Abstract
Background:
Glioblastoma multiforme (GBM) is the most common central nervous system malignant tumor in adults with 48.3% of cases. Despite it, the presence of transtentorial spread is uncommon, with few patients reported in the literature. In this study, the authors report a case of GBM transtentorial spread to cerebellopontine angle after resection and adjuvant treatment.
Case Description:
A 55-year-old male patient with GBM, previously submitted to surgical resection and adjuvant treatment with radiotherapy and quemotherapy. Fourteen months after the first surgery, he developed headaches associated with dysphagia and dysphonia. Magnetic resonance imaging showed a recurrence of the left parietal lesion and a new mass in the right cerebellopontine angle. The patient underwent successful surgical resection of both lesions. Chemotherapy was maintained after the surgery.
Conclusion:
To the best of our knowledge, there are few cases of GBM metastasis to the cerebellopontine angle reported in the literature. Surgical management should be considered in cases of intracranial hypertension and patients with good performance status.
Keywords: Glioblastoma multiforme, Pontocerebellar angle, Transtentorial spread

INTRODUCTION
Glioblastoma multiforme (GBM) represents 14.6% of the central nervous system (CNS) tumors and 48.3% of malignant brain tumors. Its presentation in the infratentorial compartment is uncommon, with a frequency of 1.2–4% of cases.[10,13,15]
Metastases to the CNS and other organs rarely are reported due to poor patient survival.[1,8] Although mechanisms of metastasis remain uncertain, two acceptable hypotheses are hematogenic dissemination and tumor cell migration through the cerebrospinal fluid (CSF).[2,4] To the best of our knowledge, there are few cases of GBM transtentorial spread to cerebellopontine angle reported in the literature. The present study aims to describe a case of cerebellopontine angle metastasis of glioblastoma.
CASE REPORT
A 55-year-old male presented with a 2-month history of progressive left frontal headache associated with nausea, vomiting, and an episode of confusion. Physical examination evidenced a discrete right homonymous hemianopsia.
A head magnetic resonance imaging (MRI) showed a mass evolving in the left parietal and occipital lobes, measuring 4.9 × 1.7 × 3.7 cm, and compressing the ipsilateral ventricle. It presented a heterogenic pattern in T1, T2-, and T1-weighted gadolinium-enhanced sequences with cystic areas and a pattern consistent with central necrosis [Figure 1]. The patient evolved without deficits after the first surgical resection of the cranial mass. The pathological specimen showed giant cells with nuclear atypia, pseudopalisading necrosis, and neovascular proliferation; it was also positive for GFAP, IDH, ATRX, and a Ki-67 index of 30%, compatible with a giant cell glioblastoma pattern [Figure 2].
Figure 1:
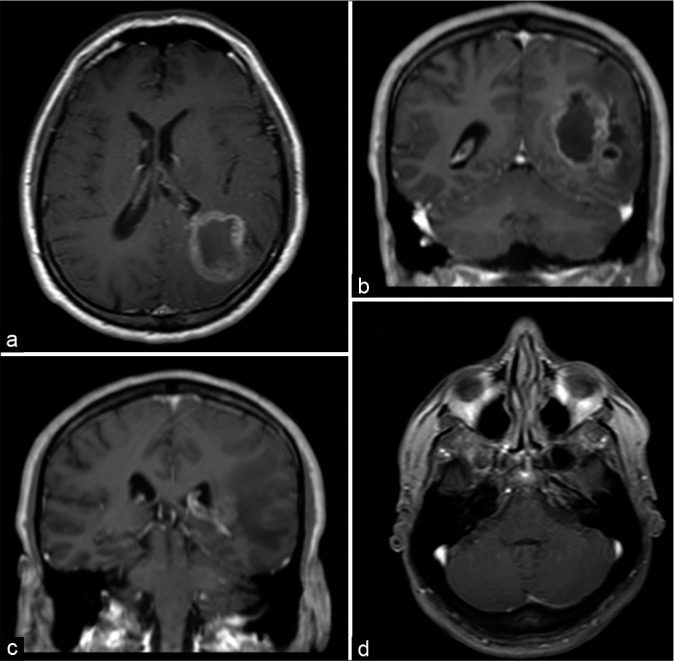
(a and b) Magnetic resonance imaging scans with axial and coronal T1-weighted postgadolinium sequences showing a heterogeneous contrast enhancement lesion that involves the parietal and occipital lobes. (c and d) The same scans did not show any lesion in the cerebellopontine angle and posterior fossa.
