Abstract
Since its inception in late December 2020 in China, novel coronavirus has affected the global socio-economic aspect. Currently, the world is seeking safe and effective treatment measures against COVID-19 to eradicate it. Many established drug molecules are tested against SARS-CoV-2 as a part of drug repurposing where some are proved effective for symptomatic relief while some are ineffective. Drug repurposing is a practical strategy for rapidly developing antiviral agents. Many drugs are presently being repurposed utilizing basic understanding of disease pathogenesis and drug pharmacodynamics, as well as computational methods. In the present situation, drug repurposing could be viewed as a new treatment option for COVID-19. Several new drug molecules and biologics are engineered against SARS-CoV-2 and are under different stages of clinical development. A few biologics drug products are approved by USFDA for emergency use in the covid management. Due to continuous mutation, many of the approved vaccines are not much efficacious to render the individual immune against opportunistic infection of SARS-CoV-2 mutants. Hence, there is a strong need for the cogent therapeutic agent for covid management. In this review, a consolidated summary of the therapeutic developments against SARS-CoV-2 are depicted along with an overview of effective management of post COVID-19 complications.
Keywords: COVID-19, SARS-CoV-2, Pandemic, Drug delivery, Clinical trials, Emergency use approval, Monoclonal antibodies, Post COVID Complications
Graphical abstract
Highlights
-
•
With continuous mutation of SARS-CoV-2, there is a strong need for the potent therapeutic agent for covid management.
-
•
Effectiveness of repurposed drugs used against COVID-19 till now it's not evident.
-
•
List of US-FDA approved drugs for COVID-19 were summarized in the article.
-
•
Several new drug molecules and biologics are engineered against SARS-CoV-2.
-
•
A consolidated summary of the therapeutic development against SARS-CoV-2 is depicted along with an overview of effective management of postCOVID-19 complications.
Abbreviations
- ACE-2
Angiotensin Converting Enzyme 2
- ARDS
Acute Respiratory Distress Syndrome
- AKI
Acute Kidney Injury
- ALI
Acute Lung Injury
- CTLs
Cytotoxic T-Lymphocytes
- COVID-19
Coronavirus diseases 2019
- CCR5
C–C chemokine receptor type 5
- EUA
Emergency Use Authorization
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- Favipiravir-RTP
Favipiravir-Ribofuranosyl Triphosphate
- HCV
Hepatitis C virus
- IL-6
Interleukin 6
- IFNγ
Interferon γ
- IP10
Interferon-γ-inducible protein
- JAK
Janus kinase
- NO
Nitric oxide
- nNOS
neuronal nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- PPHN
pulmonary hypertension in newborns
- PIKfyve
Phosphoinositide kinase for position 5 containing an FYVE finger domain
- RdRps
RNA-dependent RNA polymerases
- RCA
Regional Citrate Anticoagulation
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus-2
- TNF-α
Tumor Necrosis Factor-α
- Th
helper cells
- Treg
regulatory T cells
- VOI
Variants of Interest
- VOC
Variants of Concern
- WHO
World Health Organization
- 3CL pro
3- chymotrypsin like protease
- 2-DG
2-deoxy-D-glucose
1. Introduction
Coronavirus disease 2019 (COVID-19), which has begun in Wuhan city of China, has already marked its presence globally with reports of reinfections due to endemics caused by the viral mutations (Indari et al., 2021; Tillett et al., 2021). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 (Chavda et al., 2021e; Indari et al., 2021). The cell receptor neuropilin-1 was discovered to be involved in SARS-CoV-2 entrance (Indari et al., 2021). Based on clinical symptoms, COVID-19 is classified as mild, moderate, or severe (Smail et al., 2021). Angiotensin-converting enzyme 2 (ACE-2) is the fusion receptor responsible for the attachment of the SARS-CoV-2 with the host (Chavda et al., 2021a, Chavda et al., 2021b, Chavda et al., 2021c, Chavda et al., 2021d, Chavda et al., 2021e, Chavda et al., 2021f; Zhou et al., 2020). ACE-2 receptor is found in the lungs, heart, kidney, intestine, and endothelium, indicating that viruses can attach to a wide range of organs (Chavda et al., 2021f; Hadizadeh, 2021). Increased levels of proinflammatory cytokines like interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), IL-1, interferon-γ-inducible protein (IP10) and chemokines are observed, while decreased levels of cytotoxic T-lymphocytes (CTLs), helper (Th) cells, interferon γ (IFNγ) expressing (Th) cells characterize SARS-CoV-2 that corresponds to cytokine storm (Chavda et al., 2021b; Smail et al., 2021). This condition of hyperinflammation causes oxidative stress, which damages endothelial and alveolar cells in the lungs. Damage to these cells compromises the pulmonary membrane, resulting in vascular leakage, increased lung edema, and acute respiratory distress syndrome (ARDS)(Smail et al., 2021). Chemokines attract macrophages and neutrophils to the lungs, resulting in acute lung injury (ALI), disseminated intravascular coagulation, multiple organ dysfunction, and death in patients with severe COVID-19 (Smail et al., 2021) (see Fig. 1, Fig. 2).
Fig. 1.
COVID-19 cases reported weekly by WHO region and global deaths as of November 7, 2021 (World Health Organization, 2021a, World Health Organization, 2021b).
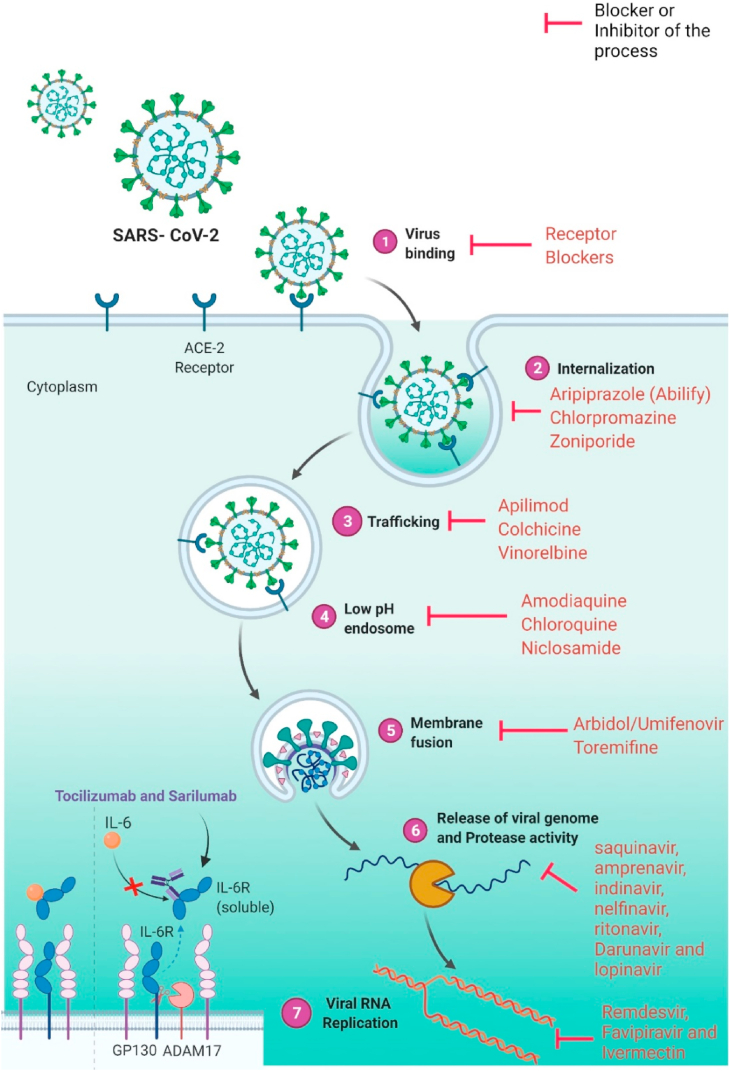
Fig. 2.
The overview diagram of SARS-CoV-2 invasion and the response of host immune system along with targeted drugs for different viral replication steps. (Adopted under CC BY 4.0 license from (Zhou et al., 2021).
Viruses constantly change through mutation (CDC, 2021; Chavda et al., 2021a). Many variants of SARS-CoV-2 have been detected all around the world during this epidemic (Chavda et al., 2021a). According to WHO, the classification of novel coronavirus variants are variants of concern (VOC) and variants of interest (VOI) (World Health Organization, 2021a, World Health Organization, 2021b). According to CDC, VOC is defined as “a variant for which there is evidence of an increase in transmissibility, more severe disease (e.g., increased hospitalizations or deaths), a significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures”, and VOI is defined as “A variant with specific genetic markers that have been associated with changes to receptor binding, reduced neutralization by antibodies generated against previous infection or vaccination, reduced efficacy of treatments, potential diagnostic impact, or predicted increase in transmissibility or disease severity” (CDC, 2021). In the current situation, the increased amount of transmissibility of SARS-CoV-2 occurs because of these variants (Chavda et al., 2021e). Most SARS-CoV-2 variants have a mutation in the spike protein of the virus (Chavda et al., 2021c, 2021d; World Health Organization, 2021a, World Health Organization, 2021b). On Aug 14, 2021,139 countries have reported VOCs like VOC 202012/01 (first found in the UK), 87 countries have reported VOC 501Y.V2 (first found in South Africa), and 54 countries have reported VOC.P1 (first found in Brazil)(World Health Organization, 2021a, World Health Organization, 2021b). VOIs like.1.617 (first found in India), B.1.616 (first found in France), B.1.526 with E484K or S477N (first found in the US), and P.3 (first found in Philippines and Japan) is spreading globally (World Health Organization, 2021a, World Health Organization, 2021b). On November 24, 2021, the World Health Organization received notification of a new variant of SARS-CoV-2, B.1.1.529 detected in South Africa on November 14, 2021. On November 26, 2021, World Health Organization (WHO) designated the B.1.1.529 (Omicron) as a Variant of Concern (VOC) (NCIRD, 2021).
There are many evolving vaccines against SARS-CoV-2 that are administered to date but the effectiveness of vaccines against these new variants is not evident and is the topic of current research interest. Hence, there is always a need for a new therapeutic tool to target SARS-CoV-2 effectively to curb the spread of SARS-CoV-2. Due to public health emergency, there are many ongoing clinical researches on a therapeutic option that includes repurposing drugs, monoclonal antibodies etc. This review will discuss the wide range of medications that can potentially be used in the treatment of COVID-19.
2. Statistics for the pandemic horizon
Up to December 22, 2021, there have been 27 million confirmed positive COVID-19 cases and 5.3 million fatalities are being reported of COVID-19 by WHO (World Health Organization (WHO), 2021). A total of 838 million doses of vaccines have been administered for COVID-19 as of December 19, 2021 (World Health Organization (WHO), 2021). Reports from WHO revealed that the positive cases of COVID-19 are increasing many folds week by week all over the world. The countries with the most cases were United States of America (48413265 cases) India (34,615,757 cases), Brazil (22,105,872 cases), United Kingdom (10,329,078 cases), and Russian Federation (9,737,037 cases)(World Health Organization, 2021a, World Health Organization, 2021b) (Fig. 1).
It is estimated that around 70% of the world's population will need to gain immunity against SARS-CoV-2 to acquire herd immunity (Dhar Chowdhury and Oommen, 2020). For achieving herd immunity against COVID-19, researchers around the world constantly developing vaccines. Most people who got fully vaccinated with both doses were United Arab Emirates (90.3%) followed by Singapore (87.88%), Portugal (86.2%), Malta (86%)(Ourworldindata.org, 2020). In WHO, research is going on for finding candidates for developing better vaccines against COVID-19 (Dhar Chowdhury and Oommen, 2020).
3. Drug repurposing for COVID-19
The process of identifying new targets for existing medications is known as drug repurposing, and it is regarded as a cost-effective and efficient method (Jang et al., 2021; Singh et al., 2020). It is estimated that 75% of already available drugs may be repurposed to treat a variety of diseases (Singh et al., 2020). Outbreaks of SARS-CoV-2, present particular obstacles to clinicians in terms of selecting suitable pharmacological drugs in a clinical setting with little time for novel drug discovery (Fig. 2) (Bakowski et al., 2021). Repurposing of existing medicines for the treatment of multiple diseases has recently become a common technique since it utilizes risk-free compounds with familiar pharmacodynamic, preclinical, and pharmacokinetic profiles that can go straight through the late phase of clinical trials, allowing the development of drug process effectively low-price and faster (Wang and Guan, 2021). Therefore, the quest for successful COVID-19 therapeutic drugs are critical and immediate (Fig. 3).
