Abstract
Streptococcus mutans strains were isolated from cohorts of Brazilian nursery school children and genotyped by arbitrarily primed PCR and restriction fragment length polymorphism analysis. Of 24 children with two to five S. mutans isolates, 29% carried two or more genotypes. The presence of matching genotypes of S. mutans among children attending one nursery suggests horizontal transmission.
Dental caries is a transmissible infectious disease in which mutans streptococci (MS) play the major role. Infective strains of Streptococcus mutans, the most prevalent species of the MS group, may persist for many years in the mouths of preschool children (1, 6, 22). Early colonization is related to high caries activity during childhood (3, 7, 11). The mechanisms by which MS colonize and accumulate on tooth surfaces are not completely clear. In addition to environmental and host factors, specific genotypes of S. mutans may be more aggressive colonizers. This is suggested by previous findings of positive relationships between the production of water-insoluble glucan from sucrose by glucosyltransferase (GTF) activities and the intensity of MS oral colonization and caries incidence (19).
Essential to the development of strategies for the prevention of dental caries is the identification of sources of MS transmission. DNA fingerprinting studies indicate that vertical transmission of bacteria from mother to child is the major route for early acquisition (2, 13, 15, 18, 21). However, detection of genotypes in children that are not found in their mothers or other family members indicates that MS may also be acquired from other sources (13, 21, 22). Since the spread of infectious agents is likely to occur in a nursery environment, we investigated the genetic similarity of MS strains isolated from Brazilian children attending nursery schools. The production of GTF isozymes was also examined to validate the genotypic similarities that we identified.
The study group included 35 MS-infected children between 12 and 30 months of age (mean ± standard deviation = 23 ± 5 months). This group accounted for 49% of the MS-colonized children previously described in a larger population (20), and it was primarily selected for the study of MS virulence factors. These children attended nine nursery schools in the city of Piracicaba, São Paulo, Brazil, for 5 days per week, 10 h per day. A total of four sucrose-rich meals were provided daily in the nurseries. Clinical exams were performed to record the number of erupted teeth and manifest caries lesions as previously described (20). Written informed consent was obtained from the parents, and all consent and experimental procedures were approved by the institutional Ethical Committee of the University of São Paulo School of Dentistry.
One to five isolates of MS were recovered from each of the 35 children. As previously described (19), oral samples were collected with tongue blades which were then pressed on the surface of mitis salivarius agar contact plates (Difco) (12) containing 2 IU of bacitracin (Sigma)/ml and 15% sucrose (Difco) (9). The number of colonies with mutans-like morphology was obtained for a predetermined area of the tongue blade impression (1.5 cm2). Individual MS colonies representative of the colonial morphologies were subcultured on mitis salivarius and tryptic soy agar plates, and pure cultures were then frozen at −70°C in 10% skim milk. These strains were identified to species level biochemically (19).
DNA from a total of 76 MS isolates (74 S. mutans and 2 Streptococcus sobrinus) was purified using the Master Pure DNA purification kit (Epicentre Technologies, Madison, Wis.) according to the manufacturer's instructions. Arbitrarily primed (AP) PCR fingerprinting was performed with the primer sequence 5′-TGCCGAGCTG-3′ as previously described (16). PCRs included 45 cycles of denaturing at 94°C (30 s), annealing at 36°C (30 s), and extending at 72°C (1 min). Amplicons were separated by electrophoresis in 1.5% agarose gels in Tris-borate-EDTA running buffer. Ethidium bromide-stained gel images were captured with a digital imaging system (Alpha IS-2000; Innotech Corp., San Leandro, Calif.). Molecular sizes for each band were computed and analyzed using Diversity Database software (Bio-Rad Laboratories, Richmond, Calif.). MS isolates from different children with very similar fingerprinting profiles (Dice coefficient, >95%) were examined by chromosomal DNA restriction fragment length polymorphism (RFLP) analysis. For this purpose, small-scale phenol extraction of chromosomal DNA was performed, and DNA was digested with HaeIII restriction endonuclease (18). The resulting fragments were electrophoretically resolved at 1.4 V/cm in Tris-borate-EDTA for 16 h in 0.55% agarose gels. Only children carrying two or more isolates of S. mutans species (n = 24) were included in the statistical analysis for comparisons of genotypic diversity regarding the other variables analyzed.