Figure 2:
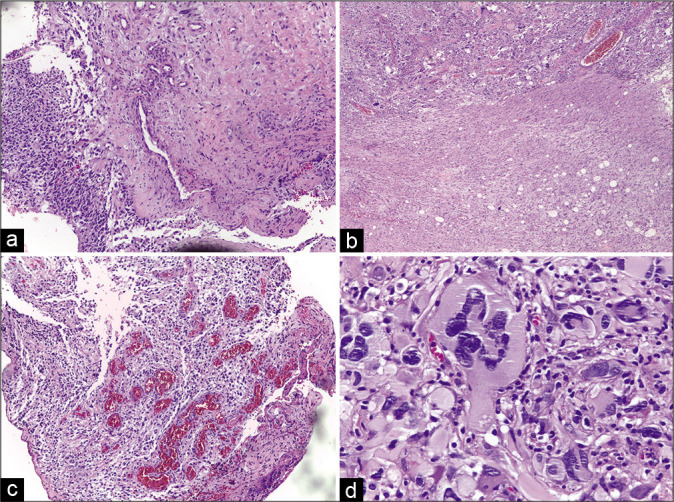
(a-c) Histopathological features of the initial tumor. A routine hematoxylin and eosin-stained specimen shows a pseudopalisading pattern, hypercellularity, and high mitotic proliferation. (d) Nuclear atypia with glomeruloid pattern, typical of glioblastoma.
The patient underwent adjuvant treatment with 60 Gy focal brain radiotherapy fractioned in 30 days and 12 cycles of chemotherapy using temozolomide.
Fourteen months later, he presented progressive headache, dizziness, right facial spasm, and dysphagia. A new MRI showed recurrence of the previous lesion and a new lesion in the right cerebellopontine angle, measuring 2.6 × 2.2 cm [Figure 3].
Figure 3:
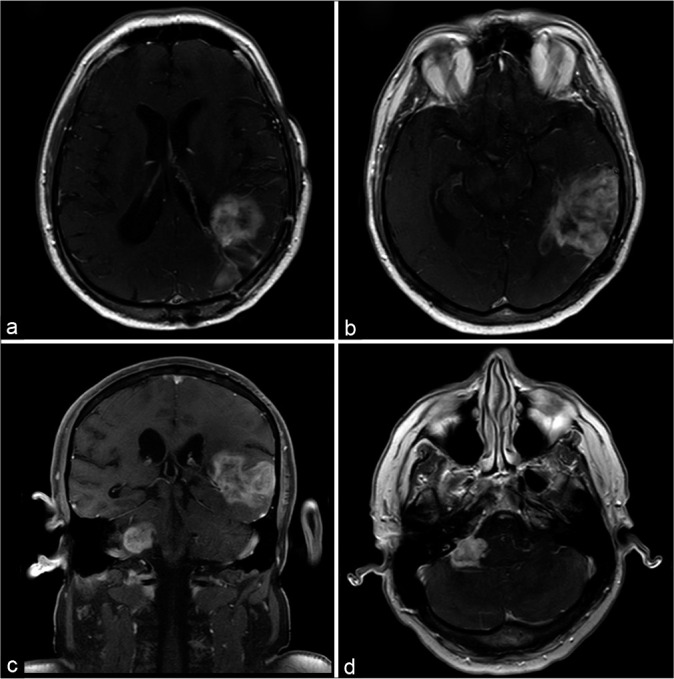
(a and b) Magnetic resonance imaging scans with axial T1-weighted postgadolinium sequence show the initial lesion in the occipital and parietal lobes relapsed. (c and d) The images of the posterior fossa show a new lesion in the contralateral cerebellopontine angle with the same pattern of the supratentorial tumor.
The resection of both lesions was undertaken in a second surgical treatment. In the intraoperative period, we observed a soft grayish lesion with dura mater invasion. The petrous surface of the cerebellum and the VII cranial nerve was infiltrated. This lesion had the same histologic pattern as the previous glioblastoma. The patient passed away 40 days after surgery due to pneumonia.
