Abstract
All effusions in serous cavities represent a pathologic processes secondary to inflammatory, neoplastic, hemodynamic, or mechanical/traumatic etiologies. This elicits reactive changes in the extremely sensitive mesothelial cells lining the serosal surfaces. The result is hypertrophy and hyperplasia which lead to broad changes with a wide range of morphological appearances. These reversible alterations may resolve entirely after the recovery of underlying pathology.
Under the tertiary care situations, neoplastic effusion specimens are encountered more frequently. Although some non-neoplastic pathologic process may demonstrate a few diagnostic features, cytologic evaluation of malignant effusions usually show diagnostic malignant cells. However, the most versatile mesothelial cells demonstrate a very wide cytomorphological spectrum secondary to reactive challenges. These mesothelial cells are usually referred to as ‘reactive mesothelial cells’. In addition other terms such as reactive mesothelial proliferation, reactive mesothelial hyperplasia, irritated mesothelial cells, activated mesothelial cells, hyperplastic mesothelial cells, hypertrophic mesothelial cells, and proliferative mesothelial cells. Rarely atypical mesothelial cells, although not recommended, is used inadvertently. Although there is a lack of general agreement defining these terms, some of these including atypical mesothelial cells, should not be preferred. With reference to this CMAS series, usually favored term ‘reactive mesothelial cells’ is preferred.
The size of reactive mesothelial cells range from 15 to 30 µm (but may be up to 50 µm). These polyhedral cells with variable amount of cytoplasm and enlarged nuclei may show variation in sizes and shapes with conspicuous nucleoli. Bi- and multi-nucleation is frequent. Cohesive groups of mesothelial cells as sheets and three dimensional groups may be present. Some floridly reactive mesothelial cells with hyperchromatic enlarged nuclei with prominent nucleoli and scant cytoplasm may resemble malignant cells. This astonishingly wide morphological spectrum of reactive mesothelial cells is a significant interpretation challenge in effusion fluid cytology. Methodical interpretation approach with appropriate knowledge about this wide spectrum is important aspect in diagnostic cytopathology of effusion fluids.
Pathologic processes affecting a serosal cavity, such as inflammation, neoplasia, and trauma, lead to reactive changes in the extremely sensitive mesothelial cells that line the serosal surfaces. The mesothelial cells react to the altered environment by hypertrophy and proliferation, resulting in a wide spectrum of morphologic appearances [Tables 1-3].[1–6] These changes may disappear completely with the resolution of the underlying pathologic process.
Table 1:
Cytomorphology of mesothelial cells —the spectrum.
| Uniform cell population |
| Monotonous, oval to round nuclei |
| Mononucleated cells with mostly centrally placed nuclei |
| Evenly distributed fine powdery chromatin |
| Inconspicuous to prominent nucleoli |
| Multinucleation with anisonucleosis |
| Moderate amount of translucent cytoplasm |
| Two-zone cytoplasm |
| Peripheral vacuoles containing glycogen |
| A faint staining thin halo along the edge (microvilli) |
| Fuzzy cell border (due to microvilli) |
| Peripheral blebs in Diff-Quik-stained smears |
| Monolayer cell aggregates |
| Doublets or triplets with clasp-like articulation |
| Mesothelial windows between the cells |
| Occasional papillary groups |
| Ballooning of cytoplasm with signet-ring-like vacuolization |
Table 3:
Features that make effusion cytology more elusive*.
| Extensive morphologic variation |
| High nucleocytoplasmic ratio |
| Nuclear hyperchromasia |
| Coarse chromatin clumping |
| Prominent macronucleoli |
| Nuclear pleomorphism |
| Irregular nuclear membrane |
| Numerous mitotic figures |
In general, non-neoplastic effusions are more common than malignant effusions even in tertiary care institutions. Cytologic evaluation is more useful for malignant effusions, which usually show diagnostic malignant cells. However, some non-neoplastic effusions may also show diagnostic morphologic features consistent with a particular nonneoplastic pathologic processes such as collagen disease related serous cavity effusions.
REACTIVE MESOTHELIAL CELLS
The mesothelial cells are one of the most versatile cells in the human body, with an extremely variable and wide morphologic spectrum. The mesothelial cells with variable cytomorphological spectrum are referred as ‘reactive mesothelial cells’. Many other terms have been used to label these cells with reactive changes: reactive mesothelial proliferation, reactive mesothelial hyperplasia, irritated mesothelial cells, activated mesothelial cells, hyperplastic mesothelial cells, hypertrophic mesothelial cells, proliferative mesothelial cells, and atypical mesothelial cells.7 General agreement on the definition and use of these terms is lacking.1,4,5 However, some of them, including atypical mesothelial cells, should be avoided. In our laboratory, we generally use the term ‘reactive mesothelial cells’, which is how they will be referred to as.