The amounts of GTF isozymes GTF-B, GTF-C, and GTF-D in culture supernatants of S. mutans isolates were analyzed with the monoclonal antibodies P72, P32, and P4, respectively (8). Fifty microliters of culture supernatant, prepared as described previously (19), was applied to nitrocellulose membranes with a dot blot apparatus (Bio-Rad). Following overnight blocking with 10% skim milk in Tris-HCl buffer (pH 7.4), the membranes were incubated for 2 h with primary antibodies P72 (1:60), P32 (1:30), or P4 (1:60) diluted in the same buffer. After a washing step with Tris-HCl buffer and incubation with anti-mouse immunoglobulin G (1:1,000) conjugated with horseradish peroxidase, the membranes were washed again and reactions were developed using the ECL system (Amersham Pharmacia Biotech, Piscataway, N.J.).
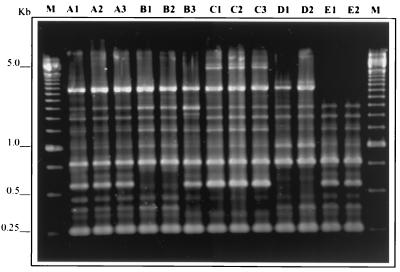
A total of 76 MS isolates from 35 children were analyzed by AP-PCR, and 45 different amplitypes were identified, 2 of which corresponded to S. sobrinus species. Figure 1 depicts the AP-PCR fingerprinting profiles observed in 5 children who attended the same nursery school and who were part of the subset of 24 children. From this subset, two or more S. mutans isolates were tested; six children (25%) were found to carry two distinct amplitypes of S. mutans and one child (4.2%) carried three different amplitypes. The characteristics of children carrying one or more amplitypes of S. mutans are shown in Table 1.
FIG. 1.
AP-PCR fingerprinting profiles of S. mutans strains isolated from five children (A, B, C, D, and E) attending the same nursery school. Child B was infected by two different amplitypes, one represented by B1 and B2 and the other by B3. Only one amplitype was identified in each of the other children. Molecular size standards are shown in lanes M.
TABLE 1.
Univariate comparisons of the distribution of 24 children with one or more S. mutans amplitypes
| Variable | No. of isolates tested | No. of amplitypes detected | No. (%) of children with:
|
OR (95% CI)a | |
|---|---|---|---|---|---|
| 1 amplitype | >1 amplitype | ||||
| Age group (mo) | |||||
| 12–18 (n = 4) | 11 | 5 | 3 (75.0) | 1 (25.0) | 1.00 |
| 19–24 (n = 8) | 22 | 12 | 5 (62.5) | 3 (37.5) | 1.80 (0.07, 71.23) |
| 25–30 (n = 12) | 31 | 15 | 9 (75.0) | 3 (25.0) | 1.00 (0.04, 36.05) |
| No. of erupted teeth | |||||
| 1–19 (n = 16) | 41 | 22 | 11 (68.8) | 5 (31.2) | 1.00 |
| 20 (n = 8) | 23 | 10 | 6 (75.0) | 2 (25.0) | 0.73 (0.07, 6.81) |
| Caries prevalence | |||||
| No manifest lesions (n = 13) | 32 | 16 | 10 (76.9) | 3 (23.1) | 1.00 |
| Manifest lesions (n = 11) | 32 | 16 | 7 (63.6) | 4 (36.4) | 1.90 (0.24, 16.23) |
| Oral levels of MS (CFU) | |||||
| 1–20 (n = 9) | 20 | 12 | 6 (66.7) | 3 (33.3) | 1.00 |
| 21–99 (n = 4) | 12 | 4 | 4 (100.0) | ||
| ≥100 (n = 11) | 32 | 16 | 7 (63.6) | 4 (36.4) | 1.14 (0.12, 10.64) |
OR, odds ratio; CI, confidence interval.
