. Asian patients being more likely to express depression somatically compared with non-Asians [48,49] may have resulted in this higher incidence of treatment-emergent severe adverse events, especially given the higher proportion of severely and extremely ill patients, based on CGI-S category, in the Asian subgroup compared with the total population at baseline (Table 1). TEAEs related to suicide, discussed below in further detail, could also be a contributing factor. The small number of events and sample sizes across the Asian, Korean, Malaysian, and Taiwanese subgroups may have contributed to the incidences observed, making it challenging to draw definitive conclusions.
TEAEs related to suicide during the treatment period was higher among Asian subjects (10.3%, n = 8) compared with the total population (5.2%, n = 42). The results seem to be not unexpected, since most of the events were reported from the Taiwanese subjects, whose baseline refractoriness was higher than the other groups (Table 1). In addition, higher lifetime suicide risk in the Asian subgroup (suicidal ideation = 28.2%; suicidal behavior = 25.6%), especially among Taiwanese subjects (suicidal ideation = 36.4%; suicidal behavior = 33.3%), was noted when compared with the total population (suicidal ideation = 25.4%; suicidal behavior = 15.4%) at baseline. The proportion of subjects with suicidal ideation in the past 6 months was also higher in the Asian (32.1%) and Taiwanese (33.3%) subgroups compared with the total population (26.9%). Although one Korean subject made a suicide attempt, no patient in the Asian subgroup completed suicide during the treatment period.
The nature and incidence of the most common TEAEs among Asian subjects was generally consistent with the total study population: dizziness (37.2% and 32.9%, respectively), nausea (29.5% and 25.1%, respectively), dissociation (28.2 and 27.6%, respectively), and headache (21.8% and 24.9%, respectively). Most TEAEs reported during the esketamine treatment period were mild to moderate in severity, reported on the day of esketamine dosing and resolved on the same day.
None of the Asian patients had a treatment-emergent dissociation that required concomitant medication (i.e., resolved spontaneously) or discontinuation of intranasal esketamine. Asian subjects (11.5%, n = 9) experienced a higher incidence of treatment-emergent sedation compared with 8.9% in the total population; with 6 out of the 9 Asians patients coming from Malaysia. Only one Asian patient discontinued esketamine because of treatment-emergent sedation. There was no report of cognitive impairment or cystitis. TEAEs related to increased blood pressure was lower in the Asian subgroup (6.5%, n = 5) compared with 12.8% in the total population, presumably due to the Asian subjects being younger. Overall, the incidence of SAEs was low; and no new safety signal or trend has been identified by this post-hoc safety analysis on the long-term use of esketamine in the Asian population.
The Physician Withdrawal Checklist (PWC-20, PWC-DS, and PWC-WS) was administered during the follow-up phase to assess withdrawal symptoms following discontinuation of long-term (up to one year) use of esketamine. A PWC assessment was conducted at treatment endpoint (last dose of esketamine) to establish a baseline prior to cessation of intranasal esketamine treatment. PWC assessments were then repeated at weeks 1, 2, and 4 of the follow-up phase post-discontinuation of esketamine. Unlike the PWC results in the non-Asian subgroup and total population that remained relatively stable throughout the follow-up phase, fluctuations did occur in the Asian subgroup, especially among Korean and Malaysian subjects (Table 5 and Fig. 1), but in a random fashion (i.e., no distinct trend observed). PWC scores were generally higher in the Asian subgroup compared with the total population during the follow-up phase, most notably at the end of treatment (i.e., baseline PWC score) when patients received their last dose of esketamine. This could be, at least partially, explained by Asians patients being more likely to report somatic symptoms rather than emotional/mood symptoms compared with their western counterparts [50,51]. PWC results should be interpreted with caution given the low number of patients in the Asian, Korean, Malaysian, and Taiwanese sub-populations resulting in wide 95% CI. The one (week 1) and two (week 2) Malaysian subjects could have skewed the results of the Asian subgroup (Fig. 1). Even so, the mean PWC-WS subscale scores were low, with a range of 0.67 to 3.08 against a maximum score of 24, suggesting no/mild withdrawal symptoms among Asian subjects (Fig. 1). Nonetheless, the 95% CI for the Asian subgroup was overlapping with the total population across all 3 timepoints in Figure 1 that may be reassuring. Overall, there was no observable trend in the PWC assessments (e.g., consistent worsening of PWC symptoms, especially between end of treatment vs. week 1 of the follow-up phase) to suggest an association between long-term (up to one year) esketamine use and withdrawal syndrome in the Asian subgroup and total population. These results concur with the findings from another long-term maintenance of effect global study which showed that the PWC-20 total scores, PWC-DS scores and PWC-WS scores remained stable in the follow-up period after cessation of esketamine treatment [23]. In addition, there was no report of overdosing, drug seeking or drug abuse of esketamine during the SUSTAIN-2 study.
