Abstract
Coronavirus disease 2019 (COVID-19) represents an unmet clinical need, due to a high mortality rate, rapid mutation rate in the virus, increased chances of reinfection, lack of effectiveness of repurposed drugs and economic damage. COVID-19 pandemic has created an urgent need for effective molecules. Clinically proven efficacy and safety profiles have made favipiravir (FVP) and remdesivir (RDV) promising therapeutic options for use against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Even though both are prodrug molecules with an antiviral role based on a similar mechanism of action, differences in pharmacological, pharmacokinetic and pharmacotoxicological mechanisms have been identified. The present study aims to provide a comprehensive comparative assessment of FVP and RDV against SARS-CoV-2 infections, by centralizing medical data provided by significant literature and authorized clinical trials, focusing on the importance of a better understanding of the interactions between drug molecules and infectious agents in order to improve the global management of COVID-19 patients and to reduce the risk of antiviral resistance.
Keywords: Favipiravir, Remdesivir, SARS-CoV-2, COVID-19, Antiviral molecules, Antiviral resistance
Graphical Abstract
1. Introduction
The respiratory disease which was discovered in Wuhan, China, at the end of the year 2019, was named COVID-19 by the World Health Organization. Due to the high infection rate and the failure to contain the virus, it rapidly became a pandemic that still affects the whole planet. The new virus was named SARS-CoV-2 since is related to the SARS (Severe Acute Respiratory Syndrome) coronavirus. SARS-CoV-2, belonging to the genus Coronavirus and the family Coronaviridae, share some structural similarities with other viruses in this family: a single-stranded RNA virus with the genome size of approximately 30 kb, they also have similar structural proteins: spike proteins, nucleocapsid proteins, envelope proteins, and membrane proteins [1]. The increased ability of biological evolution has converted SARS-CoV-2 strains into more virulent forms than previous ones. The structural proteins of the virus are mostly conserved across coronaviruses and have a 90% similarity [2], [3]. A small alteration in sequence, on the other hand, has a significant impact on the viral configuration and pathophysiology. Moreover, a minor alteration in the genomic sequence could cause a major shift in the arrangement of target proteins, rendering existing treatments ineffective. However, the mutations that may occur have to be evaluated in relation to the efficacy profiles of the anti-COVID-19 therapies and the effectiveness of vaccines as a method of prevention [4]. Updated scientific information suggests that a very effective method to stop the virus from spreading is to diagnose and isolate susceptible individuals as soon as possible [5].
The pathophysiological mechanisms for COVID-19 are not fully elucidated, but lifestyle conditions and environmental features can be considered risk factors. Furthermore, the exposure to different metals may result in biological dysfunctions, affecting different organs and contributing to the pathogenesis of several diseases [6], [7].
After the discovery of the new virus, the race to discover an effective treatment for COVID-19 had begun [8]. In-vitro studies for drug repurposing were conducted. However, this method was unable to accurately predict the physiological and pathological outcomes in humans [9]. For example, chloroquine and hydroxychloroquine showed a lot of promise in in-vitro studies, but the in-vivo studies did not show a significant effect on the disease because hydroxychloroquine interferes with only one of the two viral entry pathways (it affects only endosomal pathways but not fusional ones) [10], [11]. The increased rate of SARS-CoV-2 infections recurrence worldwide emphasizes the critical need for improved treatment strategies. Experimental studies conducted in India have detected multiple circulating mutant strains, which are more virulent than the initial ones [12].
In order to improve the overall management of COVID-19 pandemic, an overview of the therapeutic options evaluated in the scientific literature is necessary. Several drugs may be potential options in the treatment of SARS-CoV-2 infections and are currently under evaluation in clinical trials: colchicine (anti-inflammatory), galidesivir (antiviral), azithromycin (antibiotic), mefloquine (antimalarial), ivermectine (antiparasitic), clevudine (antiretroviral), tocilizumab, fedratinib (monoclonal antibodies), Rheum officinale (traditional herbal medicine) etc. Furthermore, following the assessment provided by the updated medical literature, favipiravir (FVP) and remdesivir (RDV) represent possible therapeutic options in COVID-19 patients, thus becoming essential a correct and complete characterization of the two antiviral molecules [13].
Clinical studies should find that this drug classes will have the best impact on the treatment of COVID-19: drugs that inhibit viral entry in the cell, like inhibitors of S protein, transmembrane serine protease 2 (TMPRSS2) inhibitors, endosomal entry inhibitors, heparan sulfate proteoglycans inhibitors. Other classes that could be useful in COVID-19 might be the inhibitors of viral proteases (i.e., RNA-dependent RNA-polymerase (RdRp): RDV, FVP, molnupiravir, galidesivir, ribavirin, sofosbuvir) [4]; the host proteins that help viral replication; the host importin α/β; or even agents that could support the host's natural immunity (interferons) [14], [15].
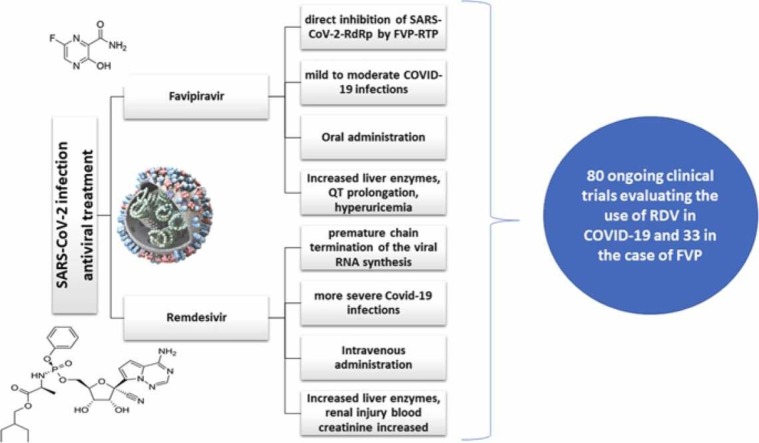
One of the medications that could be used to combat COVID-19 is FVP. The drug is a pyrazine with an aminocarbonyl group in position 2 hydroxy and fluoro- functional groups in positions 3 and 6. It is an antiviral drug that acts by inhibiting the RdRp of the virus, which is essential in the replication of the virus [16].
FVP was discovered by Toyama Chemical Company with the primary goal of treating influenza (it was approved for this use in 2014 in Japan). It also showed antiviral activity against other RNA viruses like SARS-CoV-2 [17], [18], [19].
RDV is an antiviral prodrug that acts as a nucleotide analog (for adenosine triphosphate), leading to the inhibition of RdRp and showed a lot of potential in the treatment of COVID-19 [20], [21].
The present paper aims to provide a comprehensive review of the safety and efficacy profiles of FVP and RDV, centralizing updated scientific information regarding their monographs (pharmacokinetic data, pharmacological mechanisms, management of adverse events and interactions, antiviral resistance, comparative assessment). Complex mechanisms of action are evaluated in detail to highlight major therapeutic targets that may help in reducing the damage caused by the COVID-19 pandemic. This narrative and critical review provides an overview of the therapeutic options for COVID-19 patients, keeping clinicians and researchers up to date on the results and the major role of FVP and RDV studies in a pandemic context. Furthermore, it provides a comprehensive characterization of FVP versus RDV that can contribute to a better understanding of their use and to the optimization regarding the clinical management of COVID-19.
2. Methodological approaches
This study centralizes and filters medical publications on FVP, RDV and various pharmaceutical processes between 2004 and 2022 provided by a comprehensive literature search related to chemical and physical properties, pharmacokinetics, drug interactions, pharmacology, toxicity, drug regulatory approval, clinical trials for the management of COVID-19 pandemic. Furthermore, by assessing these parameters, it is possible to outline the safety and efficacy profiles of these two antiviral molecules. Scientific literature research was conducted by searching some of the most valuable databases (i.e., MDPI, Google Scholar, Medline, ScienceDirect, Embase, Web of Science, Scopus). MeSH (Medical Subject Headings) controlled vocabulary was used for searching in PubMed and Emtree (Embase subject headings) controlled vocabulary for searching in Embase in order to obtain the most associated synonyms for the entered terms (i.e., “favipiravir”, “remdesivir”, “mechanism of action of favipiravir against SARS-CoV-2”, “mechanism of action of remdesivir against SARS-CoV-2”, “pharmacokinetic properties of favipiravir”, “pharmacokinetic properties of remdesivir”, “safety and tolerability of favipiravir”, “safety and tolerability of remdesivir”, “therapeutic management of COVID-19 infection”, “physical and chemical properties of favipiravir”, “physical and chemical properties of remdesivir”, “favipiravir drug information”, “remdesivir drug information”, “favipiravir dosage and administration”, “interactions for favipiravir”, “interactions for remdesivir”, “clinical trials to evaluate the safety and efficacy of favipiravir in the treatment of COVID-19”, “clinical trials to evaluate the safety and efficacy of remdesivir in the treatment of COVID-19 “favipiravir regulatory approval”, “remdesivir regulatory approval”, “comparative analysis of favipiravir and remdesivir”, “emerging perspectives and future trends of COVID-19 pandemic”). A total of 148 bibliographic references were cited in order to support and validate the information in this paper.
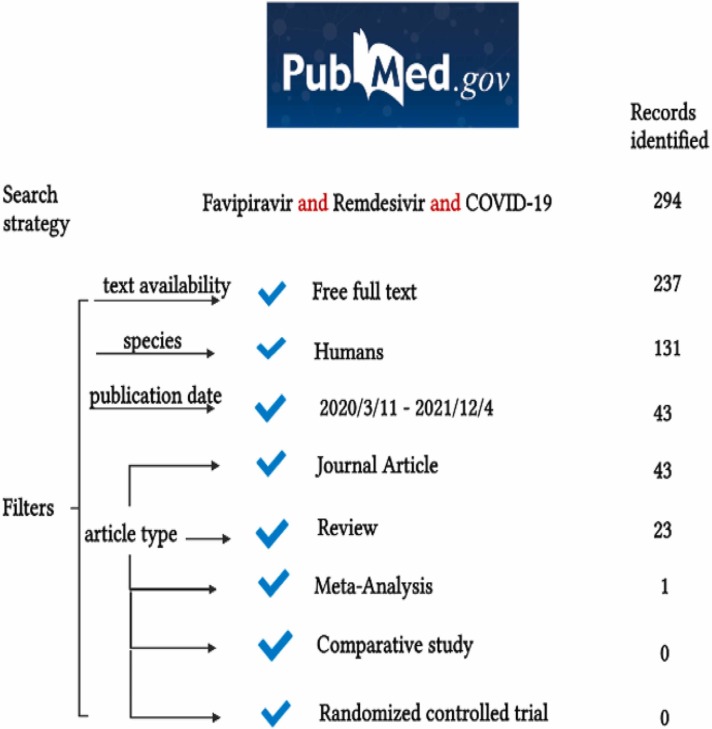
The novelty of this medical issue associated with little information from meta-analysis and comparative studies between FVP and RDV is demonstrated by a literature search algorithm. It has been performed a systematic search in one of the most important medical databases, PubMed (from March 11, 2020, to December 4, 2021). The search strategy included three terms: “Favipiravir”, “Remdesivir” and “COVID-19”, in order to assess types of publications that provide information about FVP and RDV in the context of COVID-19 pandemic. There were no restrictions concerning age, ethnicity, gender. However, only free full texts and English publications were included. The process of study search is depicted in Fig. 1 [22].
Fig. 1.
Details of the search results using AND Boolean operator.
The results presented above underline the importance of FVP and RDV studies in the context of COVID-19 pandemic, being a topic of major interest, but also the need for further research.
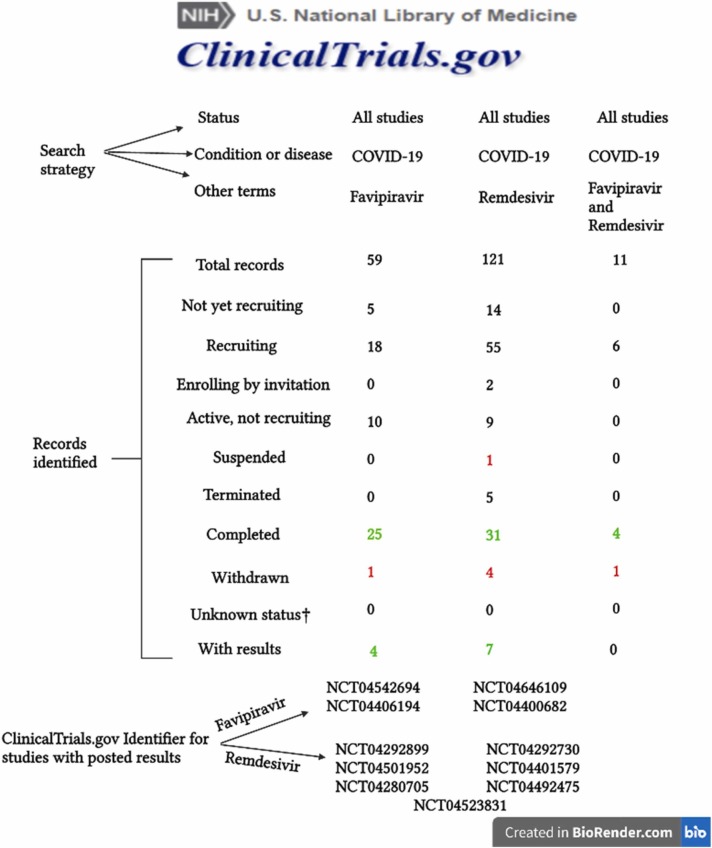
It has also been performed a search in ClinicalTrials.gov database in order to assess privately and publicly funded clinical trials from all over the world. The search algorithm implied the inclusion of all types of clinical studies and the same condition or disease (COVID-19). Three searches were performed, each time a condition was changed in the field “other terms” (Favipiravir, Remdesivir, Favipiravir and Remdesivir).