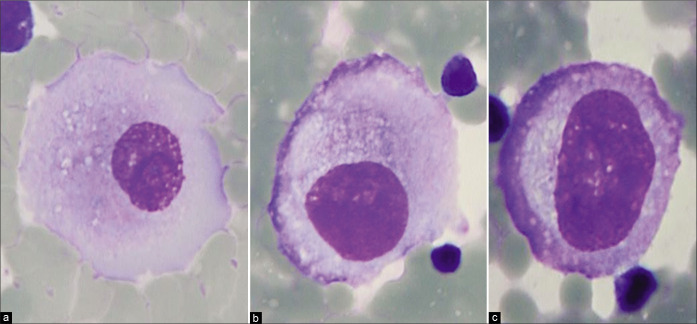
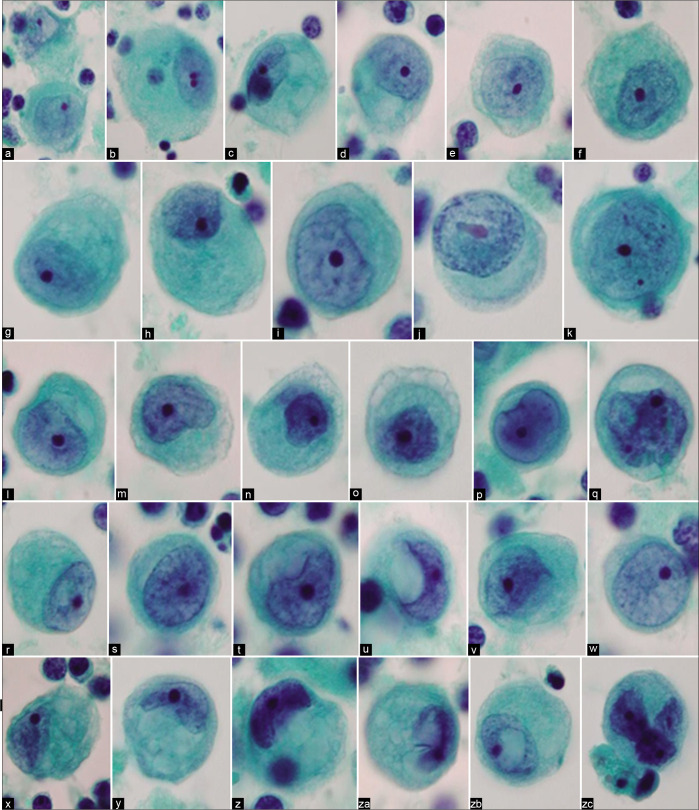
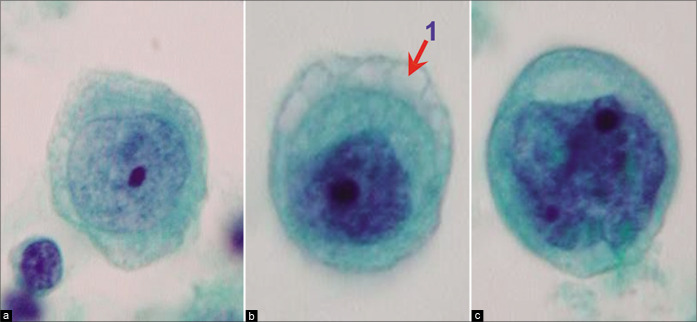
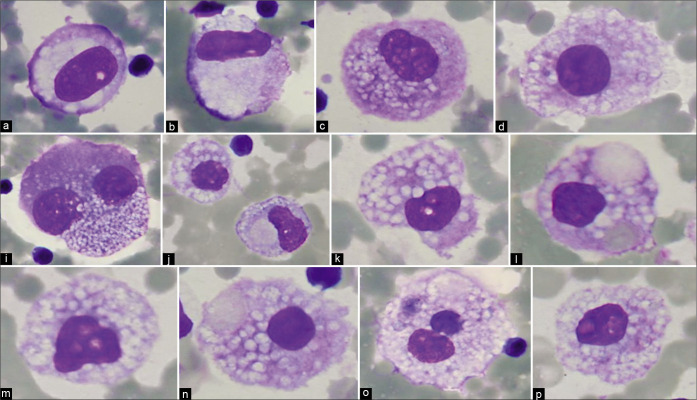
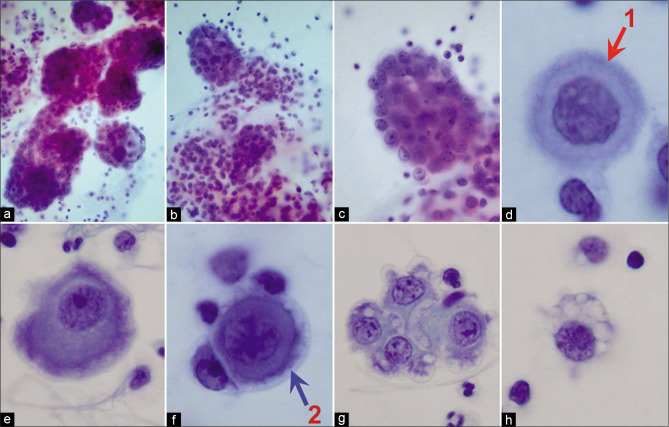
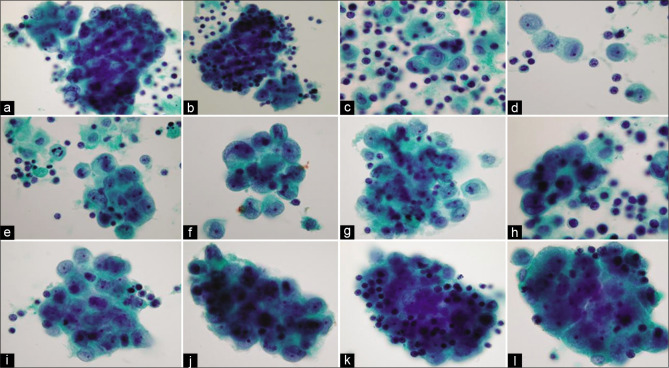
Typically, the reactive mesothelial cells range from 15 to 30 μm (but may be up to 50 µm) in diameter. They have enlarged nuclei, with some variation in their sizes and shapes. Nucleoli are usually conspicuous. The amount of cytoplasm is variable [Figures 1-7]. Binucleation and multinucleation is relatively frequent. Cohesive clusters of mesothelial cells with or without papillary configuration may be present. Some cells have scanty cytoplasm with slightly hyperchromatic enlarged nuclei with prominent nucleoli [see Figures 6,7]. This astonishingly wide morphologic spectrum of reactive mesothelial cells may overlap with the morphologic spectrum of malignant cells with increased potential for pitfalls leading to higher frequency of atypical interpretations and even false-positives [see Tables 1, 2, 3, Figures 2, 7]. This is exaggerated further by other effusion specific challenges including in vivo and in vitro fluid related physical phenomenon.
Figure 1:
Mesothelial cells with central to slightly eccentric nuclei (ascitic fluid). The cytoplasm shows a two-zone staining pattern. For additional variations see also Figures 2,3,5. [a–c, DQ-stained Cytospin smear (a–c, 100X zoomed).]
Figure 7:
Mesothelial cells with central to eccentric nuclei (ascitic fluid). Spectrum of cytomorphologic features. [a–zc, PAP-stained ThinPrep smear (a–zc, 100X zoomed).]
Figure 6:
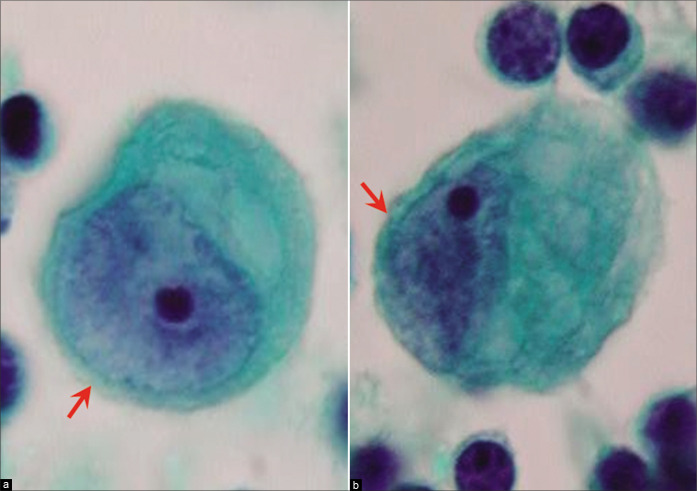
Mesothelial cells with central to slightly eccentric nuclei (ascitic fluid). The cytoplasm shows a two-zone staining pattern with peripheral vacuolation (red arrow 1). For additional ranges see also Figures 7, 8, and 10. [a–c, PAP-stained ThinPrep smear (a–c, 100X zoomed).]
Table 2:
Overlapping malignant cytomorphology of reactive mesothelial cells.
| Mesothelial cells in large groups |
| Cell groups with scalloped borders |
| Cellular molding |
| Multinucleation |
| Mitotic figures |
| Cell-to-cell apposition |
| ‘Cell-in-cell’ configuration |
| Proliferative cell balls |
| True papillary aggregates |
| Acinus-like structures |
| Degeneration of cytoplasm |
| Signet ring change |
| Spheres with collagenous cores |
| Psammoma bodies |
These features are frequent with the possible pitfall of misinterpreting reactive effusions as malignant.
