Abstract
Dysembryoplastic neuroepithelial tumors (DNTs) are benign brain tumors classified as grade 1 in the 2021 World Health Organization (WHO) classification of central nervous system tumors. DNTs rarely undergo malignant transformation and cause symptomatic intracranial hemorrhage. We report a case of malignant transformation of DNT presenting with intraventricular hemorrhage and review the literature on malignant transformation of DNTs. An 18-year-old woman with a history of epilepsy presented with a sudden headache and vomiting. Radiological examination revealed a mass lesion in the left parietal lobe and intraventricular hemorrhage. The patient underwent an emergency craniotomy for brain tumor resection. The lesion was pathologically diagnosed as a malignant transformation of DNT. She had been followed up without tumor recurrence for 2 years after surgery.
Keywords: Dysembryoplastic neuroepithelial tumor, Malignant transformation, Intraventricular hemorrhage
Introduction
Dysembryoplastic neuroepithelial tumors (DNTs) are glioneuronal neoplasms arising from the cerebral cortex of children or young adults, typically with drug-resistant focal epilepsy, characterized by the occurrence of a pathognomonic glioneuronal element that may be associated with glial nodules and activating mutations in FGFR1 [1]. DNTs are benign brain tumors classified as grade 1 in the 2021 World Health Organization (WHO) classification of central nervous system tumors. DNTs rarely undergo malignant transformation and cause symptomatic intracranial hemorrhage [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Here, we report a case of malignant transformation of DNT presenting with intraventricular hemorrhage.
Case report
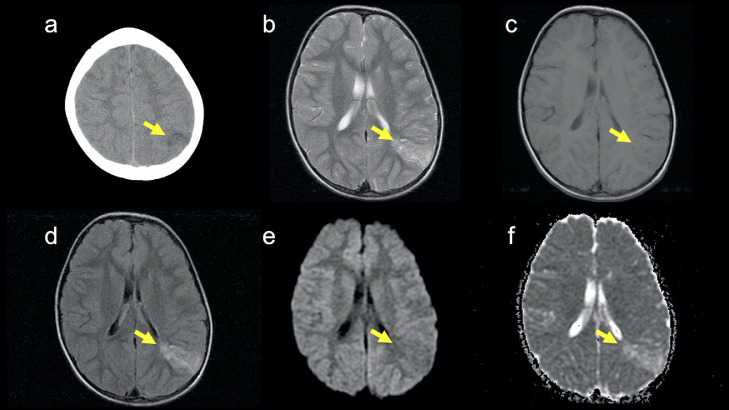
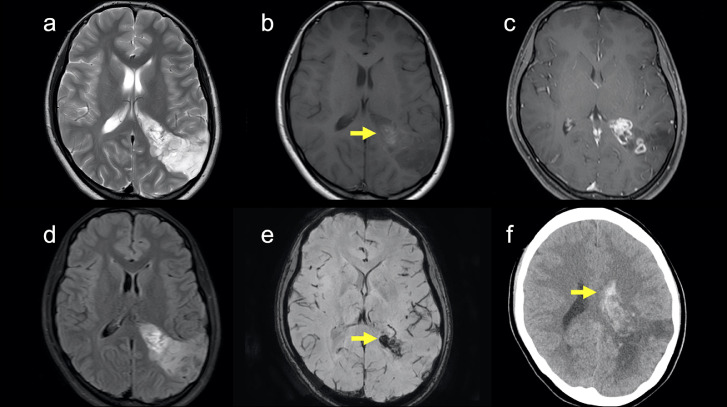
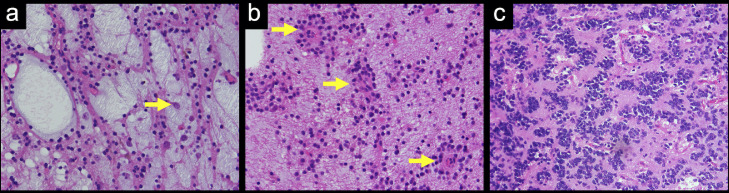
An 18-year-old woman with a history of epilepsy presented to our hospital for further investigation. When she was 4 years old, seizures occurred repeatedly. Hence, she was admitted to another hospital. Unenhanced computed tomography (CT) at the age of 4 revealed a hypoattenuation area in the left parietal lobe (Fig. 1). On magnetic resonance imaging (MRI) at the age of 4, the lesion was hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images and slightly hypointense on T1-weighted and diffusion-weighted images (Fig. 1). She was clinically diagnosed with epilepsy due to an old infarction in the left parietal lobe at another hospital and had taken antiepileptic drugs until 14 years of age. When she was 14 years old, she stopped taking antiepileptic drugs since seizures had stopped. She hoped to further investigate epilepsy at the age of 18 years, so she presented to our hospital, and imaging examinations were performed. On MRI at the age of 18 (Fig. 2), T2-weighted images revealed a hyperintense mass lesion from the left parietal cortex to the trigone of the left lateral ventricle. The lesion at the side of the trigone of the lateral ventricle was hyperintense, and the lesion at the side of the left parietal cortex was hypointense on T1-weighted images. Post-contrast-enhanced T1-weighted images showed several ring-enhanced areas in the mass lesion. The mass lesion was larger at the age of 18 years than at 4. She was suspected to have a low-grade brain tumor. Therefore, she was scheduled to undergo surgery for the mass lesion and was followed up. One month later, she was transported to our hospital because of a sudden headache and vomiting. An unenhanced CT scan after emergency transport revealed intraventricular and intratumoral