The results provided by the database showed that most of the clinical trials that were conducted were focused on RDV (121). However, the efficacy and the safety profile of FVP (59) in the context of the COVID-19 pandemic are also being studied, and even more, some clinical trials use both FVP and RDV (11) as interventional treatments. The studies that were conducted are in different statuses, some of them being suspended or withdrawn. Clinical trial with the identifier NCT04252664 named “A Trial of Remdesivir in Adults with Mild and Moderate COVID-19” is the only that has been suspended due to the fact that the epidemic of COVID-19 was well controlled at the time. In the category of withdrawn clinical trials 4 studies were included (NCT04675086 due to administrative decision, NCT04519424 due to business reasons, not safety issues, NCT04386447 due to the disapproval of the Italian Medicines agency, NCT04361461 due to a decision by the Sponsor before enrollment and was a result in all three search strategies that have been performed). Even though there are more completed clinical trials, only some of them have the results posted in the database until now. The analysis of the results may contribute to the improvement of the therapeutic management of SARS-CoV-2 infection. Synthesis of clinical trials searches that addressed the subject of COVID-19 pandemic with the possibility of using FVP and RDV as therapeutic tools are depicted in Fig. 2 [23], [24], [25].
Fig. 2.
Details of the search using ClinicalTrials.gov database.
3. FVP and RDV therapeutic approaches for SARS-CoV-2 infection
SARS-CoV-2 enters the cell by binding to the angiotensin-converting enzyme 2 (ACE2) of the host cell via spike proteins [26]. A big concentration of ACE2 receptors can be found in the epithelial cells located in the lungs or in the enterocytes located in the intestinal epithelium, being two possible ways to enter the organism [27], [28].
Due to the lack of an etiological treatment, the therapeutic approaches are focused on three possible directions: regulation of the inflammatory response (Tocilizumab, Sarilumab [29], Dexamethasone, Hydrocortisone, convalescent plasma or hyperimmune immunoglobulins [30]), oxygen supply in the situation of a low oxygen saturation in the blood as symptomatic treatment [31] and antiviral drugs with a good efficacy profile, especially in the early phase of infection when the inflammatory process has not emerged [32], [33], [34], [35].
In order to target specific proteins involved in the mechanism of viral replication, therapeutic options for managing COVID-19 disease require the use of appropriate inhibitors [36]. The genetic processes involving the transfer of viral genetic information from DNA to RNA to proteins (transcription) and from DNA to DNA (replication) are mediated through complexes of non-structural proteins (nsp1-nsp16), the most important for the management of COVID-19 being nsp12 (RdRp), nsp13 (Pol/RdRp), and cofactors (nsp7 and nsp8), which are effective for increasing the binding processes [37], [38], [39]. RdRp is thus a key biomolecular target for the suppression of viral replication and can be inhibited by the action of FVP and RDV [40].
With the onset of the COVID-19 pandemic, the pharmacological research activity in order to establish an optimal treatment has increased considerably worldwide, the proof being the numerous scientific publications that have contributed to the improvement of pharmacological management and have constantly updated the existing medical information [41], [42], [43], but also the clinical trials provided by ClinicalTrials.gov that were comprehensively reviewed.
3.1. FVP pharmacological properties and antiviral role in SARS-CoV-2 infection
Continuous advancements in drug design procedures and techniques have resulted in significant progress in pharmacological approaches to finding viable treatment options for COVID-19 disease. Moreover, the number of clinical studies targeting antiviral therapy has increased. The focus of these studies is the assessment of efficacy and safety profiles for antiviral agents, especially FVP and RDV [44].
FVP is a pyrazine carboxamide derivative (6-fluoro-3-hydroxy-2-pyrazine carboxamide) and a broad-spectrum antiviral drug, initially approved in Japan (2014) for the treatment of resistant infections with influenza viruses like A(H1N1)pdm09, A(H5N1), and A(H7N9) avian virus [16], [45], [46]. It has been included by the World Health Organization on a short list of drugs to be tested in clinical trials in order to assess their effectiveness on the Ebola virus (2014) [47]. The results of in vitro studies have been promising, but in vivo extrapolation led to statistically insignificant improvements [48], [49], [50]. In addition to these medical evidences, FVP has demonstrated therapeutic efficacy in animal models and cell cultures against arenaviruses, phleboviruses, bunyavirus, flaviviruses, Western equine encephalitis virus, Lassa virus [47]. Furthermore, due to existing medical evidences that suggested an effective antiviral profile, FVP was included in the category of repurposed drugs for COVID-19 [51] along with chloroquine, hydroxychloroquine [52], silvestrol, RDV, saracatinib, ribavirin, azithromycin, ritonavir, lopinavir, dexamethasone, chlorpromazine, bafilomycin A [53]. Drug repurposing approaches include molecular docking, transcriptional signatures, machine learning, similarity analysis, data mining, network analysis [54].
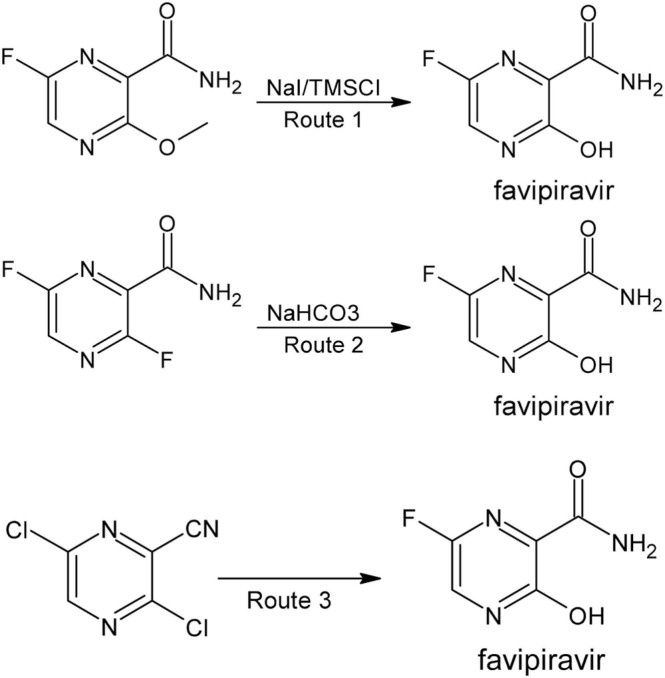
FVP (T-705) is a prodrug obtained by chemical synthesis and was first discovered by searching in the chemical library of Toyama chemicals. Three routes of chemical synthesis are possible in order to obtain FVP. The original synthesis involved the use of methyl 3-amino-6-bromopyrazine-2-carboxylate as first reactant and consisted of five steps. The second route for the synthesis of FVP involved the use of 3-hydroxypyrazine-2-carboxamide as first reactant and consisted of five reactions, while the third route used 3-aminopyrazine-2-carboxylic acid as starting reactant and consisted of six reactions. Fig. 3 shows the last step of each synthesis pathway [55].
Fig. 3.
Final reactions of the three favipiravir (FVP) synthesis pathways. NaI, sodium iodide; TMSCl, trimethylsilyl chloride; NaHCO3, sodium hydrogen carbonate; H2O, water.
The scientific explanation for testing the effectiveness of FVP in case of SARS-CoV-2 infection is based on its molecular mechanism of action starting from evidence-based medicine. SARS-CoV-2-RdRp complex is considerably more active than any other viral RdRp that has been studied and FVP is an inhibitor of this complex [56].
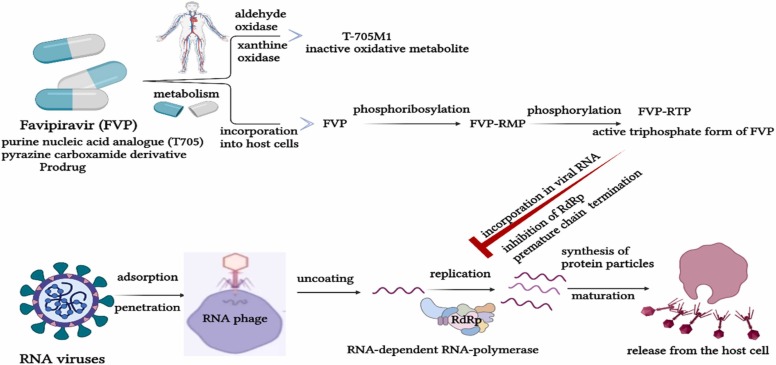
FVP is an antiviral drug, structurally equivalent to guanosine, which is biochemically transformed into FVP ribose monophosphate (FVP-RMP), and after phosphorylation into FVP ribose triphosphate (FVP-RTP) by the enzyme complexes that are present in the infected cells. Furthermore, another metabolic pathway involves the effects of aldehyde oxidase and xanthine oxidase but leads to an inactive oxidative metabolite (T-705M1) [47].
FVP-RTP binds to SARS-CoV-2-RdRp complex and integrates into the viral RNA chain, causing possible mutagenic effects in the viral genome, due to several transitions of purine and pyrimidine bases. Three molecular mechanisms for the antiviral action of FVP have been described: direct inhibition of SARS-CoV-2-RdRp by FVP-RTP, premature chain termination of the viral RNA synthesis and incorporation of FVP-RTP into viral molecules leading to genetic mutations [57]. The first mechanism is possible due to cellular processes of recognition and binding of FVP-RTP by SARS-CoV-2-RdRp that implies a suppression of the functionality of the enzyme complex. Due to the absence of RdRp in humans, FVP-RTP effector becomes highly selective on viral synthesis, without damaging other cellular structures [18]. The premature chain termination of the viral RNA synthesis is based on the inability of FVP to supply completely complementary base pairing with pyrimidine bases, due to partial analogy with purine bases. Moreover, due to this incomplete complementarity, RdRp is disrupted on the RNA template, resulting in premature cessation of RNA synthesis and the production of short RNA fragments. Viral replication is inhibited by the production of prematurely terminated fragments that will interfere with non-defective molecules [57]. The third mechanism implies the binding of FVP-RTP to RNA molecules, causing alterations in genomic structures involved in the synthesis of virions. Furthermore, mutant virions are unable to maintain the functionality of viral replication. Mutational processes are induced by ongoing transitions in the viral genome (e.g., guanine-adenine and cytosine-uracil) [47]. The main targets for FVP antiviral activity are depicted in Fig. 4.
Fig. 4.
Mechanism of action of FVP. FVP, favipiravir; FVP-RMP, favipiravir ribose monophosphate; FVP-RTP, favipiravir ribose triphosphate; RdRp, RNA-dependent RNA-polymerase.
In addition to the certain therapeutic benefits of FVP, the enhanced production of mutant virions due to its mutagenic action imply a risk of creating new hazardous viral strains with high pathogenicity for mammals, along with the development of mechanisms of resistance to antiviral compounds [58].
Medical studies have suggested three important measures for managing the negative effects of mutagenesis and to reduce the evolution of new virus strains:
-
▪
The first alternative involves structural changes in FVP in order to enhance the affinity of the nucleoside compound for RdRp and to create an irreversible complex, simultaneously with the loss of ability to incorporate into a nascent strand [21].
-
▪
The second choice implies a therapeutic management based on the use of combinations of antiviral compounds with different molecular mechanisms of action and viral targets. Medical evidence indicate that monotherapy is more likely to produce mutant variants of viral strains with increased pathogenicity, resistance and a lower efficacy profile than polytherapy [16].
-
▪
The third possibility supports dose management based on the principle of the dose-dependency effect. The extrapolation of the results that have been obtained on infected cell cultures in humans is based on pharmacokinetic parameters that allow estimates of the therapeutic concentrations, with a low risk of viral mutations [58].
Further research is needed to investigate the mutagenic effect of FVP on virus microevolution.
3.2. RDV pharmacological properties and antiviral role in SARS-CoV-2 infection
RDV was the first U.S. Food and Drug Administration (FDA) approved drug for the treatment of SARS-CoV-2 infection, and it is still the only one with intravenous administration. Molnupiravir and a combination between nirmatrelvir and ritonavir are the latest oral therapeutic options authorized by FDA to be used in COVID-19 patients [59].
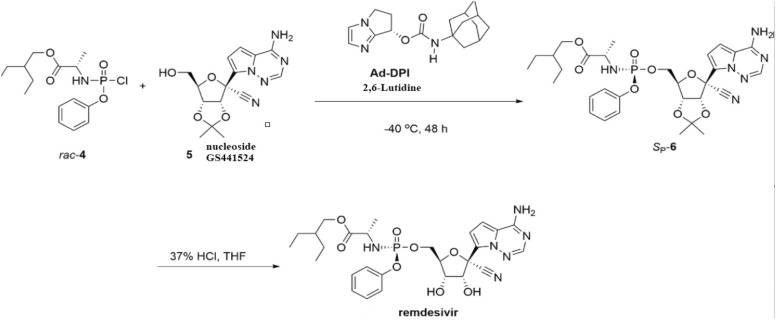
RDV is a broad-spectrum antiviral adenosine nucleotide analog, whose pharmacological activity was initially tested against Ebola virus, but later the indications were extended to several viruses (e.g., syncytial virus, SARS-CoV, Middle East respiratory syndrome coronavirus), including SARS-CoV-2. It is a SARS-CoV-2-RdRp inhibitor with antiviral activity tested on cell cultures and animal disease models [60]. Several routes of chemical synthesis of RDV are available and the catalytic asymmetric synthesis via chiral bicyclic imidazole-catalyzed phosphorylation leads to low resource waste and to high synthetic efficiency, the last steps being depicted in Fig. 5 [61], [62], [63].
Fig. 5.
Synthesis of RDV, third protocol. Rac-4, 2-ethylbutyl (chloro(phenoxy)phosphoryl)-L-alaninate; Ad-DPI, (S)-6,7-Dihydro-5H-pyrrolo[1,2-a]imidazol-7-yladamantan-1-ylcarbamate; Sp-6, protected RDV; HCl, hydrochloric acid. THF, tetrahydrofuran.
Synthesis protocols involve three main steps: synthesis of 2-ethylbutyl (chloro(phenoxy)phosphoryl)-L-alaninate rac-4, synthesis of chiral bicyclic imidazole Ad-DPI and synthesis of RDV by deprotection [62], [63].