The change in MADRS total score over time in the Asian subgroup was generally overlapping with the total population in Figure 2. Response (≥ 50% improvement) and remission (MARDS total score ≤ 12) rates among Asian subjects were consistent with the total population; with 72.7% and 46.8% in the Asian subgroup achieving response and remission at the end of the 4-week induction phase, respectively. Improvements in PHQ-9 total score, GAD-7 total score, and CGI-S score were observed at the end of the induction phase and were maintained till the end of the optimization/maintenance phase in the Asian subgroup; that was comparable to the total population. These efficacy results are consistent with the findings from previous placebo-controlled, short-term studies involving the use of esketamine in adult patients with TRD [22-24].
Most of the improvements in SDS total scores over the treatment period occurred during the 4-week induction phase and were generally maintained through the optimization/maintenance phase in both the Asian subgroup (−7.7 and 2.4, respectively) and total population (−9.3 and −1.6, respectively), albeit in a less pronounced fashion among Asian subjects. Given SDS assesses disruption of work/school, the Asian subgroup (38.5%) having a higher proportion of unemployed individuals at baseline compared with the total population (21.8%), may have contributed to these differences observed. A similar trend was observed for EQ-5D-5L health status index, EQ VAS and Sum scores with most of the improvements occurring during the 4-week induction phase. These changes in EQ-5D-5L health status index (0.166, SD 0.2351), EQ VAS (12.3, SD 24.23) and Sum (−13.0, SD 18.89) scores in the Asian subgroup were meaningful, given the threshold for clinically meaningful improvement in health status index is considered to be in the order of 0.03 to 0.07 points, and EQ VAS score in the order of 7 to 10 points [52-54].
Significant ethnic differences can occur in pharmacological profiles, particularly in metabolism, between Asians and Caucasians impacting treatment outcomes and AEs with psychotropic medications [55,56]. Genetic polymorphisms for cytochrome P450 isoenzyme system may account for the differences in metabolism between Asians and Caucasians [56]. Some genetic variations observed in Asians can lead to alteration of enzyme activity resulting in a lower capacity to metabolize certain drugs like antidepressants and antipsychotics [57-59]. AEs and tolerability issues have been demonstrated to increase proportionally with dose increment of some psychotropic medications [56]. Combination therapy used in depression may further aggravate the risk of developing AEs through drug-drug interactions [60]. Hence, dose selection in Asians is important with some literature recommending a lower dose of various psychotropic medication (e.g., atypical antipsychotics for MDD) for Asians compared with Caucasians [56].
In SUSTAIN-2, the median final daily dose of esketamine during the induction phase was the same at 56 mg for both the Asian subgroup (comprising subjects from Taiwan, Korea, and Malaysia) and the Total population. During the optimization/maintenance phase, the median final daily dose of esketamine was 56 mg in the Asian subgroup and 84 mg in the total population; with 49.1% of Asian subjects on 84 mg at the end of the optimization/ maintenance phase. This is supported by results from a population pharmacokinetic analysis involving 820 subjects, that showed esketamine maximum concentration for a typical Asian non-Japanese subject to be similar to that of a typical Caucasian subject [61]. Area under the curve 0−24 h for esketamine was around 8% higher in a typical Asian non-Japanese compared with a typical Caucasian subject. It is not surprising then to find esketamine’s long-term safety and efficacy results in the Asian subgroup to be consistent with the total population. A recent expert panel comprising senior psychiatrists from the Asia-Pacific region recommends physicians consider using esketamine when it attains approval for TRD treatment and is made available in their countries [62].
Limitations of this study include the open-label, single-arm design of the primary SUSTAIN-2 study, and the post-hoc nature of this analysis with a smaller sample size, that may have contributed to the imbalance in baseline demographics/characteristics with a higher proportion of subjects that were more ill (based on CGI-S category) and were at higher risk of suicide in the Asian subgroup vs. the total population. In SUSTAIN-2, eligible patients were recruited directly or transferred from a short-term study involving elderly (≥ 65 years) but not Asian subjects with TRD [24]. Hence, all patients in the Asian subgroup entered SUSTAIN-2 directly, resulting in a younger population with a smaller proportion of patients ≥ 65 years compared with the total study population and non-Asian subgroup. However, Asian subjects recruited from multiple sites and locations amounting to a sizable ~10% (n = 78) of the total study population, may provide scientifically meaningful data that can be extrapolated to the TRD population in Asia. Given the significant unmet need in patients suffering from TRD and a general lack of published data on the use of esketamine in Asians, especially in relation to its longer term use, the results of this post-hoc analysis is expected to provide valuable insights on the long-term use of esketamine in Asian patients with TRD.