DISCUSSION
GBM is a high-grade infiltrative astrocytic neoplasm of the CNS which only involves the cerebellum in 1.2–4% of all cases.[10,13,15] Despite being a primary tumor of the CNS, GBM can present metastases to the CNS itself, as well as to other organs.[8]
About 0.5% of GBM patients present extracranial metastases, which are more frequently reported in lungs, liver, bones, and lymph nodes. Moreover, sporadic cases to the pericardium, myocardium, oral mucosa, and eyelids were described.[8,12] On the other hand, metastases can be found in 6–25% of GBM patient autopsies which shows low diagnosis rates.[11]
Müller et al. demonstrated that 20% of GBM cases have tumor cells circulating in the bloodstream regardless of surgical intervention, which explains the potential risk of local and distant metastases by hematogenic dissemination.[9,13,14] In addition, epidermal growth factor receptor gene amplification was observed in some circulating cells showing a possible association between the mutation and GBM spread.[5,9]
Few cases of GBM transtentorial spread and metastasis outside of the brain have been reported in the literature, which can be attributed to the patient’s poor survival rate and the protection provided by the natural barrier formed by the lack of lymphatic vessels in the brain, the dura mater, and around venous sinuses.[1,6]
There are two theories regarding the etiology of GBM in the posterior fossa: first, dissemination of silent supratentorial GBMs through the CSF, which represents the transtentorial spread.[2,6] Second, the infratentorial GBM might arise without spreading from a supratentorial location. Since no supratentorial tumor could be detected in most reports on cerebellar GBMs, they presumably originate from glial cells within the cerebellum.[10,13,15]
The clinical incidence of the symptomatic spread of GBM is relatively infrequent compared to the incidental discovery of such spread at autopsy. This finding may result from the low survival rate of the affected patients, not from the biology of the tumor.[15]
Clinical diagnosis of transtentorial spread or infratentorial GBM can be difficult due to nonspecific symptoms, including ataxia, dysmetria, dizziness, and executive function alterations. Areas of necrosis and sometimes hemorrhage characterize MRI, but sometimes, due to its rarity, it can be misdiagnosed as a low-grade tumor.[11,12] In rare cases of dura mater infiltration, the tumor could mimic a meningioma dural tail.[3]
Surgical resection is the treatment of choice whenever a patient’s performance status permits. This makes it possible to confirm the diagnosis through a histopathological study, improve symptoms by reducing mass effect, and allow better outcomes.[10,13,15]
There are no specific radiotherapy protocols in cases of GBM metastases to CNS. One of the reasons for it is the metastases appearance after primary lesion irradiation, normally with local radiotherapy due to its lower toxicity. Despite it, Lahmi et al. showed in a recent retrospective study the safety use of whole-brain radiotherapy (WBRT) for multicentric and multifocal GBM in doses of 45 Gy fractioned in 1.8 Gy over 37 days. Future studies are necessary to clarify the safety and if WBRT could avoid glioblastoma metastases.[7,16]
CONCLUSION
Transtentorial spread of glioblastoma is a rare condition that shows unspecific clinical and radiological findings. It needs to be suspected if the patient with a history of GBM has posterior fossa neurological signs such as ataxia, dysmetria, dizziness, and executive function alterations. We can confirm the diagnosis through an anatomopathological study with the same characteristics of the previous glioblastoma.
Despite patient’s poor prognosis, tumor resection could be attempted to improve symptoms and confirm the diagnosis.
Footnotes
How to cite this article: Caramanti RL, Aprigio RM, Tognola WA, Laurenti MR, Rocha CE, Góes MJ. Transtentorial spread of glioblastoma multiforme to cerebellopontine angle – A rare case report. Surg Neurol Int 2022;13:5.
Contributor Information
Ricardo Lourenço Caramanti, Email: ricardocaramanti1987@gmail.com.
Raysa Moreira Aprígio, Email: raysaaprigio@gmail.com.
Waldir Antônio Tognola, Email: watognola@terra.com.br.
Matheus Rodrigo Laurenti, Email: mrlaurenti@yahoo.com.br.
Carlos Eduardo Rocha, Email: drcarlosrocha@hotmail.com.