Figure 2:
Panorama of mesothelial cells (ascitic fluid). Central to near central nuclei. Rare mesothelial cells may show eccentric nuclei touching the cell membrane, but usually there is a narrow rim of cytoplasm separating the nucleus from the cell border (arrowheads) [see also Figures 1,3,5]. [a–x, DQ-stained Cytospin smears (a–x, 100X zoomed).]
Binucleation and multinucleation
Similar to reactive histiocytes, reactive mesothelial cells may have two or more nuclei. This is considered to be an in-vivo change within the serous cavity. They are frequent in peritoneal dialysis fluids.8 However, mesothelial cells in effusion specimens may form small aggregates due to technical factors such as filtration, Cytospin preparation, centrifugation, or liquid-based cytology preparations. The cytoplasmic borders of these ‘sticky’ reactive mesothelial cells may fuse together as a degenerative change and appear multinucleated. This is usually observed as in vitro phenomenon in poorly preserved specimens left at room temperature for several hours.
Gigantic nuclei
Degeneration may also lead to fusion of nuclear membranes, with the formation of a mesothelial cell with one or more gigantic nuclei. These large nuclei, however, usually have fine powdery chromatin with evenly distributed small nucleoli. Such cells formed secondary to degenerative changes may lead to an interpretation of ‘atypical’ cells. They are not observed in biopsy specimens.
Phagocytic activity
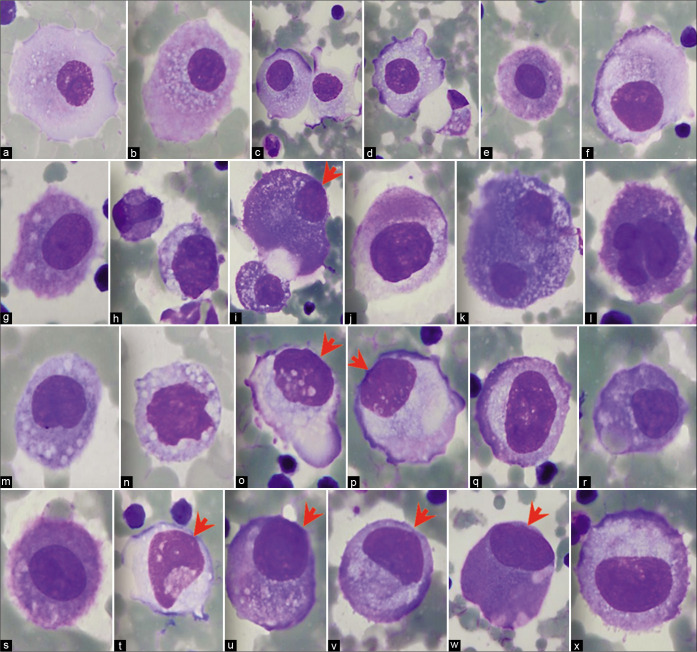
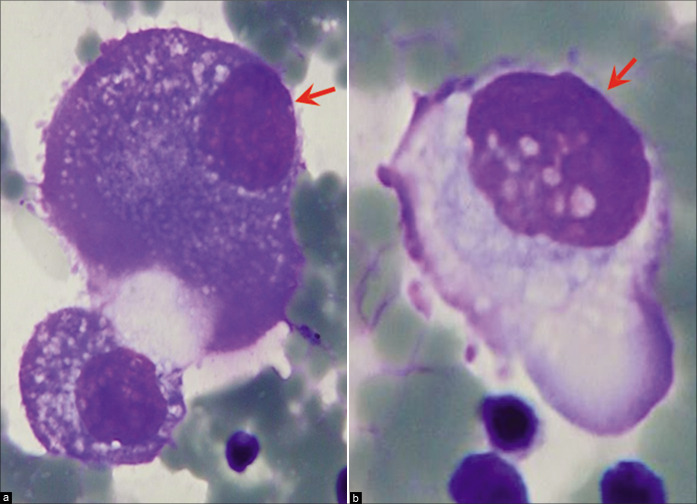
Reactive mesothelial cells with phagocytic activity may transform into foamy macrophages [see Figure 5]. Although distinguishing reactive mesothelial cells from reactive macrophages is usually of little clinical significance, this has been demonstrated by experimental models and by immunocytochemistry.
Figure 5:
Vacuolated mesothelial cells with macrophage features (pleural fluid). Panorama of cytomorphologic features with central to slightly eccentric nuclei. [a–l, DQ-stained cytospin smears (a–l, 100X zoomed).]
However, mesothelial cells with some features of macrophages may resemble malignant cells. A large cytoplasmic vacuole in a mesothelial cell may seem to have pushed its nucleus to the cell margin, thus imparting a signet ring appearance, as observed in some adenocarcinoma cells [see Figure 7u].