The results of this subgroup analysis demonstrated the long-term safety and efficacy of intranasal esketamine in patients with TRD from Korea, Malaysia, and Taiwan. Results for the Asian subgroup were consistent with the total SUSTAIN-2 patient population. Most of the benefits of esketamine occurred early during the induction phase, and were generally maintained through the optimization/ maintenance phase in both the Asian subgroup and total population. Based on this post-hoc analysis, there is no evidence to suggest a high potential for abuse with long-term (up to one year) use of esketamine in the Asian population. Overall, results from this Asian post-hoc analysis may help guide the long-term use of esketamine in Asian patients with TRD.
Supplemental Materials
Acknowledgements
The study, medical writing and editorial support were funded by Janssen Asia Pacific, a division of Johnson and Johnson Pte Ltd. Medical writing and editorial support was provided by Tech Observer Asia Pacific Pte Ltd.
Footnotes
Conflicts of Interest
Salina Abdul Aziz is currently receiving a grant from Janssen. Wilson Tan is a full-time employee of Janssen Asia-Pacific and holds stocks in Janssen Asia-Pacific awarded as part of his total compensation package. Daisy Bai is a full-time employee of Janssen. The rest of the authors declared no potential conflict of interest.
Author Contributions
Conceptualization: Daisy Bai, Wilson Tan. Data acquisition: Ahmad Hatim Sulaiman, Cheng-Ta Li, Hong Jin Jeon, Jong-Woo Paik, Po-Chung Ju, and Salina Abdul Aziz were all investigators of the primary SUSTAIN-2 study. Formal analysis: Daisy Bai, Wilson Tan. Writing−original draft: Wilson Tan. Writing−review & editing: all authors.
References
- 1.Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, et al. Morbidity in depressive disorders. Psychother Psychosom. 2017;86:65–72. doi: 10.1159/000448661. [DOI] [PubMed] [Google Scholar]
- 2.MacQueen GM, Memedovich KA. Cognitive dysfunction in major depression and bipolar disorder: assessment and treatment options. Psychiatry Clin Neurosci. 2017;71:18–27. doi: 10.1111/pcn.12463. [DOI] [PubMed] [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/S0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 4.Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of medicaid beneficiaries with treatment-resistant depression. J Manag Care Spec Pharm. 2018;24:226–236. doi: 10.18553/jmcp.2018.24.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien IC, Kuo CC, Bih SH, Chou YJ, Lin CH, Lee CH, et al. The prevalence and incidence of treated major depressive disorder among National Health Insurance enrollees in Taiwan, 1996 to 2003. Can J Psychiatry. 2003;52:28–36. doi: 10.1177/070674370705200106. [DOI] [PubMed] [Google Scholar]
- 6.Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CT, Bai YM, Huang YL, Chen YS, Chen TJ, Cheng JY, et al. Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: cohort study. Br J Psychiatry. 2012;200:45–51. doi: 10.1192/bjp.bp.110.086983. [DOI] [PubMed] [Google Scholar]
- 8.Jeng JS, Li CT, Chen MH, Lin WC, Bai YM, Tsai SJ, et al. Repeated low-grade infections predict antidepressant-resistant depression: a nationwide population-based cohort study. J Clin Psychiatry. 2018;79:17m11540. doi: 10.4088/JCP.17m11540. [DOI] [PubMed] [Google Scholar]
- 9.Chan YE, Chen MH, Tsai SJ, Bai YM, Tsai CF, Cheng CM, et al. Treatment-resistant depression enhances risks of dementia and Alzheimer's disease: a nationwide longitudinal study. J Affect Disord. 2020;274:806–812. doi: 10.1016/j.jad.2020.05.150. [DOI] [PubMed] [Google Scholar]
- 10.Kim N, Cho SJ, Kim H, Kim SH, Lee HJ, Park CHK, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in South Korea. PLoS One. 2019;14:e0221552. doi: 10.1371/journal.pone.0221552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amos TB, Witt EA, Alphs L, DiBernardo A, Tandon N. In: the Anxiety and Depression Association of America (ADAA) Annual Conference. San Francisco, CA, USA: 2017. Apr 6-9, The humanistic and economic burden of treatment-resistant depression: a PHQ-9 severity analysis. Abstract NR S2-067. [Google Scholar]
- 12.Amos TB, Tandon N, Lefebvre P, Pilon D, Kamstra RL, Pivneva I, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a us commercial claims database. J Clin Psychiatry. 2018;79:17m11725. doi: 10.4088/JCP.17m11725. [DOI] [PubMed] [Google Scholar]
