Abstract
Background
Anemic preterm infants may require red blood cell (RBC) transfusions to maintain sufficient oxygen supply to vital organs. Transfusion treatment, however, may have adverse intestinal effects. We aimed to investigate the short-term effects of RBC transfusions, hypothesizing to find signs of oxidative stress and intestinal injury, possibly related to levels of splanchnic (re-)oxygenation.
Methods
We prospectively included preterm infants (gestational age < 32 weeks). We measured urinary biomarkers for oxidative stress (8-isoprostane) and intestinal cell injury (intestinal fatty acid-binding protein, I-FABP) shortly before and after RBC transfusion. Splanchnic oxygen saturation (rsSO2) and rsSO2 variability were assessed simultaneously.
Results
Twenty-nine preterm infants received 58 RBC transfusions at various postnatal ages. Six of them developed necrotizing enterocolitis (NEC) after transfusion. Urinary 8-isoprostane and I-FABP increased following RBC transfusion (median 282–606 pg/ml and 4732–6968 pg/ml, p < 0.01), more pronounced in infants who developed NEC. Change in I-FABP correlated with change in 8-isoprostane (rho = 0.623, p < 0.01). Lower rsSO2 variability, but not higher mean rsSO2 was associated with higher 8-isoprostane and I-FABP levels after transfusion.
Conclusions
Preterm RBC transfusions are associated with concomitant signs of oxidative stress and intestinal injury, parallel with lower variability in splanchnic oxygenation. This may represent the early pathogenetic process of transfusion-associated NEC.
Impact
Red blood cell (RBC) transfusions in preterm infants are associated with a near 2-fold increase in urinary biomarkers for oxidative stress (8-isoprostane) and intestinal cell injury (intestinal fatty acid-binding protein, I-FABP).
Magnitude of change in I-FABP strongly correlated with the magnitude of 8-isoprostane change, suggesting a role for oxidative stress in the pathogenesis of intestinal injury.
Lower splanchnic oxygen saturation variability following RBC transfusion was associated with higher 8-isoprostane and I-FABP levels.
Loss of splanchnic variability after RBC transfusion may result from increased oxidative stress and its concomitant intestinal injury, possibly representing the early pathogenetic process of transfusion-associated necrotizing enterocolitis.
Introduction
The majority of very preterm infants receive at least one red blood cell (RBC) transfusion to treat anemia in the neonatal intensive care unit (NICU).1–4 RBC transfusions are beneficial to increase oxygen carrying capacity and improve tissue oxygenation of vital organs.5,6 RBC transfusion treatment, however, may have adverse effects. The RBC transfusion has been suggested to be an independent risk factor of poor clinical outcome in critically ill patients.7 Transfusions have been associated with an increased risk of death and several morbidities, one of the most severe being necrotizing enterocolitis (NEC).3,8,9 These serious risks for transfusion-associated NEC may be the result of oxidative stress and reperfusion/reoxygenation injury after hypoxia and/or ischemia in organs such as the brain and the intestines.8,10–14 Other researchers, however, did not find this association,15–20 but rather found an association between anemia severity before NEC development.4,21
Biomarkers providing insight in intestinal cell injury and oxidative stress, are intestinal fatty acid-binding protein (I-FABP) and 8-isoprostane, respectively.22,23 I-FABP is a protein, present in the intestinal epithelial cells, that is rapidly released into the circulation and excreted through the kidneys after intestinal cell injury.24 In preterm infants, urinary I-FABP has been shown to serve as a measure of intestinal injury and its severity.22,25 Intestinal injury after transfusion could be the result of oxidative stress. Isoprostanes are generated as free-radical products when oxidative stress occurs, defined as an imbalance between antioxidant capacity and reactive oxygen species (ROS) generation, and are considered reliable biomarkers of oxidative stress.23,26 Higher levels of 8-isoprostane post-treatment are most likely due to increased oxygen content and ROS levels, which makes it a systemic marker both for the detection and the progression of oxidative stress.23
The suggested role of an impaired balance between organ oxygen delivery and consumption before and after RBC transfusion can be investigated using near-infrared spectroscopy (NIRS). With NIRS, splanchnic tissue oxygen saturation (rsSO2) can be measured continuously and non-invasively.27,28 Previously, NIRS measurements have proven its added value in showing a different vasculatory response in infants developing NEC after RBC transfusion,29 contributing to the diagnosis of NEC,30,31 predicting complicated NEC,32 and predicting intestinal recovery after NEC.33 Decreased variability of rsSO2 might represent the diminished capacity for splanchnic vascular adaptation by the immature intestines.29–32
To increase the knowledge on both the beneficial and harmful short-term effects of an RBC transfusion in preterm infants, and its potential association with intestinal injury, the first aim of this study was to investigate the effect of RBC transfusions on urinary biomarkers for oxidative stress and intestinal cell damage in preterm infants. If there was such an effect, did these biomarkers correlate with each other? Secondly, we were interested in whether the course of splanchnic oxygen saturation and its variability before and after RBC transfusion were related to urinary 8-isoprostane and I-FABP levels. Thirdly, we explored whether there was a different effect of the RBC transfusion on urinary 8-isoprostane and I-FABP between infants who did and did not develop NEC within subsequent days after the transfusion.
