Abstract
Background:
Gangliocytomas are rare neuronal tumors with an incidence of <1% of all central nervous system (CNS) neoplasms. They occur mostly in the pediatric age group, localizing within the cerebral cortex, most often the temporal lobe.
Case Description:
We report a case of an intracranial gangliocytoma arising within the lateral ventricle in a 66-year-old female. Magnetic resonance imaging of the brain showed a diffusely enhancing lobulated mass situated within the frontal horn of the right lateral ventricle with extension into the foramen of Monro and obstructive hydrocephalus. The patient underwent an interhemispheric transcallosal approach with gross total resection and relief of her hydrocephalus. Pathological examination showed clusters of highly pleomorphic neuron-like cells without evidence of neoplastic glial cells. Histopathological and immunohistochemistry findings were consistent with the diagnosis of gangliocytoma (World Health Organization Grade 1).
Conclusion:
Gangliocytomas are rare low-grade CNS neoplasms that can present in an older population within unusual locations and should be included within the differential whenever a suspicious lesion is encountered.
Keywords: Adult, Central nervous system, Gangliocytoma, Ganglioglioma, Ventricle

INTRODUCTION
Gangliocytomas are rare central nervous system (CNS) neoplasms that consist of mature, large neuron-like cells.[21] They are considered low grade and are assigned a World Health Organization (WHO) Grade 1.[33] They have a predilection for pediatric and young adult populations, and are very rarely reported in the older population.[11,25,34] Gangliocytomas are most frequently reported in the cerebral hemispheres, especially within the temporal lobe.[13,16,28] We report an unusual case of gangliocytoma arising within the right frontal horn of a 66-year-old female who presented with hydrocephalus.
CASE REPORT
Clinical presentation
A 66-year-old female with a medical history of hypertension and depression presented with a 10-month history of progressive generalized weakness, fatigability, mild nonspecific headaches, and penultimately confusion. Her symptoms significantly worsened the week before her presentation, suffering multiple falls as a result. In the emergency department, she did not endorse any visual changes, seizures or seizure-like activity, any specific pattern to her headaches, nausea or vomiting, or any other constitutional symptoms. Her neurological examination was within the normal limits with no apparent focal neurological deficits.
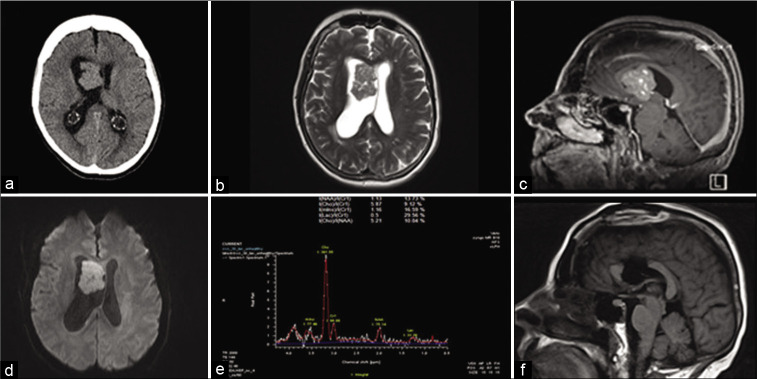
Computed tomography scan of the head showed an intraventricular mass lesion with associated obstructive hydrocephalus [Figure 1a]. Magnetic resonance imaging (MRI) of the brain with gadolinium, time-of-flight angiogram, and magnetic resonance spectroscopy [Figure 1b, c, d, e] sequences was obtained for further delineation of the lesion and operative planning. The lesion was approximately 2.8 × 4.1 × 3.9 cm in maximal transverse by anterior posterior by craniocaudal dimensions. It was localized to the inferior aspect of the right lateral ventricle, contacting the septum pellucidum, and extending through the right foramen of Monro into the third ventricle. It expanded the ventricle without evidence of parenchymal invasion. It also appeared to come into contact with the inner surface of the body of corpus callosum [Figure 1c]. The lesion appeared to have a diffuse pattern of enhancement on T1 + Gd sequences [Figure 1c]. It seemed of intermediate intensity on T2-weighted sequences [Figure 1b]. It had uniformly low-grade restricted diffusion [Figure 1d]. There was no calcification or evidence of hemorrhage. MR angiogram showed small branching vessels within and along the margin of the mass. MR spectroscopy showed markedly elevated choline peak and diminished but preserved N-acetyl aspartate (NAA) peak. [Figure 1e].