hemorrhage (Fig. 2). The patient underwent emergency craniotomy for brain tumor resection. Most of the specimens showed specific glioneuronal elements, and some specimens showed a perivascular pseudorosette. In addition, some tumor cells showed high cellularity and marked nuclear atypia (Fig. 3). In the immunochemical analysis, the tumor cells showed immunoreactivity for OLIG2, S100, synaptophysin, MAP2, and ATRX and no immunoreactivity for GFAP, NeuN, IDH1-S, IDH1-H, BRAF V600E, and p53. Genetic analysis revealed FGFR1 D650G and K654E mutations in the tumor. Thus, the mass lesion was pathologically diagnosed as an anaplastic glioneuronal tumor, including DNT, namely malignant transformation of DNT. She had been followed up without tumor recurrence for 2 years after surgery.
Fig. 1.
CT and MRI at the age of 4. (A) An unenhanced CT image shows a hypoattenuation area in the left parietal lobe (arrow). (B) A T2-weighted image shows a hyperintense area from the left parietal cortex to the left parietal subcortical white matter (arrow). (C) A T1-weighted image shows a slightly hypointense area (arrow). (D) A FLAIR image shows a hyperintense area (arrow). (E) A diffusion-weighted image shows a slightly hypointense area (arrow). (F) An apparent diffusion coefficient map shows no diffusion restriction (arrow). Post-contrast-enhanced MRI was not performed at the age of 4.
Fig. 2.
CT and MRI at the age of 18. (A) A T2-weighted image shows a hyperintense area from the left parietal cortex to the left trigone of lateral ventricle. The lesion at the age of 18 is larger than that at the age of 4. (B) A T1-weighted image shows a hyperintense area (arrow) in the side of the trigone of the left lateral ventricle and a hypointense area in the side of the left parietal cortex. (C) A post-contrast-enhanced image shows several ring-enhanced areas in the lesion. (D) A FLAIR image shows a hyperintense area in the side of the left trigone of lateral ventricle and a hypointense area in the side of the left parietal cortex. (E) A susceptibility-weighted image shows a hypointense area (arrow) in the side of the trigone of the left lateral ventricle. This finding suggests intratumoral hemorrhage. (F) An unenhanced CT after emergency transport shows intraventricular hemorrhage (arrow).
Fig. 3.
Hematoxylin and eosin staining of the tumor. (A) The tumor cells exhibit oligodendroglia-like morphology embedded in mucoid matrix with floating neurons (arrow), which is the so-called specific glioneuronal element. (B) The tumor cells are arrayed radiating towards a capillary (arrows), which is a perivascular pseudorosette. (C) The tumor cells show high cellularity and marked nuclear atypia.
Discussion
To our knowledge, only 14 cases of malignant transformation of DNTs have been reported, including the present case [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. A summary of the cases of malignant transformation of DNTs is presented in Table 1. In DNTs with WHO grade 1, 70% of cases occur in the temporal lobe and 20%-30% of cases show enhancement on post-contrast-enhanced T1-weighted images [11,[17], [18], [19]]. Contrastingly, in the above 14 cases of malignant transformation of DNTs, 9 of 14 (65%) cases occurred outside the temporal lobe, and 13 of 14 (93%) cases showed enhancement on post-contrast-enhanced T1-weighted images. Therefore, in suspected DNT cases, malignant transformation of DNTs should be considered as differential diagnoses when tumors occur outside the temporal lobe and show enhancement on post-contrast-enhanced T1-weighted images, as in our case.
Table 1.