- 13.Li CT, Bai YM, Tu PC, Lee YC, Huang YL, Chen TJ, et al. Major depressive disorder and stroke risks: a 9-year follow-up population-based, matched cohort study. PLoS One. 2012;7:e46818. doi: 10.1371/journal.pone.0046818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler RC, Berglund P, Borges G, Nock M, Wang PS. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990-1992 to 2001-2003. JAMA. 2005;293:2487–2495. doi: 10.1001/jama.293.20.2487. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Son SJ, Jang M, Kim BH, Hong SJ, Seo L, et al. Rapid symptom improvement in major depressive disorder using accelerated repetitive transcranial magnetic stimulation. Clin Psychopharmacol Neurosci. 2021;19:73–83. doi: 10.9758/cpn.2021.19.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reutfors J, Andersson TM, Brenner P, Brandt L, DiBernardo A, Li G, et al. Mortality in treatment-resistant unipolar depression: a register-based cohort study in Sweden. J Affect Disord. 2018;238:674–679. doi: 10.1016/j.jad.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 17.DiBernardo A, Lin X, Zhang Q, Xiang J, Lu L, Jamieson C, et al. Humanistic outcomes in treatment resistant depression: a secondary analysis of the STAR*D study. BMC Psychiatry. 2018;18:352. doi: 10.1186/s12888-018-1920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olchanski N, McInnis Myers M, Halseth M, Cyr PL, Bockstedt L, Goss TF, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35:512–522. doi: 10.1016/j.clinthera.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Janssen Pharmaceuticals, author. Highlights of prescribing information: SPRAVATO [Internet] U.S. Food and Drug Administration; Silver Spring (MD): 2020. [cited at 2020 Oct 11]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf. [Google Scholar]
- 20.Janssen Pharmaceuticals, author. Spravato: EPAR - product information [Internet] European Medicines Agency; Amsterdam: 2019. [cited at 2020 Oct 11]. https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf. [Google Scholar]
- 21.Muller J, Pentyala S, Dilger J, Pentyala S. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6:185–192. doi: 10.1177/2045125316631267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active- controlled study (TRANSFORM-1) Int J Neuropsychopharmacol. 2019;22:616–630. doi: 10.1093/ijnp/pyz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–438. doi: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- 24.Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28:121–141. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893–903. doi: 10.1001/jamapsychiatry.2019.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381:1–4. doi: 10.1056/NEJMp1903305. [DOI] [PubMed] [Google Scholar]
- 27.Par Pharmaceutical, author. Highlights of prescribing information: KETALAR [Internet] U.S. Food and Drug Administration; Silver Spring (MD): 2020. [cited at 2020 Oct 11]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/016812Orig1s046lbl.pdf. [Google Scholar]
- 28.Liang HJ, Ungvari GS, Lee TSH, Tang WK. Ketamine addiction. Dual Diagn Open Acc. 2018;3:37. doi: 10.21767/2472-5048.100037. [DOI] [Google Scholar]
- 29.Feng LY, Wada K, Chung H, Han E, Li JH. Comparison of legislative management for new psychoactive substances control among Taiwan, South Korea, and Japan. Kaohsiung J Med Sci. 2020;36:135–142. doi: 10.1002/kjm2.12140. [DOI] [PubMed] [Google Scholar]
- 30.Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2) J Clin Psychiatry. 2020;81:19m12891. doi: 10.4088/JCP.19m12891. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders (DSM-5) 5th ed. American Psychiatric Association; Arlington: 2013. [DOI] [Google Scholar]
- 32.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg depression rating scale (SIGMA) Br J Psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 33.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia classification algorithm of suicide assessment (C-CASA): classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/ajp.2007.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 35.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. doi: 10.2466/pr0.1962.10.3.799. [DOI] [Google Scholar]
- 36.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the observer's assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. doi: 10.1097/00004714-199008000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 38.Rickels K, Garcia-Espana F, Mandos LA, Case GW. Physician withdrawal checklist (PWC-20) J Clin Psychopharmacol. 2008;28:447–451. doi: 10.1097/JCP.0b013e31817efbac. [DOI] [PubMed] [Google Scholar]