Fig. 3.
Repurposed drugs for COVID-19 and their mechanism of action.
3.1. Potential drugs that act through SARS-CoV-2 related targets (genomic) in ongoing clinical trials
3.1.1. Remdesivir
Remdesivir has a wide range of antiviral activities against coronaviruses (Singh et al., 2020; Smith et al., 2020). Previously, remdesivir was used as an investigational drug for the ebola virus (Singh et al., 2020). The possible mechanism of action by which remdesivir may help to treat COVID-19 is by inhibition of viral replication. The enzyme RNA-dependent RNA polymerases (RdRps) are needed for SARS-CoV-2 replication. Remdesivir is a mono-phosphoramidite prodrug of remdesivir-triphosphate (RDV-TP), an adenosine analog that inhibits RdRps (Singh et al., 2020; Smith et al., 2020). Adenosine-triphosphate competes with remdesivir-TP for insertion into embryonic viral RNA chains. Since RDV-TP does not induce immediate chain termination, it terminates RNA synthesis until it is integrated into viral RNA (Singh et al., 2020; Smith et al., 2020). Several clinical trials are going on all over the world for its efficacy against COVID-19 (Table 1). A clinical study in the US reported that remdesivir improved clinical conditions of severe COVID-19 patients (Hoek et al., 2021; Wang et al., 2020a, Wang et al., 2020b). When patients with severe COVID-19 were given remdesivir, symptomatic improvement was seen in a clinical study (Ader et al., 2021; Buckland et al., 2020). Currently, remdesivir is the only USFDA approved repurposed drug for severe COVID-19 conditions (Authorization, 2021).
Table 1.
Drugs repurposing for COVID-19 under clinical development.
| The phase of clinical trial | Trade Name/Generic Name | Medication Class | Innovator | The possible mechanism in COVID-19 | Dosage form | Registered clinical trial no |
|---|---|---|---|---|---|---|
| Phase 4 | Carragelose® | Antiviral | Marinomed Biotech AG | It acts as a defensive physical shield on the oral and nasal mucosa for virus entry to the host cell. | Nasal spray | NCT04590365, NCT04681001, NCT04521322, NCT04793984 |
| Phase 3/4 | Eliquis (Apixaban) | Anticoagulant | NHLBI | Adjunctive/Supportive therapy, to mitigate immunothrombosis in COVID-19, Apixaban, a thromboxane A2 receptor antagonist will inhibit COX2 stimulated TxA2 signaling, as COX2 is upregulated by COVID 19. | Oral |
NCT04498273, NCT04650087, NCT04801940, NCT04746339, NCT04512079 |
| Phase 2/3/4 | Vascepa (icosapent ethyl) | Lipid-lowering agent | Amarin Corporation | Anti-inflammatory effects in COVID-19 | Oral | NCT04412018, NCT04460651, NCT04505098 |
| Phase 3 | Regkirona (regdanvimab, CT-P59) | Monoclonal antibody | Celltrion | Neutralize SARS-CoV-2 virus and several variants like mutated G-variant strain (D614G) by acting on viral RBD. | I.V. | NCT04525079, NCT04602000, |
| Phase 3 | AZD7442 | Monoclonal antibody | Astra Zeneca; Vanderbilt University Medical Center | Targets the viral S protein | I.V. | NCT04625725, NCT04625972, NCT04723394, NCT04518410, NCT04501978 |
| Phase 3 | Lenzilumab | Monoclonal antibody | Humanigen; Catalent | Antagonize GM-CSF signaling in COVID-19. | I.V. | NCT04583956 |
| Phase 3 | Ilaris (canakinumab) | Monoclonal antibody | Novartis | Interleukin (IL)-1β inhibitor in COVID-19 | I.V. |
NCT04362813, NCT04365153 |
| Phase 3 | Losmapimod | Mitogen-activated protein kinase (MAPK) inhibitor | Fulcrum Therapeutics | DUX4 protein inhibitor, p38 mitogen-activated protein kinase inhibitors reduces inflammatory biomarkers such as C-reactive protein and IL-6 in COVID-19. | Oral | NCT04511819 |
| Phase 3 | Actemra (tocilizumab) | IL-6 inhibitors | Roche | Anti IL-6 Receptor mAb | I.V. |
NCT04320615, NCT04372186, NCT04409262, NCT04381936 |
| Phase 3 | Bucillamine | Antirheumatic agent | Revive Therapeutics Ltd. | Prevent acute lung injury, immunomodulator in COVID-19 | Oral | NCT04504734 |
| Phase 3 | INOpulse | Nitric oxide | Bellerophon Therapeutics | iNOS hinders the coronavirus replication via intermediate peroxynitrite. | Inhaled | NCT04421508 |
| Phase 3 | Hydrocortisone | Glucocorticoid | Various | Inhibition of proinflammatory transcription factors in severe COVID-19 | Oral |
NCT04348305, NCT02517489 |
| Phase 3 | NT-300 (nitazoxanide extended release) | Antiviral | Romark Laboratories L.C. | Inhibits SARS-CoV-2 effect on interferon pathway; upregulates innate immunity and interferon pathway | Oral |
NCT04486313, NCT04359680, NCT04343248, |
| Phase 2/3 | Bamlanivimab + etesevimab | Monoclonal antibodies | Lily; Junshi Biosciences | Acts on S Protein and prevents viral entry in the host. | I.V. |
NCT04427501, NCT04497987, NCT04634409, NCT04518410, NCT04501978, NCT04411628 |
| Phase 2b/3 | Leronlimab (PRO 140) | Monoclonal antibody | CytoDyn | CCR5 antagonist which causes the downstream release of proinflammatory cytokines | I.V. |
NCT04343651, NCT04347239 |
| Phase 2/3 | Remicade (infliximab) | Monoclonal antibody | Janssen | Tumor necrosis factor inhibitor for cytokine release syndrome linked with COVID-19 | I.V. | NCT04593940 |
| Phase2/3 | Veklury (Remdesivir) | Antiviral | Gilead Sciences | Inhibits RNA polymerases (RdRps) | I.V. | ISRCTN83971151, NCT04315948, NCT04292730 ,NCT04292899, NCT04280705, NCT04401579, NCT04492475, NCT04640168, NCT04410354, NCT04582266, NCT04843761 |
| Phase 2/3 | SNG001 | Antiviral | Synairgen | Interferon-beta-1a proteins which have antiviral properties | I.V. |
NCT04385095, NCT04732949, NCT04518410 |
| Phase 2/3 | Niclocide (niclosamide), UNI91103 | Anthelmintic | Neuro Bo Pharmaceuticals; UNION therapeutics | It inhibits SKP2 (S-Phase kinase-associated protein-2) | Intra nasal |
NCT04603924, NCT04436458, NCT04749173, NCT04372082 |
| Phase 2/3 | Ivermectin | Antihelmintic | Various | It is a nuclear transport inhibitor mediated by the importinalphabeta/1 heterodimer | Oral |
NCT04529525, NCT04438850, NCT04431466, NCT04703205 |
| Phase2/3 | Zyesami(aviptadil,RLF-100) | Synthetic human vasoactive intestinal peptide | Neuro Rx; Relief Therapeutics | Vasoactive intestinal peptide (VIP) is highly localized in the lungs and prevents apoptosis and caspase-3 activation in the lungs, as well as halts the manufacturing of IL6 and TNF alpha and reversing the CD4/CD8 ratio. | Nebuliser |
NCT04360096, NCT04843761, NCT04311697, NCT04453839, NCT04536350, NCT04488081 |
| Phase 2/3 | Dexamethasone | Glucocorticoid | Various | Decreases the inflammation linked with cytokine release syndrome | I.V. | NCT04640168, NCT04327401, |
| Phase 2/3 | Pyramax (artesunate/pyronaridine) | Antimalarial | Shin Poong Pharmaceutical Co.,Ltd | Antiviral activity is depicted through the TLR4 inflammatory signaling pathway | Oral |
NCT04475107, NCT04532931, NCT04701606, NCT04695197 |
| Phase 2/3 | PTC299 | Dihydroorotate dehydrogenase (DHODH) inhibitor | PTC | DHODH inhibitor; Inhibits viral replication and suppressed IL-6, IL-17A, IL-17F, and vascular endothelial growth factors in cell culture. | Oral | NCT04439071 |
| Phase 1/3 | CPI-006 | Immunomodulatory antibody | Corvus Pharmaceuticals, Inc. | Anti-CD73 antibody; inhibition of CD73 enzymatic activity and activates CD73POS B cells directly, inducing plasmablast differentiation, immunoglobulin class switching, and antibody secretion independent of adenosine. | I.V. |
NCT04464395, NCT04734873 |
| Phase 2 | Brilacidin (PMX-30063) | Host defense protein mimetic | Innovation Pharmaceuticals | Binds to the spike proteins of SARS-CoV-2 and prevents viral entry in a host cell | I.V. | NCT04784897 |
| Phase 2 | Gimsilumab | Monoclonal antibody | Roivant Sciences | GM-CSF antagonist | I.V. | NCT04351243 |
| Phase 2 | APN01 | rhACE2 | Apeiron Biologics | Recombinant human ACE-2 enzyme that blocks ACE2 receptor, prevents SARS-CoV-2 entry to host cell | I.V. | NCT04335136 |
| Phase 2/2 | Humira (adalimumab) | Anti-TNF | University of Oxford; Pharm-Olam | Anti-tumor necrosis inhibitor in COVID-19 | I.V. | NCT04705844 |
| Phase 2 | AT-527 | Antiviral | Atea Pharmaceuticals Inc. | Inhibit viral replication by interfering with viral RNA polymerase | Oral | NCT04396106 |
| Phase 2 | STI-5656 (abivertinib) | Tyrosine kinase inhibitor | Sorrento Therapeutics | Tyrosine kinase inhibitors | I.V. |
NCT04440007, NCT04528667 |
| Phase 2 | PB1046 | VIP receptor agonist | PhaseBio | VIP agonist possesses control of cell activation and differentiation, down-regulation of proinflammatory cytokines especially IL-10 in SARS-CoV-2 | I.V. | NCT04433546 |
| Phase 2 | BLD-2660 | Small molecule protein inhibitor | Blade Therapeutics | Small molecule inhibitor of calpain for treatment in pneumonia associated with COVID-19 | Oral | NCT04334460 |
| Phase 2 | Rhu-pGSN (gelsolin) | Recombinant human plasma | BioAegis Therapeutics | Suppress cytokine release syndrome associated with COVID-19 | NCT04358406 | |
| Phase 2 | TXA127 | Angiotensin-(1–7) peptide | Constant Therapeutics | Mas receptor agonist which has demonstrated efficacy in reducing inflammation, stabilizing endothelial and epithelial barrier and reducing fibrosis in lungs | I.V. | NCT04401423 |
| Phase 2 | LAM-002A (apilimoddimesylate) | Interleukin inhibitor | AI Therapeutics, Inc. | Inhibits lipid kinase PIKfyve | Oral | NCT04446377 |
| Phase 2 | AdMSCs | Autologous adipose-derived stem cells | Celltex Therapeutics | Effectively treating pneumonia associated with COVID-19 | NCT04428801 | |
| Phase 1 | PF-07321332 | Antiviral | Pfizer | Protease inhibitor that blocks 3-CL pro in SARS-CoV-2 | Oral | NCT04756531 |
| Phase 1 | Ensovibep (MP0420) | Antiviral | Molecular Partners; Novartis | Bind to the RBD of viral S protein at three distinct locations to prevent viral entry into cells. | NCT04834856 | |
| Phase 1b | PF-00835321 (PF-07304814) | Small molecule inhibitor | Pfizer | A prodrug which is a potent inhibitor in vitro of the coronavirus family 3CL pro, especially targets human host protease. | Oral | NCT04535167 |
| Phase 1b | Takhzyro (lanadelumab) | Monoclonal antibody | Takeda (Shire) | Lanadelumab blocks the activation of bradykinin which has been theorized to be responsible for vascular dilation, vascular permeability, and hypotension when bradykinin levels increase during COVID-19 | I.V. | NCT04460105 |
| Phase 1b | DNL758 (SAR443122) | RIPK1 inhibitor | Sanofi; Denali Therapeutics | Reduce excessive inflammation associated with severe cases of COVID-19 | Oral | NCT04469621 |
3.1.2. Favipiravir
Favipiravir is approved in Japan for influenza viruses (Singh et al., 2020). Favipiravir is a prodrug of purine nucleotide which converts into active form favipiravir-ribofuranosyl triphosphate (favipiravir-RTP) within the tissue, works by inhibiting the SARS-CoV-2's RdRp enzyme. It allows favipiravir to be easily inserted into viral RNA thus sparing human DNA which results in virucidal activity (Agrawal et al., 2020). In Wuhan, where COVID-19 started, favipiravir was initially used to treat COVID-19 (Agrawal et al., 2020). In China, a clinical study reported faster clearance of viral load and amelioration in lung CT scans when patients with COVID-19 were administered with favipiravir as compared with other treatment arms (Agrawal et al., 2020). An observational study conducted in Japan reported faster recovery from favipiravir in patients with mild and moderate COVID-19(Shinkai et al., 2021). In India, DCGI approved favipiravir in mild and moderate COVID-19 patients. Emergency use of favipiravir is approved in many countries which includes Russia, Japan, Italy, Moldova, Bangladesh, Turkey, Egypt, Ukraine, Uzbekistan, and Kazakhstan (Agrawal et al., 2020). Detailed ongoing trials are summarized in Table 1.