Summary of cases of malignant transformation of DNTs.
| Age (year) | Sex | Site | Enhancement/Hemorrhage | Pathology* | |
|---|---|---|---|---|---|
| Present case | 18 | F | P | +/+ | Anaplastic glioneuronal tumor |
| Matsumura et al. [4] | 1 | F | P | + / N/A | Anaplastic glioneuronal tumor |
| Heiland et al. [5] | 28 | M | O | + / N/A | Glioblastoma |
| Aggarwal et al. [6] | 29 | M | Fr | + / N/A | Diffuse astrocytoma |
| Moazzam et al. [7] | 22 | F | T | + / N/A | Oligoastrocytoma |
| Chuang et al. [8] | 2 | F | Fr, P | + / N/A | Glioblastoma |
| Chao et al. [9] | 15 | F | T | + / N/A | Diffuse astrocytoma |
| Mano et al. [10] | 4 | F | F | + / N/A | DNT and anaplastic oligodendroglioma |
| Thom et al. [11] | 56 | M | T | + / N/A | Anaplastic glioneuronal tumor |
| Ray et al. [12] | 12 | F | Fr, P | + / N/A | Anaplastic astrocytoma |
| Tsuboi et al. [13] | 35 | F | T | + / N/A | Anaplastic oligoastrocytoma |
| Hammond et al. [14] | 29 | M | Fr | + / N/A | Glioblastoma |
| Gonzales et al. [15] | 47 | F | Fr | + / N/A | Oligoastrocytoma |
| Rushing et al. [16] | 14 | M | T, P | N/A / N/A | Anaplastic astrocytoma |
DNT, dysembryoplastic neuroepithelial tumor; F, female; Fr, frontal lobe; M, male.
MRI, magnetic resonance imaging; N/A, not applicable; O, occipital lobe; P, parietal lobe; T, temporal lobe.
In the present case and the case of Mano et al. [10], primary tumors were malignant transformation of DNTs. In the other above cases, primary tumors were DNTs with WHO grade 1 and recurrent tumors were the above brain tumors with WHO grade 2, 3, or 4.
In DNTs with WHO grade 1, 2%-3% of cases have intratumoral hemorrhage [17,20]. Some authors have reported that DNTs with WHO grade 1 cause symptomatic intracranial hemorrhage [2,3]. In contrast, no previous case reports of malignant transformation of DNTs have mentioned hemorrhage. Our case suggests that malignant transformation of DNTs can also cause symptomatic intracranial hemorrhage.
In DNTs with WHO grade 1, 58%-82% of cases have FGFR1 mutations [21,22]. Matsumura et al. [4] reported a case with an identical FGFR1 mutation between primary DNT with WHO grade 1 and recurrent malignant transformation of DNT. Our case suggests that malignant transformation of DNTs can also result in FGFR1 mutations.
Conclusion
DNTs can undergo malignant transformation, cause symptomatic intracranial hemorrhage, and have FGFR1 mutations. In cases suspected of DNTs, malignant transformation of DNTs should be considered as differential diagnoses when tumors occur outside the temporal lobe and show enhancement on post-contrast-enhanced T1-weighted images.
Patient consent
A written informed consent was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Pietsch T, Ellison DW, Jacques TS, Hirose T, Varlet P, Schüller U., WHO Classification of Tumours Editorial Board . Central nervous system tumours [Internet] International Agency for Research on Cancer; Lyon (France): 2021. Dysembryoplastic neuroepithelial tumour.https://tumourclassification.iarc.who.int/chapters/45 [cited 2021 November 24th]. (WHO classification of tumours series, 5th ed.; vol. 6). Available from: [Google Scholar]
- 2.Singh S, Kedia S, Garg A, Kumar H, Singh G. DNET presenting with bleed: an infrequent event – Histopatho-radio-surgical report. Interdiscip Neurosurg. 2021;23 doi: 10.1016/j.inat.2020.100890. [DOI] [Google Scholar]
- 3.Pollo C, Pizzolato GP, Fransen P, Cox JN, Rilliet B. Dysembryoplastic neuroepithelial tumour as a cause of coma. J Clin Neurosci. 1998;5(4):453–457. doi: 10.1016/S0967-5868(98)90288-0. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura N, Natsume A, Maeda S, Aoki K, Yamazaki T, Nobusawa S, et al. Malignant transformation of a dysembryoplastic neuroepithelial tumor verified by a shared copy number gain of the tyrosine kinase domain of FGFR1. Brain Tumor Pathol. 2020;37(2):69–75. doi: 10.1007/s10014-020-00361-3. [DOI] [PubMed] [Google Scholar]
- 5.Heiland DH, Staszewski O, Hirsch M, Masalha W, Franco P, Grauvogel J, et al. Malignant transformation of a dysembryoplastic neuroepithelial tumor (DNET) characterized by genome-wide methylation analysis. J Neuropathol Exp Neurol. 2016;75(4):358–365. doi: 10.1093/jnen/nlw007. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal A, Salunke P, Sodhi HBS, Vasishta RK, Gowda KK. Dysembryoplastic neuroepithelial tumor transforming into malignancy: a case report. Neurol India. 2014;62(3):323–325. doi: 10.4103/0028-3886.137011. [DOI] [PubMed] [Google Scholar]