Cohesive clusters
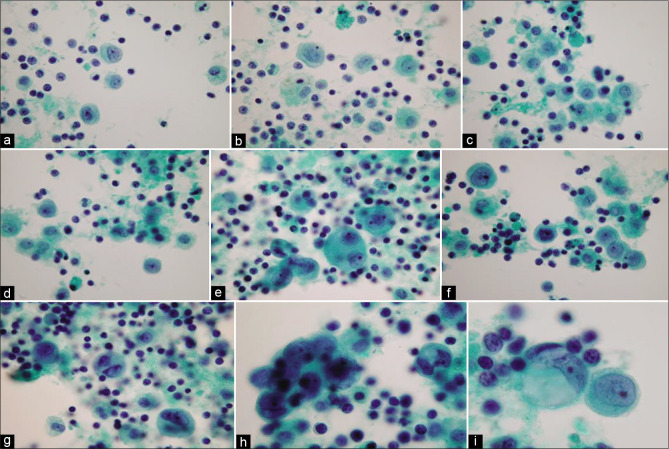
Mesothelial hyperplasia associated with effusions may show cohesive clusters (sometimes in ill-defined papillary configurations) [Figure 10, 11]. Non-neoplastic effusions usually contain fewer cohesive clusters, together with numerous solitary mesothelial cells [Figure 12].
Figure 10:
Reactive mesothelial cells in clusters mixed with chronic inflammatory cells, mostly mature lymphocytes (ascitic fluid). This consult case was initially misinterpreted as positive for malignant cells. Extensive search for the primary was negative [see also Figures 10, 11]. [PAP-stained ThinPrep smear (X100 oil, zoomed.)]
Figure 12:
Reactive mesothelial cells mixed with chronic inflammatory cells (ascitic fluid). Mesothelial cells are present as isolated cells and as small groups [see also Figures 9, 10]. [PAP-stained ThinPrep smear (100X zoomed).]
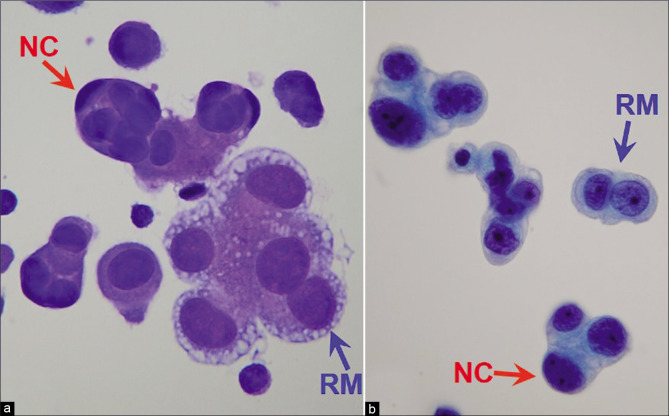
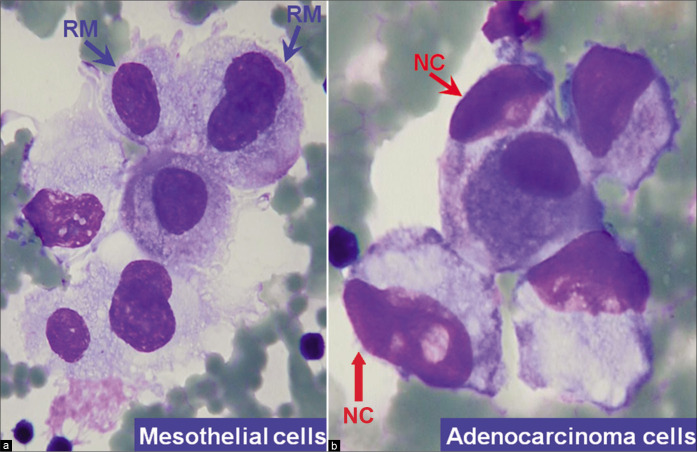
Increased number of cohesive clusters and/or papillary structures formed by mesothelial cells should arouse suspicion of mesothelioma (especially if these cluster in cell-block sections show stromal cores). Cohesive clusters of reactive mesothelial cells generally have scalloped (knobby) contours. However, similar features may also be observed in clusters of cells from various neoplasms [Figure 14]. The individual cells at the periphery of mesothelial clusters show recognizable cytoplasmic features of mesothelial cells and the nuclei do not touch the cell borders of the cells along the periphery of the group. The perimeter of such clusters of mesothelial cells (applicable to both reactive and neoplastic mesothelial cells) is formed by the cytoplasm of individual cells in the group [Figures 13g, 14] and not by the nuclear outlines usually observed in clusters of adenocarcinoma cells [Figure 14].
Figure 14:
Metastatic adenocarcinoma with a two-cell population (ascitic fluid). Blue arrow RM highlights reactive mesothelial cells (central nuclei, peripheral vacuolation, community borders of cell groups formed by cell membrane) and red arrow NC highlights neoplastic cells of adenocarcinoma (eccentric nuclei touching the cell membranes without any rim of cytoplasm between the nucleus and cell membrane; the community border of cell groups is formed by mostly nuclear contours). [a, DQ-stained Cytospin smear; b, PAP-stained ThinPrep smear (a,b, X100 oil, zoomed).]