3.1.3. Molnupiravir
Molnupiravir, the prodrug of ribonucleoside analog of β-D-N4-hydroxycytidine (Vicenti et al., 2021). It has been shown to prevent the replication of a variety of viruses with low cytotoxicity and a high degree of resistance. When the active form of a drug gets incorporated in the virus instead of uracil or cytosine during RNA synthesis which causes G to A and C to U transformations in a dose-dependent manner resulting in deadly mutagenesis through the entire genome of many viruses (Fischer et al., 2021; Vicenti et al., 2021). This possible mechanism of action of molnupiravir may also prevent viral replication via inhibitingSARS-CoV-2-RdRp in a host cell (Vicenti et al., 2021). The efficacy of molnupiravir was proved against influenza and various coronaviruses(Kabinger et al., 2021). A clinical study is going for the efficacy of molnupiravir against COVID-19 (Table 1).
3.1.4. AT-527
AT-527 is a double prodrug of a guanosine nucleotide analog that has shown effective in vitro and in vivo activity against hepatitis C virus (HCV)by specifically inhibiting RdRp (Good et al., 2021). Good et al. found that in an in vitro study, where AT-527 shows potent activity for COVID-19. Clinical trials are underway regarding the efficacy of AT-527 (Table 1).
3.1.5. Ivermectin
Ivermectin is a macrocyclic lactone with a wide-range of anthelmintic actions (Rizzo, 2020). It is a nuclear transport inhibitor facilitated by the importin αβ/1 heterodimer, which is responsible for the translocation of viral proteins needed for the replication of RNA viruses (Rizzo, 2020). It also shields S protein which binds to transmembrane receptor CD147 and ACE-2 (Kaur et al., 2021). Ivermectin's antiviral effect may also be due to allosteric regulation of the P2X4 receptor, which are cation-selective channels that are gated by extracellular ATP and serve as an ionophore (Kaur et al., 2021). Anti-inflammatory activity of ivermectin is also proved in mice study which may mitigate cytokine storm in COVID-19 (Kaur et al., 2021). Caly et al. reported that ivermectin inhibits SARS-CoV-2 replication in vitro (Caly et al., 2020). Rajter et al. in a retrospective clinical study observed a lower death rate in hospitalized cases (Rajter et al., 2021). Mahmud et al. observed that a combination of ivermectin and doxycycline was effective in patients with mild to moderate COVID-19 (Mahmud et al., 2021).
3.1.6. Niclosamide
Niclosamide is an approved drug for the treatment of tapeworm infection (Zeng et al., 2020). It works by inhibiting S-phase kinase-associated protein-2 activity by promoting autophagy which results in decreasing coronavirus replication. It may also target SARS-CoV-2 by this mechanism. At the sub molecular level, niclosamide has been shown to inhibit SARS-CoV-2 (Zeng et al., 2020). A clinical trial reported that niclosamide has been shown to mitigate cytokine storms in severe COVID-19 patients (Wu et al., 2020). Currently, clinical trials are going on for niclosamide against SARS-CoV-2 (Table 1).
3.1.7. PF-07321332
3-chymotrypsin like protease (3CLpro) is a prominent target and exciting prospect for rational-based antiviral exploration because it controls virus replication (Vandyck et al., 2021). Protease indicators are indicated generally for other viruses and it is not still FDA-approved for SARS-CoV-2. 3CL pro inhibitors have shown broad-spectrum antiSARS-CoV-2 activity in terms of viral entry through cathepsin L and other host proteases (Mellott et al., 2021). PF-07321332 from Pfizer is in the clinical trial, a second-generation drug targeting 3CLpro for inhibiting viral replication (Vandyck et al., 2021).
3.1.8. Nitric oxide
Nitric oxide (NO) is a vital agent generated endogenously by three enzymes in mammalian cells: neuronal (n NOS), endothelial (eNOS), and inducible nitric oxide synthase (iNOS)(Chavda et al., 2021e). NO is a vital element of the lungs and is essential for the regulation of pulmonary vasomotor tone (Mehta and Dhapte-Pawar, 2021). Inhaled NO therapy is explored for the treatment of acute lung injury, pulmonary hypertension in newborns (PPHN), and ARDS (Bernasconi and Beghetti, 2002). During SARS-CoV-1 infection, iNOS activity is usually increased, and NO inhibits viral replication by cytotoxic reactions involving the intermediate peroxynitrite (Chavda et al., 2021e; Mehta and Dhapte-Pawar, 2021). SARS-CoV-2, on the other hand, infects endothelial cells, which are an important source of NO synthesis. As a result, the use of inhaled NO in the treatment of SARS-CoV-2 infections may be effective by restoring endothelial function and reducing inflammatory responses (Lotz et al., 2021). It is an important molecule for severe COVID-19 patients where there is a shortage of ventilator.
3.2. Potential drugs that act through SARS-CoV-2 uptake pathways in ongoing clinical trials
3.2.1. Carragelose®
Carragelose®is a unique, widely active antiviral molecule used to treat a variety of pulmonary diseases (Powell et al., 2017). In the European Union, Australia, and parts of Asia, Carragelose®(sulfated polymer) is made from red seaweed algae that have been allowed for use in nasal sprays, throat sprays, and lozenges (Mehta and Dhapte-Pawar, 2021). It acts as a defensive physical shield on the oral and nasal mucosa, preventing pulmonary viruses from entering and/or attaching to the cells. According to Morokutti and Co-workers, a study found that treatment with Carragelose® decreased the period of symptoms in patients infected with coronavirus by 3 days when compared to placebo (Morokutti-Kurz et al., 2021). Clinical trials of Carragelose® against SARS-CoV-2 are in the process (Table 1).
3.3. Potential drugs that act through host proinflammatory cytokines in ongoing clinical trials
3.3.1. Tocilizumab
Tocilizumab was approved to treat rheumatoid arthritis and cytokine release syndrome(Singh et al., 2020). It is a humanized recombinant monoclonal antibody (mAb) that binds to the human IL-6 receptor (IL6R) and blocks its signal transduction pathway (Singh et al., 2020). It inhibits the IL6R to mitigate proinflammatory cytokines which are raised during severe COVID-19 conditions (Deb et al., 2021). A study in China reported successful treatment of the patient with COVID-19 and multiple myeloma when given tocilizumab (Singh et al., 2020). After intravenous infusion of tocilizumab, along with other treatments such as lopinavir-ritonavir, a 42-year-old patient from France with COVID-19-related respiratory failure had a fast and favorable results (Samaee et al., 2020). Deb et al. evaluated the benefits of tocilizumab in patients with critical COVID-19 (Deb et al., 2021). A clinical study from Italy recommended tocilizumab for use in critical patients including pneumonia or patients in ventilators (Deb et al., 2021).
3.3.2. Corticosteroids
Corticosteroids drugs like hydrocortisone, and dexamethasone are associated with decreased mortality in viruses like influenza A (Kifle et al., 2021). Dexamethasone has been reported to improve mortality in severe COVID-19 patients requiring mechanical ventilation when compared with patients of COVID 19 on usual treatment (Group et al., 2021). Dexamethasone had no improvement in the mortality rate in COVID-19 patients without respiratory assistance (Prescott and Rice, 2020). Their anti-inflammatory properties can help to minimize systemic inflammation, decrease fluid retention in the lungs, and avoid further alveolar injury, thus improves hypoxia and lowering the risk of respiratory system failure (Kifle et al., 2021).
3.3.3. Leronlimab
Leronlimab, humanized IgG4-κ mAb that binds to the C–C chemokine receptor type 5(CCR5) and inhibits it(Agresti et al., 2021). As a result, it reduces proinflammatory cytokines and also preventing Treg(regulatory T cells) from migrating to the infection location. It has shown effectiveness against HIV. A clinical study reported that subcutaneous leronlimab administration was shown to be safe and may have been linked to impressive recovery in four critically ill SARS-CoV-2 patients (NCT04901689).
3.3.4. Infliximab
Infliximab is a chimeric monoclonal anti-TNF antibody(Stallmach et al., 2020). Stallmach et al. reported that successful treatment in patients with severe COVID-19 and inflammatory bowel disease (IBD) when Infliximab was administered (Stallmach et al., 2020). TNF, in particular, can aggravate lymphopenia by destroying T cells directly through TNF/TNFR1 signaling. T cell dysfunction is an essential but underappreciated target for immunomodulatory involvements. So, anti-TNF targets may be encouraging to use in cytokine storm of COVID-19 but its effect can be seen only in severe COVID-19 patients (Stallmach et al., 2020). The clinical trial is ongoing for infliximab against SARS-CoV-2 (Table 1).
3.3.5. Artesunate/pyronaridine
The antimalarial drug combination artesunate and pyronaridine have had a wide range of the antiviral spectrum (Nair et al., 2021). Artesunate's antiviral effects against DNA viruses have been well documented, and they tend to be regulated by Sp1 and NF-kB, two proteins that take part in the IFN pathway (Pfeffer, 2011). Artesunate, in addition to its antiviral properties, has been shown to protect mice from lung damage. Inverno cells study showed the potency of inhibiting replication of SARS-CoV-2 by artesunate and pyronaridine (Nair et al., 2021).
3.3.6. Gimsilumab
Gimsilumab, a mAb used in ankylosing spondylitis (Saha et al., 2020). Granulocyte-macrophage colony-stimulating factor (GM-CSF), an important myelopoietic growth factor, flashed a lot of attention as a potential pharmacological drug that may use in COVID-19 (Lang et al., 2020). Gimsilumab as anti-GM-CSF may work in mitigating cytokine storm in COVID-19. In the cohort study, encouraging results are shown by anti-GM-CSF monoclonal antibodies against COVID-19 (Lang et al., 2020).
3.3.7. Miscellaneous drugs with a novel mechanism against SARS-CoV-2
-
1)
Apilimod: It may mitigate proinflammatory cytokines by inhibition of lipid kinase PIKfyve (phosphoinositide kinase for position 5 containing an FYVE finger domain) in cellular regulation (Riva et al., 2020).
-
2)
Pemziviptadil (PB1046): Vasoactive intestinal peptide agonist that controls cell activation and differentiation, as well as the downregulation of proinflammatory cytokines and reactive oxygen species and the promotion of the anti-inflammatory cytokine IL-10 (Ngo et al., 2021).
-
3)
BLD-2660: Small molecule inhibitor of calpain for treatment in pneumonia caused by COVID-19 via decreasing of proinflammatory cytokines (Apaydın et al., 2021).
-
4)
Abivertinib: Tyrosine kinase inhibitor, which specifically binds to Bruton's tyrosine kinase (BTK) receptors which causes prevention of phosphorylation of the receptor that leads to inhibition of proinflammatory cytokines like IL-6, IL-β, and TNF -α in severe COVID-19 (Jade et al., 2021).
-
5)
Lanadelumab: Increased activity of bradykinin also leads to an increase the inflammation in the lungs which can lead to acute lung inflammation (Colarusso et al., 2020). Lanadelumab, an inhibitor of bradykinin can be a potential therapy by mitigating proinflammatory cytokines (Adesanya et al., 2021).
-
6)
DNL758: Inhibition of Receptor-interacting serine/threonine kinase 1 which causes inhibition of proinflammatory cytokines in COVID-19 (Bime et al., 2021).