Figure 1:
Radiological features. (a) CT head showing right frontal lesion with ventriculomegaly. (b) Axial T2-weighted MRI showing lesion with isointense signal. (c) Sagittal T1 + Gadolinium showing diffuse enhancement of the lesion. (d) Diffusion-weighted image (DWI) showing mild diffusion restriction within the lesion. (e) MR spectroscopy showing markedly elevated choline peak and diminished NAA peak. (f) Postoperative sagittal T1-weighted MRI showing gross total resection.
Surgical intervention
Shortly after presentation, she underwent a craniotomy; interhemispheric dissection and anterior callosotomy allowed entry into the lateral ventricle. The tumor was immediately identifiable by its tan color and lobulated appearance. It had two consistencies with one part being easily aspirated and a second firmer part that required ultrasonic aspiration. The inside of the tumor contained numerous blood vessels with pronounced veins. Of note, hemostasis was easily achievable with electrocautery. Anteriorly and medially, it had some obvious attachments that required microsurgical dissection primarily from the septum pellucidum. Eventually, the dissection led down to the ipsilateral foramen of Monro and into the third ventricle. Once the tumor was freed from its attachment points, complete removal was achieved. The patient recovered well from her surgery and was neurologically intact without any focal deficits. Postoperative MRI showed gross total resection of the lesion with improving ventriculomegaly [Figure 1f]. She was placed on a 7-day course of phenytoin. She required short-term rehabilitation with physiotherapy given the degree of deconditioning in her preoperative status and was discharged home 2 weeks later. On follow-up, she continues to do well from neurological standpoint. A follow-up MRI [Figures 2a and b] done 12 months postoperatively shows no signs of tumor recurrence.
Figure 2:
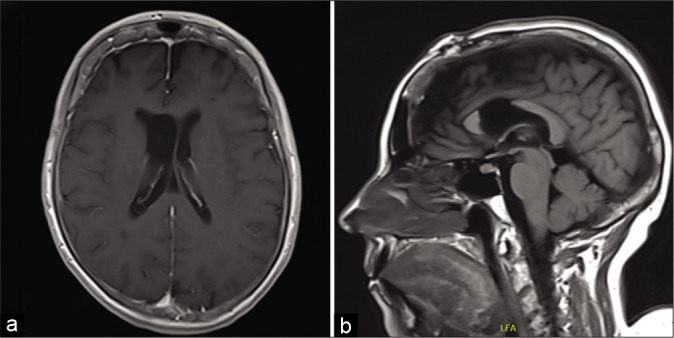
Twelve-month follow-up MRI. (a) Axial T1 + Gadolinium at the level of the lateral ventricles showing no recurrence or tumor residual. (b) Sagittal T1-weighted MRI at midline showing the corpus callosotomy and no recurrence or residual within the surgical cavity.
Pathology
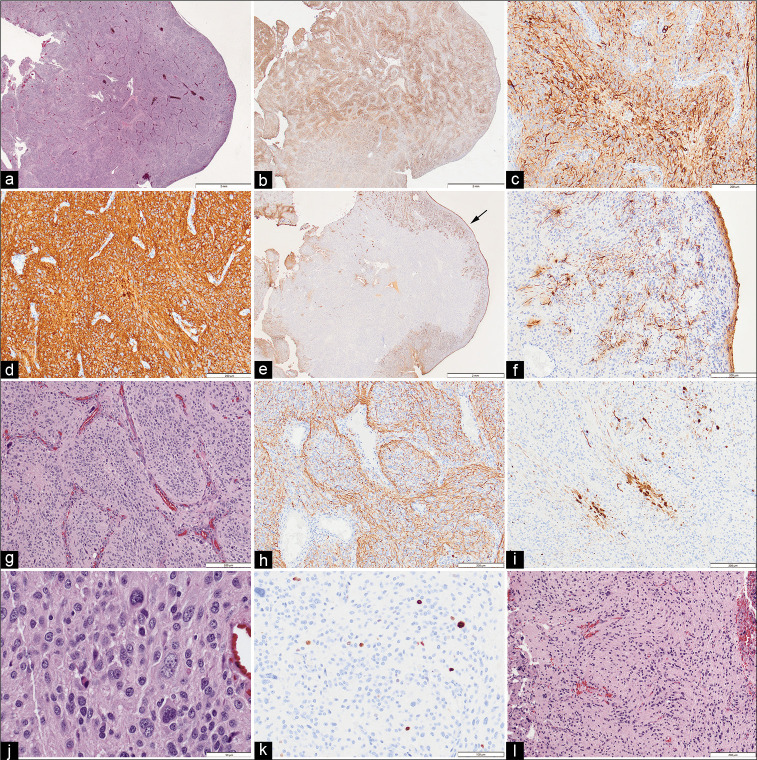
Intraoperative evaluation of smeared and frozen samples showed a pleomorphic neoplasm, without clear high-grade or papillary features. The remaining tumor specimens were fixed in formalin and embedded in paraffin. Sections were stained for hematoxylin and eosin, and a broad panel of immunohistochemical stains to determine glial and neuronal lineage. Histologically the superficial aspects of the tumor had a smooth surface [Figure 3a]. Neuronal lineage markers synaptophysin, CD56, non-phosphorylated neurofilament, and Pgp9.5 were all uniformly strong positive in most of the tumor [Figures 3b, c, and d]. GFAP was positive in reactive appearing, stellate astrocytes only in superficial areas of the tumor but was absent in the core [Figures 3e and f]. The neoplasm appeared vaguely lobulated with intervening vascular structures [Figure 3g]. Many were very large with bizarre or multiple nuclei. There were a few small foci of necrosis and microvascular proliferation, but only rare mitotic figures. There was no inflammatory cellular infiltrate and no significant reticulin network within the tumor. NeuN was focally and weakly positive in scattered cells. NF stained positively in axon-like