Methods
Study design and study participants
We performed a prospective observational cohort study (STEP trial, registered online in the Dutch Trial Registry as NL6447) from March 2019 until December 2020. Between March 2020 and May 2020 inclusion was temporarily interrupted because of the restrictions due to Covid-19. We included preterm infants born at a gestational age (GA) < 32 weeks before 7 days postnatal age (PNA), who had been admitted to the level III-IV NICU of the University Medical Center Groningen (UMCG). We excluded infants with major congenital and cardiovascular defects, with an Apgar score < 5 at 5 min, with intraventricular hemorrhage > grade II according to Papile before study enrollment, and who previously received RBC transfusions. We also excluded infants of whom parents were unable to understand the Dutch language. The Medical Ethical Review Board of the UMCG approved the study (METc 2017/363), and written parental informed consent was obtained in all cases.
For the current study, we included infants in the analyses who received at least one RBC transfusion during the study period and in whom we were able to collect urine samples before and after transfusion. Transfusion thresholds were according to the liberal, Dutch anemia transfusion protocol.34 In short, Hb thresholds were 8.0 mmol/l (=12.9 g/dl) for infants on the first day after birth, and infants on ventilator support, 7.0 mmol/l (=11.3 g/dl) for infants on non-invasive respiratory support. Thereafter, it was 6.0 mmol/l (=9.7 g/dl) for clinically stable infants during the first 4 weeks, and 4.5 mmol/l (=7.3 g/dl) after the first 4 weeks. Each RBC transfusion consisted of a leukocyte-reduced, irradiated, parvovirus-B19 negative, blood type compatible “pedipack” of erythrocytes of 15 to 20 ml/kg administered during 3–5 h. The maximum storage time of each RBC transfusion unit prior to use was 42 days.
Urinary sample collection and analyses
We collected urine samples before and after each RBC transfusion. We collected the urine samples during regular moments of care, which occurred six times a day. Urine samples were collected from an indwelling catheter, if present for clinical reasons. If not, we placed a small cotton gauze in the diaper. The cotton gauze used for the sample before RBC transfusion was placed during the first period of regular care after the indication for transfusion was made. The cotton gauze used for sampling after RBC transfusion was placed during the first period of care after completed RBC transfusion. During the next care period, the cotton was squeezed into a sterile syringe pressing the urine into the urine tube. Since regular care periods occurred six times a day (approximately every four hours), the urine samples were collected within 4–6 h before and 4–6 h after a completed RBC transfusion. Next, the urine tubes were frozen at −80 °C until batch analysis.
Both the analysis of 8-isoprostane and I-FABP levels were performed blinded for patient characteristics and moment of collection. For determining the concentrations of 8-isoprostanes we used an EIA from Cayman Chemical. The standard curve had the highest level of 5000 pg/ml. Samples were diluted 100 times in EIA Buffer obtained with the test kit. Samples that did not fit in the standard curve were repeatedly tested ten times instead of a 100 times dilution. To determine the concentration of I-FABP we used sandwich ELISA, based on the capture and biotin-labeled detection antibodies from R&D Systems (Abingdon, UK). To quantify the amount of I-FABP we used a Streptavidin-HRP and OPD substrate. The standard curve had the highest level of 4000 pg/ml. Samples were diluted two times (1:2 dilution) in 0.1% BSA/PBS Buffer. The samples that were above 4000 pg/ml were determined again in fivefold diluted samples.
Splanchnic oxygen saturation
We monitored rsSO2 non-invasively using the INVOS 5100c oximeter in combination with neonatal sensors (Medtronic, Dublin, Ireland). The sensor was placed on the central abdomen under the umbilicus. During the first weeks after birth, NIRS measurements were performed continuously in all infants. After the first weeks, we measured rsSO2 from the moment that the indication for RBC transfusion was made until 24-h after completing RBC transfusion. All rsSO2 data were collected at 1 Hz and stored for off-line analysis.
Mean rsSO2 was calculated during three periods: a 2-h period before RBC transfusion, a 2-h period directly after RBC transfusion, and a 2-h period 24-h after completed RBC transfusion. Furthermore, we calculated the variability of rsSO2 by means of the coefficient of variation (CoVar, (SD/mean)*100%) for the same three periods.35 Finally, we calculated the difference in both mean rsSO2 and CoVar of rsSO2 between before RBC transfusion and 24-h after transfusion.
Clinical variables
We assessed the presence of in-hospital mortality and the development of NEC. NEC diagnosis was based on the appearance of pneumatosis intestinalis, portal venous gas, or both on abdominal radiological examination (NEC Bell’s stage ≥ IIA). We defined NEC onset as the time of first abdominal X-ray after clinical suspicion of NEC.
Further clinical data that we collected from the infants’ medical files included GA, birth weight (BW), sex, Hb levels before and after RBC transfusion, presence of a hemodynamic significant PDA, presence of blood-culture proven sepsis, and presence of mechanical ventilation.