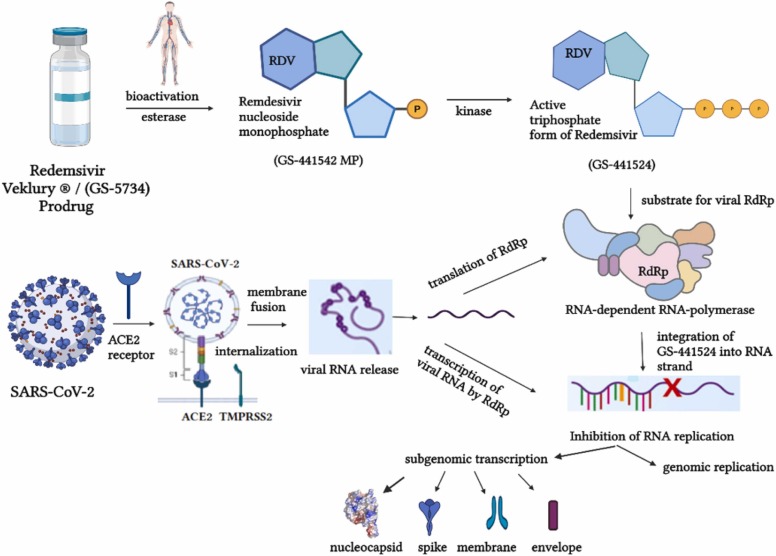
RDV (GS-5734) is a prodrug that requires bioactivation through esterase, when an intermediate compound is formed (nucleoside monophosphate GS-441542 MP), and kinases, when the active triphosphate form (GS-441542) is obtained. It is a structural analog of adenosine triphosphate (ATP) and is thus, a possible substrate for SARS-CoV-2-RdRp complex. A competitive mechanism is provided between GS-441524 and ATP for integration into the newly generated RNA strand, leading to a premature chain termination of the viral RNA synthesis [64]. Moreover, it has been shown that GS-441524 avoids being proofread by the viral exoribonuclease [65], [66]. The mechanism of action of RDV is depicted in Fig. 6.
Fig. 6.
Mechanism of action of RDV. RDV, remdesivir; ACE2, angiotensin converting enzyme 2; TMPRSS2, transmembrane protease serine type 2.
Several structural studies conducted recently showed the addition of GS-441524 to the RNA product 3′-end. Moreover, RDV monophosphate (RMP) was found in the +1 site in one compound, while in another structure RMP was found in the −1 site. Because of the cell’s inability to differentiate RMP from adenosine monophosphate, base pairs with uridine monophosphate are generated based on the Watson-Crick principle in the viral strand [60], [67], [68]. Few studies have reported significant differences between FVP and RDV concerning their mechanism of action. FVP suppresses viral replication primarily through generating mutations in the genome, while RDV inhibits viral elongation. RDV has higher molecular complexity than FVP, providing an increased destabilization of the viral replication complex. The variations concerning their mechanisms of action and subsequent effects are based on the interaction between two different heterocyclic structures (FVP and RDV) and RdRp complex [69].
The efficacy of FVP and RDV has been also tested in cell culture models infected with HCoV-NL63. The results were promising and proved that both FVP and RDV are effective in blocking the viral replication and viral biosynthesis. Moreover, RDV showed a higher inhibitory potency than FVP in HCoV-NL63 cell culture and a long-term RDV exposure has not resulted in the development of resistance mechanisms. The combination of FVP or RDV with interferon-alpha as an antiviral biomolecule, led to the observation of synergistic effects. These experimental investigations demonstrated the potential use of FVP and RDV as repurposing drugs in different disease conditions [70].
4. Pharmacokinetic properties of FVP and RDV in patients with Covid-19
In order to maximize the pharmacodynamic response, pharmacokinetic data is essential. There are four main parameters studied by this field: absorption, distribution, metabolism, and excretion (ADME). A better apprehension of these processes for each drug can contribute to better safety and efficacy profiles, with the possibility of adjusting in a context of personalized medicine. Moreover, it is essential to make correlations between equations and the clinical significance. It is outlined the flow of drugs through the body and how the body reacts to them [71].
A clinician can readily determine safe drug doses over time and the clearance rate. However, these statistical estimates depend on the dosage form, route of administration, patients’ status etc.
Absorption is the process by which a drug is transported from the administration site to the systemic circulation. It influences the speed and concentration at which a drug may reach a target location of a specific effect [71], [72].
The fraction of an initially delivered medicine that reaches system circulation is known as bioavailability and it can be an indicator of medicine absorption. When a drug is administered intravenously, the bioavailability is 100% and this administration route is the gold standard. The oral administration of drugs provides a lower bioavailability due to the processing of the drug by digestive enzymes, liver, gut [71].
The distribution of a drug throughout the body depends on the drug’s biochemical features, along with the patient’s physiology. In order to be effective, a drug must reach the desired location, which is influenced by the volume of distribution (Vd) and protein binding [72].
Metabolism is a biochemical process that occurs to convert the drug into more water-soluble compounds in order to facilitate the excretion. Furthermore, when administering a prodrug, metabolic pathways are necessary for the conversion of inactive compounds into active metabolites [71].
Excretion is the process of eliminating metabolic waste, mainly through kidneys, but can be also involved the lungs, gastrointestinal system, skin etc. The process of excretion can be examined by assessing the clearance rate and the half-time of a drug [71], [72].
Numerous reviews are present in the medical literature and have evaluated the pharmacokinetic profile of different molecules considered as therapeutic options for COVID-19 patients, including FVP and RDV [17], [73].
FVP is delivered in the organism as a prodrug. The pharmacokinetic profile makes possible the oral administration of the antiviral molecule (e.g., 94% bioavailability, 54% protein binding and 10–20 L the volume of distribution). Cmax is reached after 2 h and after a single dose. Moreover, after multiple administration, both Tmax and t½ (half-life) increase. The hydroxylated metabolite of FVP has a short t½ (2.5–5 h), resulting in fast renal clearance. Two enzymes are involved in the process of clearance, aldehyde oxidase and, to a lesser extent, xanthine oxidase. The pharmacokinetic profile of FVP is both time and dosage dependent. The cytochrome P450 family does not contribute to the metabolism of FVP, but the antiviral molecule blocks CYP2C8 [47].
The bioavailability of RDV has been assessed in animal models and low values were obtained when administered orally due to almost a 100% first-pass clearance correlated with poor hepatic stability. The results obtained in clinical trials showed that the absolute bioavailability of RDV formulations is 100% when it is administered intravenously in healthy male volunteers. Moreover, peak concentrations are reached at the end of administration [74].
In the ongoing pandemic of COVID-19, it is essential to conduct pharmacokinetic tests in order to be able to successfully manage the use of FVP and RDV.
4.1. Population pharmacokinetics of FVP in patients with SARS-CoV-2 infection
Several studies have reported pharmacokinetic data of FVP in patients with COVID-19. However, the pharmacokinetic profile of FVP is not yet fully understood.
An interventional study assessing pharmacokinetic data on healthy participants showed that the maximal plasma concentration of FVP occurred 2 h after oral administration with a rapid decline provided by a short half-time (2–5.5 h). Moreover, the plasma protein binding was 54%, mainly binding to serum albumin. The action of aldehyde oxidase and xanthine oxidase lead to an inactive metabolite (T-705M1), quickly eliminated by the kidneys [75], [76].
The results of the clinical trials showed ethnic and regional differences in pharmacokinetics since Japanese patients had a double plasma concentration of FVP than the patients in the United States [17].
A recent experimental study involving 39 patients conducted pharmacokinetic analysis on 204 serum concentrations. A total of 33 patients received 1600 mg FVP twice daily on the first day, followed by a dose of 600 mg twice daily, while 6 patients started with a dose of 1800 mg FVP twice daily, followed by 600 mg twice daily. A single-compartment model with first-order elimination has been proposed. It has been assessed the clearance/bioavailability ratio and the volume of distribution/bioavailability and the estimated means were 5.11 L/h and 41.6 L. Statistical analysis pointed out the existence of correlations between clearance/bioavailability ratio and dosage, invasive mechanical ventilation (10 patients) and body surface area [77].
A good management of SARS-CoV-2 infection requires high doses of FVP, but the dose-dependent nonlinear pharmacokinetics can cause shifts and toxicity. However, the identification of the optimum dose regimen requires further research.
4.2. Population pharmacokinetics of RDV in patients with SARS-CoV-2 infection
Due to the fact that RDV received FDA’s emergency use authorization for treatment of SARS-CoV-2 severe infections, studies on this molecule have been accelerated and diversified, including various pharmacokinetic tests [78], [79].
A randomized, blinded, placebo-controlled, phase I study assessed pharmacokinetic data from a single RDV dose administered intravenously. They were included in this study 9 cohorts of healthy volunteers (96 subjects) with no evidence of viral infections. Doses were selected according to FDA recommendations. Cohorts 1–6 received RDV (3 mg to 225 mg) administered as a 2-h infusion. The volunteers in cohorts 7 and 8 received a single dose of lyophilized RDV (75 mg cohort 7 and 150 mg cohort 8) administered intravenously for 2 h. Cohort 9 received 75 mg RDV administered intravenously for 30 min. The values of the pharmacokinetic parameters of RDV obtained from bioanalytical procedures (liquid chromatography tandem mass spectrometry) using noncompartmental methods are presented in Table 1 [80].
Table 1.
Pharmacokinetic parameters (PK) of RDV (single-dose study).
| PK | Cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Dose | 3 | 10 | 30 | 75 | 150 | 225 | 75 | 150 | 75 |
| Cmax | 57.5 | 221 | 694 | 1630 | 2280 | 4420 | 1720 | 2720 | 2930 |
| Tmax | 2.03 | 2.01 | 2.02 | 2.03 | 2.00 | 1.97 | 2.00 | 1.99 | 0.50 |
| t½ | – | 0.66 | 0.81 | 0.90 | 0.99 | 1.05 | 0.84 | 1.11 | 1.00 |
| CL | – | 755 | 700 | 661 | 863 | 719 | – | – | – |
| CLr | – | – | 48.6 | 52.1 | 78.1 | 71.4 | – | – | – |
| Vd | – | 45.1 | 48.8 | 56.3 | 73.4 | 66.5 | – | – | – |
| AUCinf | – | 230 | 774 | 2000 | 2980 | 5270 | 1840 | 3260 | 1250 |
| AUClast | 67.1 | 230 | 768 | 1990 | 2970 | 5260 | 1830 | 3270 | 1250 |
Dose administered (mg); Cmax (ng/mL), peak plasma concentration; Tmax (hours), time to peak concentration; t½ (hours), half-life; CL (mL/min), clearance; CLr (mL/min), renal clearance; Vd (L), volume of distribution; AUCinf (h*ng/mL), area under the curve vs. time extrapolated to infinity; AUClast (h*ng/mL) area under the curve from time zero to the last quantifiable concentration.
RDV exposure increased dosage proportionally over the tested range following a single dose administered intravenously.
A recent study evaluated in two severely ill COVID-19 patients, the pharmacokinetic profile of RDV in cerebrospinal fluid, plasma and bronchoalveolar aspirate. It has been administered a starting dose of 200 mg RDV in the first day, followed by 12 days of 100 mg. Cerebrospinal fluid was collected in day 7 from one patient, blood samples were collected from day 3 until day 9 and bronchoalveolar aspirate samples were collected in day 4, 7 and 9 from both patients. The analyses were performed by using a validated ultra-high performance liquid chromatography tandem mass spectrometry. The concentrations of GS-441524 in the bronchoalveolar aspirate on both patients were: (on day 4, 8.6 ng/mL vs. 9.2 ng/mL, on day 7, 2.7 ng/mL vs. 3.3 ng/mL and on day 9, 3.0 ng/mL vs. 6.1 ng/mL). Furthermore, cerebrospinal fluid concentrations in patient 2 was 22.1 ng/mL (CSF/plasma ratio is 25.7%), while the BAS/plasma ratio was 2.3% vs. 6.4% [81].
The GS-441524 metabolite was found in bronchoalveolar aspirate samples in higher concentrations than in cerebrospinal fluid samples. RDV as a parent compound was undetectable in all compartments tested. Moreover, the metabolite GS-441524 has a lower protein binding than RDV [80], [81].
A comprehensive assessment of drug’s physical, chemical, and biological properties related to pharmacokinetic processes can contribute to a better prediction of the risk of increased or decreased plasma exposure and to an improved management of possible interactions that may occur. Even though the preliminary results of the research have improved the understanding of pharmacokinetic profiles and the use of FVP and RDV for the treatment of COVID-19 patients, further studies are needed in order to confirm these outcomes [73].
5. Drug-drug interactions of FVP and RD
The initiation of a treatment for SARS-CoV-2 infection in a patient who is currently on chronic treatment should be rigorously assessed in terms of drug-drug interactions in order to minimize the risks. The presence of unstable conditions and comorbidities requires polymedication, which is widespread in geriatric and intensive care unit patients. As a result, these patients are more vulnerable to drug interactions [82].
Drug-drug interactions occur due to pharmacodynamic properties (e.g., hypoglycemic, QT prolongation) or pharmacokinetic properties (e.g., enzymatic induction or inhibition, competition in renal excretion) [83].
Acute and chronic inflammatory conditions may alter ADME and transportation processes and may influence the pharmacodynamic and pharmacokinetic aspects of therapeutic drugs used in COVID-19 treatment [84].
5.1. Drug interactions of FVP
FVP is a prodrug that requires bioactivation in order to act as a false substrate for viral RdRp. Due to the fact that it is not yet authorized by FDA and European Medicines Agency (EMA) for use in COVID-19 patients, available data from drugs interaction checkers (e.g, DrugBank, Drugs.com, WebMD, Medicine.com) is limited.
FVP is not a substrate for cytochrome P450, but it is involved in metabolic processes, mediated primarily by aldehyde oxidase. More caution is necessary when FVP is co-administered with potent aldehyde oxidase inhibitors like raloxifene, tamoxifen, ethinyl estradiol, estradiol, phenothiazines, dihydropyridine calcium blockers, tricyclic antidepressants, loratadine [85]. Since aldehyde oxidase presents genetic variations, it may be more prevalent among Asian populations. It is excreted by the kidneys in large proportions as metabolites [86], [87].