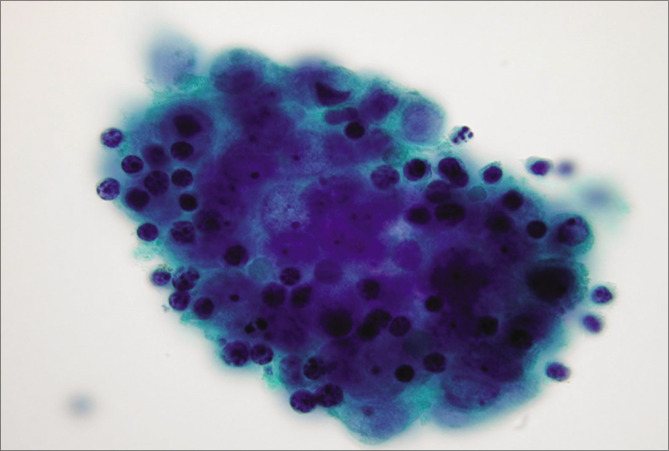
Figure 13:
Malignant epithelioid mesothelioma (pleural fluid). Mesothelioma cells show numerous large three-dimensional groups of cells. The individual mesothelioma cells do not show any significant variation from reactive mesothelial cells without remarkable features of malignancy. The mesothelioma cells, like reactive mesothelial cells, show two-zone staining (red arrow 1) with peripheral vacuolation (blue arrow 2). [a–h, PAP-stained ThinPrep smear (a,b, 20X; c, 100X; d -h, 100X zoomed).]
Two-cell foreign population
The two-cell foreign population (other than reactive mesothelial cells and inflammatory cells) approach is highly effective in interpreting the cytopathology of effusion smears for metastatic malignancy [Figure 14]. However, this approach applicable to effusion specimens, is not effective for washing specimens. All mesothelial cells, including scattered solitary reactive mesothelial cells, are seemingly of one type, although with a wide morphologic spectrum. The mesothelial cells blend morphologically with their neighbors and demonstrate a continuum in their morphological spectrum [see Figures 2, 5, 7, 11, 12]. This is different from malignant effusions due to metastatic carcinoma, in which cohesive clusters of malignant cells usually can be identified easily as an alien population [Figure 14] in Papanicolaou (PAP)- and Diff-Quik (DQ)-stained preparations. However, as mentioned previously, the two populations are detected better in DQ stained preparations with significant ease [Figure 14a] even under low magnification as compared to PAP stain [Figure 14b]. If further evaluation for malignancy is needed, ancillary techniques such as immunohistochemistry on cell-block sections may be used to confirm mesothelial versus non-mesothelial nature of the cells.
Figure 11:
Reactive mesothelial cells in clusters (ascitic fluid), mixed with chronic inflammatory cells within the groups and between the mesothelial cells in the background [see also Figures 9, 11]. [PAP-stained ThinPrep smear (X100 oil, zoomed).]
Non-neoplastic reactive conditions, such as pelvic inflammatory diseases, are frequently associated with many mesothelial cell clusters [see Figures 10, 11], some of which may show a papillary-like configuration. They may present a diagnostic pitfall for an inexperienced examiner, leading to the erroneous interpretation of malignant cells such as ovarian adenocarcinoma, especially in aspirates of the cul-de-sac.3 The consult ascitic fluid from an elderly Asian woman shown in Figures 10-12 was initially misinterpreted as positive for malignant cells. Extensive search did not show a primary.
Cell-in-cell configuration
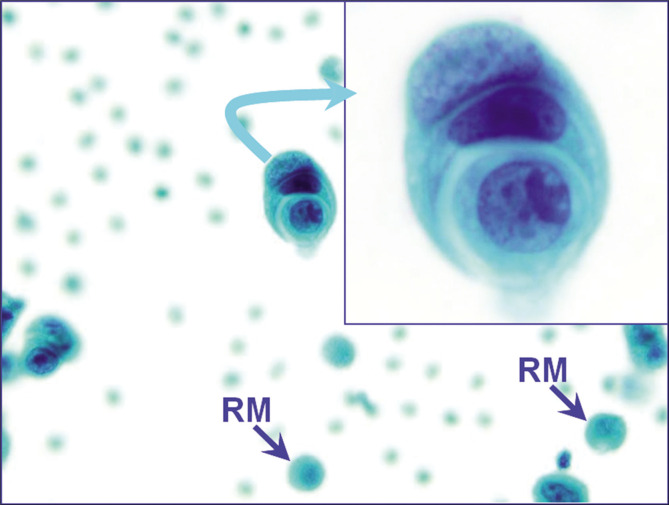
The cytoplasm of a cell may grasp the adjacent cell, with the appearance of one cell within another. This feature, although more common in reactive mesothelial cells, is highly non-specific. It may also be observed in metastatic carcinoma cells in malignant effusions [Figure 15].