- 7.Moazzam AA, Wagle N, Shiroishi MS. Malignant transformation of DNETs: a case report and literature review. NeuroReport. 2014;25(12):894–899. doi: 10.1097/WNR.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 8.Chuang NA, Yoon JM, Newbury RO, Crawford JR. Glioblastoma multiforme arising from dysembryoplastic neuroepithelial tumor in a child in the absence of therapy. J Pediatr Hematol Oncol. 2014;36(8):e536–e539. doi: 10.1097/MPH.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 9.Chao L, Tao XB, Jun YK, Xia HH, Wan WK, Tao QS. Recurrence and histological evolution of dysembryoplastic neuroepithelial tumor: A case report and review of the literature. Oncol Lett. 2013;6(4):907–914. doi: 10.3892/ol.2013.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mano Y, Kumabe T, Shibahara I, Saito R, Sonoda Y, Watanabe M, et al. Dynamic changes in magnetic resonance imaging appearance of dysembryoplastic neuroepithelial tumor with or without malignant transformation. J Neurosurg Pediatr. 2013;11(5):518–525. doi: 10.3171/2013.1.PEDS11449. [DOI] [PubMed] [Google Scholar]
- 11.Thom M, Toma A, An S, Martinian L, Hadjivassiliou G, Ratilal B, et al. One hundred and one dysembryoplastic neuroepithelial tumors: an adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J Neuropathol Exp Neurol. 2011;70(10):859–878. doi: 10.1097/NEN.0b013e3182302475. [DOI] [PubMed] [Google Scholar]
- 12.Ray WZ, Blackburn SL, Casavilca-Zambrano S, Barrionuevo C, Orrego JE, Heinicke H, et al. Clinicopathologic features of recurrent dysembryoplastic neuroepithelial tumor and rare malignant transformation: a report of 5 cases and review of the literature. J Neurooncol. 2009;94(2):283–292. doi: 10.1007/s11060-009-9849-9. [DOI] [PubMed] [Google Scholar]
- 13.Tsuboi Y, Kurimoto M, Nagai S, Kamiyama H, Endo S. Malignant transformation of oligoastrocytoma: a case report. Brain Tumor Pathol. 2007;24(2):63–68. doi: 10.1007/s10014-007-0217-1. [DOI] [PubMed] [Google Scholar]
- 14.Hammond RR, Duggal N, Woulfe JM, Girvin JP. Malignant transformation of a dysembryoplastic neuroepithelial tumor. Case report. J Neurosurg. 2000;92(4):722–725. doi: 10.3171/jns.2000.92.4.0722. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales M, Dale S, Susman M, Nolan P, Ng WH, Maixner W, et al. Dysembryoplastic neuroepithelial tumor (DNT)-like oligodendrogliomas or Dnts evolving into oligodendrogliomas: two illustrative cases. Neuropathology. 2007;27(4):324–330. doi: 10.1111/j.1440-1789.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 16.Rushing EJ, Thompson LD, Mena H. Malignant transformation of a dysembryoplastic neuroepithelial tumor after radiation and chemotherapy. Ann Diagn Pathol. 2003;7(4):240–244. doi: 10.1016/s1092-9134(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 17.Campos AR, Clusmann H, von Lehe M, Niehusmann P, Becker AJ, Schramm J, et al. Simple and complex dysembryoplastic neuroepithelial tumors (DNT) variants: clinical profile, MRI, and histopathology. Neuroradiology. 2009;51(7):433–443. doi: 10.1007/s00234-009-0511-1. [DOI] [PubMed] [Google Scholar]
- 18.Nolan MA, Sakuta R, Chuang N, Otsubo H, Rutka JT, Snead OC, et al. Dysembryoplastic neuroepithelial tumors in childhood: long-term outcome and prognostic features. Neurology. 2004;62(12):2270–2276. doi: 10.1212/01.wnl.0000130495.69512.6f. [DOI] [PubMed] [Google Scholar]
- 19.Stanescu Cosson R, Varlet P, Beuvon F, Daumas Duport C, Devaux B, Chassoux F, et al. Dysembryoplastic neuroepithelial tumors: CT, MR findings and imaging follow-up: a study of 53 cases. J Neuroradiol. 2001;28(4):230–240. [PubMed] [Google Scholar]
- 20.Daghistani R, Miller E, Kulkarni AV, Widjaja E. Atypical characteristics and behavior of dysembryoplastic neuroepithelial tumors. Neuroradiology. 2013;55(2):217–224. doi: 10.1007/s00234-013-1135-z. [DOI] [PubMed] [Google Scholar]
- 21.Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, et al. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 2016;131(6):847–863. doi: 10.1007/s00401-016-1549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131(6):833–845. doi: 10.1007/s00401-016-1539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]