- 39.Humphrey L, Arbuckle R, Moldwin R, Nordling J, van de Merwe JP, Meunier J, et al. The bladder pain/interstitial cystitis symptom score: development, validation, and identification of a cut score. Eur Urol. 2012;61:271–279. doi: 10.1016/j.eururo.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Schweitzer I, Burrows G, Tuckwell V, Polonowita A, Flynn P, George T, et al. Sustained response to open-label venlafaxine in drug-resistant major depression. J Clin Psychopharmacol. 2001;21:185–189. doi: 10.1097/00004714-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. J Psychiatr Res. 2004;38:577–582. doi: 10.1016/j.jpsychires.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 43.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 44.Guy W. ECDEU assessment manual for psychopharmacology. U.S. Department of Health, Education, and Welfare; Rockville: 1976. [DOI] [Google Scholar]
- 45.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11 Suppl 3:89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 46.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssen, author. Position paper for the Taiwan Health Authority regarding the use of esketamine nasal spray in Taiwan/Asian patients. 2019. [Google Scholar]
- 48.Parker G, Cheah YC, Roy K. Do the Chinese somatize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287–293. doi: 10.1007/s001270170046. [DOI] [PubMed] [Google Scholar]
- 49.Parker G, Gladstone G, Chee KT. Depression in the planet's largest ethnic group: the Chinese. Am J Psychiatry. 2001;158:857–864. doi: 10.1176/appi.ajp.158.6.857. [DOI] [PubMed] [Google Scholar]
- 50.Lee P, Zhang M, Hong JP, Chua HC, Chen KP, Tang SW, et al. Frequency of painful physical symptoms with major depressive disorder in Asia: relationship with disease severity and quality of life. J Clin Psychiatry. 2009;70:83–91. doi: 10.4088/JCP.08m04114. [DOI] [PubMed] [Google Scholar]
- 51.Novick D, Montgomery W, Aguado J, Kadziola Z, Peng X, Brugnoli R, et al. Which somatic symptoms are associated with an unfavorable course in Asian patients with major depressive disorder? J Affect Disord. 2013;149:182–188. doi: 10.1016/j.jad.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Gerhards SA, Huibers MJ, Theunissen KA, de Graaf LE, Widdershoven GA, Evers SM. The responsiveness of quality of life utilities to change in depression: a comparison of instruments (SF-6D, EQ-5D, and DFD) Value Health. 2011;14:732–739. doi: 10.1016/j.jval.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 54.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol. 2004;44:1083–1105. doi: 10.1177/0091270004268128. [DOI] [PubMed] [Google Scholar]
- 56.Han C, Pae CU. Do we need to consider ethno-cultural variation in the use of atypical antipsychotics for Asian patients with major depressive disorder? CNS Drugs. 2013;27 Suppl 1:S47–S51. doi: 10.1007/s40263-012-0033-y. [DOI] [PubMed] [Google Scholar]
- 57.Bertilsson L. Metabolism of antidepressant and neuroleptic drugs by cytochrome p450s: clinical and interethnic aspects. Clin Pharmacol Ther. 2007;82:606–609. doi: 10.1038/sj.clpt.6100358. [DOI] [PubMed] [Google Scholar]
- 58.Dahl ML, Yue QY, Roh HK, Johansson I, Säwe J, Sjöqvist F, et al. Genetic analysis of the CYP2D locus in relation to debrisoquine hydroxylation capacity in Korean, Japanese and Chinese subjects. Pharmacogenetics. 1995;5:159–164. doi: 10.1097/00008571-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjöqvist F, Ingelman-Sundberg M. Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol. 1994;46:452–459. [PubMed] [Google Scholar]
- 60.Chen ML. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45:957–964. doi: 10.2165/00003088-200645100-00001. [DOI] [PubMed] [Google Scholar]
- 61.Perez-Ruixo C, Rossenu S, Zannikos P, Nandy P, Singh J, Drevets WC, et al. Population pharmacokinetics of esketamine nasal spray and its metabolite noresketamine in healthy subjects and patients with treatment-resistant depression. Clin Pharmacokinet. 2021;60:501–516. doi: 10.1007/s40262-020-00953-4. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, Han C, Liu CY, Chan S, Kato T, Tan W, et al. Management of treatment-resistant depression in real-world clinical practice settings across Asia. Neuropsychiatr Dis Treat. 2020;16:2943–2959. doi: 10.2147/NDT.S264813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.