Abstract
Background
Regdanvimab (CT-P59) is a neutralizing antibody authorized in Republic of Korea for the treatment of adult patients with moderate or mild-COVID-19 who are not on supplemental oxygen and have high risk of progressing to severe disease (age ≥ 50 years or comorbidities). This study evaluated the clinical efficacy, safety and medical utilization/costs associated with real-world regdanvimab therapy.
Methods
This non-interventional, retrospective cohort study included adult patients with confirmed mild-to-moderate SARS-CoV-2 infection. Patients treated with regdanvimab were compared with controls who had received other therapies. The primary endpoint was the proportion of patients progressing to severe/critical COVID-19 or death due to SARS-CoV-2 infection up to Day 28. Propensity score matching was applied to efficacy analyses.
Results
Overall, 552 patients were included in the Safety and Efficacy Sets (regdanvimab, n = 156; control, n = 396) and 274 patients in the propensity score–matched (PSM) Efficacy Set (regdanvimab, n = 113; control, n = 161). In the PSM Set, the risk of severe/critical COVID-19 or death was significantly lower in the regdanvimab group (7.1% vs 16.1%, P = 0.0263); supplemental oxygen was required by 8.0% and 18.6% of patients in the regdanvimab and control groups, respectively (P = 0.0128). There were no unexpected safety findings in the regdanvimab group. Medical utilization analysis showed an overall cost reduction with regdanvimab compared with control treatments.
Conclusions
Regdanvimab significantly reduced the proportion of patients progressing to severe/critical disease or dying of SARS-CoV-2 infection. This study shows the potential benefits of regdanvimab in reducing disease severity and improving medical utility in patients with COVID-19.
Keywords: COVID-19, CT-P59, Pneumonia, Outcome
1. Introduction
To date, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused>265 million confirmed cases of coronavirus disease 2019 (COVID-19) and>5.2 million COVID-19–related deaths globally [1]. A highly successful, mass immunization program is currently being rolled out worldwide, but this represents a huge undertaking and it will be a number of years before the virus is controlled on a global scale [2]. Infection and mortality rates continue to increase in many regions, particularly in developing countries where immunization rates are lower owing to the lack of vaccine supply/uptake and scarcity of public health infrastructure and primary care facilities [3]. While the COVID-19 vaccines have been developed extremely quickly using new technologies, their effectiveness appears to wane over time [4], [5]. Limited duration of protection requires booster doses, yet the emergence of SARS-CoV-2 variants may reduce the effectiveness of existing vaccines against new viral strains [6], [7]. The emergence of new SARS-CoV-2 variants with higher transmissibility is therefore likely to present many ongoing challenges to public health services resulting from the consequent increase in COVID-19 cases, hospitalizations and deaths [3], [8], [9].
The urgent need for effective new treatments has led to an unprecedented research effort, with clinical evaluation of many potential therapeutic candidates, including antiviral drugs, anti-inflammatory agents, anticoagulants, antifibrotics and targeted immunomodulatory therapies [10]. One possible therapeutic approach involves targeting of the SARS-CoV-2 spike (S) protein, which is responsible for entry of the coronavirus into host cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor located on epithelial and endothelial cells [11], [12]. Indeed, proof of principle for the use of antibody therapy that targets the SARS-CoV-2 S protein has been demonstrated in several studies [13], [14], [15], [16], and clinical studies have reported reduced hospitalizations, clinical symptoms and viral titers in patients with SARS-CoV-2 infection administered antibodies to the S protein [12], [17], [18], [19].
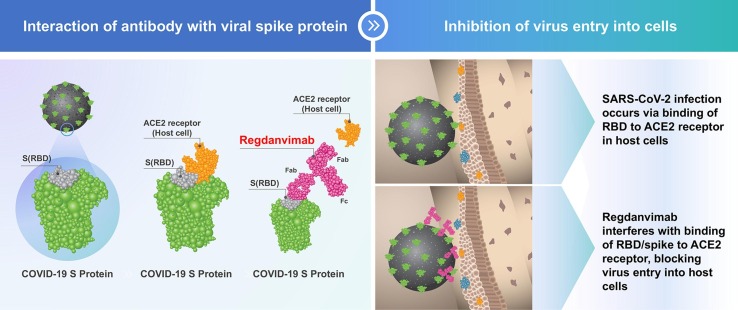
Regdanvimab (CT-P59) is a potent neutralizing antibody against various SARS-CoV-2 isolates (including the D614G S-protein variant) that blocks the interaction regions of the SARS-CoV-2 S-protein receptor-binding domain for the ACE2 receptor [20] (Fig. 1 ). Preclinical data suggest that regdanvimab is active against several variants of SARS-CoV-2 (B.1.351 [the Beta variant], B.1.526, B.1.525, B.1.617 and B.1.617.2 [the Delta variant]) [21], [22], [23].
Fig. 1.
Schematic representation of regdanvimab binding to the receptor-binding domain of SARS-CoV-2. Steric hindrance caused by regdanvimab prevents SARS-CoV-2 binding to the angiotensin-converting enzyme 2 receptor on host cells, blocking virus entry. ACE2, angiotensin-converting enzyme 2; Fab: fragment antigen binding; Fc: fragment crystallizable; RBD, receptor-binding domain; S, spike protein.
Regdanvimab has demonstrated antiviral activity and clinical efficacy, along with good tolerability and safety, in a Phase 1 single-dose study in patients with mild SARS-CoV-2 infection [24] as well as in a Phase 2/3 randomized, placebo-controlled, double-blind study in outpatients with mild-to-moderate SARS-CoV-2 infection, including high-risk patients [25], [26]. A reduced need for hospitalization or oxygen therapy up to Day 28 was demonstrated with regdanvimab in the Phase 2/3 study and led to full marketing authorization being granted by the South Korean Ministry of Food and Drug Safety on September 17, 2021, for the treatment of patients aged at least 50 years, or with at least one underlying medical condition with mild symptoms of COVID-19, and of adult patients with moderate symptoms of COVID-19 [27]. On November 12, 2021, regdanvimab was granted marketing authorization by the European Medicines Agency for treating COVID-19 in adults who do not require supplemental oxygen and who are at increased risk of their disease becoming severe [28]. Regdanvimab has also been selected by the European Commission as a preferred treatment as part of the EU strategy against COVID-19 [29]. Emergency use authorization was granted by the Indonesian National Agency for Drug and Food Control (Badan Pengawas Obat dan Makanan) [30] and the Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária) [31] in July and August 2021, respectively, for the indication of mild-to-moderate COVID-19. Provisional approval was granted by the Therapeutic Goods Administration (TGA) of Australia on Dec 2021, for the indication of mild-to-moderate COVID-19 [32].
Herein, we report 28-day results from a non-interventional, retrospective cohort study of regdanvimab for the treatment of patients with mild-to-moderate SARS-CoV-2 infection, including patients considered to be at high risk of severe disease. Using propensity score matching, the primary objective was to compare the real-world proportion of patients treated with regdanvimab versus standard of care who progress to severe/critical COVID-19 or die of SARS-CoV-2 infection.
