Abstract
A case of phaeohyphomycosis is reported in a male renal transplant recipient with a nodular lesion in the right leg who was treated with immunosuppressing drugs. The lesion consisted of a purulent cyst with thick walls. The cyst was excised surgically, and the patient did not receive any antifungal therapy. One year later he remains well. Histological study of the lesion showed a granulomatous reaction of epithelioid and multinucleate giant cells, with a central area of necrosis and pus. Fontana-Masson staining demonstrated the presence of pigmented hyphal elements. The fungus Colletotrichum crassipes was grown in different cultures from the cyst. The in vitro inhibitory activities of eight antifungal drugs against the isolate were tested. Clotrimazole and UR-9825 were the most active drugs. This case represents the first known reported infection caused by this rare species.
Within the huge number of fungal species involved in human opportunistic infections, numerous mitosporic species, which develop their asexual reproductive structures on cup-shaped (acervuli) or spherical (pycnidia) fruiting bodies (conidiomata), are frequently being reported. They are classified within the form class Coelomycetes, and up to now, at least 11 genera and 22 species have been implicated in human disease (4, 13). Colletotrichum Corda is one of the most common genera, which is characterized by causing both phaeohyphomycosis (1, 6, 7) and hyalohyphomycosis (5, 7).
Phaeohyphomycoses comprise a vast array of opportunistic fungal infections characterized by the presence of different types of melanized fungal elements in tissue (8). These elements can be clearly detected with the Fontana-Masson stain, because they become dark, in contrast to the fungal elements present in hyalohyphomycoses, which remain colorless.
The genus Colletotrichum is a typical pathogen of plants, which has been traditionally included in the coelomycetous order Melanconiales, characterized by the formation of acervular conidiomata when the fungus parasitizes the plant tissue. Species concepts are based on morphology of the fungi on natural substrate and to a lesser extent in culture, sometimes combined with host specificity or the lack of it. In culture, these fungi develop conidiomata, which consist of conidial masses supported by a superficial cushion-like mass of short conidiophores, among which erect, unbranched, and darkly pigmented sterile hyphae (setae) are developed. They also frequently develop appressoria (flat and dark-pigmented swellings at the end of a hypha), which they use to attach themselves to the host surface before penetrating the tissue. These elements are typical of plant-pathogenic fungi. The presence of these elements is an important diagnostic feature for genus recognition, and their shape is important for species separation (12, 14).
Currently, of the several hundred species described, only four species of Colletotrichum have been associated with human infections (4). These species are C. dematium, C. gloeosporioides, C. coccodes, and C. graminicola. However, these infections are very rare and are generally associated with some form of trauma. They manifest as keratitis or subcutaneous lesions, although a case of invasive infection has also been reported (9). The present report describes the first case involving Colletotrichum crassipes causing a subcutaneous phaeohyphomycotic cyst.
Case report.