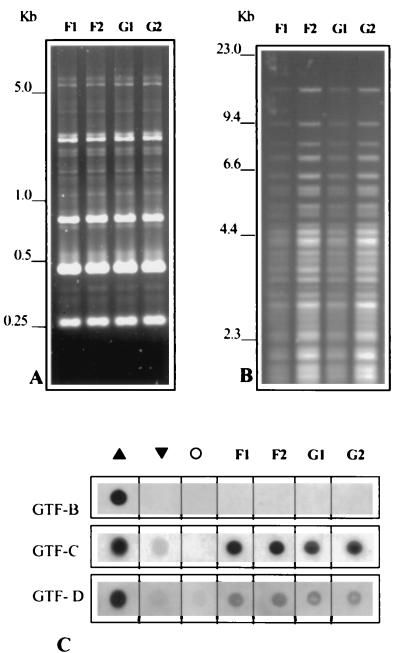
Two children who were not genetically related but attended the same nursery carried identical strains, as observed in both AP-PCR and RFLP patterns (Fig. 2). Both children were male, and they were 19 and 25 months of age. They were heavily colonized at the time of bacteriological exam. Interestingly, the patterns of GTF isozyme production in these isolates were also similar (Fig. 2). Indeed, we observed that the secreted amounts of the three GTF isozymes were very similar among strains of the same genotype. This similarity was observed in 20 (87%) out of 23 children from whom two or more S. mutans isolates represented a single genotype. In contrast, a high variability of GTF production was observed among different genotypes (data not shown). These data further indicate that the expression of GTF isozymes may be intrinsic to specific clones of S. mutans.
FIG. 2.
DNA fingerprinting profiles of S. mutans strains isolated from child F (strains F1 and F2) and child G (strains G1 and G2), both of whom attended the same nursery school. AP-PCR (A) and chromosomal DNA RFLP obtained by digestion with HaeIII (B) indicated that these two children harbored the same genotype of S. mutans. (C) Patterns of GTF-B, GTF-C, and GTF-D production were also similar among these strains. ▴ and ▾, highest and lowest isozyme producers among the total of 76 strains tested, respectively; ○, negative control (absence of primary antibody).
Clusters of clonal infection in children from day care centers have been demonstrated by matching genotypes of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, bacterial colonizers of the nasopharyngeal tract (26). MS, however, are not easily transmitted, and their establishment in the mouth is modulated by a complex group of factors, including the number of erupted teeth (4, 5), oral MS levels of the mothers (11), immunological status of the children (24), presence of enamel hypoplasia (17), and sucrose consumption (25). These factors may influence the time and intensity of the first acquisition of MS, explaining variations in MS colonization observed between different populations (5, 7, 10, 20, 24). In the present study, children were from a low-socioeconomic-level Brazilian community and received a sucrose-rich diet during the day care period. Nearly 75% of the 12- to 18-month-old children from this population were previously described as carrying detectable levels of MS, and 20% of them carried high MS levels (≥100 CFU) (20), indicating that MS colonization occurred earlier than in populations of the United States (5, 24), Japan (7), or Sweden (10). No significant associations between genotypic diversity of S. mutans and age, number of erupted teeth, MS levels, or caries prevalence were observed (Table 1). Studies comparing genotypic diversity and MS levels or caries activity have already shown conflicting results (2, 14). Despite the low number of MS isolates tested per child, 29% of those 24 children from whom two to five isolates of S. mutans were genotyped showed more than one amplitype (Table 1). This indicates a higher genotypic diversity than that observed in Sweden, where only 18% of 3-year-old children carried two distinct genotypes (21). Previous studies suggested that early colonizing MS strains may be stable in the mouth for many years, although some genotypes detected in childhood could not be recovered in later years (1, 6, 22). The frequency of matching genotypes between mother-child pairs decreases as the age of the child increases (15). The frequency of matching genotypes in mother-child pairs also varies between populations, and cultural practices may influence the degree of contact between children and their parents and other individuals. For example, matching MS genotypes were observed in 71% of the mother-child pairs in an American population (15); however, this frequency was lower (45%) among nursery children in China (18). In Swedish families, only 24% of 3-year-old children showed the same genotype as their mothers, none shared their fathers' genotypes, and 44% harbored genotypes that did not