Mário José Góes, Email: mgccc@terra.com.br.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abdallah A, Uysal ML, Abdallah BG, Emel E. Intracranial metastases of glioblastoma multiforme: A case report. J Nerv Syst Surg. 2015;5:97–100. [Google Scholar]
- 2.Czepko R, Kwinta B, Adamek D, Uhl H, Betlej M, Lopatka P. Multiple cerebral glioma or tumor dissemination via CSF pathways? Case report. Neurol Neurochir Pol. 2003;37:1307–15. [PubMed] [Google Scholar]
- 3.Doddamani RS, Meena RK, Sawarkar D. Ambiguity in the dural tail sign on MRI. Surg Neurol Int. 2018;9:62. doi: 10.4103/sni.sni_328_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goryaynov SA, Potapov AA, Ignatenko MA, Zhukov VY, Protskiy SV, Zakharova NA, et al. Glioblastoma metastases: A literature review and a description of six clinical observations. Zh Vopr Neirokhir Im N N Burdenko. 2015;79:33–43. doi: 10.17116/neiro201579233-43. [DOI] [PubMed] [Google Scholar]
- 5.Ghous G, Miller D, Doll D, Tuncer T. A rare case of glioblastoma with extensive liver metastases. Oncology. 2021;35:733–40. doi: 10.46883/ONC.2021.3511.0733. [DOI] [PubMed] [Google Scholar]
- 6.Hong CS, Hsieh JK, Edwards NA, Ray-Chaudhury A, Zaghloul KA. IDH mutations may not preclude distant, transtentorial spread in gliomas: A case report and review of the literature. World J Surg Oncol. 2016;14:1–5. doi: 10.1186/s12957-016-0814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahmi L, Idbaih A, Del Campo ER, Hoang-Xuan K, Mokhtari K, Sanson M, et al. Whole brain radiotherapy with concurrent temozolomide in multifocal and/or multicentric newly diagnosed glioblastoma. J Clin Neurosci. 2019;68:39–44. doi: 10.1016/j.jocn.2019.07.065. [DOI] [PubMed] [Google Scholar]
- 8.Mastorakos P, Hays MA, Caruso JP, Chen CJ, Ding D, Taylor DG, et al. Transtentorial dissemination of optic nerve glioblastoma: Case report. J Neurosurg. 2018;128:406–13. doi: 10.3171/2016.10.JNS161443. [DOI] [PubMed] [Google Scholar]
- 9.Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6:247ra101. doi: 10.1126/scitranslmed.3009095. [DOI] [PubMed] [Google Scholar]
- 10.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:v1–100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen J, Blau T, Grau SJ, Barbe MT, Fink GR, Galldiks N. Extracranial metastases of a cerebral glioblastoma: A case report and review of the literature. Case Rep Oncol. 2018;11:591–600. doi: 10.1159/000492111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slowik F, Balogh I. Extracranial spreading of glioblastoma multiforme. Zentralbl Neurochir. 1980;41:57–68. [PubMed] [Google Scholar]
- 13.Stark AM, Maslehaty H, Hugo HH, Mahvash M, Mehdorn HM. Glioblastoma of the cerebellum and brainstem. J Clin Neurosci. 2010;17:1248–51. doi: 10.1016/j.jocn.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Tsung AJ, Prabhu SS, Lei X, Chern JJ, Benjamin Bekele N, Shonka NA. Cerebellar glioblastoma: A retrospective review of 21 patients at a single institution. J Neurooncol. 2011;105:555–62. doi: 10.1007/s11060-011-0617-2. [DOI] [PubMed] [Google Scholar]
- 15.Walter J, Koch A, Herbold C, Schiffler S, Reichart R, Waschke A, et al. Multifocal glioblastoma multiforme in the posterior fossa mimicking cerebral metastases: Case presentation and review of the current literature. J Neurol Surg A Cent Eur Neurosurg. 2013;74:e30–5. doi: 10.1055/s-0032-1330113. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZX, Chen JX, Shi BZ, Li GH, Li Y, Xiang Y, et al. Multifocal glioblastoma-two case reports and literature review. Chin Neurosurg J. 2021;7:8. doi: 10.1186/s41016-020-00223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