-
7)
Ensovibep and MP0423: Trade name under DARPin® (Designed ankyrin repeat proteins),antivirals, which have shown to be novel potent antiSARS-CoV-2 and also showed effectiveness in mutant strains of SARS-CoV-2 (Rothenberger et al., 2021). Previously DARPin® was used in oncological studies (Stumpp et al., 2020). Rothenburger et al. revealed the novel mechanism of action of ensovibep against SARS-CoV-2 as “the viral inhibition mode of ensovibep is based on three distinct DARPin® domains targeting the RBD while MP0423 has three DARPin® domains directed to three different domains of the spike protein: the RBD, the N Terminal Domain (NTD) and the S2 domain. The tripartite binding and RBD-independent neutralization mechanism allow the MP0423 molecule to lose binding to any of its domains while maintaining a high neutralizing potency” (Rothenberger et al., 2021). Phase 1 trials are ongoing for this novel molecule against SARS-CoV-2 (Table 1).
-
8)
2-deoxy-D-glucose (2-DG):The host cells have been reported to perform metabolic reprogramming after SARS-COV-2 entry to satisfy the increased demand for nutrients and energy for viral replication, where 2-DG, a glucose antimetabolite, might be a promising therapeutic option since it works as a dual inhibitor of glycolysis and glycosylation (Balkrishna et al., 2020).
4. USFDA approved therapeutics for COVID-19
USFDA follows a systematic approach to combat COVID-19. To date, US-FDA approved only remdesivir drugs to be used in hospitalized COVID-19 patients (Authorization, 2021). US-FDA granted emergency use authorization (EUA) to neutralizing antibodies (bamlanivimab + etesevimab, and casirivimab/imdevimab), antiviral combination therapy of remdesivir + baricitinib and COVID-19 convalescent plasma (Taylor et al., 2021). The BLAZE-1 trial (NCT04427501) investigated the combination of bamlanivimab and etesevimab in COVID-19 patients who were ambulatory comparing with single monotherapy of bamlanivimab. The combination of bamlanivimab/etesevimab was accompanied by a significant decrease in viral load compared to placebo. According to virological data from 577 patients, whereas bamlanivimab monotherapy did not lead to significant decrease in the viral load. The monoclonal antibody combination was also found to decrease the number of hospitalizations and deaths in 1035 patients when compared to placebo. This result led to grant EUA to monoclonal antibody combination (bamlanivimab and etesevimab) in mild to moderate COVID-19 patients by USFDA (Hurt and Wheatley, 2021). In a phase 1/2/3 trial (NCT04425629) which is happening in many countries, casirivimab and imdevimab are being tested as a cocktail in ambulatory patients. The results of a phase 3 analysis (N = 4567) of patients with serious COVID-19 comparing 1200 mg or 2400 mg casirivimab/imdevimab to placebo have been published. As compared to placebo, the casirivimab/imdevimab combination significantly decreased the chance of mortality or hospitalization by 70% and 71% in 1200 mg dose arm and 2400 mg dose arm respectively (Hurt and Wheatley, 2021). The median period of symptoms reduced by 4 days was shown by antibody cocktail therapy when compared to placebo. When compared to placebo, interim results from the first 275 patients (phase 1/2 portion) revealed that the casirivimab/imdevimab combination showed virological effectiveness, resulting in an overall decrease (Hurt and Wheatley, 2021). These findings from the trial led to grant EUA by USFDA for the use in mild to moderate COVID-19 patients in the ambulatory setting. Convalescent plasma carrying antibodies of SARS-CoV-2 given to the patients with severe COVID-19 is the treatment approach whose observational data are very encouraging but clinical trials did not prove the evidence (Simonovich et al., 2021). Many countries grant emergency authorization to convalescent plasma in severe COVID-19 patients based on observational data (Simonovich et al., 2021). Clinical trial data (NCT04401579) of antiviral drugs (Baricitinib with remdesivir) compared to remdesivir alone showed that in COVID-19 patients who were undergoing high-flow oxygen or noninvasive ventilation, baricitinib plus remdesivir was advantageous to remdesivir alone in minimizing improvement time and fast-tracking patient's progress (Kalil et al., 2021). The combination was also linked to a lower risk of severe side effects when compared to remdesivir alone in a study of 1033 patients (Kalil et al., 2021). These outcomes led to the grant of EUA by FDA in hospitalized COVID-19 patients (Authorization, 2021). ACTT-1 trial showed that Remdesivir was shown to be significant improvement than placebo group in the hospitalized patients and also had lower chance of respiratory tract infection (Beigel et al., 2020). Furthermore, US-FDA also approved supportive/adjuvant therapy in COVID-19 which includes propofol-lipuro 1%, “REGIOCIT replacement solution that contains citrate for regional citrate anticoagulation (RCA) of the extracorporeal circuit, fresenius medical, multifiltrate PRO system, and multiBic/multiPlus solutions”(Authorization, 2021). USFDA approved therapeutic drugs are summarized in Table 2.
Table 2.
USFDA approved therapeutic drugs for COVID-19.
| Trade Name/Generic Name | Class of drug | Innovator | The possible mechanism in COVID-19 | Dosage form | USFDA Authorized use | Reference |
|---|---|---|---|---|---|---|
| Bamlanivimab + Etesevimab | Monoclonal antibodies | Lily; Junshi Biosciences | Binds to the spike proteins of SARS-CoV-2 and prevents viral entry in a host cell. | I.V. | “For the treatment of mild to moderate COVID-19 in adult and pediatric patients” | Dougan et al. (2021) |
| Casirivimab/imdevimab (REGN-COV2) | Monoclonal antibodies | Regeneron | Binds to the spike proteins of SARS-CoV-2 and prevents viral entry in a host cell. | I.V. | “Treatment to mild to moderate COVID-19 in adult and pediatric patients(12 years of age and older weighing at least 40 kg)” | Weinreich et al. (2020) |
| Baricitinib (Olumiant) in combination with remdesevir (Veklury) | Antiviral drug | Eli Lilly | JAK (Janus kinase) and RNA polymerases (RdRps) inhibitor | Oral/I.V. | “In hospitalized adults and pediatric patients of 2 years of age and older who require supplementary oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation, for the treatment of suspected or confirmed COVID-19”. | Kalil et al. (2020) |
| Remdesivir (Veklury) | Antiviral drug | Gilead Sciences | Inhibits RNA polymerases (RdRps) | I.V. | “FDA approved remdesivir for use in COVID 19 hospitalized patients (12 years of age and older and weighing at least 40 kg)” | Beigel et al. (2020) |
| Convalescent plasma | Plasma | Bratcher Bowman | Antibodies of COVID-19 | “For the treatment of hospitalized patients with COVID-19″ | Katz (2021) |
5. Novel anti SARS-CoV-2 drugs under clinical development
Vaccines may be the effective means of providing COVID-19 immunity to the majority of people. However, specific novel anti-SARS-CoV-2 medications are anticipated to play a major role in protecting people who are unvaccinated or immunocompromised, as well as at periods when vaccinations fail to protect against circulating SARS-CoV-2 variant (Chavda et al., 2021f). Few novel anti-SARS-CoV-2 molecules are already in clinical trials to target the SARS-CoV-2 virus. Monoclonal antibodies against SARS-CoV-2 are of great interest during this pandemic. Monoclonal antibodies target the spike protein of SARS-CoV-2 to neutralize it (Hurt and Wheatley, 2021). However, the concern is that the emerging variants like B.1.351 have spike substitution of E484K and K417N that is resistant to bamlanivimab and etesevimab respectively. Inhibition of B.1.351 by only 5 to 20-fold was succeeded by cocktail therapy of casirivimab and imdevimab as activity of imdevimab is unaffected by mutation of SARS-CoV-2 (Hurt and Wheatley, 2021). Still more clinical trials are warranted for efficacy of this cocktail monoclonal antibody in SARS-CoV-2 variant. US FDA already granted EUA for casirivimab/imdevimab, an artificial cocktail antibody against the spike proteins of SARS-CoV-2 (Authorization, 2021). Success of these novel molecules in clinical studies may certainly help in curb the spread of pandemic. Novel anti SARS-CoV-2 molecules are summarized in Table 3.
Table 3.
Specific novel anti SARS-CoV-2 drugs under clinical development.
| The phase of clinical trial | Trade Name/Generic Name | Medication Class | Innovator | The possible mechanism in COVID-19 | Dosage form | Registered clinical trial no |
|---|---|---|---|---|---|---|
| Phase2/3 | SAB-185 | Polyclonal antibody | SAb Biotherapeutics | Anti-SARS-CoV-2 Human immunoglobulin G | I.V. |
NCT04469179, NCT04518410 |
| Phase 2/3 | C135-LS/C144-LS | Monoclonal antibodies | The Rockefeller University/Bristol Myers Squibb | Targets two distinct epitopes on RBD | I.V. |
NCT04700163, NCT04518410 |
| Phase 1/2/3 | Casirivimab/imdevimab (REGN-COV2) | Antibody cocktail | Regeneron | Target the spike protein of SARS-CoV-2, hindered infectivity, and prevented the emergence of viral resisted mutants | I.V. |
NCT04425629, NCT04426695, NCT04519437, NCT04452318, |
| Phase 1b/2a/2/3 | VIR-7831/VIR-7832 (GSK4182136/GSK4182137) VIR-7831 | Monoclonal Antibody | Vir Biotechnology, Inc.; GSK | Binds to the spike proteins of SARS-CoV-2 and prevents viral entry in a host cell. | I.V. |
NCT04545060, NCT04634409 |
| Phase 1 | Etesevimab (LY-CoV016,JS016) | Monoclonal antibody | Lilly; Junshi Biosciences | Binds to the spike proteins of SARS-CoV-2 and prevents viral entry in a host cell. | I.V. |
NCT04427501, NCT04497987, NCT04634409 |
| Phase 1 | COVI-AMG/COVI-DROPS (STI-2020) | Monoclonal antibody | Sorrento Therapeutics | Binds to the spike proteins of SARS-CoV-2 and prevents viral entry in a host cell. | I.V. | NCT04584697 |
| Phase 1 | COVI-GUARD (STI-1499) | Monoclonal antibody | Sorrento Therapeutics | Neutralizing antibody that binds to the S1 subunit of the spike protein in SARS-CoV-2. | I.V. | NCT04454398 |
6. Coronavirus treatment acceleration program (CTAP)
“Coronavirus Treatment Acceleration Program” (CTAP), developed by the USFDA, is a distinct emergency program for potential coronavirus treatments (Manager et al., 2021). The program employs any available technique to get novel therapies to patients as soon as possible while still determining whether they are beneficial or detrimental. The FDA continues to fund clinical trials that are evaluating novel COVID drugs to collect useful information on their safety and efficacy (Manager et al., 2021; Rome and Avorn, 2020). As of Aug 4, 2021, under the CTAP program, more than 600 drugs are in the planning stages of drug development programs, 10 number COVID-19 drugs under emergency use authorization, examines more than 400 trials and 1 treatment drug is currently approved by FDA. Furthermore, under CTAP, more than 50 antiviral treatments, more than 40 cells, and gene therapies, and more than 130 immunomodulators, and more than 50 neutralizing antibodies, more than 110 therapeutics from other pharmacological categories are being studied for COVID-19 treatment. More than 100 and 330 therapeutics are in early-stage (phase 0,1,1/2) and late-stage (phase 2,2/3,3,4) respectively for COVID-19 treatment (Chen et al., 2020; Manager et al., 2021).
7. Critical evaluation of drugs used in treatment of COVID-19
Despite having strong in vitro efficacy against SARS-CoV-2 and clinical results from other human coronaviruses such as SARS and MERS, the repurposed drugs have failed to show efficacy in clinical studies (Indari et al., 2021; Martinez, 2021). Various clinical trials of repurposed drugs are reported and showed no beneficial effect in COVID-19 (Martinez, 2021). Some major repurposed drugs used from the beginning of the outbreak of COVID-19 were hydroxycholoroquine and choloroquine, favipiravir, ribavirin, lopinavir-ritonavir, interferons (Martinez, 2021; Vandyck and Deval, 2021). Based on the data of observational study on few patients and micromolar concentrations in tissue culture, antimalarial drugs like hydroxycholoroquine and chloroquine were used against SARS-COV-2 (Kamat and Kumari, 2021). However, large clinical studies have showed no benefit of hydroxychloroquine and/or chloroquine drugs against COVID-19 in reducing morbidity or mortality (Martinez, 2021). A prospective clinical trial showed that favipiravir failed to improve SARS-CoV-2 clearance in hospitalized patients (Doi et al., 2020). A large multicenter retrospective cohort study revealed that there is no clinical benefit of ribavirin/interferons in COVID-19 (Li et al., 2021). No clinical benefit was established when HIV-1 protease inhibitors like lopinavir-ritonavir was used against SARS-CoV-2 (Cao et al., 2020). Remdesivir, US-FDA approved drug for COVID -19 showed no benefit of mortality in clinical study reported by wang and colleagues (Wang et al., 2020b). Solidarity trial by WHO also revealed no effect of mortality, period of hospital stay and beginning of ventilation by remdesivir, hydroxychloroquine, lopinavir and interferons therapy in COVID-19 patients (WHO, 2020). However, clinical study in US reported that remdesivir improved clinical outcomes in severe COVID-19 patients (Singh et al., 2020). These conflicting results showed that strong evidence is lacking for remdesivir that is to be used in COVID-19. The clinical evidence of the use of ivermectin in COVID-19 is also limited (Mega, 2020). Immunomodulators like dexamethasone improved the mortality benefits among severe COVID-19 patients in hospitalized patients of COVID-19 (Martinez, 2021).