match those of any family members (21). In Japan, 31.4% of the genotypes harbored by 0- to 11-year-old children matched genotypes detected in their fathers, while 30.5% of the children studied carried genotypes not found in their parents (13). Genotypic studies with spouses have even indicated horizontal transmission of MS between adults with an established oral microbiota (23). These findings justify the investigation of other sources for MS acquisition in young children. We hypothesized that MS could be laterally transmitted among nursery cohorts with prolonged exposure to an environment that favors the spread of infectious agents (26) and shorter periods of contact with mothers. Such horizontal transmission is suggested from the present study, since two children attending the same nursery carried the same S. mutans genotype (Fig. 2). Both boys carried high levels of MS (≥100 CFU) and were within the age range (19 to 31 months) previously described as the highest-risk period for MS colonization (5). To our knowledge, this is the first report of S. mutans matching genotypes in children from unrelated families that had no contact beyond day care.
Our results support previous findings of genetic diversity of MS in young children and suggest that transmission may occur among nursery cohorts in a population exposed to high MS colonization pressure. Further prospective studies involving a higher number of MS isolates are necessary to explore the frequency of horizontal transmission in nursery environments, the stability of the infecting MS strains, and the potential means of transmission, e.g., the sharing of pacifiers. The investigation of such populations may be important to the understanding of pathways for early acquisition of MS, further directing the development of caries-preventive programs worldwide.
Acknowledgments
This study was supported by FAPESP grant 99/08278-9 and NIDR grant DE-06153.
We thank Kazuo Fukushima, Department of Microbiology, School of Dentistry at Matsudo, Nihon University, Chiba, Japan, for kindly providing the monoclonal antibodies against GTF isozymes.
REFERENCES
- 1.Alaluusua S, Alaluusua S J, Karjalainen J, Saarela M, Holttinen T, Kallio M, Hölttä P, Torkko H, Relander P, Asikainen S. The demonstration by ribotyping of the stability of oral Streptococcus mutans infection over 5 to 7 years in children. Arch Oral Biol. 1994;39:467–471. doi: 10.1016/0003-9969(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 2.Alaluusua S, Mättö J, Grönroos L, Innilä S, Torkko H, Asikainen S, Jousimies-Somer H, Saarela M. Oral colonization by more than one clonal type of mutans streptococcus in children with nursing-bottle dental caries. Arch Oral Biol. 1996;41:167–173. doi: 10.1016/0003-9969(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 3.Alaluusua S, Renkonen O V. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res. 1983;91:453–457. doi: 10.1111/j.1600-0722.1983.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz R J, Jordan H V, White G. The early establishment of Streptococcus mutans in the mouth of infants. Arch Oral Biol. 1975;20:171–174. doi: 10.1016/0003-9969(75)90005-9. [DOI] [PubMed] [Google Scholar]
- 5.Caufield P W, Cutter G R, Dasanayake A P. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 6.Caufield P W, Walker T M. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989;27:274–278. doi: 10.1128/jcm.27.2.274-278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara T, Sasada E, Mima N, Ooshima T. Caries prevalence and salivary mutans streptococci in 0–2-year-old children of Japan. Community Dent Oral Epidemiol. 1991;19:151–154. doi: 10.1111/j.1600-0528.1991.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima K, Tamami O, Ochiai K. Production, characterization, and application of monoclonal antibodies which distinguish three glucosyltransferases from Streptococcus mutans. Infect Immun. 1993;61:323–328. doi: 10.1128/iai.61.1.323-328.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold O G, Jordan H V, van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;18:1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 10.Grindefjord M, Dahllöf G, Winkner S, Höjer B, Modéer T. Prevalence of mutans streptococci in one-year-old children. Oral Microbiol Immunol. 