processes throughout lobules in most of the tumor [Figure 3h]. Calretinin staining showed scattered clusters of neuron-like cells, usually at edges of lobules [Figure 3i]. The neoplasm was composed of highly pleomorphic medium-size to large neuron-like cells [Figure 3j]. S100 was strong in astrocytes and weakly seen in patchy neuronal populations. SOX10 showed nuclear positivity in a similar distribution to GFAP. CD34 was present in blood vessels only. ATRX had retained nuclear positivity. Other immunostains (alpha-synuclein, hyperphosphorylated tau, IDH1R132H, BRAF V600E, P53, EMA, and cytokeratin AE1/AE3) were negative. Ki-67 positive cells were scattered, estimated at <2% overall [Figure 3k]. Within the ultrasonic aspirate, a few foci had a glial appearance but lacked the large gangliocytoid cells and thus was thought to represent the attachment base of the tumor [Figure 3l]. There were more GFAP positive cells in the regions thought to be transitional [Figure 3l].
Figure 3:
(a) The superficial aspects of the tumor adjacent to the lateral ventricle had a smooth surface (Hematoxylin & Eosin). (b) Immunoreactivity for nonphosphorylated neurofilament (npNF) was present throughout the tumor and at low magnification had a variegated appearance. (c) The cells of the lobules, which were of varied size, were uniformly immunoreactive for npNF. (d) Tumor cells were uniformly immunoreactive for synaptophysin (as well as CD56 and Pgp9.5, not shown). (e) Immunoreactivity for glial fibrillary acidic protein (GFAP) was restricted to the tumor surface (arrow). (f) GFAP immunoreactive glial cells had non-neoplastic stellate appearance and were restricted to the tumor surface. (g) Intermediate magnification of the tumor core showed cellular lobules outlined by narrow vascular septae (H&E). (h) Neurofilament immunostain showed axon-like processes mainly at the periphery of the lobules. (i) In some lobule cores, the neuron-like cells were also immunoreactive for calbindin 2 (calretinin). (j) High magnification shows that the majority of the tumor cells are of medium to large size, often with neuron-like appearance. (k) Scattered nuclei were immunoreactive for Ki67 (extremely rare mitotic figures were identified, not shown). (l) Rare fragments of the tumor in the CUSA sample, presumed to represent the attachment site, had a more glial appearance with scattered atypical neurons (H&E). Original magnifications - (a, b, e) x12.5; (c, d, f-i, l) x100; (j) x400; (k) x200
DISCUSSION
Gangliocytomas were described in the first edition of the WHO classification of nervous system tumors in 1979.[37] The histology consisted of mature ganglion cells with nonneoplastic glial features and a mesenchymal stroma.[37] They then began to be grouped with gangliogliomas in their definition while drawing the distinction that gangliocytomas were composed solely of mature ganglion cells, whereas gangliogliomas would have neoplastic glial cells.[4] Most recently, in the latest WHO CNS tumor classification of 2016, gangliocytomas are separately addressed, holding their definition as mature neuroepithelial neoplasms with clusters of neoplastic ganglion cells and noting possible dysplastic features.[6]