8. Post COVID-19 complications and its management
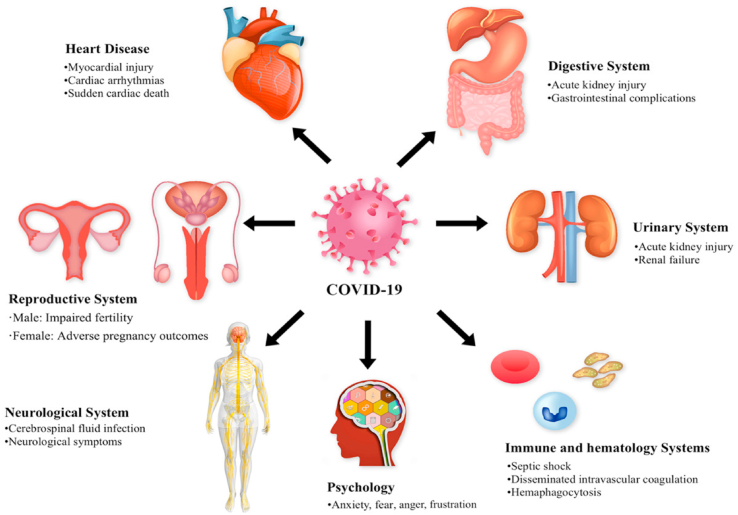
It is reasonable to expect that 80% of those who recovered from COVID-19 of a mildly symptomatic appearance will not have long-lasting consequences and will ultimately improve completely. Patients with a relatively serious symptomatic appearance that needed hospitalization but not artificial ventilation had no mid-term complications (Lai et al., 2020). Patients with serious symptomatic presentation who need artificial ventilation likely to experience long-term problems and delayed recovery aging(del Rio et al., 2020). Changes in the pathophysiology of SARS-CoV-2, inflammatory damage, and immunologic irregularities in COVID-19 are all potential pathways leading to post-COVID-19 complications. The various multiorgan system can be affected in severe COVID-19 survivors (Nalbandian et al., 2021) (Fig. 4).
Fig. 4.
Post COVID-19 complications involving different organ system. (Adopted under permission from (Zheng et al., 2021)).
8.1. Pulmonary conditions and their management after COVID-19
COVID-19 survivors have recorded a wide range of pulmonary symptoms, from dyspnea to complicated fibrotic lung injury and ventilator weaning (Nalbandian et al., 2021). The most prevalent chronic symptom outside acute COVID-19 is dyspnea, with 42–66 percent prevalence at 60–100 days follow-up, similar to the recovered patients from ARDS of different etiologies.
A tentative finding of an important radiological and symptomatic change in a sample of COVID-19 recovered patients shows that at post-COVID-19, corticosteroid treatment could be effective in a subset of patients (Myall et al., 2021). ARDS caused by severe COVID-19 and influenza A (H1N1) infection, lung transplantation was previously done for fibro proliferative lung disease (Nalbandian et al., 2021). Antifibrotic treatments are being tested in clinical trials to suppress pulmonary fibrosis after COVID-19 (Peter M George et al., 2020, George et al., 2020).
8.2. Hematologic conditions and their management after COVID-19
The prevalence of venous thromboembolism (VTE) was reported in the 5 % patients recovered from COVID-19 (Nalbandian et al., 2021). While definitive proof is lacking, provided with sustained primary thromboprophylaxis (up to 45 days), prolonged hospital discharge (up to 6 weeks), and in those managed as outpatients could have a better risk-benefit ratio in SARS-CoV-2 infection (Nalbandian et al., 2021). Anticoagulation agents such as direct oral anticoagulants and low-molecular-weight heparin are preferred in post-COVID-19 infection (Barnes et al., 2020). Similar to triggered VTE, anticoagulation drugs are prescribed for those with imaging-confirmed venous thromboembolism (up to 3 months) (Bai et al., 2020; Moores et al., 2020).
8.3. Cardiovascular conditions and their management after COVID-19
A Chinese study reported that 20% of COVID-19 recovered patients at 60 days' follow-up documented chest pain while 9% and 5% of COVID-19 recovered patients shown continuing palpitations and chest pain respectively at 6 months follow-up (Carvalho-Schneider et al., 2021; Nalbandian et al., 2021).
In those with cardiovascular problems after an acute infection or recurrent heart symptoms, assessment with electrocardiogram and echocardiogram for serial clinical and imaging at 4–12 weeks can be considered (Desai et al., 2021; Peter M. George et al., 2020, George et al., 2020). Abstaining from competitive activities or physical exercise up to 6 months before myocarditis is resolved by cardiac magnetic resonance imaging or troponin normalization. It is also recommended for sportsperson with COVID-19-related cardiovascular complications (Hendren et al., 2020; Maron et al., 2015). In persons with stable cardiovascular disease, renin-angiotensin-aldosterone system (RAAS) inhibitors are useful and should be sustained, despite initial theoretical worries about the possibility of acute COVID-19 and increased levels of ACE2 with their use (Hendren et al., 2020; Lopes et al., 2021). Instead, abruptly stopping RAAS inhibitors may be dangerous (Lopes et al., 2021). Low-dose beta-blockers can help patients to regulate their heart rates and diminish adrenergic activity (Raj et al., 2009; Vaduganathan et al., 2020). The use of medications in patients like anti-arrhythmic agents (like amiodarone)with fibrotic pulmonary changes following COVID-19 needs special attention (Lopes et al., 2021; Nalbandian et al., 2021; Raj et al., 2009).
8.4. Neuropsychiatric conditions and their management after COVID-19
COVID-19 survivors have documented recurrent malaise, diffuse myalgia, sleep disturbance, and depressive symptoms (Nalbandian et al., 2021; Raj et al., 2009). Migraine-like headaches and late-onset headaches linked to elevated cytokine levels are other COVID-19 post-acute manifestations (Arca and Starling, 2020; Belvis, 2020; Nalbandian et al., 2021). A clinical study reported around 10% of patients, having loss of taste and smell with recurrent headache (Huang et al., 2021; Nalbandian et al., 2021). Nearly 30–40% of patients reported clinically severe depression and anxiety (Nalbandian et al., 2021). For neurologic conditions such as migraine, standard treatments may be used with the consultation of a physician (Do et al., 2019). In patients with cognitive dysfunction, a neuropsychological assessment should be considered in the post-acute disease environment (Nalbandian et al., 2021).
8.5. Renal conditions and their management after COVID-19
Extreme acute kidney injury (AKI) affects 5–6% of all hospitalized patients with up to 30% of critically COVID-19 patients which demanding renal replacement therapy (RRT), particularly those with severe infections requiring mechanical ventilation (Robbins-Juarez et al., 2020; Stevens et al., 2020). Although the prevalence of dialysis-dependent AKI at discharge is poor, the degree of renal function improvement is not surfaced. Therefore, COVID-19 recovered patients with persistently compromised renal function in the post-COVID-19 infectious process can benefit from nephrologists in AKI survivor clinics, which has been linked to better outcomes in the past (Gupta et al., 2020; Nalbandian et al., 2021; Stevens et al., 2020).
8.6. Endocrine conditions and their management after COVID-19
Patients without diabetes mellitus have developed diabetic ketoacidosis, weeks to months after the COVID-19 signs have resolved (Sathish and Chandrika Anton, 2021). COVID-19 can also exacerbate autoimmune thyroid diseases, such as Hashimoto's thyroiditis or Graves' disease (Chng et al., 2018; Mateu-Salat et al., 2020).
In patients with recently diagnosed diabetes mellitus who do not have typical risk factors for type 2 diabetes, serologic tests for type 1 diabetes-related autoantibodies and repeat post-prandial C-peptide analyses should be done at follow-up, while it is appropriate to handle patients with such risk factors as if they had ketosis-prone type 2 diabetes (DiMeglio et al., 2018). Corticosteroids can be used to control hyperthyroidism caused by SARS-CoV-2-related disruptive thyroiditis (Ruggeri et al., 2021).
8.7. Gastrointestinal and hepatobiliary conditions and their management after COVID-19
COVID-19 can change the gut microbiota, favoring opportunistic infectious agents thus reducing beneficial commensals (Donati Zeppa et al., 2020; Zuo et al., 2020). The gut microbiota's capacity to influence the progression of respiratory infections (gut–lung axis) has previously been recognized in influenza and other respiratory infections (Bradley et al., 2019). Faecalibacterium prausnitzii, a butyrate-producing anaerobe associated with good health, was shown to be inversely linked to disease severity in COVID-19 (Miquel et al., 2013; Nalbandian et al., 2021). Still, clinical research is going on regarding post-COVID-19 effects on gastrointestinal and hepatobiliary systems (Nalbandian et al., 2021).
8.8. Dermatologic conditions and their management after COVID-19
Dermatic manifestations of COVID-19 emerged after (64%) or concurrently with (15%) other post-COVID-19 symptoms in a worldwide sample of 716 individuals with COVID-19, with an estimated delay of 7.9 days in adults from the onset of upper respiratory symptoms to dermatologic outcomes (Freeman et al., 2020; Mirza et al., 2021). In the Chinese trial of recovered COVID-19 patients, only 3% of patients shown skin rash after 6 months (Huang et al., 2021). Hair loss was the most common dermatologic complaint, with about 20% of patients reporting it. Hair loss can be caused by telogen effluvium, which is caused by a viral infection or a stress factor. Still, clinical study is going worldwide regarding the effects of post-COVID-19 on dermatologic conditions (Nalbandian et al., 2021; Sun et al., 2021).
8.9. Secondary infections associated with post-COVID-19 and their management
Mucormycosis, also known as zygomycosis or phycomycosis, is a rare and deadly fungal illness that mainly affects people who have a compromized immune system (Chavda and Apostolopoulos, 2021; Valour et al., 2014). The ethmoids are the most often affected sinuses, followed by the maxillary sinus (Sharma et al., 2021). According to the study, 8% of secondary bacterial or fungal infections were developed among COVID-19 patients or recovered COVID-19 patients while in the hospital, despite extensive usage of steroids and antibiotics (Rawson et al., 2020). The use of a lot of steroids and broad-spectrum antibiotics to treat COVID-19 might induce or worsen fungal illness (Sharma et al., 2021). In a study of 135 COVID-19 infected patients, White et al. reported a 26.7 percent incidence of invasive fungal infections (White et al., 2020).
Once the diagnosis is established, surgical debridement of the fungal-infected region should be undertaken urgently. An intensive surgical method in mucormycosis has a great success rate. To begin, amphotericin-B deoxycholate is the antifungal therapy of choice, with liposomal formulations favored due to lower nephrotoxicity. Posaconazole is a viable alternative to amphotericin treatment in circumstances when it is refractory or intolerant (Sharma et al., 2021).
8.10. Multiple inflammatory syndromes in children (MIS-C)
MIS-C is defined by the following syndromes such as multiple organ dysfunction, fever, diarrhea, vomiting, dermatological disorders like rashes, increased inflammatory markers with neurological, and cardiovascular complications which may happen in children with -post-COVID-19 infection (Morita et al., 2007). Metanalysis study of children with MIS-C reported a survival rate of 91.1% and a mortality rate of 3.5% (Jiang et al., 2020). Current treatment of MIS-C includes immunoglobulin I.V., supportive glucocorticoids, and a low dosage of aspirin (Nalbandian et al., 2021).