1991;6:280–283. doi: 10.1111/j.1399-302x.1991.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 11.Köhler B, Andréen I. Influence of caries-preventive measures in mothers on cariogenic bacteria and caries experience in their children. Arch Oral Biol. 1994;39:907–911. doi: 10.1016/0003-9969(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 12.Köhler B, Bratthall D. Practical method to facilitate estimation of Streptococcus mutans levels in saliva. J Clin Microbiol. 1979;9:584–588. doi: 10.1128/jcm.9.5.584-588.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozai K, Nakayama R, Tedjosasongko U, Kuwahara S, Suzuki J, Okada M, Nagasaka N. Intrafamilial distribution of mutans streptococci in Japanese families and possibility of father-to-child transmission. Microbiol Immunol. 1999;43:99–106. doi: 10.1111/j.1348-0421.1999.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 14.Kreulen C M, de Soet H J, Hogeveen R, Veerkamp J S J. Streptococcus mutans in children using nursing bottles. J Dent Child. 1997;64:107–111. [PubMed] [Google Scholar]
- 15.Li Y, Caufield P W. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 1995;74:681–685. doi: 10.1177/00220345950740020901. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Caufield P W. Arbitrarily primed polymerase chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Oral Microbiol Immunol. 1998;13:17–22. doi: 10.1111/j.1399-302x.1998.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Navia J M, Caufield P W. Colonization by mutans streptococci in the mouth of 3- and 4-year-old Chinese children with or without enamel hypoplasia. Arch Oral Biol. 1994;39:1057–1062. doi: 10.1016/0003-9969(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Wang W, Caufield P W. The fidelity of mutans streptococci transmission and caries status correlate with breast-feeding experience among Chinese families. Caries Res. 2000;34:123–132. doi: 10.1159/000016579. [DOI] [PubMed] [Google Scholar]
- 19.Mattos-Graner R O, Smith D J, King W F, Mayer M P A. Water-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12- to 30-month-old children. J Dent Res. 2000;79:1371–1377. doi: 10.1177/00220345000790060401. [DOI] [PubMed] [Google Scholar]
- 20.Mattos-Graner R O, Zelante F, Line R C S R, Mayer M P A. Association between caries prevalence and clinical, microbiological and dietary variables in 1.0 to 2.5-year-old Brazilian children. Caries Res. 1998;32:319–323. doi: 10.1159/000016466. [DOI] [PubMed] [Google Scholar]
- 21.Redmo Emanuelson I, Li Y, Bratthall D. Genotyping shows different strains of mutans streptococci between father and child and within parental pairs in Swedish families. Oral Microbiol Immunol. 1998;13:271–277. doi: 10.1111/j.1399-302x.1998.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 22.Redmo Emanuelson I, Thornqvist E. Genotypes of mutans streptococci tend to persist in their host for several years. Caries Res. 2000;34:133–139. doi: 10.1159/000016580. [DOI] [PubMed] [Google Scholar]
- 23.Saarela M, von Troil-Lindén B, Torkko H, Stucki A M, Alaluusua S, Jousimies-Somer H, Asikainen S. Transmission of oral bacterial species between spouses. Oral Microbiol Immunol. 1993;8:349–354. doi: 10.1111/j.1399-302x.1993.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith D J, King W F, Akita H, Taubman M A. Association of salivary immunoglobulin A antibody and initial mutans streptococcal infection. Oral Microbiol Immunol. 1998;13:278–285. doi: 10.1111/j.1399-302x.1998.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Houte J, Gibbs G, Butera C. Oral flora of children with “nursing bottle caries.”. J Dent Res. 1982;61:382–385. doi: 10.1177/00220345820610020201. [DOI] [PubMed] [Google Scholar]
- 26.Yano H, Suetake M, Kuga A, Irinoda K, Okamoto R, Kobayashi T, Inoue M. Pulsed-field gel electrophoresis analysis of nasopharyngeal flora in children attending a day care center. J Clin Microbiol. 1999;38:625–629. doi: 10.1128/jcm.38.2.625-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]