Data analyses and statistics
We used SPSS Statistics version 24.0 (IBM Corp., Armonk, NY) for statistical analyses. Patient characteristics were described as the median, interquartile range (IQR), or n, percentage. We calculated differences between time periods using the Wilcoxon signed-rank test. Next, we assessed the association between urinary biomarkers using Spearman’s rank correlation test. Finally, we performed linear regression analyses to test whether changes in both Hb-level, splanchnic oxygen saturation, variability of rsSO2, and pre-transfusion storage time of the administered RBC transfusions were related to (the change in) urinary 8-isoprostane and I-FABP after RBC transfusion. A p-value < 0.05 was considered statistically significant.
Results
Participants and RBC transfusions
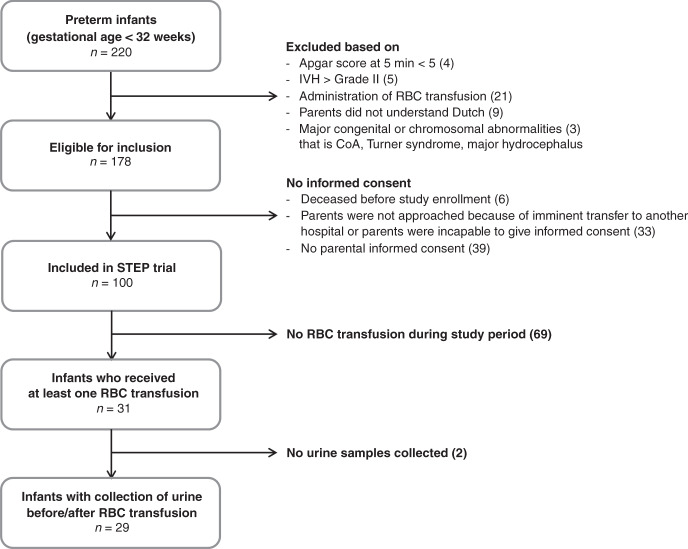
We included 29 infants in this study (Fig. 1). Their GAs ranged from 24.9 to 31.0 weeks, and their BWs from 630 to 1850 g. In fourteen infants, an RBC transfusion was given more than once. The study sample, therefore, consisted of 58 instances of RBC transfusion, which were administered between days 2 and 31 after birth. Pre-transfusion storage time varied between 2 and 8 days. Infants had a postmenstrual age between 25.0 and 32.3 weeks on the day of transfusion. Table 1 includes the baseline characteristics. Table 2 provides characteristics of the administered RBC transfusions.
Fig. 1. Flowchart of participants.
IVH intraventricular hemorrhage, RBC red blood cell, CoA coarctation of the aorta, NICU neonatal intensive care unit, STEP Study on Transfusion Effects in Preterm infants.
Table 1.
Clinical characteristics of participants.
| Median (P25–P75) or n (%) n = 58 RBC transfusions in 29 infants |
|
|---|---|
| Gestational age, weeks | 27.3 (25.9–27.9) |
| Birth weight, g | 865 (750–1128) |
| SGA | 8 (28%) |
| Sex, male | 14 (48%) |
| Apgar score at 5 min | 8 (6–8) |
| RBC transfusion volume, ml/kg | 15 (14–16) |
| PNA at day of RBC transfusion | 17 (10–24) |
| Mechanical ventilationa | 21 (72%) |
| During RBC transfusion | 37 (64%) |
| PDA during NICU stayb | 13 (45%) |
| NEC within 72 h after RBC transfusion | 6 (21%) |
| Blood-culture proven sepsis during NICU stay | 15 (54%) |
| Survival during NICU stay | 24 (83%) |
SGA small-for-gestational age (<10th percentile), RBC red blood cell, PNA postnatal age, PDA patent ductus arteriosus, NEC necrotizing enterocolitis, NICU neonatal intensive care unit.
aMechanical ventilation indicates synchronized intermittent positive pressure ventilation, synchronized intermittent mechanical ventilation, or high-frequency oscillation;
bPDA indicates a hemodynamically significant left-to-right shunt that, according to the team of neonatologists and cardiologists, required treatment, i.e., a symptomatic PDA.
Table 2.
Red blood cell transfusion characteristics.
| Median (P25–P75) or n (%) n = 58 red blood cell transfusions in 29 infants |
|
|---|---|
| Leukocyte-reduced | 58 (100%) |
| Irradiateda | 57 (98%) |
| Parvovirus-B19 negative | 58 (100%) |
| Pre-transfusion storage time, days | 4 (3–5) |
| Use of same pedipackb | 10/29 subsequent transfusions (34%) |
aIrradiated shortly before transfusing.
bPedi-pack indicates a maximum of four units from one donor.