9. Concluding remarks and future prospects
The COVID-19 pandemic continues to be a global issue. Although the exceptionally great report of millions of people receiving safe and efficient vaccines every day, the novel coronavirus will continue to spread until global herd immunity is achieved - a goal that may take a long time to achieve. Even then, public health officials will have to be on the lookout for viral mutants. We will be able to effectively control future occurrences if we have numerous reliable medicines in our COVID-19 toolbox. As per USFDA's CTAP, Antivirals drugs, steroids, cell and gene therapies, and mAbs are just a few of the therapeutics that could effectively cure COVID-19.
More than 300 clinical trials are underway in COVID-19 patients to assess the safety and efficacy of various medication options. A swarm of studies published recently in various scientific journals on COVID-19 genesis, pathology, clinical therapies, and drug discovery, as well as repurposing initiatives, which are serving as beacons of hope that a vaccine and/or successful treatment may be available soon. Because SARS-CoV-2 targets the respiratory tract, the medicines are administered in non-invasive ways. Future cellular therapies may also help with COVID-19 treatment. AlloVir and Baylor College of Medicine are collaborating to develop virus-specific T cells (VSTs), which could help in combating a variety of viral infections, including COVID-19.
There are many newer drugs and repurposed drugs being investigated for the COVID-19 treatment since last 2 years. As of December 2021, more than 5782 ongoing clinical trials registered on ClinicalTrials.gov, encompasses 189 vaccine, 1387 drug, and 516 mapped drug names under investigation. For the newer version of the antiviral drugs against COVID-19 following points to be considered:
-
1)
The newer versions of the antiviral should have ease of accessibility in all the affected area. It should be available as the over the counter drug if possible. This will make the treatment option more patient friendly.
-
2)
Currently, majority of the drugs for COVID-19 management are given by IV route which required trained medical professionals for drug delivery. In order to tackle the pandemic situationthe newer versions of the antiviral should have preferential route of administration either oral (tablet or capsule) or nasal (intranasal spray) for better patient compliance (Chavda et al., 2021e).
-
3)
The newer versions of the antiviral should have better specificity for the specific viral targets. It is highly desirable that these drugs have sufficient concentration at target side and can easily disrupt the viral replication process. Similarly, viral mutation should also be targeted to get the better efficacy profile as a part of standard treatment.
-
4)
The newer versions of the antiviral should have better safety margin with proved efficacy in the large diverse population range. It can easily be given in the initial phase of the infection that prevent the severity of the COVD-19 with less side effects and speedy recovery of the patient without hospitalization.
To date, only government regulators such as the Food and Drug Administration (FDA) have approved a few medications, with the vast majority still under research. The magnitude of the COVID-19 crisis provides a once-in-a-lifetime chance to acquire data on the efficacy of potential therapeutics. This data sheds light not only on the monitoring of coronavirus pathogens, but also on the recent success of various solutions to identifying candidate therapeutics for an emerging disease.
CRediT authors contribution statement
Debdoot Basu: Writing – original draft, written the first draft of the manuscript. VPC and AM prepared the article plot and critically revised the manuscript. All authors have read the final version of the manuscript and approved the same.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Fig. 3 was prepared using templates from BioRender.com. We are very thankful to Dr. Lalit Vora (Queen's University Belfast, UK) for his support in the figure preparation. All authors want to dedicate this work to L M College of pharmacy as a part of the 75th year celebration of the college.
References
- Ader F., Bouscambert-Duchamp M., Hites M., Peiffer-Smadja N., Poissy J., Belhadi D., Diallo A., Lê M.-P., Peytavin G., Staub T., Greil R., Guedj J., Paiva Jose-Artur, Costagliola D., Yazdanpanah Y., Burdet C., Mentré F., Egle A., Greil R., Joannidis M., Lamprecht B., Altdorfer A., Belkhir L., Fraipont V., Hites M., Verschelden G., Aboab J., Ader F., Ait-Oufella H., Andrejak C., Andreu P., Argaud L., Bani-Sadr F., Benezit F., Blot M., Botelho-Nevers E., Bouadma L., Bouchaud O., Bougon D., Bouiller K., Bounes-Vardon F., Boutoille D., Boyer A., Bruel C., Cabié A., Canet E., Cazanave C., Chabartier C., Chirouze C., Clere-Jehl R., Courjon J., Crockett F., Danion F., Delbove A., Dellamonica J., Djossou F., Dubost C., Duvignaud A., Epaulard O., Epelboin L., Fartoukh M., Faure K., Faure E., Ferry T., Ficko C., Figueiredo S., Gaborit B., Gaci R., Gagneux-Brunon A., Gallien S., Garot D., Geri G., Gibot S., Goehringer F., Gousseff M., Gruson D., Hansmann Y., Hinschberger O., Jaureguiberry S., Jeanmichel V., Kerneis S., Kimmoun A., Klouche K., Lachâtre M., Lacombe K., Laine F., Lanoix J.-P., Launay O., Laviolle B., Le Moing V., Le Pavec J., Le Tulzo Y., Le Turnier P., Lebeaux D., Lefevre B., Leroy S., Lescure F.-X., Lessire H., Leveau B., Loubet P., Makinson A., Malvy D., Marquette C.-H., Martin-Blondel G., Martinot M., Mayaux J., Mekontso-Dessap A., Meziani F., Mira J.-P., Molina J.-M., Monnet X., Mootien J., Mourvillier B., Murris-Espin M., Navellou J.-C., Nseir S., Oulehri W., Peiffer-Smadja N., Perpoint T., Pialoux G., Pilmis B., Piriou V., Piroth L., Poissy J., Pourcher V., Quenot J.-P., Raffi F., Reignier J., Revest M., Richard J.-C., Riu-Poulenc B., Robert C., Roger P.-A., Roger C., Rouveix-Nordon E., Ruch Y., Saidani N., Sayre N., Senneville E., Sotto A., Stefan F., Tacquard C., Terzi N., Textoris J., Thiery G., Timsit J.-F., Tolsma V., Turmel J.-M., Valour F., Wallet F., Wattecamps G., Yazdanpanah Y., Zerbib Y., Berna M., Reuter J., Staub T., Braz S., Ferreira Ribeiro J.-M., Paiva José-Artur, Roncon-Albuquerque R., Bouscambert-Duchamp M., Gaymard A., Lê M.-P., Lina B., Peytavin G., Tubiana S., Couffin-Cadièrgues S., Esperou H., Belhadi D., Burdet C., Costagliola D., Dechanet A., Delmas C., Diallo A., Fougerou C., Guedj J., Mentré F., Mercier N., Noret M., Saillard J., Velou P. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesanya T.M.A., Campbell C.M., Cheng L., Ogbogu P.U., Kahwash R. C1 esterase inhibition: targeting multiple systems in COVID-19. J. Clin. Immunol. 2021;41:729–732. doi: 10.1007/s10875-021-00972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76:370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti N., Lalezari J.P., Amodeo P.P., Mody K., Mosher S.F., Seethamraju H., Kelly S.A., Pourhassan N.Z., Sudduth C.D., Bovinet C., ElSharkawi A.E., Patterson B.K., Stephen R., Sacha J.B., Wu H.L., Gross S.A., Dhody K. Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: case series of four critically ill patients treated with leronlimab. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaydın Ç.B., Çınar G., Cihan-Üstündağ G. Small-molecule antiviral agents in ongoing clinical trials for COVID-19. Curr. Drug Targets. 2021 doi: 10.2174/1389450122666210215112150. [DOI] [PubMed] [Google Scholar]
- Arca K.N., Starling A.J. Treatment-refractory headache in the setting of COVID-19 pneumonia: migraine or meningoencephalitis? Case report. SN compr. Clin. Med. 2020:1–4. doi: 10.1007/s42399-020-00369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authorization E.U. 2021. Emergency Use Authorization for Vaccines Explained what Is an Emergency Use Authorization (EUA)? Are the COVID-19 Vaccines Rigorously Tested ? what Safety and Effectiveness Data Are Required to Be Submitted to FDA for an EUA Request for a Vaccine Intend 2–4. [Google Scholar]
- Bai C., Chotirmall S.H., Rello J., Alba G.A., Ginns L.C., Krishnan J.A., Rogers R., Bendstrup E., Burgel P.-R., Chalmers J.D., Chua A., Crothers K.A., Duggal A., Kim Y.W., Laffey J.G., Luna C.M., Niederman M.S., Raghu G., Ramirez J.A., Riera J., Roca O., Tamae-Kakazu M., Torres A., Watkins R.R., Barrecheguren M., Belliato M., Chami H.A., Chen R., Cortes-Puentes G.A., Delacruz C., Hayes M.M., Heunks L.M.A., Holets S.R., Hough C.L., Jagpal S., Jeon K., Johkoh T., Lee M.M., Liebler J., McElvaney G.N., Moskowitz A., Oeckler R.A., Ojanguren I., O'Regan A., Pletz M.W., Rhee C.K., Schultz M.J., Storti E., Strange C., Thomson C.C., Torriani F.J., Wang X., Wuyts W., Xu T., Yang D., Zhang Z., Wilson K.C. Updated guidance on the management of COVID-19: from an American thoracic society/European respiratory society coordinated international task force (29 July 2020) Eur. Respir. Rev. 2020;29 doi: 10.1183/16000617.0287-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski M.A., Beutler N., Wolff K.C., Kirkpatrick M.G., Chen E., Nguyen T.-T.H., Riva L., Shaabani N., Parren M., Ricketts J., Gupta A.K., Pan K., Kuo P., Fuller M., Garcia E., Teijaro J.R., Yang L., Sahoo D., Chi V., Huang E., Vargas N., Roberts A.J., Das S., Ghosh P., Woods A.K., Joseph S.B., Hull M.V., Schultz P.G., Burton D.R., Chatterjee A.K., McNamara C.W., Rogers T.F. Drug repurposing screens identify chemical entities for the development of COVID-19 interventions. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-23328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Thakur P., Singh S., Dev S.N., Jain V., Varshney A., Sharma R. 2020. Glucose antimetabolite 2-Deoxy-D-Glucose and its derivative as promising candidates for tackling COVID-19: insights derived from in silico docking and molecular simulations. [DOI] [Google Scholar]
- Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A., Dager W.E., Deitelzweig S.B., Ellsworth S., Garcia D., Kaatz S., Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020;60:1422–1426. doi: 10.1111/head.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi A., Beghetti M. Inhaled nitric oxide applications in paediatric practice. Images Paediatr. Cardiol. 2002;4:4–29. [PMC free article] [PubMed] [Google Scholar]
- Bime C., Casanova N.G., Nikolich-Zugich J., Knox K.S., Camp S.M., Garcia J.G.N. Strategies to DAMPen COVID-19-mediated lung and systemic inflammation and vascular injury. Transl. Res. 2021;232:37–48. doi: 10.1016/j.trsl.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K.C., Finsterbusch K., Schnepf D., Crotta S., Llorian M., Davidson S., Fuchs S.Y., Staeheli P., Wack A. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28:245–256. doi: 10.1016/j.celrep.2019.05.105. e4. [DOI] [PubMed] [Google Scholar]
- Buckland M.S., Galloway J.B., Fhogartaigh C.N., Meredith L., Provine N.M., Bloor S., Ogbe A., Zelek W.M., Smielewska A., Yakovleva A., Mann T., Bergamaschi L., Turner L., Mescia F., Toonen E.J.M., Hackstein C.-P., Akther H.D., Vieira V.A., Ceron-Gutierrez L., Periselneris J., Kiani-Alikhan S., Grigoriadou S., Vaghela D., Lear S.E., Török M.E., Hamilton W.L., Stockton J., Quick J., Nelson P., Hunter M., Coulter T.I., Devlin L., Bradley J.R., Smith K.G.C., Ouwehand W.H., Estcourt L., Harvala H., Roberts D.J., Wilkinson I.B., Screaton N., Loman N., Doffinger R., Lyons P.A., Morgan B.P., Goodfellow I.G., Klenerman P., Lehner P.J., Matheson N.J., Thaventhiran J.E.D., Collaboration C.-N.C.-19B., Consortium M.-T.U.C.-19. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat. Commun. 2020;11:6385. doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang Jingli, Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li Huadong, Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang Juan, Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyère F., Stefic K., Gaudy-Graffin C., Grammatico-Guillon L., Bernard L. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC COVID-19 : SARS-CoV-2 variant classifications and definitions variant classifications. 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html [WWW Document]. URL. 7.5.21.