Figure 15:
The cell-in-cell pattern of neoplastic cells (metastatic mammary carcinoma, pleural fluid). A similar pattern may also be observed in reactive mesothelial cells. In this case, the morphology of these cells resembled other cancer cells seen as a second population without overlap with nuclear morphology of reactive mesothelial cells (blue arrows RM). [PAP-stained ThinPrep smear (X100 oil; inset, zoomed)].
Cells in sheets
These are a common finding in pelvic or peritoneal washings, but are rarely seen in effusions. Their presence is due to mechanical detachment of the serosal membrane during operative incision or lavage procedures, or both. These mesothelial cells in sheets may resemble clusters of squamous cells, but they usually do not present a diagnostic challenge.
Groups of reactive mesothelial cells
Groups of reactive mesothelial cells [see Figures 10, 11] in effusions are likely to be the most important diagnostic pitfall. The conditions [Table 4] associated with groups of reactive mesothelial cells in effusions are as follows:
Table 4:
Conditions associated with significant reactive changes in mesothelial cells.
| Liver disease (cirrhosis) |
| Underlying neoplasia (hamartoma, subserosal implants, fibroids) |
| Foreign substance (talc, asbestos) |
| Traumatic irritation (hemodialysis, operative procedures) |
| Chemoradiation therapy |
| Pancreatic disease (pancreatitis) |
| Inflammation and infection (pleuritis, pericarditis) |
| Infarction (lung infarct, pulmonary embolism) |
| Collagen disorders (rheumatic fever, lupus erythematosus) |
| Renal disease (uremia) |
| Chronic inflammation (pelvic inflammatory disease) |
Hepatomegaly related to congestive heart failure and chronic active hepatitis related cirrhosis, leading to peritoneal effusion with exfoliation of reactive mesothelial cells from the surface of the congested liver.
Ischemic conditions such as pulmonary infarction, ischemic colitis, and occlusion of mesenteric blood vessels frequently show reactive changes in the serosal membranes surrounding the ischemic areas. These reactive mesothelial cells may exfoliate in groups into the effusions and may contain intracytoplasmic hemosiderin granules or red blood cells or both [Figure 9].
Trauma to organs covered with mesothelium such as spleen, liver, and lung, etc.
Large retroperitoneal masses: slowly growing retroperitoneal masses, including benign retroperitoneal neoplasms growing close to the peritoneum, may elicit reactive changes in the overlying mesothelium. This mesothelium tends to exfoliate into the peritoneal cavity and seen as groups in cytology preparations of peritoneal effusions.
Postoperative: following laparotomy and thoracotomy. Artifactual desquamation in this situation is secondary to surgical trauma.
Figure 9:
Mesothelial cells with phagocytosed erythrocyte (red arrow in a) and hemosiderin (red arrows in b) [a–b, PAP-stained ThinPrep (X100 oil, zoomed)]
These conditions may be associated with groups and sheets of reactive mesothelial cells in effusions that may simulate groups of neoplastic cells. In the absence of proper clinical information, this kind of pitfall may lead to a cytomorphologic misinterpretation of malignancy. Presence of sheets of mesothelial cells does not bear any diagnostic significance in the interpretation of pelvic washing cytology.
‘ATYPICAL’ MESOTHELIAL CELLS
Floridly reactive changes in mesothelial cells in some nonneoplastic effusions may show significant atypia and may resemble malignant cells [Table 5].6 These mesothelial cells demonstrate: nuclear enlargement, coarse clumped chromatin, high nucleocytoplasmic ratios, nuclear hyperchromasia, and prominent macronucleoli. They may be seen as cells in large cohesive groups with scalloped edges with molding of the cells with extensive morphologic variation. Such cells are sometimes referred to as ‘atypical mesothelial cells’ [see Figure 6c]. However, this terminology should be avoided.7 If mesothelioma is excluded and these cells are confirmed as mesothelial cells (cytomorphologically with or without immunocharacterization) such cells should be interpreted as floridly reactive mesothelial cells. If these cells are definitively confirmed as non-mesothelial cells and the features are suspicious for neoplastic cells, then they may be characterized as atypical cells (not atypical mesothelial cells).