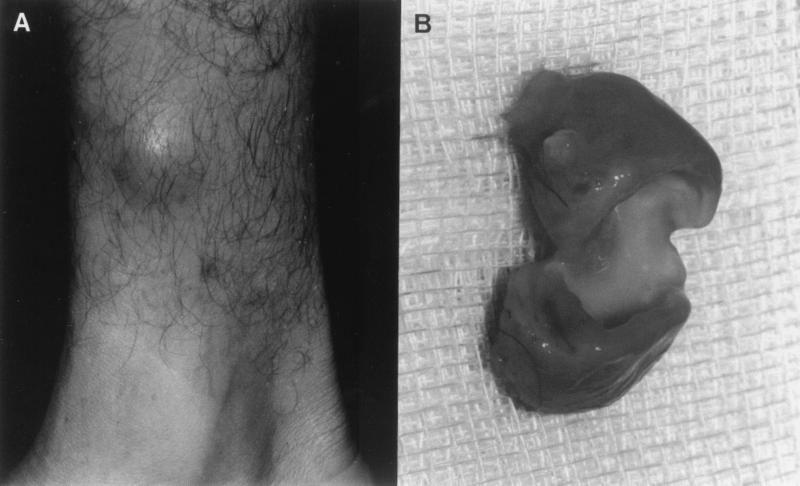
A 34-year-old Brazilian male gardener, resident of São Paulo, had a renal transplant 2 years earlier due to chronic and progressive renal failure. The patient was immunocompromised secondary to cyclosporine, prednisone, azathioprine, and captopril treatment but otherwise in good condition. One year after transplantation, he noticed a small area of swelling on the anterior face of the right leg, which progressed slowly to a painless nodule with no noticeable antecedent trauma. On examination in September 1998, he showed a subcutaneous nodule (2.5 cm in diameter) which was not painful, and the overlying skin appeared completely normal, with no sign of inflammation (Fig. 1A). The nodule was surgically excised and found to encompass a flesh-colored cystic lesion. The cyst had a thick wall and was purulent (Fig. 1B). Part of the nodule was fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, Groccott-Gomori methanamine silver, and Fontana-Masson stains. Three different portions of the nodule were each homogenized separately in sterile saline with a sterile mortar and pestle and used for microbiological analysis. Each homogenized specimen was cultured by standard techniques on routine media for aerobic or anaerobic bacterial isolation and on Sabouraud dextrose agar (Oxoid, Basinstoke, England), incorporating chloramphenicol (0.05%) with and without cycloheximide (0.04%), for fungal isolation. All cultures were incubated at 35 to 37°C.
FIG. 1.
(A) Nodular lesion on the anterior face of the right leg. (B) Purulent and thick-walled cyst.
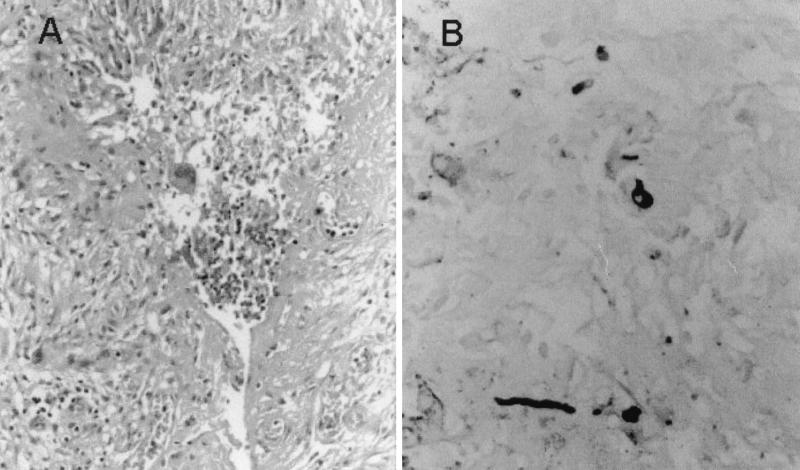
Examination of hematoxylin- and eosin-stained tissue section of the excised nodule revealed a granulomatous lesion (Fig. 2A). The subcutaneous tissue was fibrotic, and in the areas of fibrosis there were circumscribed small foci of mixed granulomatous and suppurative inflammation. Palisading epithelioid cells, multinucleate giant cells, and lymphocytes surrounded central microabscesses of polymorphonuclear leukocytes. Grocott-Gomori stain revealed the presence of a few distorted hyphal elements (Fig. 2B), and Fontana-Masson stain revealed the presence of melanine in these hyphae. Routine bacteriological cultures were negative, while those for fungi were positive. Multiple dark-colored colonies, apparently belonging to the same fungus, grew in all Sabouraud petri dishes. For identification purposes, the fungus was cultured on potato carrot agar (PCA; 20 g of potato, 20 g of carrot, 18 g of agar, 1,000 ml of tap water, homemade) and oatmeal agar (OA; 30 g of oat flakes, 1 g of MgSO4 · 7H2O, 1.5 g of KH2PO4, 15 g of agar, 1,000 ml of tap water, homemade) and incubated at 25, 37, 40, and 45°C in the dark.
FIG. 2.