Urinary biomarkers
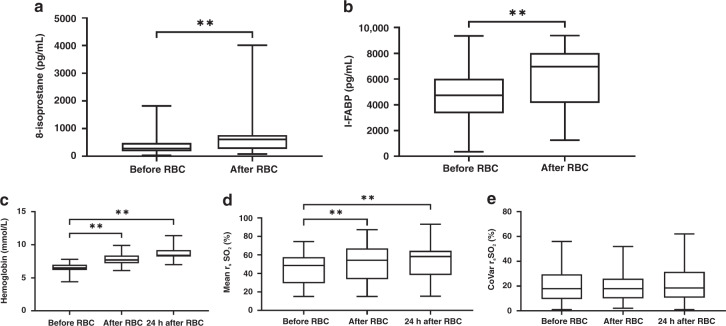
Figure 2a shows an increase in urinary 8-isoprostane levels following RBC transfusion, from median 282 to 606 pg/ml, p < 0.01 (Fig. 2a). Median increase was 235 pg/ml (IQR 27–540). Urinary I-FABP levels after RBC transfusion also increased compared with before RBC transfusion (median 4732 pg/ml versus 6968 pg/ml, p < 0.01, Fig. 2b). Median increase was 1975 pg/ml (IQR 934–3270).
Fig. 2. Urinary 8-isoprostane and I-FABP, hemoglobin level, and splanchnic oxygen saturation at several time points before and after red blood cell transfusion.
a 8-isoprostane before and after red blood cell transfusion. b I-FABP before and after red blood cell transfusion. c Hemoglobin level before, after, and 24 h after red blood cell transfusion. d Mean splanchnic oxygen saturation before, after and 24 h after red blood cell transfusion. e CoVar of splanchnic oxygen saturation before, after and 24 h after red blood cell transfusion. Data are presented as box-and-whisker plots; **p < 0.01; RBC red blood cell transfusion, I-FABP intestinal fatty acid-binding proteins, rsSO2 splanchnic tissue oxygen saturation, CoVar coefficient of variation (calculated as (SD/mean)*100%).
Urinary 8-isoprostane after RBC transfusion correlated with I-FABP after RBC transfusion (Spearman’s ρ = 0.276, p = 0.04). The magnitude of change (delta of 8-isoprostane levels following the transfusion) also correlated with the magnitude I-FABP levels changed (Spearman’s ρ = 0.623, p < 0.01).
Hemoglobin level, splanchnic oxygen saturation, and variability
In Fig. 2c, we present Hb-level before, after, and 24-h after RBC transfusion. Hemoglobin level increased after transfusion, and further increased up to 24-h later, p < 0.01. Median Hb gain after 24-h was 2.1 mmol/l (IQR 1.7–2.4).
Figure 2d, shows the course of rsSO2 from before RBC transfusion until 24-h after RBC transfusion. Median baseline rsSO2 was 49%. Following RBC transfusion, rsSO2 significantly increased and a further increase occurred 24-h after RBC transfusion. Median rsSO2 increase from before to 24-h after transfusion was 11% (IQR −3 to 21). In 6 (10%) cases, rsSO2 was lower after transfusion compared with before. In 15 (26%) cases, rsSO2 decreased 24-h after RBC transfusion compared with before transfusion.
Variability of rsSO2 as expressed by CoVar is presented in Fig. 2e. Median CoVar of rsSO2 neither differed between before and after RBC transfusion, nor between before RBC transfusion and 24-h after RBC transfusion.
Relation between hemoglobin levels, splanchnic oxygen saturation, and urinary biomarkers
Hemoglobin level before RBC transfusion correlated with the magnitude of change (delta) in 8-isoprostane levels (Spearman’s ρ = 0.364, p = 0.01). Regarding splanchnic oxygen saturation before RBC transfusion, the correlation coefficient with a magnitude of change of 8-isoprostane levels following RBC transfusion just failed to reach significance (Spearman’s ρ = −0.276, p = 0.06).
In Table 3A, we present the results of the analyses on urinary 8-isoprostane. We found no significant associations between changes before and after transfusion of Hb or rsSO2 and urinary 8-isoprostane levels. Decreased CoVar of rsSO2 was associated with both higher levels of urinary 8-isoprostane after RBC transfusion and a higher increase of urinary 8-isoprostane after transfusion.
Table 3.
(A) Hemoglobin level, splanchnic oxygen saturation, and pre-transfusion storage time in relation to change in urinary 8-isoprostane level during RBC transfusion, as analyzed by univariable linear regression analyses. (B) Hemoglobin level, splanchnic oxygen saturation, and pre-transfusion storage time in relation to change in urinary I-FABP level during RBC transfusion, as analyzed by univariable linear regression analyses.