Table 5:
Atypical mesothelial cells*.
| Mesothelial cells with cytomorphology overlapping that of malignant cells. The cells show: |
| •Enlargednuclei |
| •Highnucleocytoplasmicratios |
| •Nuclearhyperchromasia |
| •Clumpedchromatin |
| • Macronucleoli |
| •Extensivemorphologicvariability |
| •Thecellsmaybeinlargegroupswithscallopedborders •*Predominantdifferentialdiagnosisinthissituationismesothelioma. |
Floridly reactive mesothelial cells are frequent in the effusions associated with cirrhosis of the liver, pulmonary infarction, viral pericarditis (due to exaggerated reactive process secondary to friction with every heart beat)collagen vascular diseases, renal failure with uremia, pancreatitis,2 bile peritonitis, therapeutic radiation, chemotherapy, and large benign intra-abdominal masses with floridly reactive mesothelial hyperplasia of the overlying serosa [Table 4].6
Such floridly reactive mesothelial cells with morphologic features approaching ‘atypical’ mesothelial cells are a serious pitfall in effusion cytology [Tables 2, 3, 5, Figure 6c]. Their enlarged nuclei, high nucleo-cytoplasmic ratios, and conspicuous nucleoli may suggest adenocarcinoma. However, the endoplasm–ectoplasm pattern with dense cytoplasm around the nuclei and paler lacy (or microvesicular) cytoplasm at the periphery is consistent with mesothelial cells [Figures 4, 6, 8]. Some reactive mesothelial cells may show a reverse pattern: foamy paranuclear cytoplasm and dense peripheral cytoplasm with prominent blebs [see Figure 3].
Figure 4:
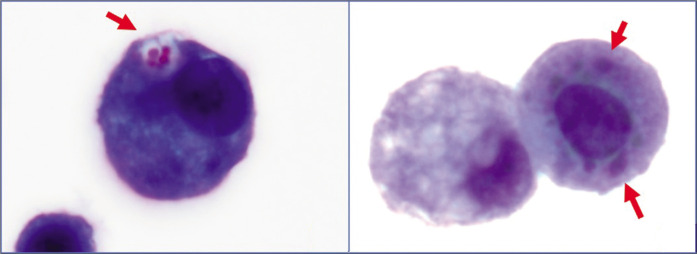
Mesothelial cells versus adenocarcinoma cells (ascitic fluid). a. Mesothelial cells with central to eccentric nuclei. A thin rim of cytoplasm separates the nuclear border from the cell border (blue arrows RM). b. Compared to mesothelial cells the adenocarcinoma cells with eccentric nuclei appose the cell border without a distinct rim of intervening cytoplasm (red arrows NC). [a,b, DQ-stained Cytospin smear (a,b, 100X zoomed).]
Figure 8:
Mesothelial cells with eccentric nuclei (ascitic fluid); [see more cells in Figure 7b,d,g,h,i,j,m, etc.]. Careful scrutiny usually shows a narrow rim of cytoplasm separating the nucleus from the cell border (arrows). [l,x, PAP-stained ThinPrep smear (100X zoomed).]
Figure 3:
Mesothelial cells with eccentric nuclei (ascitic fluid) [see also Figures 2g,i,o,p,r,t,u,v,w]. Careful scrutiny usually shows a narrow rim of cytoplasm separating the nucleus from the cell border (arrows) with blebs [see also Figures 1,2,5]. [i,o, DQ-stained Cytospin smears (i,o,100X zoomed).]
Footnotes
How to cite this article: Shidham VB. The panorama of different faces of mesothelial cells . CytoJournal 2021;18:31.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/CMAS_02_02_2021
References
- 1.Bedrossian CWM. New York: Igaku-Shoin; 1994. Malignant Effusions: A Multimodal Approach to Cytologic Diagnosis. [DOI] [Google Scholar]
- 2.Kutty CPK, Remeniuk E, Verkey B. Malignant-appearing cells in pleural effusion due to pancreatitis: Case report and literature review. Acta Cytol. 1981;25:412–416. [PubMed] [Google Scholar]
- 3.Kern WH. Benign papillary structures with psammoma bodies in culdocentesis fluid. Acta Cytol. 1969;13:178–180. [PubMed] [Google Scholar]
- 4.McGowan L. Morphology of mesothelial cells in peritoneal fluid from normal women. Acta Cytol. 1974;18:205–209. [PubMed] [Google Scholar]
- 5.Spriggs AI, Boddington MM. Boston: Kluwer; 1989. Atlas of Serous Fluid Cytopathology. [DOI] [Google Scholar]
- 6.Soendergaard K. On the interpretation of atypical cells in pleural and peritoneal effusion. Acta Cytol. 1977;21:413–416. [PubMed] [Google Scholar]
- 7.Bedrossian CW. Diagnostic problems in serous effusions. Diagn Cytopathol. 1998;19:131–137. doi: 10.1002/(SICI)1097-0339(199808)19:2<131::AID-DC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Selvaggi SM, Migdal S. Cytologic features of atypical mesothelial cells in peritoneal dialysis fluid. Diagn Cytopathol. 1990;6:22–26. doi: 10.1002/dc.2840060107. [DOI] [PubMed] [Google Scholar]