2. Methods
2.1. Study design and participants
This non-interventional, retrospective cohort study included patients with confirmed mild-to-moderate SARS-CoV-2 infection who were admitted to and received medical treatment at one of three hospitals in the Republic of Korea (Kyungpook National University Chilgok Hospital, Kyungpook National University Hospital or Pusan National University Hospital). The first patient with COVID-19 reported in Korea occurred in January 2020, and the date of the first patient admitted to the hospital among analyzed patients in this study was March 2020. Regdanvimab acquired conditional marketing approval on February 5, 2021 in Korea. From March 3, 2020 to February 4, 2021 before acquiring conditional marketing authorization, only the patients in control group were enrolled in this study. After approval of conditional marketing authorization of regdanvimab in Korea, both control and regdanvimab patients were enrolled in the study and the last patient was enrolled on July 15, 2021. The protocol and all applicable amendments were reviewed and approved by local institutional review boards (IRBs) prior to study initiation. In accordance with Article 16 of the Bioethics and Safety Act of the Republic of Korea, formal informed consent was not required for this retrospective analysis [33]. The medical records of all patients admitted to the study centers were reviewed to determine eligibility for the study. Data were recorded electronically for all eligible patients and anonymized on electronic data capture forms.
Eligible participants were adults (aged ≥ 18 years) with mild-to-moderate disease as defined by National Institutes of Health (NIH) guidance [34], who had been diagnosed with SARS-CoV-2 infection at screening by means of reverse transcription–polymerase chain reaction. Individuals were required to have oxygen saturation of > 94% on room air, no need for supplemental oxygen, and onset of symptoms ≤ 7 days before study drug administration or admission for COVID-19 treatment. High-risk patients were included among the study population; in accordance with the Korean prescribing information of regdanvimab at the time of IRB submission, high risk was defined as age ≥ 60 years or having mild disease with underlying medical conditions (≥1 of the following: cardiovascular disease, chronic respiratory disease, diabetes or hypertension) [35].
Patients who had received a COVID-19 vaccine or who had severe or critical illness at screening (per US National Institutes of Health [NIH] classification [34]) were excluded from the study.
Regdanvimab was administered according to the dose and schedule approved by the Korean Ministry of Food and Drug Safety at the time when patients received treatment: 40 mg/kg as an intravenous infusion over 90 min, not later than 7 days after symptom onset [35].
2.2. Study objectives
The primary study objective was to evaluate the clinical efficacy of regdanvimab compared with a propensity score–matched control cohort receiving standard of care in a real-world setting, according to the proportion of patients progressing to severe/critical COVID-19 or death due to SARS-CoV-2 infection. Secondary objectives were to evaluate mortality, oxygen therapy and rescue treatment, and safety endpoints related to regdanvimab. Medical utilization of regdanvimab was evaluated as an exploratory objective.
2.3. Study endpoints
The primary efficacy endpoint was the proportion of patients developing severe or critical disease (based on the NIH severity categories for SARS-CoV-2 infection) [34] or death due to SARS-CoV-2 infection, up to Day 28. The NIH definitions are as follows: severe illness – individuals who have SpO2 of < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen of < 300 mmHg, respiratory frequency > 30 breaths/minute or lung infiltrates > 50%; and critical illness – individuals who have respiratory failure, septic shock and/or multiple organ dysfunction [34].
Secondary efficacy endpoints (analyzed up to Day 28 except where noted) were: proportion of patients developing severe or critical disease or death due to SARS-CoV-2 infection, up to Day 10; proportion of patients requiring mechanical ventilation due to SARS-CoV-2 infection; duration of mechanical ventilation in each patient due to SARS-CoV-2 infection; proportion of patients requiring supplemental oxygen due to SARS-CoV-2 infection; duration of supplemental oxygen therapy in each patient due to SARS-CoV-2 infection; duration of hospitalization in survivors (i.e., patients who survived up to Day 28), including patients transferred to other hospitals; proportion of patients requiring corticosteroid therapy due to SARS-CoV-2 infection; proportion of patients requiring remdesivir due to SARS-CoV-2 infection; duration of fever (defined as body temperature > 38 °C); and proportion of patients with all-cause mortality (at any time throughout the study).
Safety was analyzed according to reported adverse events (AEs), including serious AEs, infusion-related reactions (IRRs) and pregnancy test analyses. AEs were classified using the Common Terminology Criteria for Adverse Events, Version 5.0. Medical utilization and costs were also analyzed as exploratory endpoints. The medical utilization and cost endpoints were length of inpatient stay for each patient and total medical costs for each patient during hospitalization.
2.4. Statistical analysis
The proposed sample size was approximately 450 patients; this was based on the estimated number of patients admitted for COVID-19 at the study centers during the data collection period who would meet enrollment criteria, rather than a formal statistical hypothesis. The intention was for the final ratio of study participants (regdanvimab:no regdanvimab) to be approximately 1:2. Patients in the “no regdanvimab” group (hereafter referred to as the control cohort) were propensity score–matched to the “regdanvimab” cohort, using the following factors: sex, age, body mass index (BMI), Charlson Comorbidity index score and presence of pneumonia upon admission. Patients were also equally matched according to study center.
All statistical analyses were conducted using Statistical Analysis System (SAS) software, Version 9.4 (SAS Institute Inc., Cary, NC). The analysis populations were the Propensity Score–Matched (PSM) Efficacy Set (patients who received a full dose of regdanvimab or who were admitted for the treatment of COVID-19, had at least one post-admission evaluation for efficacy, and met the criteria for propensity score matching), the Safety Set (all patients who received a full or partial dose of regdanvimab or who were admitted for the treatment of COVID-19), and the Medical Utilization Set (all patients in the PSM Efficacy Set for whom information on medical billing was available).
Efficacy endpoints were summarized by cohort (regdanvimab and control groups) using descriptive statistics or frequency tables. For categorical variables, the chi-squared test or Fisher exact test was used. For continuous variables, the Student t test was used. A P value of < 0.05 was determined to indicate statistical significance. Medical utilization results were summarized by cohort (regdanvimab and control groups) using descriptive statistics or frequency tables.