It has been shown that FVP is a mild inhibitor of various metabolic pathways and transportation processes, but its impact on CYP2C8 is the only one that potentially have therapeutic implications for substrates with increased exposure [17]. Careful monitoring is required when FVP is co-administered with CYP2C8 substrate drugs like paclitaxel, montelukast, pioglitazone, repaglinide, cerivastatin, enzalutamide, rosiglitazone, imatinib, loperamide [86].
The risk of pharmacodynamic interactions concerning the risk of QT prolongation is low. However, when combined with other OT-prolonging medications, rigorous monitoring is required. The most potent QT-prolonging drug are amiodarone, ondansetron, haloperidol, and metronidazole [88].
The interaction between FVP and anticancer drugs may exacerbate the hepatotoxic processes. Furthermore, the association of FVP with paracetamol requires a maximum daily dose of 3 g paracetamol, due to a potential risk of hepatoxicity. However, studies have pointed out that the degree of interaction between FVP and paracetamol is weak, with an increase in AUC of up to 15% [86].
Further studies are needed for a better management of clinically significant drug-drug interactions between FVP as a potential COVID-19 therapeutic option and drugs that are utilized in patients suffering from other diseases.
5.2. Drug interactions of RDV
RDV is a prodrug authorized by FDA and EMA for the treatment of SARS-CoV-2 infection under specific conditions. Medical data obtained from studies simulating drug-drug interaction behavior of RDV in patients affected by COVID-19 are more numerous than with FVP.
Towards FVP, RDV is a substrate for several cytochrome P450 enzymes (e.g., CYP3A4, CYP2D6, CYP2C8), P-glycoprotein and for organic anion transporting polypeptide (OATP1B1) [73]. Furthermore, RDV is a mild inhibitor of OATP1B1, OATP1B3, multidrug resistance-associated protein 4 (MRP4), bile salt export pump (BSEP), sodium taurocholate co-transporting polypeptide (NTCP). However, RDV metabolism is predominantly conducted via hydrolases rather than enzymes of the cytochrome P450 [89].
The drug interactions of RDV were searched on the interactions checkers provided by Liverpool Drug Interaction Group (LDIG) and Drugs.com, including sources from IBM Watson Micromedex [90], [91]. The drugs listed for the study of RDV interactions are molecules frequently prescribed and are related to various effects on the cytochrome P450 system (e.g., inducers, inhibitors, substrates), and the results are presented in Table 2.
Table 2.
RDV drug interactions from LDIG and Drugs.com interactions checkers.
| Interacting drug | Co-medication | Type of interaction |
Consequence | |
|---|---|---|---|---|
| LDIG | Drugs.com | |||
| RDV | Chloroquine | Do not co-administer | Major | Avoid combinations |
| Hydroxychloroquine | Do not co-administer | Major | ||
| Rifampicin | Potential weak interaction | Moderate | Usually avoid combinations | |
| Voriconazole | No interaction expected | |||
| Carbamazepine | Potential weak interaction | |||
| Itraconazole | No interaction expected | |||
| Phenobarbital | Potential weak interaction | |||
| Amiodarone | No interaction expected | |||
| Phenytoin | Potential weak interaction | |||
| Montelukast | No interaction expected | – | No interactions reported | |
| Clopidogrel | ||||
| Omeprazole | ||||
| Paroxetine | ||||
The interactions checker provided by Drugs.com included in list of RDV interactions, 2 major drug interactions, 358 moderate drug interactions, 1 moderate interaction with ethanol due to an increased risk of hepatotoxicity and 1 major interaction with renal dysfunction due to the lack of information concerning this condition and due to the presence of betadex sulfobutyl ether sodium as excipient, which has a high rate of renal excretion and can accumulate at patients with renal dysfunctions.
Major interactions should be totally avoided because the potential harm is higher than the potential benefits. There are two major interactions possible when RDV is co-administered with hydroxychloroquine or chloroquine. In vitro studies have reported a dose-dependent antagonistic effect of hydroxychloroquine or chloroquine on the antiviral activity of RDV, including the alteration of intracellular metabolic activation [92]. Moderate interactions may be clinically significant; therefore, it is likely to require additional monitoring, compared to minor interactions where it is unlikely to be required dosage adjustment or additional action [90].
The role of each isoenzyme of cytochrome P450 or transportation proteins in the overall metabolism of RDV and metabolites is not yet fully understood.
The period of co-administration, the doses utilized and the existence of organ failure and other polymedication features can influence the degree of interaction correlated with the clinical significance.
In order to optimize the treatment for COVID-19, in vitro studies should be correlated with more clinical studies assessing pharmacokinetics, safety and efficacy profiles of FVP and RDV.
6. Clinical features of FVP and RDV
Although FVP and RDV are two antiviral molecules with inhibitory effects on viral RNA replication, the efficacy and safety profiles are not similar, which has led to some differences between the decisions of regulatory authorities. Moreover, an improvement in clinical data management may contribute to the clarification of the use pattern of FVP and RDV in COVID-19 patients.
6.1. FVP drug information
FVP (T-705), C5H4FN3O2, is an RdRp inhibitor that was approved by the Ministry of Health, Labour and Welfare Japan (MHLW) in 2014 for the treatment of pandemic influenza virus infections. It is a pyrazine carboxamide-structured guanine analog with reduced activity in the presence of purine nucleosides. Furthermore, the prodrug FVP enters via endocytosis in the infected cells and undergoes in order to be active phosphor ribosylation and phosphorylation [16].
In vitro efficacy tests showed potential in inhibiting the replication of several viruses, including SARS-CoV-2 [93].
6.1.1. Pharmaceutical form, therapeutic indications, dosage regimen
FVP is authorized in Russia, Kenya, Thailand, Pakistan, China, Jordan, Egypt, Hungary, India, Poland, Saudi Arabia, and Serbia, for oral use in COVID-19 patients. One of the most common trade names is Avigan®. FVP is available as a 200 mg light-yellow, film-coated tablet and it is used to treat novel and re-emerging influenza virus infections, but also mild to moderate COVID-19 infections [94], [95].
The Japanese guidelines recommend for the treatment of COVID-19 infections 1800 mg twice daily on the first day, then 13 days 800 mg twice daily. The Ministry of Health of the Russian Federation recommend a dosage regimen depending on the patient’s weight: patients weighing < 75 kg, a dose of 1600 twice daily on day 1, then 600 mg twice daily until day 10; patients weighing between 75 kg to 90 kg, a dose of 2000 mg twice daily on day 1, then 600 mg twice daily until day 10; patients weighing > 90 kg, a dose of 2400 mg twice daily on day 1, then 1000 mg twice daily until day 10. Furthermore, for pediatric patients the dose is correlated according to their weight [96].
The protocol of Saudi Arabia included FVP in mild to moderate infections (1600 mg twice daily on day 1, then 600 mg twice daily until day 10). However, it can be also recommended for in pediatric patients or severe cases.
FVP has been recommended by Thailand’s Department of Disease Control for mild to moderate COVID-19 cases in both adults and children, while the recommendations coming from India included mild symptomatic COVID-19 patients, with or without comorbidities [95]. It has been recommended an initial therapeutic dose of 1800 mg twice daily on day 1, followed by 7 days of 800 mg administration, which can continue up to 14 days if necessary.
6.1.2. Adverse events, contraindications, drug toxicity
The analysis of existing FVP safety data is critical in determining whether FVP can be used globally for COVID-19 patients in the near future. Several studies, including meta-analysis, have been performed to evaluate the safety profile of FVP.
A recent study aimed to assess the adverse drug events (ADEs) after the use of FVP for COVID-19 patients reported in VigiBase®, an international pharmacovigilance database maintained by World Health Organization (WHO). The reports were evaluated in correlation with gender, age, and severity of ADEs, starting from 2015 until 2020. The results of the study showed 194 ADEs reported from 93 patients. Furthermore, the most frequent ADEs were intentional product use issue, elevated liver enzymes, nausea and vomiting, tachycardia, diarrhea, long QT syndrome, headache, pruritus, rash, erythema. The distribution of ADEs across continents is uneven (e.g., 91.23% Asia, 3.09% Europe, 2.06% Americas, 2.06% Oceania and 1.54% Africa). Severe ADEs occurred frequently in men and the elderly (> 64 years) and the most common manifestations were:
-
•
Injury, poisoning and procedural complications (65 cases);
-
•
Gastrointestinal and hepatobiliary disorders (32 cases);
-
•
Skin and subcutaneous tissue disorders (20 cases);
-
•
Cardiac disorders (14 cases);
-
•
Blood and lymphatic system disorders (5 cases) [97].
However, medical evidence has been obtained on a small number of patients, therefore a more complete assessment of the ADEs should be conducted.
A study conducted in March 2020 assessed 29 studies (6 were phase 2 or 3 studies), including 4299 participants, in order to report significant data about the safety profile of FVP. The results showed some safety issues regarding teratogenicity, QT prolongation and hyperuricemia [98].
A retrospective study aimed to provide information about the occurrence of FVP-related adverse events (AEs) in patients with renal dysfunction. Medical data from 228 hospitalized patients have been assessed and the results showed that 131 patients had experienced AEs. The most common AE was an increase in serum transaminases (e.g., aspartate aminotransferase and alanine aminotransferase, 57%), anemia (16.2%), hyperuricemia (10.5%). Moreover, the estimated glomerular filtration rate did not significantly influence the incidence of AEs, but further research is needed in order to be applied on larger populations [99]. It has been reported in a meta-analysis of 9 studies assessing 827 patients that the most frequent AEs identified were nausea, chest pain, vomiting, hyperuricemia, diarrhoea [100].
The teratogenic potential assessed in animal studies has made FVP contraindicated in lactating and pregnant women. Moreover, it is contraindicated for people with hypersensitivity, severe hepatic, or renal impairment. Due to the detection of FVP in sperm, contraceptive measures are required for both partners. FVP should be used with caution when the patients are susceptible to gout or hyperuricemia [35], [95].
Single-dose toxicity studies demonstrated that the lethal dose for intravenous and oral administration via certain pharmaceutical forms is predicted to be > 2000 mg/kg in mice and rats, while in the case of dogs and monkeys is > 1000 mg/kg. The overdosage symptoms include weight loss, decreased locomotor activity and vomiting. Serious AEs, including a decrease of the red blood cells and increased vacuolization in hepatic cells have been observed in repeat-dose toxicity studies using animal models (e.g., monkeys, dogs, rats) [101].
Early embryonic deaths in animal models (rats, mice) and teratogenicity in various species were found in the medical data from developmental and reproductive toxicity studies conducted during the drug registration procedure, with exposure levels similar to those in human patients (1200 mg-4800 mg). FVP as a therapeutic option may have a higher teratogenic risk in clinical practice than other antiviral molecules. Moreover, FVP causes reversible histological alterations in the testis and defective sperm, but only after a long period of treatment [102].
FVP has a good safety profile in terms of major adverse events, but additional investigations are needed to determine the treatment’s long-term effects. There are still insufficient data about the toxicity of FVP, being noticed differences in the medical literature regarding the safety profile of FVP [98], [99].
6.1.3. Clinical studies assessing the efficacy and safety of FVP in COVID-19 patients
Clinical trials evaluating the efficacy and safety profile of FVP in the global management of COVID-19 have been conducted all over the world in the last 2 years. FVP is an antiviral molecule that has been repurposed to treat COVID-19 patients because of its quick viral clearance, availability as an oral medicine, increased clinical recovery rates, and a clinical documented safety profile. Some of the most promising studies are summarized in Table 3.
Table 3.
Clinical trials investigating the efficacy and safety of FVP for COVID-19 patients.