| (A) | Urinary 8-isoprostane levels after RBC transfusion | Delta urinary 8-isoprostane levels before–after RBC transfusion | ||
|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | |
| Delta hemoglobin before – 24 h after RBC transfusion, mmol/L | −194 (−486; 99) | 0.19 | −186 (−443; 71) | 0.15 |
| Delta mean rsSO2 before – 24 h after RBC transfusion, per 10% | 87 (−53; 228) | 0.22 | −14 (−137; 110) | 0.82 |
| Delta CoVar rsSO2 before – 24 h after RBC transfusion, per 10% | −186 (−348; −24) | 0.02 | −157 (−295; −19) | 0.03 |
| Pre-transfusion storage time, days | 70 (−141; 273) | 0.53 | 102 (−85; 289) | 0.28 |
| (B) | Urinary I-FABP levels after RBC transfusion | Delta urinary I-FABP levels before–after RBC transfusion | ||
|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | |
| Delta hemoglobin before – 24 h after RBC transfusion, mmol/l | −917 (−1705; −129) | 0.02 | −954 (−1661; −246) | <0.01 |
| Delta mean rsSO2 before – 24 h after RBC transfusion, per 10% | −149 (−513; 215) | 0.41 | −400 (−738; −62) | 0.02 |
| Delta CoVar rsSO2 before – 24 h after RBC transfusion, per 10% | −479 (−895; −63) | 0.03 | −187 (−612; 238) | 0.38 |
| Pre-transfusion storage time, days | 442 (−123; 1006) | 0.12 | 256 (−288; 801) | 0.35 |
B unstandardized coefficient, CI confidence interval, RBC red blood cell, I-FABP intestinal fatty acid-binding protein, rsSO2 splanchnic tissue oxygen saturation, CoVar coefficient of variation.
Statistically significant p = 0.05 values are in bold.
In Table 3B, we present the results of the regression analyses on urinary I-FABP. We found that the magnitude of increase (delta) of Hb and rsSO2 was associated with lower urinary I-FABP after RBC transfusion. Furthermore, we found that a decrease in CoVar of rsSO2 was associated with higher actual levels of urinary I-FABP after transfusion.
The pre-transfusion storage time of RBC transfusions did not influence the degree of oxidative stress or intestinal injury (Table 3A, B).
Development of NEC within subsequent days
In Table 4, we present the median (IQR), minimum, and maximum change in hemoglobin level, splanchnic oxygen saturation, and urinary biomarkers between before and after RBC transfusion, both for all infants, for infants who did not develop NEC within 72-h after RBC transfusion, and for infants who did develop NEC within 72-h after RBC transfusion.
Table 4.
Change in hemoglobin level, splanchnic oxygen saturation, and urinary biomarkers after RBC transfusions, compared with before transfusion.
| Median (IQR) | Smallest; greatest | Median (IQR) | Smallest; greatest | Median (IQR) | Smallest; greatest | |
|---|---|---|---|---|---|---|
| All RBC transfusions in all infants | BC transfusions in infants, who did not develop NEC < 72 h after RBCT | RBC transfusions in infants, who developed NEC < 72 h after RBCT | ||||
| Hemoglobin level, mmol/l (before – 24 h after) | 2.1 (1.7; 2.4) | 0.7; 3.9 | 2.2 (1.7; 2.7) | 0.7; 3.9 | 1.8 (1.1; 1.9) | 0.9; 2.3 |
| Mean rsSO2, % (before – 24 h after) | 11 (−3; 21) | −38; 47 | 12 (−9; 21) | −38; 47 | 14 (−5; 21) | −10; 21 |
| CoVar rsSO2, % (before – 24 h after) | −2 (−6; 9) | −36; 41 | −1 (−5; 10) | −14; 39 | −22 (−34; 2) | −36; 8 |
| Urinary 8-isoprostane, pg/ml (before–after) | 235 (27; 540) | −727; 2816 | 146 (−1; 491) | −727; 1512 | 1105 (706; 2410) | 603; 2816 |
| Urinary I-FABP, pg/ml (before–after) | 1975 (934; 3270) | −3519; 4523 | 1446 (201; 2646) | −3519; 3896 | 3174 (2205; 4344) | 2137; 4479 |
IQR interquartile range, RBC red blood cell, I-FABP intestinal fatty acid-binding protein, rsSO2 splanchnic tissue oxygen saturation, CoVar coefficient of variation.
Six infants developed NEC within 6–72-h after RBC transfusion. In infants who subsequently developed NEC, urinary 8-isoprostane increased after RBC transfusion with a median 1105 pg/ml (IQR 706–2411) compared with 111 pg/ml (IQR −90–491) in infants who did not develop NEC, p < 0.01. Median increase in urinary I-FABP was 3174 pg/ml (IQR 2205–4333) in infants who developed NEC after RBC transfusion versus 1446 pg/ml (IQR 201–2646) in infants who did not develop NEC, p = 0.02.
Infants who developed NEC after transfusion had a higher loss of splanchnic oxygen saturation variability 24-h after RBC transfusion compared with infants who did not develop NEC. Change in CoVar of rsSO2 24-h after transfusion compared with before was median −22% in NEC cases (IQR −32 to 2) versus −1% (IQR −5 to 10) in infants who did not develop NEC, p = 0.03.
Discussion
In this study, we demonstrated an increase in urinary 8-isoprostane and I-FABP following RBC transfusion in anemic preterm infants. The magnitude in which both biomarkers increased, strongly correlated with each other. Splanchnic oxygen saturation also increased after RBC transfusion, even up to 24-h later. A higher increase in both hemoglobin and splanchnic oxygen saturation 24-h after RBC transfusion was associated with a lower rise in urinary I-FABP after RBC transfusion, but not with rise in urinary 8-isoprostane. Furthermore, we found that particularly diminished rsSO2 variability after RBC transfusion was associated with higher actual levels of both urinary I-FABP and 8-isoprostane after transfusion, and a higher rise of 8-isoprostane after RBC transfusion. Finally, the magnitude in which urinary 8-isoprostane and I-FABP increased after RBC transfusion was more pronounced in infants who developed NEC within subsequent days than in those who did not.