Ganglion cell tumors have a controversial nosology. They are regarded as a spectrum of neoplasms predominated by neuronal cells. Gangliogliomas and gangliocytomas occupy opposite ends of the spectrum, mainly based on the presence of neoplastic glial cells.[5,17] In some cases, however, the distinction between the two is not definite, and the term ganglion cell tumor is reserved for those transitional forms.[15] Ganglion cell tumors can arise within the central and peripheral nervous systems. Histologically, a glial neuropil forms the stroma of those in a central location, and Schwann cells form the stroma of the peripherally occurring tumors.[27] Gangliocytomas are low-grade, slow-growing lesions with a histological WHO Grade 1.[33] There are, however, reports of dissemination and change of behavior.[17,36]
Their histogenesis remains elusive. Some have been observed to have common cytological abnormalities with certain neuronal migration disorders, such as hemimegalencephaly.[17] Genes involved in signaling pathways for glioneuronal differentiation and migration might be implicated in their pathogenesis.[5] Particularly, cyclin-dependent kinase 5 and doublecortin genes had reduced expression in some of the analyzed ganglioglioma specimens.[3,5] These genes play a key role within the Reelin signaling cascade in neuronal development.[3,10,22] Moreover, at some point in the discovery of ganglion cell tumors, their true neoplastic nature was doubted and the notion of possible hamartomatous origin was entertained.[7]
Epidemiology
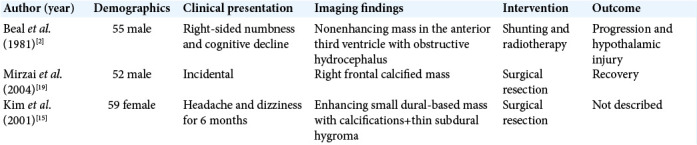
Ganglion cell tumors are rare, accounting for 1–3% of CNS tumors with gangliogliomas more common than gangliocytomas.[21] In two surgical epilepsy series, gangliocytomas accounted for 0.4–3.2% of examined pathological specimens.[31,33] It is, however, difficult to ascertain the exact incidence of gangliocytomas as they are often grouped with gangliogliomas. In terms of age distribution, gangliocytomas have been mostly observed in the pediatric and young adult populations[34] with an average age of diagnosis that ranged from 8.5 to 25 years with majority occurring before the third decade.[11,25,34] Even prenatal diagnosis has been reported.[8] Reports of detection in the older adult population are exceedingly rare, including one described case of a 50-year-old man with an asymptomatic highly calcified right frontal gangliocytoma.[19] Another report describes a parieto-occipital lesion with dural attachment and histopathological features of gangliocytoma with inflammatory cell infiltrates in a female patient within her late 50s, presenting with headaches.[15] There are also few reports of the third ventricular gangliocytomas occurring beyond the fourth decade of life [Table 1].[2]
Table 1:
Summary of cases of older adults with intracranial gangliocytomas described in the literature.
CNS location and clinical presentation
Ganglion cell tumors are reported throughout the CNS. Gangliocytomas specifically are reported to arise within the cerebral hemispheres, temporal lobe especially, and the spinal cord.[13,16,28] The third ventricle and hypothalamic involvement is another frequently reported location for ganglion cell tumors.[2] The floor of the fourth ventricle is another reported location, presenting with bulbar involvement.[35] They are occasionally reported in the cerebellum as the dysplastic gangliocytoma subtype seen in Lhermitte-Duclos disease.[33] Diffuse forms have been described,[26] as well as leptomeningeal dissemination.[36] Although gangliogliomas have rarely been reported in the ventricles, to the best of our knowledge, there are no reports of gangliocytomas within the lateral ventricle.