3. Results
3.1. Patient population and baseline characteristics
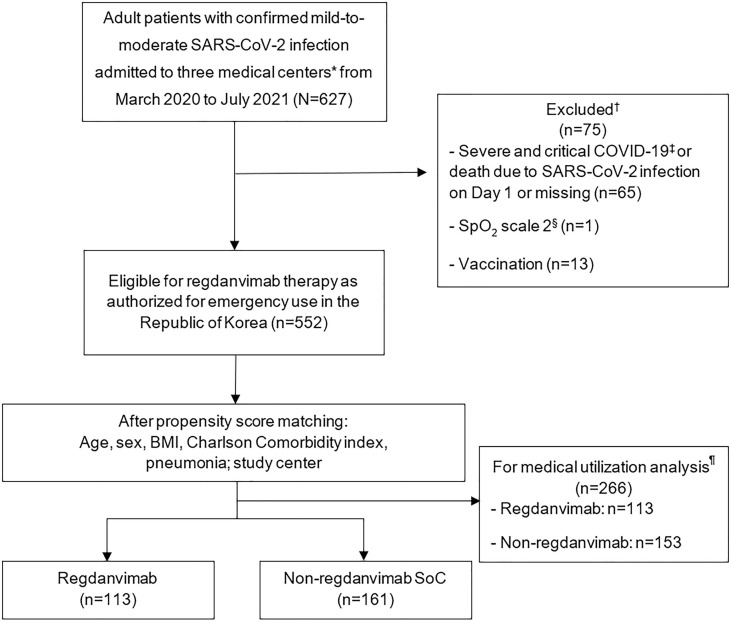
A total of 627 patients with a confirmed COVID-19 diagnosis were admitted to one of the three study centers between March 3, 2020, and July 17, 2021 (Fig. 2 ). After exclusions, 552 patients were eligible for regdanvimab therapy, and of these, 156 received regdanvimab and 396 received other treatments for COVID-19 and were included in the Efficacy and Safety Sets. The PSM Efficacy Set consisted of 274 patients (113 treated with regdanvimab and 161 treated with other COVID-19 medication; Fig. 2). The Medical Utilization Set also comprised 113 patients treated with regdanvimab and 153 treated with other COVID-19 medication(s).
Fig. 2.
Patient flow diagram. *The three medical centers were Kyungpook National University Chilgok Hospital, Kyungpook National University Hospital and Pusan National University Hospital. †Some patients were excluded based on more than one factor. ‡Severe illness – individuals with oxygen saturation (SpO2) < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen < 300 mmHg, respiratory frequency > 30 breaths/minute or lung infiltrates > 50%; and critical illness – individuals with respiratory failure, septic shock and/or multiple organ dysfunction. §SpO2 is for use in patients with hypercapnic respiratory failure who have clinically recommended oxygen saturation of 88–92%. BMI, body mass index; SoC, standard of care. ¶Patients without medical cost information were excluded from the medical utilization analysis.
Baseline demographic and disease characteristics are summarized for the PSM Efficacy Set in Table 1 and for the Efficacy and Safety Sets in Supplementary Table S1. Although the regdanvimab group tended to be older (median age: 66 vs 61 years), with a higher BMI (mean: 24.0 vs 23.3 kg/m2) and higher prevalence of pneumonia at baseline (37.8% vs 12.6%), due to propensity score matching, the differences between the regdanvimab and control groups were not statistically significant for the efficacy analyses, except for the presence of pneumonia (P = 0.0263). Therefore, baseline characteristics in the PSM Efficacy Set were well balanced across the two treatment groups (Table 1); overall, 116 (42.3%) patients were female, the median age was 63.5 years (range: 25–97) and the mean BMI was 24.0 kg/m2 (standard deviation: 3.46). Eighty (29.2%) patients had moderate disease and no patients had severe or critical disease.
Table 1.
Baseline demographics and disease characteristics (Propensity Score–Matched Efficacy Set).
| Characteristic | Regdanvimab (n = 113) | Control (n = 161) | P value* |
|---|---|---|---|
| Age, years | |||
| Median (range) | 64.0 (26–90) | 63.0 (25–97) | 0.9221 |
| Female, n (%) | 41 (36.3) | 75 (46.6) | 0.0894 |
| BMI, kg/m2 | |||
| Mean (SD) | 24.2 (3.41) | 23.9 (3.50) | |
| <25, n (%) | 67 (59.3) | 92 (57.1) | 0.9873 |
| 25–29, n (%) | 27 (23.9) | 40 (24.8) | |
| >29, n (%) | 9 (8.0) | 14 (8.7) | |
| Missing, n (%) | 10 (8.8) | 15 (9.3) | |
| CCI, mean (SD) | 2.8 (1.95) | 2.7 (1.93) | 0.7522 |
| Severity of disease, n (%) | |||
| Asymptomatic or pre-symptomatic | 0 | 10 (6.2) | NA |
| Mild disease | 74 (65.5) | 110 (68.3) | |
| Moderate disease | 39 (34.5) | 41 (25.5) | |
| Symptom incidence, n (%) | |||
| Feeling feverish | 24 (21.2) | 34 (21.1) | NA |
| Shortness of breath or difficulty breathing | 35 (31.0) | 49 (30.4) | |
| Cough | 48 (42.5) | 59 (36.6) | |
| Diarrhea | 0 | 2 (1.2) | |
| Sputum | 20 (17.7) | 21 (13.0) | |
| Sore throat | 18 (15.9) | 30 (18.6) | |
| Loss of taste of smell | 2 (1.8) | 3 (1.9) | |
| Fatigue | 0 | 3 (1.9) | |
| Myalgia | 8 (7.1) | 19 (11.8) | |
| Widespread pain | 2 (1.8) | 2 (1.2) | |
| Nausea or vomiting | 1 (0.9) | 1 (0.6) | |
| Others | 7 (6.2) | 21 (13.0) | |
| Presence of pneumonia, n (%) | 39 (34.5) | 36 (22.4) | 0.0263 |
BMI, body mass index; CCI, Charlson Comorbidity Index; NA, not available; SD, standard deviation.
P value for covariates after propensity score matching, calculated from Student t test for continuous variables and chi-squared test for categorical variables.
3.2. Efficacy
All efficacy analyses were conducted using the PSM Efficacy Set unless otherwise specified.
3.2.1. Primary endpoints
Table 2 summarizes the incidence of severe/critical COVID-19 (NIH-defined) or death due to SARS-CoV-2 infection, up to Day 28. Compared with controls, patients treated with regdanvimab had a significantly lower risk of severe/critical disease or death (7.1% vs 16.1%, P = 0.0263). The incidence of severe/critical COVID-19 (NIH-defined) or death due to SARS-CoV-2 infection, up to Day 28, was also lower for the regdanvimab group versus the control group when analyzed prior to propensity score matching (7.7% vs 12.6%, respectively [P = 0.1023]; Supplementary Table S2).
Table 2.
Primary efficacy endpoint analysis (Propensity Score–Matched Efficacy Set).
| Regdanvimab (n = 113) | Control (n = 161) | Difference | P-value* | |
|---|---|---|---|---|
| Patients with severe/critical disease or death due to SARS-CoV-2 infection up to Day 28, n (%) [95% CI] | 8 (7.1) [3.6%,13.4%†] | 26 (16.1) [11.3%, 22.6%†] | −9.1% [−16.7%, −0.6%‡] | 0.0263 |
CI, confidence interval.
P values derived from Fisher’s exact test.
Wilson (score) 95% CI is presented.
Farrington and Manning score exact 95% CI for proportion difference is presented.