We found higher levels of urinary 8-isoprostane and I-FABP after RBC transfusion compared with before. The increase in 8-isoprostane was strongly associated with the increase in I-FABP, suggesting that oxidative stress plays an important role in the pathogenesis of intestinal injury. The higher levels of 8-isoprostane after RBC transfusion might show hypoxia-reoxygenation injury of the transfusion. Oxidative stress can typically occur during post-hypoxic reperfusion, such as during and after RBC transfusion. Excess production of ROS has been shown following hypoxia–reperfusion and has been suggested to be one of the most likely contributors to reperfusion-induced oxidative stress.36,37
The change in splanchnic oxygen saturation was not associated with a level of oxidative stress as assessed by 8-isoprostane. Our results suggest that an increase in rsSO2 may not (fully) explain the oxidative stress following RBC transfusion. This may be caused by the fact that rsSO2 is the result of the balance between oxygen supply and consumption. When intestinal oxygen consumption diminishes, for example as a result of intestinal injury, rsSO2 will rise, while oxygen supply does not. Another explanation for not finding the relation between rsSO2 increase and oxidative stress, might be that isoprostane levels have been associated with several other complications of prematurity, related to injury by free radicals, such as a PDA, intraventricular hemorrhage, and bronchopulmonary dysplasia.38,39 We, however, found a significant increase in a relatively short period, which supports the notion that the increased oxidative stress is, at least partly, associated with the RBC transfusion. Furthermore, in contrast to the actual levels of rsSO2, a decrease in rsSO2 variability, as expressed by the CoVar, was associated with both higher actual levels of 8-isoprostane after RBC transfusion and the magnitude in which 8-isoprostane increased after transfusion. We speculate that, as a result of the oxidative stress, the injured intestinal wall shows a reduced variability in rsSO2.
The higher levels of urinary I-FABP we found after RBC transfusion compared with before, are suggestive for intestinal injury following RBC transfusion. Elevated levels of I-FABP represent intestinal epithelial cell injury,22 supporting the hypothesis of the intestines being vulnerable for injury following RBC transfusion, possibly worsened by anemia severity before RBC transfusion. These results suggest that RBC transfusions cause intestinal injury. In our series, the magnitude by which I-FABP increased may reflect a predilection toward NEC. In case of NEC development, however, previously it was demonstrated that the increase in I-FABP particularly occurred shortly before or just after clinical symptoms of NEC,24,25 which in our population occurred in six infants within 72 h after RBC transfusion. Furthermore, the urinary I-FABP levels we measured were rather low compared to I-FABP levels previously related to NEC development.25,33 Even so, I-FABP has a short half-life, making it a relatively time-sensitive marker, implying that elevated levels of I-FABP after RBC transfusion represent either mild or more severe intestinal injury, in some cases followed by NEC.
We contemplated on how we could explain our findings pathogenetically. A decrease in rsSO2 variability, was associated with higher levels of urinary 8-isoprostane and I-FABP after RBC transfusion. Intestinal injury in preterm infants has previously been reported to be associated with a diminished rsSO2 variability.29–31,40 Our results are in line with those associations, as the presence of reduced variability co-occurred with higher urinary levels of biomarkers for intestinal cell injury after the RBC transfusion. This indicates that intestinal epithelial injury is reflected by an inadequate splanchnic vasculatory response, possibly due to an inability to adapt to changes in the balance between oxygen supply and demand. Whether the diminished variability of splanchnic oxygenation is the cause or the result from intestinal injury cannot be deducted from our data. Our data, however, do indicate that oxidative stress plays a key role, because it is also related to markers of intestinal injury and diminished splanchnic variability after RBC transfusion. We speculate therefore that increased oxidative stress and its concomitant intestinal injury are part of the pathogenesis of intestinal epithelial injury following RBC transfusion, potentially resulting in NEC.
Concerning splanchnic oxygenation during and after RBC transfusion, we confirmed previously published results on increased rsSO2 following RBC transfusions in preterm infants.27,30 The increased rsSO2 lasted even up to 24-h after completing RBC transfusion. Remarkably, we found that a higher increase in splanchnic oxygenation was associated with a lower increase or decrease in urinary I-FABP after RBC transfusion. We speculate that high or low actual levels of splanchnic oxygen saturation might be less sensitive for oxygen supply, possibly as a result of the constant changing balance between splanchnic oxygen supply and demand. The conflicting results on high and low rsSO2 in infants who developed (un-)complicated NEC may support this hypothesis.24,32,41,42 Alternative explanations for the association between less increase in rsSO2 and more increase in urinary I-FABP following RBC transfusion may be either ongoing anemia/anemic hypoxia or that the splanchnic circulation is already affected as a result of intestinal injury due to the earlier hypoxic state.