Seizures are a common presentation of CNS gangliocytomas.[31] Thus, it is plausible that the relatively nonepileptogenic intraventricular location of our patient’s lesion contributed to her late presentation. Her presentation correlated with the development of obstructive hydrocephalous due to the progressive narrowing of CSF outflow from tumor growth.
Radiological features
Gangliocytomas can resemble gangliogliomas in their imaging features.[30] Reports on their radiological appearance on MRI have been inconsistent. Some showed them to be hypointense on T1-weighted images and hyperintense on T2-weighted images with frequent enhancement.[15,24,29,30] Another report describes them as having mixed signal intensity on T1 and hypointense on T2.[1,30]
Our patient’s MRI findings [Figure 1] were that of an isointense T1 lesion with intermediate signal intensity on T2-weighted images. The lesion exhibited diffuse low-grade restricted diffusion as well as diffuse enhancement due to their cellularity and vascularity. MR spectroscopy showed markedly elevated choline peak, which is usually observed in tumors with high membrane turnover.[12] Likewise, ganglion cell tumors are recognized to have an elevated choline peak.[32] The tumor also appeared to have a preserved but diminished NAA peak, which indicates a disruption to neuronal integrity[12] that is likely related to its neoplastic processes. Similar MR spectroscopy findings are reported in another gangliocytoma case.[18]
Pathology
Ganglion cell tumors usually appear as well-circumscribed, firm, nodular, and frequently calcified lesions with some having associated cysts.[18,32] The histopathology of ganglion cell tumors is highly variable.[14] The specific microscopic features of gangliocytomas include polymorphic groups of neurons, mostly having dysplastic features with varying degrees of differentiation, and a nonneoplastic glial stroma.[6] Immunohistochemical staining should also be employed to prove the neuronal differentiation of the cells, such as neurofilament protein, synaptophysin, and NeuN. However, some of the older reports of supratentorial gangliocytomas lack the immunohistochemical findings to prove their neuronal lineage, with only few reports exhibiting those specific features.[2,18]
The lesion in our case exhibited features of a glioneuronal neoplasm with its ganglionic cell elements. The tumor core is purely neuronal, with demonstrated positivity for synaptophysin, CD56, nonphosphorylated neurofilament, Pgp9.5, NF, and NeuN as to be expected of a gangliocytoma. Furthermore, the core lacked a glial component, and the GFAP-positive cells were confined to the tumor attachment and the surfaces exposed to the ventricles, which would be atypical of ganglioglioma.
Other glioneuronal lesions such as focal cortical dysplasia, dysembryoplastic neuroepithelial tumors, and gangliogliomas have exhibited aberrant CD34-immunoreactivity, speculated to relate to undifferentiated neural progeny.[5] CD34 was only present in blood vessels of the tumor presented here. Moreover, some of the available reports in the literature described certain histopathological findings including a reticulin network[9] and lymphocytic infiltration;[18] such features are not observed in our tumor.
Molecular pathology
MAP kinase pathway activation might be implicated in gangliogliomas with reports of positive BRAF V600E mutations in up to 67% in specimens from certain age groups and locations, specifically the pediatric population.[20,23] The specific molecular pathology of gangliocytomas is not clear but might have a genetic link to gangliogliomas.[6] The tumor in our case was negative for BRAF V600E.
CONCLUSION
Gangliocytomas of the CNS of adults are very rare. As with our patient, they can arise from unusual locations such as the lateral ventricles and present at an older age. It is important to keep a broad differential whenever a suspicious lesion is encountered, especially when the lesion can be cured surgically, given its low-grade pathology.
Footnotes
How to cite this article: Alarifi N, Del Bigio MR, Beiko J. Adult gangliocytoma arising within the lateral ventricle: A case report and review of the literature. Surg Neurol Int 2022;13:11.
Contributor Information
Norah Alarifi, Email: alarifi.noraha@gmail.com.
Marc R. Del Bigio, Email: marc.delbigio@umanitoba.ca.