3.2.2. Secondary endpoints
Secondary endpoint data were analyzed up to Day 28 (except where noted) and are summarized in Table 3 . Up to Day 10, 7 (6.2%) patients in the regdanvimab group experienced severe/critical COVID-19, death due to SARS-CoV-2 infection, or death from any cause, compared with 21 (13.0%) patients in the control group (P = 0.0654). No patients in the regdanvimab group required mechanical ventilation, compared with 1 (0.6%) patient in the control group. Supplemental oxygen was required by 8.0% and 18.6% of patients in the regdanvimab and control groups, respectively (P = 0.0128). There was a trend toward the duration of supplemental oxygen therapy being longer in the control group, but this did not reach statistical significance. The mean duration of hospitalization was significantly shorter in the regdanvimab group than in the control group (12.4 vs 14.6 days, P < 0.0001). Significantly lower percentages of patients in the regdanvimab arm than the control arm required treatment with a corticosteroid or with remdesivir. The mean duration of fever was 2.3 days in the regdanvimab group versus 2.5 days in the control group (P = 0.5252). No patients in the regdanvimab group died during the study, compared with 2 (1.2%) patients in the control group.
Table 3.
Secondary efficacy endpoint data (Propensity Score–Matched Efficacy Set).
| Endpoint | Regdanvimab (n = 113) | Control (n = 161) | P value* |
|---|---|---|---|
| Patients with severe symptoms or death due to SARS-CoV-2 infection up to Day 10, n (%) | 7 (6.2) | 21 (13.0) | 0.0654 |
| Patients requiring mechanical ventilation up to Day 28, n (%) | 0 | 1 (0.6) | 1.0000† |
| Duration of mechanical ventilation, days, mean (SD) | 0 | 22.79 (NC) | NC |
| Patients requiring supplemental oxygen therapy up to Day 28, n (%) | 9 (8.0) | 30 (18.6) | 0.0128 |
| Duration of supplemental oxygen therapy, days, mean (SD) | 4.7 (7.1) | 8.6 (6.1) | 0.1070 |
| Duration of hospitalization up to Day 28‡, days, mean (SD) | 12.4 (3.7) | 14.6 (5.6) | <0.0001 |
| Patients requiring corticosteroid therapy up to Day 28, n (%) | 0 | 20 (12.4) | <0.0001 |
| Patients requiring remdesivir therapy up to Day 28, n (%) | 1 (0.9) | 23 (14.3) | 0.0001 |
| Duration of fever, days, mean (SD) | 2.3 (1.1) | 2.5 (1.7) | 0.5252 |
| Patients dying of any cause, n (%) | 0 | 2 (1.2) | 0.5136† |
NC, not calculated; SD, standard deviation.
P-value is derived from Student t-test for continuous variables and chi-squared test for categorical variables.
P-value derived from Fisher’s exact test, since chi-squared test may not be valid due to 50% of the cells having expected counts<5.
Two patients in the control group who died were not included for the purpose of this endpoint (regdanvimab = 113; control = 153). Duration of hospitalization for patients hospitalized over Day 28 was calculated as being hospitalized for 28 days. Patients transferred to other hospitals were included in this analysis, with duration calculated as the duration of admission at the study site.
3.3. Safety
All safety analyses were conducted using the Safety set unless otherwise specified. In the regdanvimab group, 7 (4.5%) patients experienced treatment-emergent AEs (TEAEs). All TEAEs were mild in severity (Grade 1) and patients recovered within 2 days of onset. Two IRRs (Grade 1 hypoxia and Grade 1 hypertension, both possibly treatment related) were observed separately in two patients who had received regdanvimab. No pregnancies were recorded in patients who had received regdanvimab. No new safety events were identified that would have affected the safety profile of regdanvimab.
3.4. Medical utilization
Medical utilization differed significantly between treatment groups (Table 4 ). Mean total medical costs per patient during hospitalization were 7,449,060.64 Korean Won (₩; 6260.77 US dollars [$]) in patients who had received regdanvimab and ₩9,536,197.84 ($8014.96) in those who had received other therapies for COVID-19 (P = 0.0032). The mean duration of hospitalization used for estimating medical utilization comprised the total length of hospitalization for patients (i.e., including patients hospitalized for>28 days); this differed from the estimation of the secondary efficacy endpoint of “duration of hospitalization up to Day 28”, which did not count days over Day 28. There was a statistically significant difference between the two groups, with a mean duration of hospitalization per patient of 12.7 days in patients receiving regdanvimab versus 15.4 days in patients receiving other therapies for COVID-19 (P = 0.0011).
Table 4.
Medical utilization data, by treatment group (Medical Utilization Set**).
| Endpoint | Regdanvimab (n = 113) | Control (n = 153) | P value |
|---|---|---|---|
| Mean duration of hospitalization,* days | 12.67 | 15.42 | 0.0011 |
| Mean total cost of medical bills per patient during hospitalization, ₩ | 7,449,060.64 | 9,536,197.84 | 0.0032 |
₩, Korean Won.
The mean duration of hospitalization used for estimating medical utilization comprised the total length of hospitalization for patients (i.e., including those hospitalized for>28 days); this differed from the estimation of the secondary efficacy endpoint “duration of hospitalization up to Day 28”, which did not count days over Day 28. Two patients who died during the study were not included in the calculation of duration of hospitalization.
Patients with no information on medical billing in the PSM Efficacy Set were excluded from the Medical Utilization Set.
4. Discussion
4.1. Study overview
This non-interventional, propensity score–matched, retrospective cohort study of Korean patients demonstrates that regdanvimab is effective in the treatment of mild-to-moderate COVID-19. The primary analysis showed that regdanvimab significantly reduced the proportion of patients progressing to severe or critical disease severity or dying of SARS-CoV-2 infection. Regdanvimab was associated with significant reductions in the percentages of patients requiring treatment with supplemental oxygen, corticosteroids or remdesivir. In addition, a modest but statistically significant reduction in the duration of hospitalization was observed with regdanvimab, with no notable safety issues.
A crucial attribute of any COVID-19 treatment is preventing disease deterioration and death in patients with moderate disease. Increased disease severity is associated with worsened outcomes and increased healthcare and economic burden. In one study of hospitalized patients with COVID-19, the mortality rate among those who required mechanical ventilation was 88.1%, compared with 11.7% in individuals not requiring ventilation [36]. A second study, also conducted in hospitalized patients with COVID-19, showed marked increases in in-hospital mortality, length of hospital stay and hospital charges in patients who required admission to the intensive care unit (ICU) or invasive mechanical ventilation (IMV) [37].
Identifying patterns in the course of COVID-19 disease deterioration may highlight optimal timepoints for interventions to prevent deterioration, and a Chinese observational study of patients hospitalized for moderate COVID-19 has provided important information in this regard. Among patients who deteriorated to severe or critical status, the median time to deterioration was 11 days from symptom onset; exacerbation to a critical condition occurred after a median of 3 days in a severe condition [38]. Initiating treatment early in the disease course before patients experience disease deterioration would therefore appear to be advantageous, and this is supported by the efficacy observed across both arms of our study, in which patients were required to have symptom onset within 7 days of study drug administration.