Infants who subsequently developed NEC after transfusion showed a larger increase in urinary 8-isoprostane and I-FABP compared to infants who did not develop NEC. These findings, combined with the lower gain in splanchnic oxygen saturation, may demonstrate the susceptibility of the preterm splanchnic vasculature to intestinal injury and might support the hypothesis of NEC development after RBC transfusion. Of course, multiple factors are related to NEC etiology including prematurity, enteral feeding, diminished splanchnic perfusion, an altered intestinal microbiota, and excessive inflammation responses.43–45 Moreover, preterm infants that receive an RBC transfusion are mostly the smaller, younger, and sicker ones. Therefore, they already have a higher risk of developing NEC, as can be observed in the high NEC incidence in our selected population.
We are aware of several limitations of our study. First, we performed the study with a relatively small sample size. The single-center design allowed us to minimize variations in sample collection and laboratory procedures. Second, two infants could not be included in this analysis because of missing urine samples due to logistic reasons. All data, however, were collected prospectively. Third, we cannot rule out other factors that may have affected urinary 8-isoprostane and I-FABP levels. We, however, compared the measurements within a relatively short period before and after RBC transfusion.
Conclusion
RBC transfusions are associated with an increase in both 8-isoprostane and I-FABP, urinary biomarkers for oxidative stress and intestinal injury, in preterm infants. The extent to which both biomarkers increased strongly correlated, suggesting a role for oxidative stress in the pathogenesis of intestinal injury following RBC transfusion. RBC transfusions increased splanchnic oxygen saturation even up to 24-h after transfusion, but splanchnic oxygenation was not related to the level of oxidative stress. However, a lower splanchnic oxygen saturation variability after RBC transfusion was associated with higher levels of urinary 8-isoprostane and I-FABP and its changes before and after, suggesting reduced vascular adaptation capabilities. Loss of splanchnic variability may thus be a result of the increased oxidative stress and its concomitant intestinal injury following transfusion. This may represent the early pathogenetic process of transfusion-associated NEC.
Acknowledgements
We greatly acknowledge the patients and their parents for participating. We also acknowledge Prof. Dr. E.M. Dempsey for the critical revision of the manuscript for important intellectual content. Furthermore, we would like to thank the nurses and medical staff for their help with data collection. Finally, we thank Ms. A.J. Olthuis, Ms. H.A. Bouma, and Ms. D.H. Baptist for their help with the inclusion of infants and data collection. This study was part of the research program of the Graduate School of Medical Sciences, Research Institute SHARE, University of Groningen.
Author contributions
W.S.K. conceptualized and designed the study, collected data, carried out and interpreted the analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript for important intellectual content. A.F.B. conceptualized and designed the study, supervised and interpreted the analyses, and critically reviewed and revised the manuscript for important intellectual content. K.A.B. conceptualized and designed the study, and critically reviewed and revised the manuscript for important intellectual content. W.v.O. performed laboratory analyses, and critically reviewed and revised the manuscript for important intellectual content. J.B.F.H. conceptualized and designed the study, and critically reviewed and revised the manuscript for important intellectual content. E.M.W.K. conceptualized and designed the study, coordinated and supervised data collection, supervised and interpreted the analyses, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
Ms. W.S. Kalteren received a grant from the Tekke Huizinga Fonds, Groningen, the Netherlands. Ms. W.S. Kalteren also received financial support from the Junior Scientific Master Class of the University of Groningen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare no competing interests.
Consent statement
All parents or guardians of neonates provided written informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J. Pediatr. 2009;155:331–337. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss RG. Anaemia of prematurity: pathophysiology and treatment. Blood Rev. 2010;24:221–225. doi: 10.1016/j.blre.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dos Santos AMN, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight infants. J. Pediatr. 2011;159:371–376. doi: 10.1016/j.jpeds.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Patel RM, et al. Association of red blood cell transfusions, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–897. doi: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank JP, et al. The role of RBC transfusion in the premature infant. Am. J. Dis. Child. 1984;138:831–833. doi: 10.1001/archpedi.1984.02140470031010. [DOI] [PubMed] [Google Scholar]
- 6.Van Hoften JCR, Verhagen EA, Keating P, ter Horst HJ, Bos AF. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch. Dis. Child. Fetal Neonatal Ed. 2010;95:F352–F358. doi: 10.1136/adc.2009.163592. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y-C, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr. Neonatol. 2017;58:216–222. doi: 10.1016/j.pedneo.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–540. doi: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 9.Ghirardello S, et al. Effects of red blood cell transfusions on the risk of developing complications or death: an observational study of a cohort of very low birth weight infants. Am. J. Perinatol. 2017;34:88–95. doi: 10.1055/s-0036-1584300. [DOI] [PubMed] [Google Scholar]