| Study design | Medication in intervention/ sample size | Medication in Comparison/ sample size | Main findings | Ref. |
|---|---|---|---|---|
| Open -label, non- randomized, before-and-after controlled study |
Oral FVP (Day 1: 1600 mg twice daily; Days 2–14: 600 mg twice daily) + interferon (IFN)-a (5 million U twice daily)/35 | LPV/RTV (Days 1–14: 400 mg/100 mg twice daily) plus IFN-a by aerosol inhalation (5 million U twice daily)/45 | A shorter viral clearance median time was found for the FVP arm versus the control arm (4 d (interquartile range (IQR): 2.5–9) vs. 11 d (IQR: 8–13), P < 0.001); the FVP arm also showed significant improvement in chest CT compared with the control arm, with an improvement rate of 91.43% versus 62.22% (P = 0.004) | [103] |
| Prospective, randomized, controlled, open-label multicenter trial | FVP (1600 mgx2/first day followed by 600 mgx2/day) for 10 days/116 | Umifenovir (Arbidol) (200 mg*3/day)/120 | Clinical recovery rate of Day 7 does not significantly differ between FVP group (71/116) and Arbidol group (62/120) (P = 0.1396, difference of recovery rate: 0.0954; 95% CI: −0.0305 to 0.2213); FVP led to shorter latencies to relief for both pyrexia and cough | [104] |
| Prospective, randomized, open-label, multicenter trial | Early FVP: 1800 mg orally at least four hours apart on the first day, followed by 800 mg orally twice a day, for a total of up to 19 doses/33 | Late FVP: FVP was dosed at 1800 mg orally at least four hours apart on the first day, followed by 800/ 33 | Viral clearance occurred within 6 days in 66.7% and 56.1% of the early and late treatment groups (adjusted hazard ratio [aHR], 1.42; 95% confidence interval [95% CI], 0.76 to 2.62); during therapy, 84.1% developed transient hyperuricemia; FVP did not significantly improve viral clearance as measured by reverse transcription-PCR (RT-PCR) by day 6 | [105] |
| Multicenter, open label, randomized, phase 2 and 3 clinical trial | AVIFAVIR® 1600/1800 mg BID on Day 1 followed by 600/800 mg BID on Days 2–14/20 | Standard of care of Russian guidelines for treatment of COVID-19/20 | Both dosing regimens of AVIFAVIR® demonstrated similar virologic response; on Day 5, the viral clearance was achieved in 25/40 (62.5%) patients on AVIFAVIR® and in 6/20 (30.0%) patients on SOC (P = 0.018); by Day 10 the viral clearance was achieved in 37/40 (92.5%) patients | [96] |
| Open label randomized controlled study | FVP 1600 mg on day 1 followed by 600 mg twice a day for a maximum of 10 days, and interferon beta-1b at a dose of 8 million IU (0.25 mg) twice a day was given for 5 days/44 | Standard of care of Oman guidelines for treatment of COVID-19: HCQ 400 mg twice per day on day 1, then 200 mg twice per day for 7 days/45 | There were also no significant differences between the two groups with regards to the overall LOS (7 vs 7 days; p = 0.948), transfers to the ICU (18.2% vs 17.8%; p = 0.960), discharges (65.9% vs 68.9%; p = 0.764) and overall mortality (11.4% vs 13.3%; p = 0.778) | [106] |
| Randomized, open-label, parallel-arm, multicentre, phase 3 study | Oral FVP (1800 mg BID loading dose on day 1; 800 mg BID thereafter plus standard supportive care/68 | Standard supportive care alone that included antipyretics, cough suppressants, antibiotics, and vitamins/68 | Median time to the cessation of viral shedding was 5 days (95% CI: 4 days, 7 days) versus 7 days (95% CI: 5 days, 8 days), P = 0.129; adverse events were observed in 36% of FVP and 8% of control patients; one control patient died due to worsening disease | [107] |
| Exploratory single center, open label, randomized, controlled trial | FVP group: 1600 mg or 2200 mg orally, followed by 600 mg each time, three times a day, and the duration of administration was not more than 14 days/9 | Baloxavir marboxil group: 80 mg once a day orally on Day 1 and Day 4; for patients who are still positive in virological test, they can be given again on Day 7/10 | A total of 24 (82.8%) patients turned viral negative (defined as two consecutive tests with viral RNA undetectable results) within 14 days after the initiation of the trial; the percentage of patients who turned viral negative after 14-day treatment was 70%, 77%, and 100% in the baloxavir marboxil, FVP, and control group respectively, of which the control group was higher than that of the other two treatment groups | [108] |
| Control group: LPV/RTV (400 mg/100 mg, BID) or darunavir/cobicistat (800 mg/150 mg) and arbidol (200 mg)/10 | A total of 15 (51.7%) patients turned viral negative within 7 days after the initiation of the trial (60%, 44% and 50% in the baloxavir marboxil, FVP, and control group, respectively); one patient in the baloxavir marboxil group, and two patients in the FVP group were transferred to ICU within seven days after trial initiation |
NR, not reported; FVP, Favipiravir; LPV, Lopinavir; RTV, Ritonavir; HCQ, Hydroxychloroquine; LOS, length of hospital days; ICU, intensive care units; BID, twice a day.
6.2. RDV drug information
Gilead Sciences developed RDV (GS-5734) in 2015, in cooperation with the US Army Medical Research Institute of Infectious Diseases (USAMRIID) and the US Centers for Disease Control and Prevention (CDC) as a potential treatment for RNA-based viruses with worldwide pandemic potential, such as Ebola virus or novel coronaviruses causing Middle East respiratory syndrome (MERS) and SARS [109].
RDV is a carboxylic ester functioning as a prodrug that is metabolized via several processes into an active nucleoside triphosphate compound, with high potential to inhibit viral replication via RdRp [110]. The efficacy of RDV against SARS-CoV-2 infections is based on in vitro and in vivo studies using animal models [111].
6.2.1. Pharmaceutical form, therapeutic indications, dosage regimen
FDA has approved RDV under the name of Veklury® for emergency use, and EMA has granted conditional approval for COVID-19 patients. Due to a scarcity of data in the medical field, the benefit-risk ratio is still under evaluation [112]. RDV is available as 100 mg powder for concentrate for solution for infusion and is indicated for the treatment of SARS-CoV-2 infections in adults and adolescents (12–18 years, weighing more than 40 kg), with pneumonia requiring supplemental oxygen and for adults who are at risk of developing severe forms [113].
In search of an optimal pharmaceutical form and dosage regimen, a study assessed the possibility of a combination between pulmonary and intravenously administration in order to obtain additional benefit. A pulmonary administration may result in higher lung concentrations of RDV, along with a reduction of systemic toxicity. However, further research is needed to correlate a theoretical calculation to results obtained on animal models or humans [114].
COVID-19 treatment guidelines recommend a starting dose of 200 mg RDV administered intravenously once daily, followed by 100 mg RDV administered intravenously once daily for 4 days. The doses should be adjusted according to renal function. Moreover, the association between RDV and Dexamethasone may have potential benefits for COVID-19 patients that have recently been intubated. However, this recommendation is optional, due to the C level of evidence (e.g., expert opinion) [115].
6.2.2. Adverse events, contraindications, drug toxicity
RDV was the first antiviral drug specifically approved for the treatment of COVID-19 patients, and as its use increased so did the side effects, causing clinicians to be concerned [116], [117].
A recent study assessed potential ADEs provided by medical studies in COVID-19 patients and WHO VigiBase® from 2015 to 2020. The results included 1004 ADEs from 439 COVID-19 patients, with a higher prevalence in the Americas (67.7%) and in men (>45 years). Furthermore, the most frequently reported ADEs were hepatic enzyme increase (32.11%), renal injury (14.4%), blood creatinine increased (11.2%), medication error (7.7%), product use in unapproved condition (6.6%), respiratory failure (6.4%), tachyarrhythmia or bradyarrhythmia (5.9%). Serious and fatal ADEs were reported more in the elderly group (>64years), as well as urinary disorders [118].
Transaminase elevations have been reported in several studies assessing the use of RDV in COVID-19 patients, being one of the first side effects identified [32], [119], [120].
A randomized, double-blind, placebo-controlled, multicentre trial from ten hospitals in China evaluated the efficacy profile of RDV in 158 patients. The results showed that ADEs were reported in 102 patients and the treatment for 18 patients was stopped due to the presence of serious ADEs. The most prevalent ADEs among the patients were constipation (14%), hypoalbuminemia (13%), hypokalaemia (12%), anemia (12%), while the most frequently serious ADEs reported were respiratory failure (10%), cardiopulmonary failure (5%), pulmonary embolism (1%), recurrence of COVID-19 (1%), septic shock (1%) [121].
A retrospective study conducted between May and October 2020 assessed the ADEs in 164 patients who received RDV. The results showed an increased prevalence of allergic-type reactions (13.7%) and a decrease in the estimated glomerular filtration rate (6 patients) [122]. However, further research is needed for an improved perspective on the safety profile of RDV. A meta-analysis of five randomized controlled trials including 13544 COVID-19 patients also contributed to this statement, suggesting that there is no evidence of a higher risk of ADEs after RDV treatment [123].
According to EMA regulations, Veklury® is contraindicated only when cases of hypersensitivity to RDV or any of the excipients (e.g., betadex sulfobutyl ether sodium, hydrochloric acid, sodium hydroxide) are suspected [113]. RDV had no toxic effect on embryonic development in pregnant animals, according to non-clinical reproductive toxicity trials. Furthermore, RDV has not been comprehensively examined in pregnant or lactating women, but due to the blood detection of GS-441524 metabolite in rats’ pups, the presence of compounds in milk can be expected [124].
Pre-registration toxicity studies evaluated several processes. Renal toxicity was observed after RDV was administered intravenously to rhesus monkeys and rats for short periods of time. After the administration of 5, 10, and 20 mg/kg/day for 7 days, renal tubular atrophy, basophilia and increased creatinine levels were observed. Furthermore, doses of more than 3 mg/kg/day in rats for 1 month resulted in renal damage. M27 is an unidentified metabolite that was found to be present in human plasma. As a result, animal studies may not be helpful in determining the potential malfunctions connected with this metabolite [113].
Toxicity studies updated in various medical reviews have provided a good safety profile of RDV, but it remains essential to monitor certain biochemical parameters [113], [116], [123].
6.2.3. Clinical studies assessing the efficacy and safety of RDV in COVID-19 patients
The safety and efficacy profile of RDV according to the studies conducted worldwide allowed it to be authorized for the treatment of COVID-19 in many states. However, there are still unmet needs that can be evaluated in an evidence-based medicine context. Numerous studies have been conducted to have an optimal control of COVID-19 patients treated with RDV, but limitations may occur. Therefore, assessing the risk of biases may be essential [125], [126]. Some of the most relevant randomized controlled trials (RCT) are presented in Table 4.
Table 4.
Clinical trials investigating the efficacy and safety of RDV for COVID-19 patients.
| Study design | Interventions/sample size | Results and interpretation | Ref. |
|---|---|---|---|
| Randomized, double-blind, placebo- controlled trial |
200 mg RDV IV on day 1, then 100 mg daily for 2 days/279 Placebo up to 3 days/283 |
2 patients (0.7%) in the RDV group and 15 patients (5.3%) in the placebo group were hospitalized by day 28; all hospitalizations occurred by day 14; no patient died by day 28; by day 28, AE had occurred in 118 patients (42.3%) in the RDV group and in 131 patients (46.3%) in the placebo group; the most prevalent non-serious AE that occurred in at least 5% of patients in both groups were cough, nausea, and headache | [127] |
| Multinational, placebo- controlled, double-blind RCT | 200 mg RDV IV on day 1, followed by 100 mg RDV once daily up to 9 days/541 Placebo for up to 10 days/521 |
RDV reduced time to recovery compared to placebo (10 days vs. 15 days; rate ratio for recovery 1.29; 95% CI, 1.12–1.49; P < 0.001); no differences concerning time to recovery were reported for patients on high-flow oxygen, ECMO and MV at enrollment; | [117] |
| Randomized, open-label trial | 200 mg RDV IV on day 1, then 100 mg daily up to 5 days/199 200 mg RDV IV on day 1, then 100 mg daily up to 10 days/197 SOC/200 |
After treatment, patients in the 5-day RDV group had statistically significantly higher probabilities of a better clinical status distribution than those receiving SOC (odds ratio, 1.65; 95% CI, 1.09-2.48); by day 28, 9 patients had died, 2 (1%) in the 5-day RDV group, 3 (2%) in the 10-day RDV group, and 4 (2%) in the SOC group; nausea (10% vs 3%), and headache (5% vs 3%) were more frequent among RDV-treated patients compared with SOC | [128] |
| Multinational, open-label, RCT | 200 mg RDV IV on day 1, followed by 100 mg RDV daily for 4 days/200 200 mg RDV IV on day 1, then 100 mg RDV daily for 9 days/197 |
Day 14 distribution in clinical status was comparable between groups (P = 0.14); time to clinical improvement was similar between groups (10 days in 5-day arm vs. 11 days in 10-day arm); in hospitalized patients with a severe SARS-CoV-2 infection without receiving MV or ECMO, the using of RDV for 5 or 10 days had comparable clinical benefits | [129] |
| Multinational, open-label, adaptive RCT | 200 mg RDV IV on day 1, then 100 mg RDV daily for 9 days/2743 SOC/2708 |
In-hospital mortality: 11.0% in RDV group vs. 11.2% in SOC arm (rate ratio 0.95; 95% CI, 0.81–1.11); initiation of MV: 10.8% in RDV group vs. 10.5% in SOC arm; RDV did not decrease in-hospital mortality or the need for MV compared to SOC | [130] |
RDV, remdesivir; IV, intravenous; SOC, standard of care; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation.
7. FVP versus RDV as therapeutic options for COVID-19 patients
FVP and RDV are antiviral prodrugs with varied results in clinical trials, repurposed to treat SARS-CoV-2 infections. RDV is indicated in hospitalized COVID-19 patients who require supplementary oxygen, while FVP is authorized for use in mild to moderate forms. A comprehensive characterization of FVP versus RDV may contribute to a better understanding of their use. Moreover, the ongoing studies are likely to shed further insight on their activity in COVID-19 patients [131].
7.1. Physico-chemical, pharmacological, and regulatory considerations of FVP versus RDV
A comparative analysis of different characteristics of FVP and RDV is presented in Table 5 [19], [20], [21], [131], [132], [133], [134].
Table 5.
Comparative analysis of various properties of FVP versus RDV.
| Characteristics | FVP | RDV |
|---|---|---|
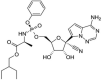
| Chemical structure | 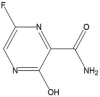 |
 |
| Molecular formula | C5H4FN3O2 | C27H35N6O8P |
| Molecular weight | 157.10 | 602.6 |
| XLogP3-AA | -0.6 | 1.9 |
| IUPAC name | 5-fluoro-2-oxo-1 H-pyrazine-3-carboxamide | 2-ethylbutyl (2S-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate |
| ATC Code | J05AX27 | J05AB16 |
| Drug regulatory approval (COVID-19) | Approved in Russia, China, Hungary, Serbia, India, Thailand, Turkey, Poland, Korea, Saudi Arabia, Jordan, Egypt, Portugal | FDA and EMA approved |
| Trade names | Avigan®, Avifavir®, Areplivir®, FabiFlu®, Favipira®, Reeqonus®, Qifenda® | Veklury® |
| Indication | Treatment of mild to moderate COVID-19 disease in adults under restricted emergency use | Treatment of adult and pediatric patients aged 12 years for COVID-19 infection requiring hospitalization |
| Mechanism of action | Nucleoside analog competing with endogenous guanosine triphosphate for incorporation into RdRp | Nucleoside analog competing with adenosine triphosphate for incorporation into RdRp |
| Administration route | Oral | Intravenous |
| Adverse drug events | Increased liver enzymes QT prolongation, hyperuricemia |
Increased liver enzymes, renal injury blood creatinine increased |
| Drug-drug interactions | Raloxifene, Tamoxifen, Paclitaxel, Montelukast, Pioglitazone | Chloroquine, Hydroxychloroquine |
| Cost ($) | 0.5-1per tablet | 390 per 100 mg vial |
IUPAC, International Union of Pure and Applied Chemistry; XLogP3-AA, atom-additive method that calculates logP; ATC code, Anatomical, Therapeutical, Chemical classification system.