- Chavda V.P., Apostolopoulos V. Mucormycosis – an opportunistic infection in the aged immunocompromised individual: a reason for concern in COVID-19. Maturitas. 2021 doi: 10.1016/j.maturitas.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Feehan J., Apostolopoulos V. Vaccines; 2021. A Veterinary Vaccine for SARS-CoV-2: the First COVID-19 Vaccine for Animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Gajjar N., Shah N., Dave D.J. Darunavir ethanolate: repurposing an anti-HIV drug in COVID-19 treatment. Eur. J. Med. Chem. Reports. 2021;3 doi: 10.1016/j.ejmcr.2021.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Hossain M.K., Beladiya J., Apostolopoulos V. Nucleic acid vaccines for COVID-19: a paradigm shift in the vaccine development arena. Biol. 2021 doi: 10.3390/biologics1030020. [DOI] [Google Scholar]
- Chavda V.P., Pandya R., Apostolopoulos V. DNA vaccines for SARS-CoV-2: towards third generation vaccination era. Expert Rev. Vaccines. 2021 doi: 10.1080/14760584.2021.1987223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Vora L.K., Pandya A.K., Patravale V.B. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov. Today. 2021;26:2619–2636. doi: 10.1016/j.drudis.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Vora L.K., Vihol D.R. COVAX-19Ⓡ Vaccine: completely blocks virus transmission to non-immune individuals. Clin. Complement. Med. Pharmacol. 2021;1 doi: 10.1016/j.ccmp.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.-L., Lee N.-Y., Cia C.-T., Ko W.-C., Hsueh P.-R. A review of treatment of coronavirus disease 2019 (COVID-19): therapeutic repurposing and unmet clinical needs. Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.584956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng C.L., Tan H.C., Too C.W., Lim W.Y., Chiam P.P.S., Zhu L., Nadkarni N.V., Lim A.Y.Y. Diagnostic performance of ATA, BTA and TIRADS sonographic patterns in the prediction of malignancy in histologically proven thyroid nodules. Singap. Med. J. 2018;59:578–583. doi: 10.11622/smedj.2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colarusso C., Terlizzi M., Pinto A., Sorrentino R. A lesson from a saboteur: high-MW kininogen impact in coronavirus-induced disease 2019. Br. J. Pharmacol. 2020;177:4866–4872. doi: 10.1111/bph.15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb P., Molla M.M.A., Saif-Ur-Rahman K.M. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf. Heal. 2021;3:87–91. doi: 10.1016/j.bsheal.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A.D., Boursiquot B.C., Melki L., Wan E.Y. Management of arrhythmias associated with COVID-19. Curr. Cardiol. Rep. 2021;23:1–9. doi: 10.1007/s11886-020-01434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar Chowdhury S., Oommen A.M. Epidemiology of COVID-19. J. Dig. Endosc. 2020;11:3–7. doi: 10.1055/s-0040-1712187. [DOI] [Google Scholar]
- DiMeglio L.A., Evans-Molina C., Oram R.A. Type 1 diabetes. Lancet (London, England) 2018;391:2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do T.P., Remmers A., Schytz H.W., Schankin C., Nelson S.E., Obermann M., Hansen J.M., Sinclair A.J., Gantenbein A.R., Schoonman G.G. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2019;92:134–144. doi: 10.1212/WNL.0000000000006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Hibino M., Hase R., Yamamoto M., Kasamatsu Y., Hirose M., Mutoh Y., Homma Y., Terada M., Ogawa T., Kashizaki F., Yokoyama T., Koba H., Kasahara H., Yokota K., Kato H., Yoshida J., Kita T., Kato Y., Kamio T., Kodama N., Uchida Y., Ikeda N., Shinoda M., Nakagawa A., Nakatsumi H., Horiguchi T., Iwata M., Matsuyama A., Banno S., Koseki T., Teramachi M., Miyata M., Tajima S., Maeki T., Nakayama E., Taniguchi S., Lim C.K., Saijo M., Imai T., Yoshida H., Kabata D., Shintani A., Yuzawa Y., Kondobb M. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati Zeppa S., Agostini D., Piccoli G., Stocchi V., Sestili P. Gut microbiota status in COVID-19: an unrecognized player? Front. Cell. Infect. Microbiol. 2020;10:576551. doi: 10.3389/fcimb.2020.576551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M., Nirula A., Azizad M., Mocherla B., Gottlieb R.L., Chen P., Hebert C., Perry R., Boscia J., Heller B., Morris J., Crystal C., Igbinadolor A., Huhn G., Cardona J., Shawa I., Kumar P., Adams A.C., Van Naarden J., Custer K.L., Durante M., Oakley G., Schade A.E., Holzer T.R., Ebert P.J., Higgs R.E., Kallewaard N.L., Sabo J., Patel D.R., Dabora M.C., Klekotka P., Shen L., Skovronsky D.M. Bamlanivimab plus etesevimab in mild or moderate covid-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Eron J.J., Holman W., Cohen M.S., Fang L., Szewczyk L.J., Sheahan T.P., Baric R., Mollan K.R., Wolfe C.R., Duke E.R., Azizad M.M., Borroto-Esoda K., Wohl D.A., Loftis A.J., Alabanza P., Lipansky F., Painter W.P. Molnupiravir, an oral antiviral treatment for COVID-19. medRxiv prepr. Serv. Heal. Sci. 2021 doi: 10.1101/2021.06.17.21258639. 2021.06.17. [DOI] [Google Scholar]
- Freeman E.E., McMahon D.E., Lipoff J.B., Rosenbach M., Kovarik C., Desai S.R., Harp J., Takeshita J., French L.E., Lim H.W., Thiers B.H., Hruza G.J., Fox L.P. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. medRxiv. 2020 doi: 10.1101/2020.05.24.20111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Peter M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., Gibbons M.A., Hart N., Jenkins R.G., McAuley D.F., Patel B.V., Thwaite E., Spencer L.G. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- George Peter M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good S.S., Westover J., Jung K.H., Zhou X.-J., Moussa A., La Colla P., Collu G., Canard B., Sommadossi J.-P. AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02479-20. e02479-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., Reiser J., Bansal A., Srivastava A., Zhou Y., Sutherland A., Green A., Shehata A.M., Goyal N., Vijayan A., Velez J.C.Q., Shaefi S., Parikh C.R., Arunthamakun J., Athavale A.M., Friedman A.N., Short S.A.P., Kibbelaar Z.A., Abu Omar S., Admon A.J., Donnelly J.P., Gershengorn H.B., Hernán M.A., Semler M.W., Leaf D.E. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med. 2020:1436–1447. doi: 10.1001/jamainternmed.2020.3596. 02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadizadeh F. Supplementation with vitamin D in the COVID-19 pandemic? Nutr. Rev. 2021;79:200–208. doi: 10.1093/nutrit/nuaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J.M., Field S.M., de Vries Y.A., Linde M., Pittelkow M.-M., Muradchanian J., van Ravenzwaaij D. Rethinking remdesivir for COVID-19: a Bayesian reanalysis of trial findings. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Yeming, Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Yanping, Li C., Peng L., Li Yong, Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Ying, Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt A.C., Wheatley A.K. Neutralizing antibody therapeutics for COVID-19. Viruses. 2021;13:628. doi: 10.3390/v13040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indari O., Jakhmola S., Manivannan E., Jha H.C. An update on antiviral therapy against SARS-CoV-2: how far have we come? Front. Pharmacol. 2021;12:1–15. doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jade D., Ayyamperumal S., Tallapaneni V., Joghee Nanjan C.M., Barge S., Mohan S., Nanjan M.J. Virtual high throughput screening: potential inhibitors for SARS-CoV-2 PL(PRO) and 3CL(PRO) proteases. Eur. J. Pharmacol. 2021;901:174082. doi: 10.1016/j.ejphar.2021.174082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W.D., Jeon S., Kim S., Lee S.Y. Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118 doi: 10.1073/pnas.2024302118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., Klein J.D., Bhutta Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., Höbartner C., Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M., Kim E.-S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.-C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/nejmoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M., Kim E.-S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.-C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 2020;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat S., Kumari M. Repurposing chloroquine against multiple diseases with special attention to SARS-CoV-2 and associated toxicity. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.576093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L.M. (A little) clarity on convalescent plasma for covid-19. N. Engl. J. Med. 2021;384:666–668. doi: 10.1056/NEJMe2035678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Shekhar N., Sharma S., Sarma P., Prakash A., Medhi B. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacol. Rep. 2021:1–14. doi: 10.1007/s43440-020-00195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifle Z.D., Ayele A.G., Enyew E.F. Drug repurposing approach, potential drugs, and novel drug targets for COVID-19 treatment. J. Environ. Public Health. 2021;2021:6631721. doi: 10.1155/2021/6631721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105924. 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F.M., Lee K.M.C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xiong N., Li C., Gong Y., Liu L., Yang H., Tan X., Jiang N., Zong Q., Wang J., Lu Z., Yin X. Efficacy of ribavirin and interferon-α therapy for hospitalized patients with COVID-19: a multicenter, retrospective cohort study. Int. J. Infect. Dis. 2021;104:641–648. doi: 10.1016/j.ijid.2021.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes R.D., Macedo A.V.S., De Barros E Silva P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L., Feldman A., D'Andréa Saba Arruda G., De Albuquerque D.C., Camiletti A.S., De Sousa A.S., De Paula T.C., Giusti K.G.D., Domiciano R.A.M., Noya-Rabelo M.M., Hamilton A.M., Loures V.A., Dionísio R.M., Furquim T.A.B., De Luca F.A., Dos Santos Sousa Í.B., Bandeira B.S., Zukowski C.N., De Oliveira R.G.G., Ribeiro N.B., De Moraes J.L., Petriz J.L.F., Pimentel A.M., Miranda J.S., De Jesus Abufaiad B.E., Gibson C.M., Granger C.B., Alexander J.H., De Souza O.F. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz C., Muellenbach R.M., Meybohm P., Mutlak H., Lepper P.M., Rolfes C.B., Peivandi A., Stumpner J., Kredel M., Kranke P., Torje I., Reyher C. Effects of inhaled nitric oxide in COVID-19–induced ARDS – is it worthwhile? Acta Anaesthesiol. Scand. 2021;65:629–632. doi: 10.1111/aas.13757. [DOI] [PubMed] [Google Scholar]
- Mahmud R., Rahman M.M., Alam I., Ahmed K.G.U., Kabir A.K.M.H., Sayeed S.K.J.B., Rassel M.A., Monayem F.B., Islam M.S., Islam M.M., Barshan A. Das, Hoque M.M., Mallik M.U., Yusuf M.A., Hossain M.Z. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J. Int. Med. Res. 2021;49 doi: 10.1177/03000605211013550. 3000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manager R.P., Regulatory C., Manager P. 2021. Coronavirus Treatment Acceleration Program (CTAP) Frequently Asked Questions 19–21. [Google Scholar]
- Maron B.J., Udelson J.E., Bonow R.O., Nishimura R.A., Ackerman M.J., Estes N.A.M., Cooper L.T., Link M.S., Maron M.S. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientif. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- Martinez M.A. Lack of effectiveness of repurposed drugs for COVID-19 treatment. Front. Immunol. 2021;12:635371. doi: 10.3389/fimmu.2021.635371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu-Salat M., Urgell E., Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID-19. J. Endocrinol. Invest. 2020;43:1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega E.R. Latin America's embrace of an unproven COVID treatment is hindering drug trials. Nature. 2020 doi: 10.1038/d41586-020-02958-2. [DOI] [PubMed] [Google Scholar]
- Mehta P.P., Dhapte-Pawar V.S. Novel and evolving therapies for COVID-19 related pulmonary complications. Am. J. Med. Sci. 2021;S0002–9629(21) doi: 10.1016/j.amjms.2021.02.019. 00072–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott D.M., Tseng C.-T., Drelich A., Fajtová P., Chenna B.C., Kostomiris D.H., Hsu J., Zhu J., Taylor Z.W., Kocurek K.I., Tat V., Katzfuss A., Li L., Giardini M.A., Skinner D., Hirata K., Yoon M.C., Beck S., Carlin A.F., Clark A.E., Beretta L., Maneval D., Hook V., Frueh F., Hurst B.L., Wang H., Raushel F.M., O'Donoghue A.J., de Siqueira-Neto J.L., Meek T.D., McKerrow J.H. A clinical-stage cysteine protease inhibitor blocks SARS-CoV-2 infection of human and monkey cells. ACS Chem. Biol. 2021;16:642–650. doi: 10.1021/acschembio.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel S., Martín R., Rossi O., Bermúdez-Humarán L.G., Chatel J.M., Sokol H., Thomas M., Wells J.M., Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Mirza F.N., Malik A.A., Omer S.B., Sethi A. Dermatologic manifestations of COVID-19: a comprehensive systematic review. Int. J. Dermatol. 2021;60:418–450. doi: 10.1111/ijd.15168. [DOI] [PubMed] [Google Scholar]
- Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B., Jimenez D., Le Gal G., Rali P., Wells P. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Konishi M., Fukuoka T., Imura T., Kitamoto H.K., Kitamoto D. Characterization of the genus Pseudozyma by the formation of glycolipid biosurfactants, mannosylerythritol lipids. FEMS Yeast Res. 2007;7:286–292. doi: 10.1111/j.1567-1364.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Morokutti-Kurz M., Fröba M., Graf P., Große M., Grassauer A., Auth J., Schubert U., Prieschl-Grassauer E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS One. 2021;16 doi: 10.1371/journal.pone.0237480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myall K.J., Mukherjee B., Castanheira A.M., Lam J.L., Benedetti G., Mak S.M., Preston R., Thillai M., Dewar A., Molyneaux P.L., West A.G. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann. Am. Thorac. Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M.S., Huang Y., Fidock D.A., Polyak S.J., Wagoner J., Towler M.J., Weathers P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021;274 doi: 10.1016/j.jep.2021.114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCIRD Omicron variant: what you need to know. 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html [WWW Document]. CDC. URL.
