Abstract
Background
The relationship between acute respiratory distress syndrome (ARDS)/acute lung injury (ALI) and levels of certain inflammatory factors remains controversial. The purpose of this meta-analysis was to summarize the available studies evaluating the association between levels of inflammatory factors and ARDS/ALI incidence.
Methods
We searched the PubMed, EmBase, and Cochrane databases for studies published up to July 2017. For each inflammatory factor, a random effects model was employed to pool results from different studies.
Results
We identified 63 studies that included 6243 patients in our meta-analysis. Overall, the results indicated that the levels of angiopoietin (ANG)-2 (standard mean difference, SMD: 1.34; P < 0.001), interleukin (IL)-1β (SMD: 0.92; P = 0.012), IL‑6 (SMD: 0.66; P = 0.005), and tumor necrosis factor (TNF)-α (SMD: 0.98; P = 0.001) were significantly higher in patients with ARDS/ALI than in unaffected individuals. No significant differences were observed between patients with ARDS/ALI and unaffected individuals in terms of the levels of IL‑8 (SMD: 0.61; P = 0.159), IL-10 (SMD: 1.10; P = 0.231), and plasminogen activator inhibitor (PAI)-1 (SMD: 0.70; P = 0.060).
Conclusions
ARDS/ALI is associated with a significantly elevated levels of ANG‑2, IL-1β, IL‑6, and TNF‑α, but not with IL‑8, IL-10, and PAI‑1 levels.
Supplementary Information
The online version of this article (10.1007/s00508-021-01971-3) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Acute lung injury, Acute respiratory distress syndrome, Systematic review, Meta-analysis
Background
Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are pulmonary diseases characterized by inflammatory pulmonary edema, acute hypoxemia, and accumulation of bilateral pulmonary infiltrates [1]. In the USA the annual number of ARDS cases is more than 140,000 [2] and the reported rates of ARDS and ALI are 59 and 79 cases for every 100,000 individuals, respectively, with reported mortality rates ranging from 22% to 41% [3]. ARDS and ALI are triggered by several factors that are associated with either direct or indirect injury. Direct injuries that trigger ARDS/ALI include serious pulmonary infection, aspiration of foreign bodies, pulmonary contusion, and oxygen poisoning. Indirect injuries associated with ARDS/ALI include severe systemic infection, severe non-pulmonary trauma, acute pancreatitis, major blood transfusion, and disseminated intravascular coagulation [4, 5]. Patients exposed to the abovementioned factors are at high risk for developing ARDS/ALI. Although the mechanisms underlying ARDS/ALI pathogenesis remain unclear, inflammation has been considered as one of the major inducing factors.
The pathology of ARDS is characterized by diffuse pulmonary interstitial and alveolar edema due to capillary endothelial and alveolar epithelial cell injuries that lead to acute respiratory insufficiency or failure [6]. In the early stages of ARDS, edema fluid is concentrated in the alveolar interstitial spaces, and neutrophils adhere to the surface of damaged vascular endothelial cells and migrate to pulmonary interstitial and alveolar cavities. Afterwards, proinflammatory factors, such as inflammatory cytokines and proteases, are released and promote neutrophil-mediated lung injury [7]. In the later stage of ARDS, the injured lung is characterized by severe fibrosis and alveolar destruction and reconstruction [8]. The majority of ARDS/ALI patients show rapid disease progression because of this acute inflammatory process.
Various inflammatory mediators that activate the inflammatory cascade and induce secondary diffuse lung parenchymal injury are the primary causes of ARDS/ALI [9, 10]; however, even low levels of inflammatory factors that do not contribute to the amplification of inflammation are associated with the onset of ARDS/ALI, suggesting their possible role as critical drivers of the disease. Therefore, inflammation-related factors can potentially serve as reliable predictors of ARDS/ALI risk.
A previous study systematically reviewed the relationship between plasma biomarkers and ARDS onset and found that levels of Krebs von den Lungen‑6 (KL-6), lactate dehydrogenase (LDH), the soluble receptor for advanced glycation end products (RAGE), and von Willebrand factor (vWF) were significantly correlated with ARDS incidence [11]; however, the study did not investigate the inflammatory factors present in the pulmonary edema fluid, and conclusions were based on a relatively small number of studies for each inflammatory factor. Therefore, further validation of these putative associations is required. This study provided update results by analyzing recently published literature that investigated the relationships between specific inflammatory biomarkers and the onset of ARDS/ALI. Furthermore, these relationships were analyzed according to different characteristics.
Methods
We conducted the meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12].