Numerous therapeutic candidates for COVID-19 have been evaluated in clinical trials and several are under evaluation for EU marketing authorization as treatments for COVID-19, including anakinra, baricitinib, and tocilizumab. However, the only drug that has so far been approved in the US and European Union (EU) for the treatment of COVID-19, besides monoclonal antibody (mAb) treatment, is remdesivir [39], [40]. Clinical studies evaluating the efficacy of remdesivir have not consistently demonstrated a statistically significant difference in comparison with control in terms of mean time to clinical improvement and mortality [41], [42]. The large, international, Solidarity study, conducted by the WHO, found in-hospital mortality rates with remdesivir and control to be comparable, and that remdesivir treatment did not reduce initiation of ventilation or the duration of hospitalization, as compared with control [43]. As a result, the WHO has recommended against the use of remdesivir for treatment of COVID-19, citing insufficient supporting evidence [44]. More recently, in December 2021, nirmatrelvir with ritonavir and molnupiravir, become the first oral antiviral treatments for SARS-CoV-2 to be issued emergency use authorizations by the US FDA [45]. However, some caution is still needed with these agents as they may not be suitable for all patients. Hepatotoxicity has been observed with ritonavir [46] and metabolism of the co-administered agent nirmatrelvir is highly dependent on CYP3A enzymes. The nirmatrelvir with ritonavir combination is therefore contraindicated with certain medications that are dependent on hepatic clearance [46]. Molnupiravir may affect bone and cartilage growth in adolescents and cause fetal harm in pregnant women [47].
Unlike these antiviral drugs, the safety of antibody drugs have been well demonstrated in both clinical studies and accumulating real world data. Several antibody treatments including casirivimab/imdevimab and sotrovimab have received EU Marketing Authorizations based on evidence of efficacy in preventing progression, reducing mortality or accelerating recovery from COVID-19 [48], [49]. Additionally, data on the therapeutic effectiveness and safety of antibody drugs has been accumulated through real world data, including studies of bamlanivimab and casirivimab/imdevimab [50], [51], [52], [53], [54], [55], and this has influenced key regulatory decisions in this setting. With these strengths, mAb prescription remains feasible in patients with limited capacity for oral drugs.
4.2. Evaluation of medical utilization
A medical utilization framework developed in the US suggests that treatments that reduce mortality in hospitalized patients with COVID-19 are likely to be cost-effective [59]. In this study, the incidence of mortality was too low to come to a definitive conclusion (n = 2, both in the control group) regarding whether regdanvimab reduced mortality. [60]COVID-19 has greatly increased the medical cost burden, especially when ICU admission and IMV have been required. In 2020, median hospital charges in the US (from a US COVID-19 database comprising records for almost 174,000 patients), per hospitalized patient, were $34,634 for patients without ICU admission and IMV, and $198,394 for those admitted to the ICU and who had received IMV. Likewise, median hospital costs (i.e., costs incurred by hospitals) were six-fold higher for patients with ICU admission and IMV usage ($9,504 vs $54,402, respectively) [37]. In addition, the disability caused by the duration and severity of COVID-19 may incur further social and economic burdens and may represent up to 30% of the total health burden [61]. According to the South Korean National Health Insurance Service, the average cost of treating patients with mild COVID-19 disease in hospital was ₩4.78 million ($4,176), whereas the cost was ₩70 million ($62,197) for treating patients with severe disease [62], [63]. At the time of our study, treatment guidelines in the Republic of Korea recommended the hospitalization of patients with non-severe COVID-19 (Patients with COVID-19 infection are hospitalized regardless of their severity). Evidence continues to accumulate demonstrating regdanvimab’s effect in decreasing the risk of progression to severe disease [60]; however, this is the first study which has also analyzed medical utilization cost in propensity score matched cohorts. Regdanvimab proved to be cost-effective over control by reducing the duration of hospitalization, as well as the proportion of patients progressing to severe/critical disease or death. In this study, mean total medical costs per patient during hospitalization were ₩7,449,060.64 ($6,260.77) in patients who had received regdanvimab compared with ₩9,536,197.84 ($8,014.96) in the control group. With regards to other COVID-19 therapies, the Institute for Clinical and Economic Review (ICER) in the US has commented that remdesivir is cost-effective at a benchmark price of $4580 to $5080, but there have been conflicting results regarding the cost-effectiveness of remdesivir in practice [64], [65], [66]. As more COVID-19 treatment options become available, ICER is planning to assess the health and economic outcomes of several available therapies and therapies under development for treatment of mild-to-moderate COVID-19 (casirivimab/imdevimab, sotrovimab, molnupiravir, PF-07321332/ritonavir, fluvoxamine) [65], [67].
4.3. Strengths and limitations
Limitations of this study include the non-interventional, retrospective study design; the specific nature of the patient population (Korean patients admitted to one of three hospitals for treatment of mild-to-moderate SARS-CoV-2 infection; although the research institutes are representative hospitals in each region, patients were only recruited from two hospitals, not from multiple institutions) and the relatively short follow-up of 28 days. In addition, no information on SARS-CoV-2 variants was collected during the study.
The key strengths of our study are the large number of patients analyzed and the real-world setting. Propensity score matching adjusted for differences between the two treatment groups and ensured that the study groups were well balanced, thus reducing the risk of confounding factors. Therefore, we consider our results to be highly applicable to real-world clinical practice in Korea. The inclusion of cost and medical utilization data is another strength, enabling the financial implications of using regdanvimab to be evaluated.
4.4. Conclusions
In conclusion, this retrospective analysis demonstrates that regdanvimab reduces the risk of severe/critical disease or death due to SARS-CoV-2 infection among patients with high risk mild-or-moderate COVID-19. Propensity score matching ensured that the study groups were well balanced and therefore that the outcomes were robust. The study results are consistent with those of a randomized Phase 2/3 outpatient study in showing the potential benefits of regdanvimab in the treatment of COVID-19. Additionally, we demonstrated that medical utilization costs were significantly lower in patients who received regdanvimab than in patients who did not receive regdanvimab. Regdanvimab may help to ease the considerable burden of SARS-CoV-2 on healthcare systems.
Data sharing
All data are available upon request. Please contact the corresponding author Ki Tae Kwon for enquiries.
Ethics approval
The Daegu Joint IRB (DGIRB 2021–07-002) and Pusan NU IRB (2109–012-107) reviewed and approved the study protocol.
Declaration of interest
SWL, SOL and JEL have been an investigator for clinical trials sponsored by Daewoong Pharmaceutical Co., Ltd., Pfizer, Chong Kun Dang Pharmaceutical and Shin Poong Pharmaceutical Co., Ltd., outside the scope of the submitted work.
K-HK has been an investigator for clinical trials sponsored by SD Biosensor, Inc., Abbott and Kogene Biotech Co., Ltd, outside the scope of the submitted work.
SHL has been an investigator for clinical trials sponsored by GlaxoSmithKline plc., Gilead Sciences, and Merck Sharp & Dohme Corp., outside the scope of the submitted work.