7.2. Clinical trials on the efficacy and safety of FVP versus RDV
To properly evaluate the efficacy and safety profiles of FVP and RDV, numerous clinical trials have been conducted around the world. A comprehensive search of the ClinicalTrials.gov database has been performed and a list of ongoing clinical trials assessing the use of FVP and RDV in COVID-19 patients was proposed in Table 6 [23], [24], [25].
Table 6.
Registered clinical trials on the use of FVP, RDV and their combination in Covid-19 patients.
| Drug | ClinicalTrials.gov Identifier | Title | Status | n | Country |
|---|---|---|---|---|---|
| FVP | NCT04336904 | Clinical Study to Evaluate the Performance and Safety of Favipiravir in COVID-19 | Active, not recruiting | 100 | Italy |
| NCT04474457 | Efficacy and Safety of Favipiravir in the Treatment of COVID-19 Patients Over 15 Years of Age | Active, not recruiting | 1000 | Turkey | |
| NCT04359615 | Favipiravir in Hospitalized COVID-19 Patients (FIC) | Not yet recruiting | 40 | Iran | |
| NCT04425460 | A Multi-center, Randomized, Double-blind, Placebo-controlled, Phase 3 Study Evaluating Favipiravir in Treatment of COVID-19 | Not yet recruiting | 256 | China, Germany, Romania | |
| NCT04529499 | Clinical Trial Evaluating the Efficacy and Safety of Favipiravir in Moderate to Severe COVID-19 Patients | Active, not recruiting | 780 | Kuwait | |
| NCT04434248 | An Adaptive Study of Favipiravir Compared to Standard of Care in Hospitalized Patients With COVID-19 | Active, not recruiting | 330 | Russia | |
| RDV | NCT04713176 | Efficacy and Safety of DWJ1248 With Remdesivir in Severe COVID-19 Patients | Recruiting | 1022 | South Korea |
| NCT04582266 | PK and Safety of Remdesivir for Treatment of COVID-19 in Pregnant and Non-Pregnant Women in the US | Recruiting | 40 | USA | |
| NCT04647695 | IFN-beta 1b and Remdesivir for COVID19 | Recruiting | 100 | China | |
| NCT04854837 | Safety of Remdesivir Treatment in COVID-19 Patients Requiring Hemodialysis (REM-HD) | Recruiting | 60 | Hungary | |
| NCT04738045 | Comparison of Remdesivir Versus Lopinavir/ Ritonavir and Remdesivir Combination in COVID-19 Patients | Recruiting | 90 | Egypt | |
| FVP and RDV | NCT04694612 | Efficacy of Favipiravir in Treatment of Mild & Moderate COVID-19 Infection | Recruiting | 676 | Nepal |
| NCT04784559 | Trial to Determine the Efficacy/Safety of Plitidepsin vs Control in Patients with Moderate COVID-19 Infection (Neptuno) | Recruiting | 609 | Argentina, Brazil | |
| NCT05041907 | Finding Treatments for COVID-19: A Trial of Antiviral Pharmacodynamics in (PLATCOV) | Recruiting | 750 | Thailand |
n, estimated enrollment.
There are 80 ongoing clinical trials evaluating the use of RDV in COVID-19 and 33 in the case of FVP. Some relevant ongoing clinical trials included in ClinicalTrials.gov database that focus on the activity of FVP, RDV or both in COVID-19 have been presented above.
The effect of RDV on mortality reduction is still unclear [117], while some results of the clinical trials evaluating FVP treatment showed no significant differences in some clinical indicators (e.g., hospitalization days, clinical recovery) [132].
7.3. SARS-CoV-2 resistance to FVP versus RDV
A major obstacle to the global use of FVP and RDV, similar to other medication regimens, is the potential development of resistance mechanisms among coronaviruses. Earlier studies demonstrated that mutations in the RdRp of influenza A virus or Ebola virus may lead to increased resistance to FVP or RDV [135], [136]. Moreover, this risk can also be correlated with the FVP and RDV use in COVID-19 patients [137].
A total of 18 mutations were found in SARS-CoV-2 sub-populations that infected hamsters, subsequently treated with FVP. Mutations were found in the nsp14 coding area, which is implicated in the RdRp proofreading activity and in the N7 MTase region, which is implicated in the viral RNA capping [138]. By comparison, in the case of resistance to RDV, mutations are more likely to occur in the nsp12 coding sequence, because RDV can elude nsp14 region, thus the virus is less likely to develop resistance to its action [139].
A case report identified a mutation on the catalytic site of RdRp (e.g, D484Y), which led to a resistant form to RDV action in a 76 years old immunocompromised female with persistent SARS-CoV-2 infection [140], [141].
However, there is still insufficient data on the mechanisms of resistance via different types of mutations that may develop when using FVP or RDV for the treatment of COVID-19 patients, so additional research is needed to prevent these medical issues.
8. Conclusions and future directions
Given the severity of the current global pandemic, numerous studies are conducted to develop antiviral drugs or vaccines as primary prevention methods. Several protein-based molecules are essential for the functionality and pathogenesis of the virus and can be considered promising therapeutic targets such as spike (S) protein, membrane (M) protein, TMPRSS2, ACE2 receptors, N-terminal RNA binding domain (NRBD), C-terminal RNA binding domain (CRBD), Nsp-7-Nsp8 complex, helicase, Nsp14-Nsp16 complex. Various studies tested the interaction between various drug molecules and these possible targets [142], [143], [144]. Moreover, anti-SARS-CoV-2 antibodies have made significant progress in reducing the morbidity and mortality rates due to COVID-19 infection [145].
COVID-19 management is divided into two directions: prevention and treatment. Regarding the preventive methods, several vaccines have been developed and are used worldwide in order to maximize the immune response and to minimize the pathogenic effects of SARS-CoV-2 infections. From a therapeutic point of view, several antiviral molecules have been authorized in various countries: RDV, FVP, umifenovir, molnupiravir, nirmatrelvir-ritonavir combination. In addition, the present approaches include the introduction of monoclonal antibodies into therapy (e.g., bamlanivimab, etesevimab, casirivimab, imdevimab, sotrovimab) [146], [147].
Being a topic of major interest intensely researched, the future directions regarding the management of COVID-19 are promising because numerous new therapeutic targets are being studied and potential therapeutic agents are in various stages of testing (e.g., SSAA09E2 and CP-1 peptide interfere with ACE2 recognition, tideglusib and PX-12 inhibit 3 C-like protease, bananin and SSYA10–001 inhibit the helicase, camostat and nafamostat inhibit TMPRSS2, SID 26681509 and SSAA09E1 target cathepsin L, nintedanib inhibits adapter-associated kinase 1 and cyclin G-associated kinase, YM201636 and apilimod interfere with phosphatidylinositol 3-phosphate 5-kinase) [148].
In this review, a comparative evaluation of FVP and RDV was presented as therapeutic options for COVID-19 patients. There was a particular focus on the aspects that can have a significant impact on the therapeutic management of COVID-19 such as pharmacological properties, antiviral role, pharmacokinetics, drug-drug interactions, safety, and efficacy clinical trials involving the use of FVP or RDV.
Despite the fact that RDV failed to show statistically significant improvements on mortality rate, it is still a promising therapeutic tool due to the reduction of the recovery time and the risk of complications. Moreover, the assessment of medical information from clinical trials showed no significant improvement in clinical recovery after the use of FVP but contributed to the relief of fever and cough [147].
One of the important deductions suggested by the medical studies conducted so far implies the possibility of choosing several therapeutic approaches, but with an accurate analysis of the benefit-risk balance and with continuous monitoring of COVID-19 patients.
Several therapy guidelines currently recommend RDV for the treatment of COVID-19 patients. There are fewer accurate clinical trials based on the use of FVP in COVID-19. However, FVP has proved to be useful in mild to moderate cases of COVID-19, and RDV in more severe forms. Both antiviral drugs may have a significant role in the management of COVID-19 pandemic, due to their suitability for distinct groups of patients.
There are still insufficient data to allow a completely accurate management of SARS-CoV-2 infection, highlighting the need for further investigations, larger RCTs, antiviral drug resistance tests and strategies to improve mortality in COVID-19.
Funding
This research was funded by the Romanian Ministry of Research, Innovation and Digitisation through Programme 1 – Development of the National Research and Development System, Subprogramme 1.2 - Institutional Performance – Projects for funding the excellence in RDI, Contract No. 29 PFE / 30.12.2021 with University of Oradea.
Author contributions
All authors contributed equally to this manuscript.
CRediT authorship contribution statement
Simona Gabriela Bungau: Conceptualization, Supervision, Funding acquisition. Paul Andrei Negru: Writing – original draft, Writing – review & editing. Andrei-Flavius Radu: Writing – original draft, Writing – review & editing. Delia Mirela Tit: Writing – original draft, Writing – review & editing. Cosmin Mihai Vesa: Writing – original draft, Writing – review & editing. Tapan Behl: Writing – review & editing. Mohamed M. Abdel-Daim: Writing – review & editing. Aurelia Cristina Nechifor: Writing – review & editing. Laura Endres: Writing – review & editing. Manuela Stoicescu: Writing – review & editing. Bianca Pasca: Writing – review & editing.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
The authors thank the University of Oradea, considering the logistic facilities they used and for supporting the publication of this manuscript.
References
- 1.Isgrò C., Sardanelli A.M., Palese L.L. Systematic search for SARS-CoV-2 main protease inhibitors for drug repurposing: ethacrynic acid as a potential drug. Viruses. 2021;13:106. doi: 10.3390/V13010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammad T., Choudhury A., Habib I., Asrani P., Mathur Y., Umair M., Anjum F., Shafie A., Yadav D.K., Hassan M.I. Genomic variations in the structural proteins of SARS-CoV-2 and their deleterious impact on pathogenesis: a comparative genomics approach. Front. Cell. Infect. Microbiol. 2021;11:951. doi: 10.3389/FCIMB.2021.765039/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asrani P., Eapen M.S., Chia C., Haug G., Weber H.C., Hassan M.I., Sohal S.S. Diagnostic approaches in COVID-19: clinical updates. Expert Rev. Respir. Med. 2020;15:197–212. doi: 10.1080/17476348.2021.1823833. [DOI] [PubMed] [Google Scholar]
- 4.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/J.BBADIS.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asrani P., Hussain A., Nasreen K., Alajmi M.F., Amir S., Sohal S.S., Hassan M.I. Guidelines and safety considerations in the laboratory diagnosis of SARS-CoV-2 infection: a prerequisite study for health professionals. Risk Manag. Healthc. Policy. 2021;14:379–389. doi: 10.2147/RMHP.S284473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skalny A.V., Lima T.R.R., Ke T., Zhou J.C., Bornhorst J., Alekseenko S.I., Aaseth J., Anesti O., Sarigiannis D.A., Tsatsakis A., et al. Toxic metal exposure as a possible risk factor for COVID-19 and other respiratory infectious diseases. Food Chem. Toxicol. 2020;146 doi: 10.1016/J.FCT.2020.111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabir M.T., Uddin M.S., Zaman S., Begum Y., Ashraf G.M., Bin-Jumah M.N., Bungau S.G., Mousa S.A., Abdel-Daim M.M. Molecular mechanisms of metal toxicity in the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 2020;58:1–20. doi: 10.1007/S12035-020-02096-W. [DOI] [PubMed] [Google Scholar]
- 8.Negrut N., Codrean A., Hodisan I., Bungau S., Tit D.M., Marin R., Behl T., Banica F., Diaconu C.C., Nistor‑Cseppento D.C. Efficiency of antiviral treatment in COVID‑19. Exp. Ther. Med. 2021;21:1–7. doi: 10.3892/ETM.2021.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics – developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 10.Ou T., Mou H., Zhang L., Ojha A., Choe H., Farzan M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLOS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murgolo N., Therien A.G., Howell B., Klein D., Koeplinger K., Lieberman L.A., Adam G.C., Flynn J., McKenna P., Swaminathan G., et al. SARS-CoV-2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLOS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asrani P., Eapen M.S., Hassan M.I., Sohal S.S. Implications of the second wave of COVID-19 in India. Lancet Respir. Med. 2021;9:e93–e94. doi: 10.1016/S2213-2600(21)00312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asrani P., Tiwari K., Eapen M.S., McAlinden K.D., Haug G., Johansen M.D., Hansbro P.M., Flanagan K.L., Hassan M.I., Sohal S.S. Clinical features and mechanistic insights into drug repurposing for combating COVID-19. Int. J. Biochem. Cell Biol. 2022;142 doi: 10.1016/J.BIOCEL.2021.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Şimşek-Yavuz S., Komsuoğlu Çelikyurt F.I. Antiviral treatment of COVID-19: an update. Turk. J. Med. Sci. 2021 doi: 10.3906/SAG-2106-250. (Advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian L., Qiang T., Liang C., Ren X., Jia M., Zhang J., Li J., Wan M., YuWen X., Li H., et al. RNA-dependent RNA polymerase (RdRp) inhibitors: the current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 2021;213 doi: 10.1016/J.EJMECH.2021.113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013;100:446–454. doi: 10.1016/J.ANTIVIRAL.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020;108:242–247. doi: 10.1002/CPT.1844. [DOI] [PubMed] [Google Scholar]
- 18.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/PJAB.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Biotechnology Information, PubChem Compound Summary for CID 492405, Favipiravir. Available online: 〈https://pubchem.ncbi.nlm.nih.gov/compound/Favipiravir〉. (Accessed 4 December 2021).
- 20.National Center for Biotechnology Information, PubChem Compound Summary for CID 121304016, Remdesivir. Available online: 〈https://pubchem.ncbi.nlm.nih.gov/compound/Remdesivir〉. (Accessed 4 December 2021).
- 21.Sreekanth Reddy O., Lai W.F. Tackling COVID-19 using remdesivir and favipiravir as therapeutic options. ChemBioChem Eur. J. Chem. Biol. 2021;22:939–948. doi: 10.1002/CBIC.202000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Search Strategy on PubMed.gov. Available online: 〈https://pubmed.ncbi.nlm.nih.gov/?term=Favipiravir+and+Remdesivir+and+COVID-19〉 (Accessed 4 December 2021).