- Ngo B.T., Marik P., Kory P., Shapiro L., Thomadsen R., Iglesias J., Ditmore S., Rendell M., Varon J., Dubé M., Nanda N., In G., Arkfeld D., Chaudhary P., Campese V.M., Hanna D.L., Sawcer D.E., Ehresmann G., Peng D., Smogorewski M., Armstrong A., Dasgupta R., Sattler F., Brennan-Rieder D., Mussini C., Mitja O., Soriano V., Peschanski N., Hayem G., Confalonieri M., Piccirillo M.C., Lobo-Ferreira A., Bello Rivero I., Turkia M., Vinjevoll E.H., Griffin D., Hung I.F. The time to offer treatments for COVID-19. Expet Opin. Invest. Drugs. 2021;30:505–518. doi: 10.1080/13543784.2021.1901883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourworldindata.org . 2020. Coronavirus (COVID-19) Testing - Statistics and Research - Our World in Data. [Google Scholar]
- Pfeffer L.M. The role of nuclear factor κB in the interferon response. J. Interferon Cytokine Res. 2011;31:553–559. doi: 10.1089/jir.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K.L., Thomas E., Cockerill G.S. Antiviral drugs for acute infections. Compr. Med. Chem. III. 2017:665–681. doi: 10.1016/B978-0-12-409547-2.12408-4. [DOI] [Google Scholar]
- Prescott H.C., Rice T.W. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. 2020;324:1292–1295. doi: 10.1001/jama.2020.16747. [DOI] [PubMed] [Google Scholar]
- Raj S.R., Black B.K., Biaggioni I., Paranjape S.Y., Ramirez M., Dupont W.D., Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.-J. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021;159:85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L., Burgstaller-Muehlbacher S., De Jesus P.D., Teriete P., Hull M.V., Chang M.W., Chan J.F.-W., Cao J., Poon V.K.-M., Herbert K.M., Cheng K., Nguyen T.-T.H., Rubanov A., Pu Y., Nguyen C., Choi A., Rathnasinghe R., Schotsaert M., Miorin L., Dejosez M., Zwaka T.P., Sit K.-Y., Martinez-Sobrido L., Liu W.-C., White K.M., Chapman M.E., Lendy E.K., Glynne R.J., Albrecht R., Ruppin E., Mesecar A.D., Johnson J.R., Benner C., Sun R., Schultz P.G., Su A.I., García-Sastre A., Chatterjee A.K., Yuen K.-Y., Chanda S.K. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:1153–1156. doi: 10.1007/s00210-020-01902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins-Juarez S.Y., Qian L., King K.L., Stevens J.S., Husain S.A., Radhakrishnan J., Mohan S. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int. Reports. 2020;5:1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome B.N., Avorn J. Drug evaluation during the covid-19 pandemic. N. Engl. J. Med. 2020;382:2282–2284. doi: 10.1056/NEJMp2009457. [DOI] [PubMed] [Google Scholar]
- Rothenberger S., Walser M., Malvezzi F., Mayor J., Ryter S., Moreno H., Liechti N., Hälg S., Bosshart A., Iss C., Calabro V., Cornelius A., Hospodarsch T., Neculcea A., Looser T., Mangold S., Reichen C., Radom F., Dawson K.M., Lewis S., Steiner D., Amstutz P., Engler O., Stumpp M.T. Multi-specific DARPin® therapeutics demonstrate very high potency against mutated SARS-CoV-2 variants in vitro. bioRxiv. 2021 2021.02.03. [Google Scholar]
- Ruggeri R.M., Campennì A., Siracusa M., Frazzetto G., Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Basel) 2021;20:219–221. doi: 10.1007/s42000-020-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R.P., Sharma A.R., Singh M.K., Samanta S., Bhakta S., Mandal S., Bhattacharya M., Lee S.-S., Chakraborty C. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front. Pharmacol. 2020;11:1258. doi: 10.3389/fphar.2020.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaee H., Mohsenzadegan M., Ala S., Maroufi S.S., Moradimajd P. Tocilizumab for treatment patients with COVID-19: recommended medication for novel disease. Int. Immunopharm. 2020;89:107018. doi: 10.1016/j.intimp.2020.107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish T., Chandrika Anton M. Newly diagnosed diabetes in patients with mild to moderate COVID-19. Diabetes Metab. Syndr. 2021;15:569–571. doi: 10.1016/j.dsx.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021:1–6. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai M., Tsushima K., Tanaka S., Hagiwara E., Tarumoto N., Kawada I., Hirai Y., Fujiwara S., Komase Y., Saraya T., Koh H., Kagiyama N., Shimada M., Kanou D., Antoku S., Uchida Y., Tokue Y., Takamori M., Gon Y., Ie K., Yamazaki Y., Harada K., Miyao N., Naka T., Iwata M., Nakagawa A., Hiyama K., Ogawa Y., Shinoda M., Ota S., Hirouchi T., Terada J., Kawano S., Ogura T., Sakurai T., Matsumoto Y., Kunishima H., Kobayashi O., Iwata S. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect. Dis. Ther. 2021;10:2489–2509. doi: 10.1007/s40121-021-00517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., Gamarnik A.V., Ojeda D.S., Santoro D.M., Camino P.J., Antelo S., Rainero K., Vidiella G.P., Miyazaki E.A., Cornistein W., Trabadelo O.A., Ross F.M., Spotti M., Funtowicz G., Scordo W.E., Losso M.H., Ferniot I., Pardo P.E., Rodriguez E., Rucci P., Pasquali J., Fuentes N.A., Esperatti M., Speroni G.A., Nannini E.C., Matteaccio A., Michelangelo H.G., Follmann D., Lane H.C., Belloso W.H., Group P.S. A randomized trial of convalescent plasma in covid-19 severe pneumonia. N. Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T.U., Parida S., Lingaraju M.C., Kesavan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020;72:1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smail S.W., Saeed M., Alkasalias Twana, Khudhur Z.O., Younus D.A., Rajab M.F., Abdulahad W.H., Hussain H.I., Niaz K., Safdar M. Inflammation, immunity and potential target therapy of SARS-COV-2: a total scale analysis review. Food Chem. Toxicol. 2021;150 doi: 10.1016/j.fct.2021.112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T., Bushek J., Leclaire A., Prosser T. Elseivier; 2020. COVID-19 Drug Therapy what ’ s been updated : Highlights; pp. 1–25. [Google Scholar]
- Stallmach A., Kortgen A., Gonnert F., Coldewey S.M., Reuken P., Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure - a cautionary case series. Crit. Care. 2020;24:1–3. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.S., King K.L., Robbins-Juarez S.Y., Khairallah P., Toma K., Verduzco H.A., Daniel E., Douglas D., Moses A.A., Peleg Y., Starakiewicz P., Li M.T., Kim D.W., Yu K., Qian L., Shah V.H., O'Donnell M.R., Cummings M.J., Zucker J., Natarajan K., Perotte A., Tsapepas D., Krzysztof K., Dube G., Siddall E., Shirazian S., Nickolas T.L., Rao M.K., Barasch J.M., Valeri A.M., Radhakrishnan J., Gharavi A.G., Husain S.A., Mohan S. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS One. 2020;15:1–14. doi: 10.1371/journal.pone.0244131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpp M.T., Dawson K.M., Binz H.K. Beyond antibodies: the DARPin® drug platform. BioDrugs. 2020;34:423–433. doi: 10.1007/s40259-020-00429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Tang N., Peluso M.J., Iyer N.S., Torres L., Donatelli J.L., Munter S.E., Nixon C.C., Rutishauser R.L., Rodriguez-Barraquer I., Greenhouse B., Kelly J.D., Martin J.N., Deeks S.G., Henrich T.J., Pulliam L. Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. 2021;10:386. doi: 10.3390/cells10020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.C., Adams A.C., Hufford M.M., de la Torre I., Winthrop K., Gottlieb R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A., Laverdure C., Verma S.C., Rossetto C.C., Jackson D., Farrell M.J., Van Hooser S., Pandori M. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect. Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valour F., Sénéchal A., Dupieux C., Karsenty J., Lustig S., Breton P., Gleizal A., Boussel L., Laurent F., Braun E., Chidiac C., Ader F., Ferry T. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 2014;7:183–197. doi: 10.2147/IDR.S39601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandyck K., Abdelnabi R., Gupta K., Jochmans D., Jekle A., Deval J., Misner D., Bardiot D., Foo C.S., Liu C., Ren S., Beigelman L., Blatt L.M., Boland S., Vangeel L., Dejonghe S., Chaltin P., Marchand A., Serebryany V., Stoycheva A., Chanda S., Symons J.A., Raboisson P., Neyts J. ALG-097111, a potent and selective SARS-CoV-2 3-chymotrypsin-like cysteine protease inhibitor exhibits in vivo efficacy in a Syrian Hamster model. Biochem. Biophys. Res. Commun. 2021;555:134–139. doi: 10.1016/j.bbrc.2021.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandyck K., Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr. Opin. Virol. 2021;49:36–40. doi: 10.1016/j.coviro.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenti I., Zazzi M., Saladini F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin. Ther. Pat. 2021;31:325–337. doi: 10.1080/13543776.2021.1880568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Guan Y. COVID-19 drug repurposing: a review of computational screening methods, clinical trials, and protein interaction assays. Med. Res. Rev. 2021;41:5–28. doi: 10.1002/med.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yeming, Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Yi, Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yeming, Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Yi, Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., Pandey M., Whitaker H., May A., Morgan M., Wise M.P., Healy B., Blyth I., Price J.S., Vale L., Posso R., Kronda J., Blackwood A., Rafferty H., Moffitt A., Tsitsopoulou A., Gaur S., Holmes T., Backx M. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2020;ciaa1298 doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Repurposed antiviral drugs for covid-19 — interim WHO solidarity trial results. N. Engl. J. Med. 2020;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization COVID-19 Weekly Epidemiological Update 22. World Heal. Organ. 2021:1–3. [Google Scholar]
- World Health Organization (WHO) 2021. WHO-COVID-19-global-data. [Google Scholar]
- Wu R., Wang L., Kuo H.-C.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J., Amorosa L., Brunetti L., Kong A.-N. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. reports. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Song X., Ma T., Pan X., Zhou Y., Hou Y., Zhang Z., Li K., Karypis G., Cheng F. Repurpose open data to discover therapeutics for COVID-19 using deep learning. J. Proteome Res. 2020;19:4624–4636. doi: 10.1021/acs.jproteome.0c00316. [DOI] [PubMed] [Google Scholar]
- Zheng K.I., Feng G., Liu W.-Y., Targher G., Byrne C.D., Zheng M.-H. Extrapulmonary complications of COVID-19: a multisystem disease? J. Med. Virol. 2021;93:323–335. doi: 10.1002/jmv.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X. Lou, Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R Di, Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.-W., Xie Y., Tang L.-S., Pu D., Zhu Y.-J., Liu J.-Y., Ma X.-L. Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies. Signal Transduct. Target. Ther. 2021;6:317. doi: 10.1038/s41392-021-00733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., Lai C.K.C., Chen Z., Tso E.Y.K., Fung K.S.C., Chan V., Ling L., Joynt G., Hui D.S.C., Chan F.K.L., Chan P.K.S., Ng S.C. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]