SYH and KTK have been investigators for clinical trials sponsored by Pfizer, Chong Kun Dang Pharmaceutical, SK Bioscience Co., Ltd. and Celltrion, Inc., outside the scope of the submitted work.
SW-K, YJK and SHB have been investigators for clinical trials sponsored by Dong Wha Pharmaceutical Co., Ltd., Daewoong Pharmaceutical Co., Ltd., ImmunMed, Inc., Green Cross Corporation, Shin Poong Pharmaceutical Co., Ltd. and Gilead Sciences, outside the scope of the submitted work.
H-HC has been an investigators for clinical trials sponsored by Green Cross Corporation, Celltrion, Inc., Shin Poong Parmaceutical Co., Ltd., and Gilead Sciences, outside the scope of the submitted work and also reports consulting fees from Green Cross Corporation.
A-SK declares no conflict of interest.
Funding
Celltrion, Inc. funded the study and contributed to statistical analyses. Celltrion, Inc. reviewed the manuscript throughout development for scientific accuracy.
Authors Contribution
SOL, SWL and KTK contributed to the hypothesis, data gathering, and revising of the final manuscript before submission. Especially, KTK contributed to correspondence. All the authors reviewed and confirmed the manuscript before submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all patients and investigators involved in the study. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking and referencing) was provided by Duncan Campbell, PhD, CMPP, at Aspire Scientific (Bollington, UK), and funded by Celltrion, Inc. (Incheon, Republic of Korea).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2022.108570.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.World Health Organization, WHO coronavirus disease (COVID-19) dashboard, 2021. https://covid19.who.int/ (accessed 27 June 2021).
- 2.The Lancet Microbe [Editorial], COVID-19 vaccines: the pandemic will not end overnight, Lancet Microbe 2 (2021) e1. [DOI] [PMC free article] [PubMed]
- 3.The Lancet [Editorial], India's COVID-19 emergency, Lancet 397 (2021) 1683. [DOI] [PMC free article] [PubMed]
- 4.Forni G., Mantovani A., Forni G., Mantovani A., Moretta L., Rappuoli R., et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, Science Brief: COVID-19 Vaccines and Vaccination, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fmore%2Ffully-vaccinated-people.html (accessed 15 December 2021). [PubMed]
- 7.Nanduri Srinivas, Pilishvili Tamara, Derado Gordana, Soe Minn Minn, Dollard Philip, Wu Hsiu, Li Qunna, Bagchi Suparna, Dubendris Heather, Link-Gelles Ruth, Jernigan John A., Budnitz Daniel, Bell Jeneita, Benin Andrea, Shang Nong, Edwards Jonathan R., Verani Jennifer R., Schrag Stephanie J. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(34):1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCabe R., Schmit N., Christen P., D'Aeth J.C., Løchen A., Rizmie D., et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020;18:329. doi: 10.1186/s12916-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sah P., Vilches T.N., Moghadas S.M., Fitzpatrick M.C., Singer B.H., Hotez P.J., et al. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2019;324(2020):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann Markus, Kleine-Weber Hannah, Schroeder Simon, Krüger Nadine, Herrler Tanja, Erichsen Sandra, Schiergens Tobias S., Herrler Georg, Wu Nai-Huei, Nitsche Andreas, Müller Marcel A., Drosten Christian, Pöhlmann Stefan. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrente-Lopez A., Hermosilla J., Navas N., Cuadros-Rodriguez L., Cabeza J., Salmeron-Garcia A. The relevance of monoclonal antibodies in the treatment of COVID-19. Vaccines. 2021;9:557. doi: 10.3390/vaccines9060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Lihong, Wang Pengfei, Nair Manoj S., Yu Jian, Rapp Micah, Wang Qian, Luo Yang, Chan Jasper F.-W., Sahi Vincent, Figueroa Amir, Guo Xinzheng V., Cerutti Gabriele, Bimela Jude, Gorman Jason, Zhou Tongqing, Chen Zhiwei, Yuen Kwok-Yung, Kwong Peter D., Sodroski Joseph G., Yin Michael T., Sheng Zizhang, Huang Yaoxing, Shapiro Lawrence, Ho David D. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 14.Shi Rui, Shan Chao, Duan Xiaomin, Chen Zhihai, Liu Peipei, Song Jinwen, Song Tao, Bi Xiaoshan, Han Chao, Wu Lianao, Gao Ge, Hu Xue, Zhang Yanan, Tong Zhou, Huang Weijin, Liu William Jun, Wu Guizhen, Zhang Bo, Wang Lan, Qi Jianxun, Feng Hui, Wang Fu-Sheng, Wang Qihui, Gao George Fu, Yuan Zhiming, Yan Jinghua. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 15.Wu Yan, Wang Feiran, Shen Chenguang, Peng Weiyu, Li Delin, Zhao Cheng, Li Zhaohui, Li Shihua, Bi Yuhai, Yang Yang, Gong Yuhuan, Xiao Haixia, Fan Zheng, Tan Shuguang, Wu Guizhen, Tan Wenjie, Lu Xuancheng, Fan Changfa, Wang Qihui, Liu Yingxia, Zhang Chen, Qi Jianxun, Gao George Fu, Gao Feng, Liu Lei. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zost Seth J., Gilchuk Pavlo, Case James Brett, Binshtein Elad, Chen Rita E., Nkolola Joseph P., Schäfer Alexandra, Reidy Joseph X., Trivette Andrew, Nargi Rachel S., Sutton Rachel E., Suryadevara Naveenchandra, Martinez David R., Williamson Lauren E., Chen Elaine C., Jones Taylor, Day Samuel, Myers Luke, Hassan Ahmed O., Kafai Natasha M., Winkler Emma S., Fox Julie M., Shrihari Swathi, Mueller Benjamin K., Meiler Jens, Chandrashekar Abishek, Mercado Noe B., Steinhardt James J., Ren Kuishu, Loo Yueh-Ming, Kallewaard Nicole L., McCune Broc T., Keeler Shamus P., Holtzman Michael J., Barouch Dan H., Gralinski Lisa E., Baric Ralph S., Thackray Larissa B., Diamond Michael S., Carnahan Robert H., Crowe James E. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinreich David M., Sivapalasingam Sumathi, Norton Thomas, Ali Shazia, Gao Haitao, Bhore Rafia, Musser Bret J., Soo Yuhwen, Rofail Diana, Im Joseph, Perry Christina, Pan Cynthia, Hosain Romana, Mahmood Adnan, Davis John D., Turner Kenneth C., Hooper Andrea T., Hamilton Jennifer D., Baum Alina, Kyratsous Christos A., Kim Yunji, Cook Amanda, Kampman Wendy, Kohli Anita, Sachdeva Yessica, Graber Ximena, Kowal Bari, DiCioccio Thomas, Stahl Neil, Lipsich Leah, Braunstein Ned, Herman Gary, Yancopoulos George D. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Peter, Nirula Ajay, Heller Barry, Gottlieb Robert L., Boscia Joseph, Morris Jason, Huhn Gregory, Cardona Jose, Mocherla Bharat, Stosor Valentina, Shawa Imad, Adams Andrew C., Van Naarden Jacob, Custer Kenneth L., Shen Lei, Durante Michael, Oakley Gerard, Schade Andrew E., Sabo Janelle, Patel Dipak R., Klekotka Paul, Skovronsky Daniel M. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C., Ryu D.K., Lee J., Kim Y.I., Seo J.M., Kim Y.G., et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021;12:288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu Dong-Kyun, Song Rina, Kim Minsoo, Kim Young-Il, Kim Cheolmin, Kim Jong-In, Kwon Ki-Sung, Tijsma Aloys SL., Nuijten Patricia M., van Baalen Carel A., Hermanus Tandile, Kgagudi Prudence, Moyo-Gwete Thandeka, Moore Penny L., Choi Young Ki, Lee Soo-Young. Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant. Biochem. Biophys. Res. Commun. 2021;566:135–140. doi: 10.1016/j.bbrc.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celltrion Healthcare, Celltrion confirms neutralising potency against emerging SARS-CoV-2 variants with anti-COVID-19 monoclonal antibody treatment regdanvimab (CT-P59), 2021. https://www.celltrionhealthcare.com/en-us/board/newsdetail?modify_key=482&pagenumber=1&keyword=&keyword_type= (accessed 2 July 2021).