Jason Beiko, Email: jbeiko@hsc.mb.ca.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Altman NR. MR and CT characteristics of gangliocytoma: A rare cause of epilepsy in children. AJNR Am J Neuroradiol. 1988;9:917–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Beal MF, Kleinman GM, Ojemann RG, Hochberg FH. Gangliocytoma of third ventricle: Hyperphagia, somnolence, and dementia. Neurology. 1981;31:1224–8. doi: 10.1212/wnl.31.10.1224. [DOI] [PubMed] [Google Scholar]
- 3.Becker AJ, Klein H, Baden T, Aigner L, Normann S, Elger CE, et al. Mutational and expression analysis of the reelin pathway components CDK5 and doublecortin in gangliogliomas. Acta Neuropathol. 2002;104:403–8. doi: 10.1007/s00401-002-0570-4. [DOI] [PubMed] [Google Scholar]
- 4.Becker AJ. Ganglioglioma and Gangliocytoma. WHO Classification of Tumours of the Central Nervous System. 2007. pp. 103–5.
- 5.Blümcke I, Wiestler OD. Gangliogliomas: An intriguing tumor entity associated with focal epilepsies. J Neuropathol Exp Neurol. 2002;61:575–84. doi: 10.1093/jnen/61.7.575. [DOI] [PubMed] [Google Scholar]
- 6.Capper D, Becker AJ, Giannini C, Figarella-Branger D, Huse JT, Rosemblum MK, et al. 4th ed. Lyon: International Agency for Research on Cancer; 2016. Gangliocytoma. WHO Classification of Tumours of the Central Nervous System, Revised; p. 136. [Google Scholar]
- 7.Cavanagh JB. On certain small tumours encountered in the temporal lobe. Brain. 1958;81:389–405. doi: 10.1093/brain/81.3.389. [DOI] [PubMed] [Google Scholar]
- 8.Chung SN, Rosemond RL, Graham D. Prenatal diagnosis of a fetal intracranial tumor. J Ultrasound Med. 1998;17:521–3. doi: 10.7863/jum.1998.17.8.521. [DOI] [PubMed] [Google Scholar]
- 9.Ebina K, Suzuki S, Takahashi T, Iwabuchi T, Takei Y. Gangliocytoma of the pineal body a case report and review of the literature. Acta Neurochir. 1985;74:134–40. doi: 10.1007/BF01418803. [DOI] [PubMed] [Google Scholar]
- 10.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–56. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 11.Hirose T, Scheithauer BW, Lopes MB, Gerber HA, Altermatt HJ, van den Berg SR. Ganglioglioma. Cancer. 1997;79:989–1003. [PubMed] [Google Scholar]
- 12.Horská A, Barker PB. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin N Am. 2010;20:293–310. doi: 10.1016/j.nic.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh Y, Yagishita S, Chiba Y. Cerebral gangliocytoma. Acta Neuropathol. 1987;74:169–78. doi: 10.1007/BF00692848. [DOI] [PubMed] [Google Scholar]
- 14.Johannsson JH, Rekate HL, Roessmann U. Gangliogliomas: Pathological and clinical correlation. J Neurosurg. 1981;54:58–63. doi: 10.3171/jns.1981.54.1.0058. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Lee HK, Jeong AK, Shin JH, Choi CG, Khang SK. Supratentorial gangliocytoma mimicking extra-axial tumor: A report of two cases. Korean J Radiol. 2001;2:108–12. doi: 10.3348/kjr.2001.2.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komotar RJ, O’Toole JE, Mocco J, Khandji AG, Keller CE, Connolly ES, et al. Gangliocytoma of the spinal cord. Neurosurgery. 2007;60:895–900. doi: 10.1227/01.NEU.0000255429.54667.39. [DOI] [PubMed] [Google Scholar]
- 17.Love S, Louis D, Ellison DW. 8th ed. Vol. 2. Boca Raton, Florida, United States: CRC Press; 2008. Greenfield’s Neuropathology. [Google Scholar]
- 18.Menon G, Patro SN, Krishnakumar K, Kesavadas C, Nair S, Radhakrishnan VV. Subfrontal gangliocytoma masquerading as olfactory groove meningioma. Br J Neurosurg. 2009;23:79–82. doi: 10.1080/02688690802308695. [DOI] [PubMed] [Google Scholar]
- 19.Mirzai H, Isisag A, Selcuki M. Incidental gangliocytoma in a middle-age adult: A case report. Ann Neurosurg. 2004;4:1–6. [Google Scholar]
- 20.Mohila CA, Rauch RA, Adesina AM. Gangliocytoma and ganglioglioma. In: Adesina AM, Tihan T, Fuller CE, Poussaint TY, editors. Atlas of Pediatric Brain Tumors. Cham. Springer; 2016. pp. 185–94. [Google Scholar]