- 23.Search Strategy on ClinicalTrials.gov. Favipiravir. Available online: 〈https://clinicaltrials.gov/ct2/results?cond=CVOVID-19&term=Favipiravir&cntry=&state=&city=&dist=〉 (Accessed 4 December 2021).
- 24.Search Strategy on ClinicalTrials.gov. Remdesivir. Available online: 〈https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=Remdesivir&cntry=&state=&city=&dist=〉. (Accessed 4 December 2021).
- 25.Search Strategy on ClinicalTrials.gov. Favipiravir and Remdesivir. Available online: 〈https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=Favipiravir+and+Remdesivir&cntry=&state=&city=&dist=〉. (Accessed 4 December 2021).
- 26.Behl T., Kaur I., Aleya L., Sehgal A., Singh S., Sharma N., Bhatia S., Al-Harrasi A., Bungau S. CD147-spike protein interaction in COVID-19: get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022;808 doi: 10.1016/J.SCITOTENV.2021.152072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/J.CELL.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asrani P., Hassan M.I. SARS-CoV-2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol. Cell. Biochem. 2021;476:675–687. doi: 10.1007/S11010-020-03935-Z/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayvargiya P., Esquer Garrigos Z., Castillo Almeida N.E., Gurram P.R., Stevens R.W., Razonable R.R. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof) Mayo Clin. Proc. 2020;95:1454–1466. doi: 10.1016/J.MAYOCP.2020.04.027/ATTACHMENT/20E369A6-12B9-4F3A–8E70-FB5477ACB832/MMC2.MP4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/JAMA.2020.6019. [DOI] [PubMed] [Google Scholar]
- 31.Dondorp A.M., Hayat M., Aryal D., Beane A., Schultz M.J. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am. J. Trop. Med. Hyg. 2020;102:1191–1197. doi: 10.4269/AJTMH.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., et al. Compassionate use of Remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMOA2007016/SUPPL_FILE/NEJMOA2007016_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Liu C., Liu G., Luo W., Xia N. COVID-19: progress in diagnostics, therapy and vaccination. Theranostics. 2020;10:7821–7835. doi: 10.7150/THNO.47987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borbone N., Piccialli G., Roviello G.N., Oliviero G. Nucleoside analogs and nucleoside precursors as drugs in the fight against SARS-CoV-2 and other coronaviruses. Molecules. 2021;26:986. doi: 10.3390/MOLECULES26040986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 2018;153:85–94. doi: 10.1016/J.ANTIVIRAL.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A., Pradhan A., Maurya V.K., Kumar S., Theengh A., Puri B., Saxena S.K. Therapeutic approaches for SARS-CoV-2 infection. Methods. 2021;195:29–43. doi: 10.1016/J.YMETH.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9:1267. doi: 10.3390/CELLS9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav R., Chaudhary J.K., Jain N., Chaudhary P.K., Khanra S., Dhamija P., Sharma A., Kumar A., Handu S. Role of structural and non-structural proteins and therapeutic targets of SARS-CoV-2 for COVID-19. Cells. 2021;10:821. doi: 10.3390/CELLS10040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Almeida S.M.V., Santos Soares J.C., dos Santos K.L., Alves J.E.F., Ribeiro A.G., Jacob Í.T.T., da Silva Ferreira C.J., dos Santos J.C., de Oliveira J.F., de Carvalho Junior L.B., et al. COVID-19 therapy: what weapons do we bring into battle? Bioorg. Med. Chem. 2020;28 doi: 10.1016/J.BMC.2020.115757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Instiaty Sri, Darmayani I.G.A.A.P., Marzuki J.E., Angelia F., William Siane A., Sary L.D., Yohanes L., Widyastuti R., Nova R., et al. Antiviral treatment of COVID-19: a clinical pharmacology narrative review. Med. J. Indones. 2020;29:332–345. doi: 10.13181/MJI.REV.204652. [DOI] [Google Scholar]
- 42.Jorgensen S.C.J., Kebriaei R., Dresser L.D. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020;40:659–671. doi: 10.1002/PHAR.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jomah S., Asdaq S.M.B., Al-Yamani M.J. Clinical efficacy of antivirals against novel coronavirus (COVID-19): a review. J. Infect. Public Health. 2020;13:1187–1195. doi: 10.1016/J.JIPH.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saini M., Rana M., Bhatti K., Das R., Mehta D.K., Chidurala R.M. Clinical efficacy of Remdesivir and Favipiravir in the treatment of Covid-19 patients: scenario so far. Curr. Drug Res. Rev. 2021;13 doi: 10.2174/2589977513666210806122901. (Advance online publication) Online ahead print. [DOI] [PubMed] [Google Scholar]
- 45.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209 doi: 10.1016/J.PHARMTHERA.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayden F.G., Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr. Opin. Infect. Dis. 2019;32:176–186. doi: 10.1097/QCO.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76:370–376. doi: 10.1016/J.MJAFI.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oestereich L., Lüdtke A., Wurr S., Rieger T., Muñoz-Fontela C., Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 2014;105:17–21. doi: 10.1016/J.ANTIVIRAL.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Smither S.J., Eastaugh L.S., Steward J.A., Nelson M., Lenk R.P., Lever M.S. Post-exposure efficacy of Oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antivir. Res. 2014;104:153–155. doi: 10.1016/J.ANTIVIRAL.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Kerber R., Lorenz E., Duraffour S., Sissoko D., Rudolf M., Jaeger A., Cisse S.D., Camara A.M., Miranda O., Castro C.M., et al. Laboratory findings, compassionate use of favipiravir, and outcome in patients with Ebola virus disease, Guinea, 2015–a retrospective observational study. J. Infect. Dis. 2019;220:195–202. doi: 10.1093/INFDIS/JIZ078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020;38:379–381. doi: 10.1038/D41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 52.Tagde P., Tagde S., Tagde P., Bhattacharya T., Monzur S.M., Rahman M.H., Otrisal P., Behl T., Hassan S.S.U., Abdel-Daim M.M., et al. Nutraceuticals and herbs in reducing the risk and improving the treatment of COVID-19 by targeting SARS-CoV-2. Biomedicines. 2021;9:1266. doi: 10.3390/BIOMEDICINES9091266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De P., Chakraborty I., Karna B., Mazumder N. Brief review on repurposed drugs and vaccines for possible treatment of COVID-19. Eur. J. Pharmacol. 2021;898 doi: 10.1016/J.EJPHAR.2021.173977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hema Sree G.N.S., Saraswathy G.R., Manikanta M., Mamatha K. An update on drug repurposing: re-written saga of the drug’s fate. Biomed. Pharmacother. 2019;110:700–716. doi: 10.1016/J.BIOPHA.2018.11.127. [DOI] [PubMed] [Google Scholar]
- 55.Guo Q., Xu M., Guo S., Zhu F., Xie Y., Shen J. The complete synthesis of favipiravir from 2-aminopyrazine. Chem. Pap. 2019;73:1043–1051. doi: 10.1007/S11696-018-0654-9/SCHEMES/5. [DOI] [Google Scholar]
- 56.Pathania S., Rawal R.K., Singh P.K. RdRp (RNA-dependent RNA polymerase): a key target providing anti-virals for the management of various viral diseases. J. Mol. Struct. 2022;1250 doi: 10.1016/J.MOLSTRUC.2021.131756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhirnov O.P., Chernyshova A.I. Favipiravir: the hidden threat of mutagenic action. J. Microbiol. Epidemiol. Immunobiol. 2021;98:213–220. doi: 10.36233/0372-9311-114. [DOI] [Google Scholar]
- 58.Procko E. Deep mutagenesis in the study of COVID-19: a technical overview for the proteomics community. Expert Rev. Proteom. 2020;17:633–638. doi: 10.1080/14789450.2020.1833721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cully M. A tale of two antiviral targets – and the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 2022;21:3–5. doi: 10.1038/D41573-021-00202-8. [DOI] [PubMed] [Google Scholar]
- 60.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Höbartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12:279. doi: 10.1038/S41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schooley R.T., Carlin A.F., Beadle J.R., Valiaeva N., Zhang X.Q., Clark A.E., McMillan R.E., Leibel S.L., McVicar R.N., Xie J., et al. Rethinking remdesivir: synthesis, antiviral activity, and pharmacokinetics of oral lipid prodrugs. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.01155-21/SUPPL_FILE/AAC.01155-21-S0001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M., Zhang L., Huo X., Zhang Z., Zhang W. Chemical synthesis of the anti-COVID-19 drug Remdesivir. Curr. Protoc. 2021;1 doi: 10.1002/CPZ1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar Palli K., Ghosh P., Krishna Avula S., Sridhara Shanmukha Rao B., Patil A.D., Ghosh S., Sudhakar G., Raji Reddy C., Mainkar P.S., Chandrasekhar S. Total synthesis of remdesivir. Tetrahedron Lett. 2022;88 doi: 10.1016/J.TETLET.2021.153590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ni X., Schröder M., Olieric V., Sharpe M.E., Hernandez-Olmos V., Proschak E., Merk D., Knapp S., Chaikuad A. Structural insights into plasticity and discovery of Remdesivir metabolite GS-441524 binding in SARS-CoV-2 macrodomain. ACS Med. Chem. Lett. 2021;12:603–609. doi: 10.1021/ACSMEDCHEMLETT.0C00684/SUPPL_FILE/ML0C00684_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Chakraborty C. Probable molecular mechanism of remdesivir for the treatment of COVID-19: need to know more. Arch. Med. Res. 2020;51:585–586. doi: 10.1016/J.ARCMED.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uzunova K., Filipova E., Pavlova V., Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020;131 doi: 10.1016/J.BIOPHA.2020.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368:1499–1504. doi: 10.1126/SCIENCE.ABC1560/SUPPL_FILE/ABC1560_YIN_SM_CORRECTED.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung L.S., Gund T.M., Narayan M. Comparison of binding site of Remdesivir and its metabolites with NSP12-NSP7-NSP8, and NSP3 of SARS CoV-2 virus and alternative potential drugs for COVID-19 treatment. Protein J. 2020;39:619–630. doi: 10.1007/S10930-020-09942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao L., Zhong W. Mechanism of action of favipiravir against SARS-CoV-2: mutagenesis or chain termination? Innovations. 2021;2 doi: 10.1016/J.XINN.2021.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y., Li P., Rajpoot S., Saqib U., Yu P., Li Y., Li Y., Ma Z., Baig M.S., Pan Q. Comparative assessment of favipiravir and remdesivir against human coronavirus NL63 in molecular docking and cell culture models. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-02972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chappell M., Payne S. Pharmacokinet. Biosyst. Biorobot. 2021;24:61–72. doi: 10.1007/978-3-030-39705-0_5. [DOI] [Google Scholar]
- 72.Starkey E.S., Sammons H.M. Practical pharmacokinetics: what do you really need to know? Arch. Dis. Child. Educ. Pract. 2015;100:37–43. doi: 10.1136/ARCHDISCHILD-2013-304555. [DOI] [PubMed] [Google Scholar]
- 73.Deb S., Reeves A.A., Hopefl R., Bejusca R. ADME and pharmacokinetic properties of Remdesivir: its drug interaction potential. Pharmaceuticals. 2021;14:655. doi: 10.3390/PH14070655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Humeniuk R., Mathias A., Kirby B.J., Lutz J.D., Cao H., Osinusi A., Babusis D., Porter D., Wei X., Ling J., et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of Remdesivir, a SARS-CoV-2 replication inhibitor. Clin. Pharmacokinet. 2021;60:569–583. doi: 10.1007/S40262-021-00984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madelain V., Nguyen T.H.T., Olivo A., de Lamballerie X., Guedj J., Taburet A.M., Mentré F. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016;55:907–923. doi: 10.1007/S40262-015-0364-1/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irie K., Nakagawa A., Fujita H., Tamura R., Eto M., Ikesue H., Muroi N., Tomii K., Hashida T. Pharmacokinetics of Favipiravir in critically Ill patients With COVID‐19. Clin. Transl. Sci. 2020;13:880–885. doi: 10.1111/CTS.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irie K., Nakagawa A., Fujita H., Tamura R., Eto M., Ikesue H., Muroi N., Fukushima S., Tomii K., Hashida T. Population pharmacokinetics of favipiravir in patients with COVID‐19. Pharmacomet. Syst. Pharmacol. 2021;10:1161–1170. doi: 10.1002/PSP4.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sörgel F., Malin J.J., Hagmann H., Kinzig M., Bilal M., Eichenauer D.A., Scherf-Clavel O., Simonis A., El Tabei L., Fuhr U., et al. Pharmacokinetics of remdesivir in a COVID-19 patient with end-stage renal disease on intermittent haemodialysis. J. Antimicrob. Chemother. 2021;76:825–827. doi: 10.1093/JAC/DKAA500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corcione S., De Nicolò A., Montrucchio G., Scabini S., Avataneo V., Bonetto C., Mornese Pinna S., Cusato J., Canta F., Urbino R., et al. Real-life study on the pharmacokinetic of remdesivir in ICU patients admitted for severe COVID-19 pneumonia. Br. J. Clin. Pharmacol. 2021;87:4861–4867. doi: 10.1111/BCP.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Humeniuk R., Mathias A., Cao H., Osinusi A., Shen G., Chng E., Ling J., Vu A., German P. Safety, tolerability, and pharmacokinetics of Remdesivir, an antiviral for treatment of COVID‐19, in healthy subjects. Clin. Transl. Sci. 2020;13:896–906. doi: 10.1111/CTS.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tempestilli M., Caputi P., Avataneo V., Notari S., Forini O., Scorzolini L., Marchioni L., Bartoli T.A., Castilletti C., Lalle E., et al. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J. Antimicrob. Chemother. 2020;75:2977–2980. doi: 10.1093/JAC/DKAA239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/S12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palleria C., Di Paolo A., Giofrè C., Caglioti C., Leuzzi G., Siniscalchi A., De Sarro G., Gallelli L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013;18:601–610. [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Y., Figler B., Huang H., Tu Y.C., Wang J., Cheng F. Characterization of the mechanism of drug-drug interactions from PubMed using MeSH terms. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0173548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Obach R.S., Huynh P., Allen M.C., Beedham C. Human liver aldehyde oxidase: inhibition by 239 drugs. J. Clin. Pharmacol. 2004;44:7–19. doi: 10.1177/0091270003260336. [DOI] [PubMed] [Google Scholar]
- 86.Lemaitre F., Solas C., Grégoire M., Lagarce L., Elens L., Polard E., Saint-Salvi B., Sommet A., Tod M., Barin-Le Guellec C. Potential drug–drug interactions associated with drugs currently proposed for COVID-19 treatment in patients receiving other treatments. Fundam. Clin. Pharmacol. 2020;34:530–547. doi: 10.1111/FCP.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar D., Trivedi N. Disease-drug and drug-drug interaction in COVID-19: Risk and assessment. Biomed. Pharmacother. 2021;139 doi: 10.1016/J.BIOPHA.2021.111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armahizer M.J., Seybert A.L., Smithburger P.L., Kane-Gill S.L. Drug-drug interactions contributing to QT prolongation in cardiac intensive care units. J. Crit. Care. 2013;28:243–249. doi: 10.1016/J.JCRC.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 89.Yang K. What do we know about Remdesivir drug interactions? Clin. Transl. Sci. 2020;13:842–844. doi: 10.1111/CTS.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.COVID-19 Drug Interactions, University of Liverpool. Available online: 〈https://www.covid19-druginteractions.org/checker〉. (Accessed 6 January 2022).