- 23.Business Wire, Celltrion’s monoclonal antibody treatment for COVID-19, regdanvimab (CT-P59), demonstrates strong neutralising activity against delta variant, 2021. https://www.businesswire.com/news/home/20210715006147/en/%C2%A0Celltrion%E2%80%99s-Monoclonal-Antibody-Treatment-for-COVID-19-regdanvimab-CT-P59-Demonstrates-Strong-Neutralising-Activity-Against-Delta-Variant (accessed 1 November 2021).
- 24.Kim J.Y., Jang Y.R., Hong J.H., Jung J.G., Park J.H., Streinu-Cercel A., et al. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection. Clin Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ison Michael G, Kim Jin Yong, Sandulescu Oana, Preotescu Liliana-Lucia, Martinez Norma Erendira Rivera, Dobryanska Marta, Birlutiu Victoria, Miftode Egidia Gabriela, Gaibu Natalia, Caliman-Sturdza Olga Adriana, Florescu Simin-Aysel, Streinu-Cercel Anca, Lee Sang Joon, Kim Sung Hyun, Chang Il Sung, Bae Yun Ju, Suh Jee Hye, Kim Mi Rim, Chung Da Re, Kim Sun Jung, Lee Seul Gi, Park Ga Hee, Eom Joong Sik. 546. Therapeutic Effect of Regdanvimab in Patients with Mild to Moderate COVID-19: Day 28 Results from a Multicentre, Randomised, Controlled Pivotal Trial. Open Forum Infectious Diseases. 2021;8(Supplement_1):S375. [Google Scholar]
- 26.The Korean Economic Daily, Celltrion’s COVID-19 treatment reduces incidence of severity by 70%, 2021. https://www.kedglobal.com/newsView/ked202107130018 (accessed 15 December 2021).
- 27.Business Wire, Celltrion’s monoclonal antibody treatment for COVID-19, regdanvimab (CT-P59) becomes the first authorized COVID-19 treatment approved from the Korean Ministry of Food and Drug Safety (MFDS), 2021. https://www.businesswire.com/news/home/20210918005026/en/%C2%A0Celltrion%E2%80%99s-monoclonal-antibody-treatment-for-COVID-19-regdanvimab-CT-P59-becomes-the-first-authorized-COVID-19-treatment-approved-from-the-Korean-Ministry-of-Food-and-Drug-Safety-MFDS (accessed 18 November 2021).
- 28.European Medicines Agency, Regkirona: summary of product characteristics, 2021. https://www.ema.europa.eu/en/documents/product-information/regkirona-epar-product-information_en.pdf (accessed 30 November 2021).
- 29.L. Burger [Reuters], EU picks antibody treatments, arthritis drug as preferred COVID-19 therapies, 2021. https://www.reuters.com/business/healthcare-pharmaceuticals/eu-picks-antibody-treatments-arthritis-drug-preferred-covid-19-therapies-2021-06-29/ (accessed 2 November 2021).
- 30.Indonesian National Agency for Drug and Food Control (Badan Pengawas Obat dan Makanan - Republik Indonesia), Regkirona (regdanvimab) product information [in Indonesian], 2021. http://pionas.pom.go.id/sites/default/files/obat_baru/Produk%20Informasi_Regkirona%20Larutan%20Injeksi%2060%20mg%2Cml_Regdanvimab_EUA2154100449A1_2021.pdf (accessed 18 November 2021).
- 31.Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária - Anvisa), Anvisa authorizes emergency use of new drug for Covid-19 [in Portuguese], 2021. https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/anvisa-autoriza-uso-emergencial-de-novo-medicamento-para-covid-19 (accessed 18 November 2021).
- 32.Australian Government Department of Health Therapeutic Goods Administration, TGA Provisional Approval of Celltrion Healthcare Australia Pty Ltd COVID-19 treatment, regdanvimab (REGKIRONA), 2021. https://www.tga.gov.au/media-release/tga-provisional-approval-celltrion-healthcare-australia-pty-ltd-covid-19-treatment-regdanvimab-regkirona (accessed 15 December 2021).
- 33.Statutes of the Republic of Korea. Bioethics and Safety Act, 2014. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=33442&type=part&key=36 (accessed 18 November 2021).
- 34.National Institutes of Health, COVID-19 treatment guidelines. Clinical spectrum of SARS-CoV-2 infection 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed 26 May 2021).
- 35.Korean Ministry of Food and Drug Safety, Regkirona 960 mg (regdanvimab; monoclonal antibody, recombinant) [Korean prescribing information], 2021. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=202101124 (accessed 1 November 2021).
- 36.Richardson Safiya, Hirsch Jamie S., Narasimhan Mangala, Crawford James M., McGinn Thomas, Davidson Karina W., Barnaby Douglas P., Becker Lance B., Chelico John D., Cohen Stuart L., Cookingham Jennifer, Coppa Kevin, Diefenbach Michael A., Dominello Andrew J., Duer-Hefele Joan, Falzon Louise, Gitlin Jordan, Hajizadeh Negin, Harvin Tiffany G., Hirschwerk David A., Kim Eun Ji, Kozel Zachary M., Marrast Lyndonna M., Mogavero Jazmin N., Osorio Gabrielle A., Qiu Michael, Zanos Theodoros P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Fusco Manuela, Shea Kimberly M., Lin Jay, Nguyen Jennifer L., Angulo Frederick J., Benigno Michael, Malhotra Deepa, Emir Birol, Sung Anita H., Hammond Jennifer L., Stoychev Sophia, Charos Apostolos. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J. Med. Econ. 2021;24(1):308–317. doi: 10.1080/13696998.2021.1886109. [DOI] [PubMed] [Google Scholar]
- 38.Chen S.L., Feng H.Y., Xu H., Huang S.S., Sun J.F., Zhou L., et al. Patterns of deterioration in moderate patients with COVID-19 from Jan 2020 to Mar 2020: a multi-center, retrospective cohort study in China. Front. Med. (Lausanne) 2020;7 doi: 10.3389/fmed.2020.567296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.European Medicines Agency, Veklury: summary of product characteristics, 2020. https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_en.pdf (accessed 2 July 2021).