Search strategy
We systematically searched the PubMed, EmBase, and Cochrane Central Register of Controlled Trials databases for publications up to July 2017 using the keywords “acute respiratory distress syndrome,” “acute lung injury,” “inflammation,” “C-reactive protein,” “interleukin,” “tumour necrosis factor,” “cytokines,” “interferon,” “transforming growth factor,” and “risk factor.” The search strategy used for the PubMed database is described in Supplementary information (searching strategy in PubMed). We restricted our search to reports published in English. We also included relevant articles cited as references of the studies.
Data selection and extraction
Literature search and selection were independently performed by two researchers, and any inconsistencies were resolved by group discussion. A study was eligible for inclusion if the following criteria were met: (1) the study included patients with ARDS/ALI; (2) participants in the control group were not diagnosed with ARDS/ALI; (3) the primary outcomes of interest included Angiopoietin (ANG)‑2, Interleukin (IL)-1β, IL‑6, IL‑8, IL-10, Plasminogen activator inhibitor (PAI)‑1, and Tumor necrosis factor (TNF)‑α, while the secondary outcomes included albumin, ANG‑1, Clara cell secretory protein (CC16), C-reactive protein (CRP), endotoxin, granulocyte colony-stimulating factor (G‑CSF), intercellular cell adhesion molecule (ICAM), IL‑2, IL‑4, IL-12, KL‑6, Lactate dehydrogenase (LDH), myeloperoxidase (MPO), nuclear factor (NF)-κβ, procalcitonin (PCT), protein C, RAGE, sE-selectin, surfactant protein (SP‑D), transforming growth factor (TGF)-β1, tissue factor (TF), Tumor necrosis factor receptor (TNFR)‑1, TNFR‑2, vascular endothelial growth factor (VEGF), and vWF. Reviews, editorials, non-human studies, letters, and conference papers were excluded because of insufficient data.
The following parameters were extracted from the articles: first author, country, publication year, study design, sample size, and average age, gender, underlying disease of participants, patient disease status, method of ARDS/ALI diagnosis, specimen source, test time, and follow-up. The Newcastle-Ottawa Scale (NOS) was used to evaluate the methodological quality of each study [13]. The NOS is based on the following three subscales: selection of the study group (four categories), comparability of the groups (one category), and outcome assessment (three categories). Data extraction and quality assessment were conducted independently by two authors, and results were examined and adjudicated by an additional author who referred to the original study.
Statistical analysis
In this meta-analysis, the standard mean difference (SMD) and 95% confidence interval (CI) were determined to evaluate the effect of sample size across studies [14]. We pooled the SMDs for each inflammatory factor using a random effects model [15]. The I2 statistic was used to assess heterogeneity of the SMDs across multiple studies [16]. Means and variances were estimated from medians and ranges as previously described [17]. A sensitivity analysis was performed by sequentially removing each individual study from the meta-analysis [16]. Meta-regression was also conducted for ANG‑2, IL-1β, IL6, IL‑8, IL10, PAI‑1, and TNF‑α based on sample size and mean age. Furthermore, stratified analyses were conducted based on sample size, mean age, patient status, and sample origin. Visual inspection of funnel plots from the Egger’s [18] or Begg’s test [19] was conducted to evaluate publication bias. All tests were two-tailed, and P-value < 0.05 was considered statistically significant. Data analyses were performed using STATA software (version 10.0; Stata Corporation, College Station, TX, USA).
Results
Literature search
In this study, a total of 302 articles were retrieved from PubMed, 704 from EmBase, and 28 from the Cochrane Library. After removing duplicates, 851 articles passed the inclusion criteria in the meta-analysis. A total of 757 articles were excluded because they were considered irrelevant after scanning the titles and abstracts. Furthermore, articles that were considered to be unrelated based on full-text assessment (n = 5), a duplicate publication (n = 1), mortality-related studies (n = 18), studies with undesired outcomes (n = 4), and studies with control groups without ARDS/ALI risk (n = 3) were also excluded. Finally, 63 studies that studied a total of 6243 patients were included in our systematic review (Fig. 1; [20–82]).
Fig. 1.