- 21.Nelson JS. Pathology and Genetics of Tumours of the Nervous System. Berlin, Germany: Springer; 2000. Ganglioglioma and gangliocytoma; pp. 96–8. [Google Scholar]
- 22.Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/ Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–8. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 23.Pekmezci M, Villanueva-Meyer JE, Goode B, van Ziffle J, Onodera C, Grenert JP, et al. The genetic landscape of ganglioglioma. Acta Neuropathol Commun. 2018;6:47. doi: 10.1186/s40478-018-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peretti-Viton P, Perez-Castillo AM, Raybaud C, Grisoli F, Bernard F, Poncet M, et al. Magnetic resonance imaging in gangliogliomas and gangliocytomas of the nervous system. J Neuroradiol. 1991;18:189–99. [PubMed] [Google Scholar]
- 25.Prayson RA, Khajavi K, Comair YG. Cortical architectural abnormalities and MIB1 immunoreactivity in gangliogliomas. J Neuropathol Exp Neurol. 1995;54:513–20. doi: 10.1097/00005072-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ratilal B, McEvoy A, Sisodiya S, Thom M, Toma A. Diffuse cerebral gangliocytoma in an adult with late-onset refractory epilepsy. Neuropathol Appl Neurobiol. 2007;33:706–9. doi: 10.1111/j.1365-2990.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein LJ. Washington, DC: AFIP; 1972. Tumors of the Central Nervous System; pp. 349–60. [Google Scholar]
- 28.Russo CP, Katz DS, Corona RJ, Jr, Winfield JA. Gangliocytoma of the cervicothoracic spinal cord. AJNR Am J Neuroradiol. 1995;16:889–91. [PMC free article] [PubMed] [Google Scholar]
- 29.Sherazi ZA. Gangliocytoma-magnetic resonance imaging characteristics. Singapore Med J. 1998;39:373–5. [PubMed] [Google Scholar]
- 30.Shin JH, Lee HK, Khang SK, Kim DW, Jeong AK, Ahn KJ, et al. Neuronal tumors of the central nervous system: Radiologic findings and pathologic correlation. Radiographics. 2002;22:1177–89. doi: 10.1148/radiographics.22.5.g02se051177. [DOI] [PubMed] [Google Scholar]
- 31.Thom M, Blümcke I, Aronica E. Long-term epilepsy-associated tumors: Long-term epilepsy associated tumors. Brain Pathol. 2012;22:350–79. doi: 10.1111/j.1750-3639.2012.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weis S, Sonnberger M, Dunzinger A, Voglmayr E, Aichholzer M, Kleiser R, et al. Ganglioglioma/gangliocytoma. In: Weis S, Sonnberger M, Dunzinger A, Voglmayr E, Aichholzer M, Kleiser R, et al., editors. Imaging Brain Diseases. A Neuroradiology Nuclear Medicine, Neurosurgery, Neuropathology and Molecular Biology-Based Approach. Vienna: Springer; 2019. pp. 1553–65. [Google Scholar]
- 33.Wen PY, Huse JT. 2016 World Health Organization classification of central nervous system tumors. Continuum (Minneap Minn) 2017;23:1531–47. doi: 10.1212/CON.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 34.Wolf HK, Müler MB, Spänle M, Zenther J, Schramm J, Wiestler OD. Ganglioglioma: A detailed histopathological and immunohistochemical analysis of 61 cases. Acta Neuropathol. 1994;88:166–73. doi: 10.1007/BF00294510. [DOI] [PubMed] [Google Scholar]
- 35.Yagyu K, Sueda K, Shiraishi H, Asahina N, Sakurai K, Kohsaka S, et al. Direct correlation between the facial nerve nucleus and hemifacial seizures associated with a gangliocytoma of the floor of the fourth ventricle: A case report. Epilepsia. 2011;52:e204–6. doi: 10.1111/j.1528-1167.2011.03299.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Komori T, Shibata N, Toyoda C, Kobayashi M. Multifocal neurocytoma/gangliocytoma with extensive leptomeningeal dissemination in the brain and spinal cord. Am J Surg Pathol. 1996;20:363–70. doi: 10.1097/00000478-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Zulch KJ. International Histological Classification of Tumours No 21. 1979. Histological typing of tumours of the central nervous system; pp. 19–24. [Google Scholar]