- 91.Drugs.com Interactions Checker. Available online: 〈https://www.drugs.com/drug_interactions.html〉. (Accessed 6 January 2022).
- 92.Drugs.com Interactions Checker, Drug Interactions between Chloroquine, Hydroxychloroquine and Remdesivir. Available online: 〈https://www.drugs.com/interactions-check.php?drug_list=593-0,4146-0,1298-0〉. (Accessed 6 January 2022).
- 93.Wu R., Wang L., Kuo H.C.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., et al. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020;6:1–15. doi: 10.1007/S40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avigan® Tablets 200 mg, Prescribing Information. Available online: 〈https://www.cdc.gov.tw/Uploads/9401abe5-274f-4090-a67e-70d76d9d425c.pdf〉. (Accessed 6 January 2022).
- 95.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., Patil S., Barkate H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021;102:501–508. doi: 10.1016/J.IJID.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., Azarova V.N., Blinow A.A., Egorova A.N., Gordeev I.G., Ilin A.P., Karapetian R.N., Kravchenko D.V., et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin. Infect. Dis. 2021;73:531–534. doi: 10.1093/CID/CIAA1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaur R.J., Charan J., Dutta S., Sharma P., Bhardwaj P., Sharma P., Lugova H., Krishnapillai A., Islam S., Haque M., et al. Favipiravir use in COVID-19: analysis of suspected adverse drug events reported in the WHO database. Infect. Drug Resist. 2020;13:4427–4438. doi: 10.2147/IDR.S287934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir – a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020;6:45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gök S., Bahçecioğlu Ö.F., Durmuş M., Gün Z.Ü., Ersoy Y., Aytemur Z.A., Ulutaş Ö. The safety profile of favipiravir in COVID-19 patients with severe renal impairment. Int. J. Clin. Pract. 2021;75 doi: 10.1111/IJCP.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hassanipour S., Arab-Zozani M., Amani B., Heidarzad F., Fathalipour M., Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-90551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Avigan® 200mg (Favipiravir). Review Report. Available online: 〈https://www.pmda.go.jp/files/000210319.pdf〉. (Accessed 6 January 2022).
- 102.Łagocka R., Dziedziejko V., Kłos P., Pawlik A. Favipiravir in therapy of viral infections. J. Clin. Med. 2021;10:273. doi: 10.3390/JCM10020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020;6:1192–1198. doi: 10.1016/J.ENG.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen P.J., Chao C.M., Lai C.C. Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients. J. Infect. 2021;82:186–230. doi: 10.1016/J.JINF.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doi Y., Hibino M., Hase R., Yamamoto M., Kasamatsu Y., Hirose M., Mutoh Y., Homma Y., Terada M., Ogawa T., et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khamis F., Al Naabi H., Al Lawati A., Ambusaidi Z., Al Sharji M., Al Barwani U., Pandak N., Al Balushi Z., Al Bahrani M., Al Salami I., et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int. J. Infect. Dis. 2021;102:538–543. doi: 10.1016/J.IJID.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Udwadia Z.F., Singh P., Barkate H., Patil S., Rangwala S., Pendse A., Kadam J., Wu W., Caracta C.F., Tandon M. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int. J. Infect. Dis. 2021;103:62–71. doi: 10.1016/J.IJID.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lou Y., Liu L., Yao H., Hu X., Su J., Xu K., Luo R., Yang X., He L., Lu X., et al. Clinical outcomes and plasma concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur. J. Pharm. Sci. 2021;157 doi: 10.1016/J.EJPS.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Warren T., Jordan R., Lo M., Soloveva V., Ray A., Bannister R., Mackman R., Perron M., Stray K., Feng J., et al. Nucleotide prodrug GS-5734 is a broad-spectrum filovirus inhibitor that provides complete therapeutic protection against the development of Ebola virus disease (EVD) in infected non-human primates. Open Forum Infect. Dis. 2015;2:67–70. doi: 10.1093/OFID/OFV130.02. [DOI] [Google Scholar]
- 110.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6:672–683. doi: 10.1021/ACSCENTSCI.0C00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/S41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gérard A.O., Laurain A., Fresse A., Parassol N., Muzzone M., Rocher F., Esnault V.L.M., Drici M.D. Remdesivir and acute renal failure: a potential safety signal from disproportionality analysis of the WHO safety database. Clin. Pharmacol. Ther. 2021;109:1021–1024. doi: 10.1002/CPT.2145. [DOI] [PubMed] [Google Scholar]
- 113.Veklury® 100 mg Powder for Concentrate for Solution for Infusion. Summary of Product Characteristics. Available online: 〈https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_en.pdf〉. (Accessed 6 January 2022).
- 114.Sun D. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional benefit. AAPS J. 2020;22:77. doi: 10.1208/S12248-020-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.COVID-19 Treatment Guidelines Panel, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: 〈https://www.covid19treatmentguidelines.nih.gov/〉. (Accessed 6 January 2022).
- 116.Fan Q., Zhang B., Ma J., Zhang S. Safety profile of the antiviral drug remdesivir: an update. Biomed. Pharmacother. 2020;130 doi: 10.1016/J.BIOPHA.2020.110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., et al. Remdesivir for the treatment of Covid-19 – final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMOA2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Charan J., Kaur R.J., Bhardwaj P., Haque M., Sharma P., Misra S., Godman B. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev. Clin. Pharmacol. 2020;14:1–9. doi: 10.1080/17512433.2021.1856655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marc F., Moldovan C., Hoza A., Restea P., Sachelarie L., Romila L.E., Suteu C., Farcas D.M. Evaluation of hepatic biochemical parameters during antiviral treatment in COVID-19 patients. Biology. 2022;11:13. doi: 10.3390/biology11010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elshaboury R.H., Monk M.M., Bebell L.M., Bidell M.R., Adamsick M.L., Gandhi R.G., Paras M.L., Hohmann E.L., Letourneau A.R. Remdesivir use and outcomes during the FDA COVID-19 emergency use authorization period. Ther. Adv. Infect. Dis. 2021;8:1–11. doi: 10.1177/20499361211046669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai C.C., Chen C.H., Wang C.Y., Chen K.H., Wang Y.H., Hsueh P.R. Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2021;76:1962–1968. doi: 10.1093/JAC/DKAB093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh A.K., Singh A., Singh R., Misra A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab. Syndr. 2020;14:641–648. doi: 10.1016/J.DSX.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al-Abdouh A., Bizanti A., Barbarawi M., Jabri A., Kumar A., Fashanu O.E., Khan S.U., Zhao D., Antar A.A.R., Michos E.D. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Contemp. Clin. Trials. 2021;101 doi: 10.1016/J.CCT.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gholamhoseini M.T., Yazdi-Feyzabadi V., Goudarzi R., Mehrolhassani M.H. Safety and efficacy of Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. J. Pharm. Pharm. Sci. 2021;24:237–245. doi: 10.18433/JPPS31870. [DOI] [PubMed] [Google Scholar]
- 127.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., Oguchi G., Ryan P., Nielsen B.U., Brown M., et al. Early Remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 2021 doi: 10.1056/NEJMOA2116846/SUPPL_FILE/NEJMOA2116846_DATA-SHARING.PDF. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1–10. doi: 10.1001/JAMA.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.-Y., Nahass R.G., et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020;383:1827–1837. doi: 10.1056/NEJMOA2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Consortium W.S.T. Repurposed antiviral drugs for Covid-19 – interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMOA2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soman S.O., Raveendran A. Remdesivir and Favipiravir for COVID-19: an update. Ann. Clin. Cardiol. 2020;2:51–54. doi: 10.4103/2666-6979.297511. [DOI] [Google Scholar]
- 132.Qomara W.F., Primanissa D.N., Amalia S.H., Purwadi F.V., Zakiyah N. Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 treatment: a systematic review. Int. J. Gen. Med. 2021;14:8557–8571. doi: 10.2147/IJGM.S332458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumar R., Kumar Srivastava J., Singh R., Haris Siddiqui M., Mansouri R.A., Abdulhakim J.A., Bin-Jumah M.N., Alkahtani S., Abdel-Daim M.M., Uddin M.S. Available compounds with therapeutic potential against covid-19: Antimicrobial therapies, supportive care, and probable vaccines. Front. Pharmacol. 2020;11 doi: 10.3389/FPHAR.2020.582025/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kivrak A., Ulaş B., Kivrak H. A comparative analysis for anti-viral drugs: their efficiency against SARS-CoV-2. Int. Immunopharmacol. 2021;90 doi: 10.1016/J.INTIMP.2020.107232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lo M.K., Albariño C.G., Perry J.K., Chang S., Tchesnokov E.P., Guerrero L., Chakrabarti A., Shrivastava-Ranjan P., Chatterjee P., McMullan L.K., et al. Remdesivir targets a structurally analogous region of the Ebola virus and SARS-CoV-2 polymerases. Proc. Natl. Acad. Sci. USA. 2020;117:26946–26954. doi: 10.1073/PNAS.2012294117/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goldhill D.H., Te Velthuis A.J.W., Fletcher R.A., Langat P., Zambon M., Lackenby A., Barclay W.S. The mechanism of resistance to favipiravir in influenza. Proc. Natl. Acad. Sci. USA. 2018;115:11613–11618. doi: 10.1073/PNAS.1811345115/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Szemiel A.M., Merits A., Orton R.J., MacLean O.A., Pinto R.M., Wickenhagen A., Lieber G., Turnbull M.L., Wang S., Furnon W., et al. In vitro selection of Remdesivir resistance suggests evolutionary predictability of SARS-CoV-2. PLoS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Driouich J.S., Cochin M., Lingas G., Moureau G., Touret F., Petit P.R., Piorkowski G., Barthélémy K., Laprie C., Coutard B., et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat. Commun. 2021;12:1735. doi: 10.1038/S41467-021-21992-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nili A., Farbod A., Neishabouri A., Mozafarihashjin M., Tavakolpour S., Mahmoudi H. Remdesivir: a beacon of hope from Ebola virus disease to COVID-19. Rev. Med. Virol. 2020;30:1–13. doi: 10.1002/RMV.2133. [DOI] [PubMed] [Google Scholar]
- 140.Martinot M., Jary A., Fafi-Kremer S., Leducq V., Delagreverie H., Garnier M., Pacanowski J., Mékinian A., Pirenne F., Tiberghien P., et al. Emerging RNA-dependent RNA polymerase mutation in a remdesivir-treated B-cell immunodeficient patient with protracted coronavirus disease 2019. Clin. Infect. Dis. 2021;73:e1762–e1765. doi: 10.1093/CID/CIAA1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Majumdar P., Niyogi S. SARS-CoV-2 mutations: the biological trackway towards viral fitness. Epidemiol. Infect. 2021;149 doi: 10.1017/S0950268821001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajarshi K., Khan R., Singh M.K., Ranjan T., Ray S., Ray S. Essential functional molecules associated with SARS-CoV-2 infection: potential therapeutic targets for COVID-19. Gene. 2021;768 doi: 10.1016/J.GENE.2020.145313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiu S., Dick A., Ju H., Mirzaie S., Abdi F., Cocklin S., Zhan P., Liu X. Inhibitors of SARS-CoV-2 entry: current and future opportunities. J. Med. Chem. 2020;63:12256–12274. doi: 10.1021/ACS.JMEDCHEM.0C00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C.H., Lu C.H., Wong S.H., Lin L.T. Update on antiviral strategies against COVID-19: unmet needs and prospects. Front. Immunol. 2021;11 doi: 10.3389/FIMMU.2020.616595/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boggiano C., Eisinger R.W., Lerner A.M., Anderson J.M., Woodcock J., Fauci A.S., Collins F.S. Update on and future directions for use of anti–SARS-CoV-2 antibodies: National Institutes of Health Summit on Treatment and Prevention of COVID-19. Ann. Intern. Med. 2021;2 doi: 10.7326/M21-3669. (Advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.National Institute of Health, COVID-19 Treatment Guidelines. Anti-SARS-CoV-2 Monoclonal Antibodies. Available online: 〈https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/〉. (Accessed 26 January 2022).
- 147.Kabir M.A., Ahmed R., Chowdhury R., Iqbal S.M.A., Paulmurugan R., Demirci U., Asghar W. Management of COVID-19: current status and future prospects. Microbes Infect. 2021;23 doi: 10.1016/J.MICINF.2021.104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M.Á., Urquiza J., Ramírez D., Alonso C., Campillo N.E., et al. COVID-19: drug targets and potential treatments. J. Med. Chem. 2020;63:12359–12386. doi: 10.1021/ACS.JMEDCHEM.0C00606/SUPPL_FILE/JM0C00606_SI_001.PDF. [DOI] [PubMed] [Google Scholar]