- 10.Blau J, et al. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J. Pediatr. 2011;158:403–409. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Josephson CD, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? J. Pediatr. 2010;157:972–978. doi: 10.1016/j.jpeds.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul DA, et al. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–641. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 13.Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS. Transfusion-associated necrotizing enterocolitis in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2013;98:F10–F14. doi: 10.1136/fetalneonatal-2011-301282. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham KE, Okolo FC, Baker R, Mollen KP, Good M. Red blood cell transfusion in premature infants leads to worse necrotizing enterocolitis outcomes. J. Surg. Res. 2017;213:158–165. doi: 10.1016/j.jss.2017.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallenstein MB, et al. Red blood cell transfusion is not associated with necrotizing enterocolitis: a review of consecutive transfusions in a tertiary neonatal intensive care unit. J. Pediatr. 2014;165:678–682. doi: 10.1016/j.jpeds.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R, et al. Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J. Perinatol. 2014;34:858–862. doi: 10.1038/jp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirpalani H, Zupancic JA. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Semin. Perinatol. 2012;36:269–276. doi: 10.1053/j.semperi.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Hyung N, et al. The relationship of red blood cell transfusion to intestinal mucosal injury in premature infants. J. Pediatr. Surg. 2017;52:1152–1155. doi: 10.1016/j.jpedsurg.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Le VT, Klebanoff MA, Talavera MM, Slaughter JL. Transient effects of transfusion and feeding advances (volumetric and caloric) on necrotizing enterocolitis development: a case-crossover study. PLoS ONE. 2017;12:e0179724. doi: 10.1371/journal.pone.0179724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay S, Zupancic JAF, Flannery DD, Kirpalani H, Dukhovny D. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin. Perinatol. 2017;41:80–91. doi: 10.1053/j.semperi.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J. Perinatol. 2011;31:176–182. doi: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schurink M, et al. Intestinal fatty acid-binding protein as a diagnostic marker for complicated and uncomplicated necrotizing enterocolitis: a prospective cohort study. PLoS ONE. 2015;10:e0121336. doi: 10.1371/journal.pone.0121336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belik J, Gonzalez-Luis GE, Perez-Vizcaino F, Villamor E. Isoprostanes in fetal and neonatal health and disease. Free Radic. Biol. Med. 2010;48:177–188. doi: 10.1016/j.freeradbiomed.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Schat TE, et al. The relation between splanchnic ischaemia and intestinal damage in necrotizing enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2016;101:F533–F539. doi: 10.1136/archdischild-2015-309838. [DOI] [PubMed] [Google Scholar]
- 25.Schurink M, et al. Intestinal fatty acid-binding protein in neonates with imminent necrotizing enterocolitis. Neonatology. 2014;106:49–54. doi: 10.1159/000358582. [DOI] [PubMed] [Google Scholar]
- 26.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid. Redox Signal. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 27.Seidel D, et al. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J. Perinatol. 2013;33:282–287. doi: 10.1038/jp.2012.108. [DOI] [PubMed] [Google Scholar]
- 28.Mintzer JP, Moore JE. Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions. Pediatr. Res. 2019;86:296–304. doi: 10.1038/s41390-019-0466-9. [DOI] [PubMed] [Google Scholar]
- 29.Kalteren WS, et al. Red blood cell transfusions affect intestinal and cerebral oxygenation differently in preterm infants with and without subsequent necrotizing enterocolitis. Am. J. Perinatol. 2018;35:1031–1037. doi: 10.1055/s-0038-1636532. [DOI] [PubMed] [Google Scholar]
- 30.Cortez J, et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern. Fetal Neonatal Med. 2011;24:574–582. doi: 10.3109/14767058.2010.511335. [DOI] [PubMed] [Google Scholar]
- 31.Van der Heide, M., Hulscher, J. B. F., Bos, A. F. & Kooi, E. M. W. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr. Res. 90, 148–155 (2021). [DOI] [PubMed]
- 32.Schat TE, et al. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS ONE. 2016;11:e0154710. doi: 10.1371/journal.pone.0154710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuik SJ, et al. Predicting intestinal recovery after necrotizing enterocolitis in preterm infants. Pediatr. Res. 2020;87:903–909. doi: 10.1038/s41390-019-0634-y. [DOI] [PubMed] [Google Scholar]
- 34.Von Lindern JS, Brand A, Lopriore E. Revision of the guideline ‘Blood transfusion’: for the newborn. Ned. Tijdschr. Geneeskd. 2012;156:A4795. [PubMed] [Google Scholar]
- 35.Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinat. Med. 2014;7:199–206. doi: 10.3233/NPM-14814035. [DOI] [PubMed] [Google Scholar]
- 36.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 37.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahola T, Fellman V, Kjellmer I, Raivio KO, Lapatto R. Plasma 8-isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatr. Res. 2004;56:88–93. doi: 10.1203/01.PDR.0000130478.05324.9D. [DOI] [PubMed] [Google Scholar]
- 39.Coviello C, et al. Isoprostanes as biomarker for patent ductus arteriosus in preterm infants. Front. Pediatr. 2020;8:3–6. doi: 10.3389/fped.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near-infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001;27:1401–1407. doi: 10.1007/s001340100994. [DOI] [PubMed] [Google Scholar]
- 41.Patel AK, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr. Crit. Care Med. 2014;15:735–741. doi: 10.1097/PCC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 42.Schat TE, et al. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early Hum. Dev. 2019;131:75–80. doi: 10.1016/j.earlhumdev.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 44.Neu J, Walker WA. Necrotizing enterocolitis. N. Engl. J. Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin. Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]