(A) Hematoxylin and eosin stain of the nodular tissue, showing a granulomatous lesion. (B) Groccott-Gomori methanamine silver stain, showing a few hyphal elements. Magnification, ×400.
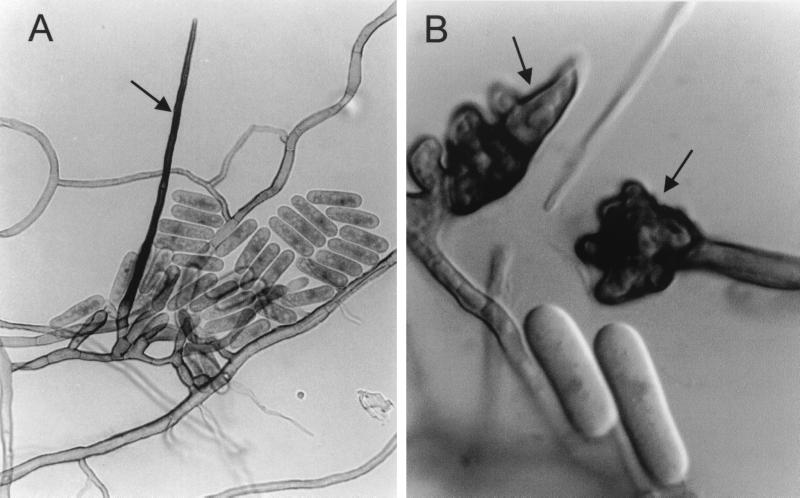
On PCA and OA at 25°C, similar colonies grew very quickly, covering the surface of the petri dishes in 10 days. They were cottony, olivaceous brown with a black reverse. The best growth and sporulation was obtained in PCA. On this medium the fungus produced abundant stiff, erect, sterile hyphae (setae) and appressoria (Fig. 3). The setae were acicular, septate, brown to dark brown, thick-walled, and up to 150 μm long. The appressoria were irregular and dark brown with crenate or deeply lobed walls, measuring, on average, 18 μm long by 10 μm wide. The conidia, emerging from slightly clavate conidiogenous cells, were slimy, hyaline, mostly cylindrical and measuring 11 to 18 μm long by 6.5 to 8 μm wide. At 37°C the colonies attained a diameter of 12 to 15 mm in 10 days. The fungus did not grow at 40°C.
FIG. 3.
C. crassipes FMR 6728. (A) Seta (arrow), conidiogenous cells and conidia. Magnification, ×640. (B) Conidia and appressoria (arrows). Magnification by Nomarski optics, ×1,600.
Based on morphology of the conidiophores and conidia and the presence of setae and numerous appressoria, the fungus was identified as a Colletotrichum sp. We first thought that it was a strain of C. gloeosporioides, the most common species of the genus, which we recently isolated from a subcutaneous infection in Brazil (5). However, a more detailed study, mainly taking into account the morphology of the appressoria with crenate or deeply lobed margins, helped us to identify it as C. crassipes. A comparison of this clinical isolate with other strains from different origins (CBS 159.75 and IMI 302450) confirmed its identity. The isolate is maintained in our mycology laboratory at the Medicine Faculty of the Rovira i Virigli University in Reus, Spain, as FMR 6728. Other living cultures have been deposited in the Centraalbureau voor Schimmelcultures of the Netherlands as CBS 109355 and in the Belgian Coordinated Collections of Microorganisms/Institute of Hygiene and Epidemiology Mycology (BCCM/IHEM).
C. coccodes, C. dematium, C. gloeosporioides, and C. graminicola, the other opportunistic species of the genus, are clearly distinguished from C. crassipes mainly by the shape and size of the conidia. C. dematium and C. graminicola have falcate conidia, while in C. coccodes they are fusiform and measure 16 to 22 μm long by 3 to 4 μm wide. In C. gloeosporioides, the closest species morphologically to C. crassipes, the conidia are also cylindrical but narrower (3 to 4.5 μm) and generally longer (up to 24 μm). Furthermore, in C. gloeosporioides the appressoria show an irregular edge but are never deeply lobed. C. crassipes might be a composite species consisting of a number of separate taxa (2). Baxter et al. (3) described many variations in the colony colors. This fungus has been reported as parasitizing legumes in Malaysia, Zambia, and India (2).