- 40.U.S. Food and Drug Administration, Veklury prescribing information, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf (accessed 2 July 2021).
- 41.Garibaldi B.T., Wang K., Robinson M.L., Zeger S.L., Bandeen-Roche K., Wang M.C., et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw. Open. 2021;4:e213071. doi: 10.1001/jamanetworkopen.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Abdouh A., Bizanti A., Barbarawi M., Jabri A., Kumar A., Fashanu O.E., et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Contemp. Clin. Trials. 2021;101 doi: 10.1016/j.cct.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO Solidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for Covid-19 - interim WHO Solidarity Trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rochwerg B., Siemieniuk R.A., Agoritsas T., Lamontagne F., Askie L., Lytvyn L., et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370 doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 45.National Institutes of Health, The COVID-19 Treatment Guidelines Panel's Statement on Therapies for High-Risk, Nonhospitalized Patients With Mild to Moderate COVID-19, 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-therapies-for-high-risk-nonhospitalized-patients/ (accessed December 11 2022).
- 46.Food and Drug Administration, Fact sheet for healtcare providers: Emergency use authorization for Paxlovid (nirmatreivir tablets; ritonavir tablets), 2021. https://www.fda.gov/media/155050/download (accessed January 11 2022).
- 47.Food and Drug Administration, Fact sheet for healthcare providers: Emergency use authorization for Molnuprivir, 2021. https://www.fda.gov/media/155054/download (accessed January 11 2022).
- 48.European Medicines Agency, Ronapreve: summary of product characteristics, 2021. https://www.ema.europa.eu/en/documents/product-information/ronapreve-epar-product-information_en.pdf (accessed 8 December 2021).
- 49.European Medicines Agency, Xevudy: summary of product characteristics, 2021. https://www.ema.europa.eu/en/documents/product-information/xevudy-epar-product-information_en.pdf (accessed January 14 2022).
- 50.Kumar R.N., Wu E.L., Stosor V., Moore W.J., Achenbach C., Ison M.G., et al. Real-world experience of bamlanivimab for COVID-19: a case-control study. Clin. Infect. Dis. [Epub ahead of print] 2021 doi: 10.1093/cid/ciab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rainwater-Lovett Kaitlin, Redd John T, Stewart Miles A, Calles Natalia Elías, Cluff Tyler, Fang Mike, Panaggio Mark J, Lambrou Anastasia S, Thornhill Jonathan K, Bradburne Christopher, Imbriale Samuel, Freeman Jeffrey D, Anderson Michael, Kadlec Robert P. Real-world effect of monoclonal antibody treatment in COVID-19 patients in a diverse population in the United States. Open Forum Infect. Dis. 2021;8(8) doi: 10.1093/ofid/ofab398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razonable R.R., Pawlowski C., O'Horo J.C., Arndt L.L., Arndt R., Bierle D.M., et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bariola J Ryan, McCreary Erin K, Wadas Richard J, Kip Kevin E, Marroquin Oscar C, Minnier Tami, Koscumb Stephen, Collins Kevin, Schmidhofer Mark, Shovel Judith A, Wisniewski Mary Kay, Sullivan Colleen, Yealy Donald M, Nace David A, Huang David T, Haidar Ghady, Khadem Tina, Linstrum Kelsey, Seymour Christopher W, Montgomery Stephanie K, Angus Derek C, Snyder Graham M. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect. Dis. 2021;8(7) doi: 10.1093/ofid/ofab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tulledge‐Scheitel Sidna, Bell Sarah J., Larsen Jennifer J., Bierle Dennis M., Takahashi Paul, Moehnke Darcie E., Destro Borgen Molly J., Springer Donna J., Reinschmidt Karen J., Baumbach Lori J., Matoush Jennifer A., Heyliger Alexander, Hanson Sara N., Razonable Raymund R., Ganesh Ravindra. A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities. J. Am. Geriatr. Soc. 2021;69(4):868–873. doi: 10.1111/jgs.17090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webb Brandon J, Buckel Whitney, Vento Todd, Butler Allison M, Grisel Nancy, Brown Samuel M, Peltan Ithan D, Spivak Emily S, Shah Mark, Sakata Theadora, Wallin Anthony, Stenehjem Eddie, Poulsen Greg, Bledsoe Joseph. Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19, Open Forum. Infect. Dis. 2021;8(7) doi: 10.1093/ofid/ofab331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheinson Daniel, Dang Joseph, Shah Anuj, Meng Yang, Elsea David, Kowal Stacey. A cost-effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv. Ther. 2021;38(4):1811–1831. doi: 10.1007/s12325-021-01654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J.Y., Lee J.Y., Ko J.H., Hyun M., Kim H.A., Cho S., et al. Effectiveness of Regdanvimab Treatment in High-Risk COVID-19 Patients to Prevent Progression to Severe Disease. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.772320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Briggs Andrew, Vassall Anna. Count the cost of disability caused by COVID-19. Nature. 2021;593(7860):502–505. doi: 10.1038/d41586-021-01392-2. [DOI] [PubMed] [Google Scholar]
- 62.Pulse, COVID-19 treatment in Korea cost $57,562 per critically-ill, bill charged on state, 2020. https://pulsenews.co.kr/view.php?year=2020&no=471089 (accessed 25 June 2021).
- 63.Lee Doowon, Choi Bobae. Policies and innovations to battle Covid-19 – A case study of South Korea. Health Policy Technol. 2020;9(4):587–597. doi: 10.1016/j.hlpt.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Institute for Clinical and Economic Review, ICER comments on Gilead's pricing for remdesivir, 2021. https://icer.org/news-insights/commentaries/icer-comments-on-gileads-pricing-for-remdesivir/ (accessed 18 November 2021).
- 65.Congly S.E., Varughese R.A., Brown C.E., Clement F.M., Saxinger L. Treatment of moderate to severe respiratory COVID-19: a cost-utility analysis. Sci. Rep. 2021;11:17787. doi: 10.1038/s41598-021-97259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo Y., Jamieson L., Edoka I., Long L., Silal S., Pulliam J.R.C., et al. Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. Open Forum Infect. Dis. 2021;8:ofab040. doi: 10.1093/ofid/ofab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Institute for Clinical and Economic Review, Treatments for COVID-19, 2021. https://icer.org/wp-content/uploads/2021/08/ICER_COVID_Draft_Scope_082621.pdf (accessed 18 November 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.