Antifungal susceptibility testing of the isolate was accomplished by a previously described microdilution method (11) mainly according to the guidelines of the National Committee for Clinical Laboratory Standards for molds (10), using RPMI 1640 medium buffered to pH 7 with 0.165 M morpholinepropanesulfonic acid (MOPS), an inoculum of 105 CFU/ml, an incubation temperature of 30°C, an incubation period of 72 h, and an additive drug dilution procedure. MICs were 1 μg/ml for amphotericin B, >128 μg/ml for 5-fluorocytosine, 2 μg/ml for itraconazole, voriconazole, and terbinafine, 4 μg/ml for miconazole, >16 μg/ml for fluconazole, 8 μg/ml for ketoconazole, 0.5 μg/ml for clotrimazole, and 0.125 μg/ml for UR-9825.
This fungus was less susceptible to antifungals than the other three opportunistic Colletotrichum species tested previously (5). Their MICs were low, with a few exceptions. In this study, eight antifungals displayed MICs higher than 1 μg/ml against C. crassipes; only clotrimazole and UR-9825, the latter being a new potent triazole from Uriach S.A., Barcelona, showed lower MICs. The present case, however, was resolved without antifungal therapy.
Acknowledgments
We are indebted to Arvind A. Padhye (Centers for Disease Control and Prevention, Atlanta, Ga.) for reviewing the manuscript.
This study was supported by CICYT (Ministerio de Educación y Cultura, Spain) grant PM98-0059.
REFERENCES
- 1.Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243–251. doi: 10.1007/BF00419488. [DOI] [PubMed] [Google Scholar]
- 2.Bailey J A, Jegger M J, editors. Colletotrichum: biology, pathology and control. Wallingford, U.K: CAB International; 1992. [Google Scholar]
- 3.Baxter A P, van der Westhuizen G C A, Eicker A. Morphology and taxonomy of South African isolates of Colletotrichum. S Af J Bot. 1983;2:259–289. [Google Scholar]
- 4.De Hoog G S, Guarro J, Gené J, Figueras M J. Atlas of clinical fungi. 2nd ed. Utrecht, The Netherlands: Centraalbureau voor Schimmelcultures; 2000. [Google Scholar]
- 5.Guarro J, Svidzinski T E, Zaror L, Forjaz M H, Gené J, Fischman O. Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides. J Clin Microbiol. 1998;36:3060–3065. doi: 10.1128/jcm.36.10.3060-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto T, Ajello L. Agents of phaeohyphomycosis. In: Ajello L, Hay R J, editors. Collier, Ballows, Sussman, Topley & Wilson's microbiology and microbial infections. 9th ed. Vol. 4. London, U.K: Arnold; 1998. pp. 503–504. [Google Scholar]
- 7.Matsumoto T, Ajello L, Matsuda T, Szanislo P J, Walsh T J. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(Suppl. 1):329–349. doi: 10.1080/02681219480000951. [DOI] [PubMed] [Google Scholar]
- 8.McGinnis M R. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis and mycology. J Am Acad Dermatol. 1983;8:1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 9.Midha N K Y, Mirzanejad Y, Soni M. Colletotrichum spp.: plant or human pathogen? Antimicrob Infect Dis Newsl. 1996;15:26–27. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Pujol I, Guarro J, Llop C, Soler L, Fernández-Ballart J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2106–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton B C. The coelomycetes. Kew, U.K: Commonwealth Mycological Institute; 1980. [Google Scholar]
- 13.Sutton D A. Coelomycetous fungi in human disease, a review: clinical entities, pathogenesis, identification and therapy. Rev Iberoam Micol. 1999;16:171–179. [PubMed] [Google Scholar]
- 14.Von Arx J A. Plant pathogenic fungi. Beih Nova Hedwigia. 1987;87:1–288. [Google Scholar]