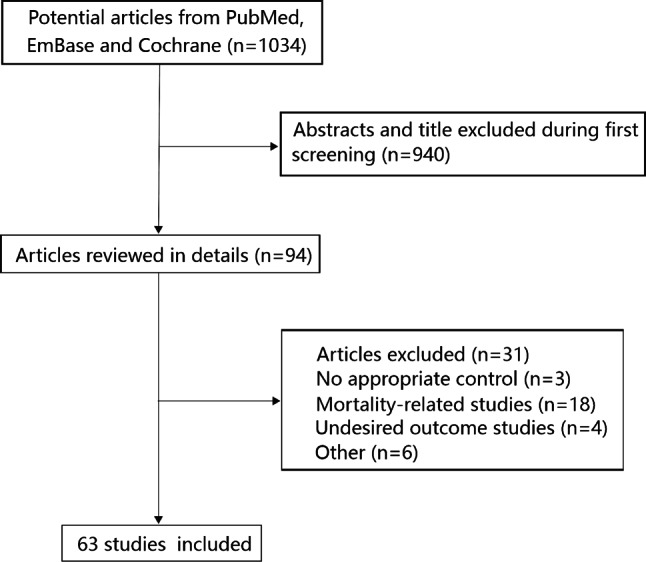
PRISMA flowchart illustrating the process of study selection in our analysis
Study characteristics
A total of 59 studies implemented a prospective design, while the remaining 4 studies employed a retrospective design. Most studies included patient groups with an average age ranging from 40 to 60 years. All participants enrolled in the included studies were at risk of ARDS/ALI, and the majority of samples were collected less than 1 day after study recruitment. All included studies employed standardized American-European Consensus Conference (AECC) criteria, Berlin definition of ARDS, lung injury score (LIS), and oxygenation index (PaO2/FiO2) for ARDS/ALI diagnosis. In earlier studies, there were no general criteria for defining ARDS, so the criteria were defined by the authors of each study. The NOS quality analysis for all studies returned scores ranging from 6 to 8, indicating a good overall quality of the included studies (Table 1).
Table 1.
Characteristics of subjects in eligible studies
| Authors | Country | Year | Study design | Sample size | Mean or median age (years) | Gender (m/f) |
Underlying disease | Patient status | Judgment method of ARDS/ALI | Specimen source | Test time | Follow-up | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoeboer [12] | Netherlands | 2015 | Prospective | 101 | 64.0 | 69/32 | Fever | ARDS | Berlin definition/LIS | Blood | 7 days | 7 days | 8 |
| Roubinian [13] | U.S | 2015 | Prospective | 317 | 58.0 | 153/164 | Pulmonary transfusion reactions hypoxemia | ALI | Defined | Blood | 1 day | NA | 6 |
| Jones [14] | U.S | 2013 | Prospective | 43 | 45.6 | 40/9 | Inhalation and burns | ALI | PaO2/FiO2 | BALF | 3 days | 3 days | 7 |
| Agrawal [15] | U.S | 2013 | Prospective | 230 | 65.0 | 79/88 | Critically ill | ALI | Berlin definition | Blood | 1 day | 60 days | 7 |
| Schultz [16] | Netherlands | 2012 | Retrospective | 20 | 59.0 | 13/7 | Mechanical ventilation | ALI | LIS | BALF | 6 days | 6 days | 7 |
| Quesnel [17] | France | 2012 | Retrospective | 122 | 49.0 | 79/43 | Critically ill | ALI/ARDS | AECC | BALF | NA | 28 days | 6 |
| Osaka [18] | Japan | 2011 | Prospective | 27 | 50.0 | 12/15 | Pneumonia | ALI | PaO2/FiO2 | Blood | 1 day | 10 days | 8 |
| Guervilly [19] | France | 2011 | Prospective | 74 | 58.0 | 53/21 | Critically ill | ALI | AECC | Blood/BALF | 1 day | 28 days | 7 |
| Jabaudon [20] | France | 2011 | Prospective | 64 | 59.0 | 41/23 | Severe Sepsis | ALI | AECC | Blood | 1 day | 28 days | 7 |
| Aman [21] | Netherlands | 2010 | Prospective | 83 | 60.0 | 65/18 | Mechanical ventilation | ALI/ARDS | AECC | Blood | 1 day | NA | 7 |
| Kohno [22] | Japan | 2011 | Prospective | 20 | 71.0 | 15/5 | Thoracic aortic aneurysm repair | ARDS | PaO2/FiO2 | Blood | 1–4 days | 22 days | 7 |
| Determann [23] | Netherlands | 2010 | Prospective | 36 | 58.0 | 22/14 | Mechanical ventilation | ALI/ARDS | LIS | Blood | 2 days | 2 days | 8 |
| Determann [24] | Netherlands | 2010 | Prospective | 150 | 61.0 | 99/51 | Mechanical ventilation | ALI | LIS | Blood/BALF | 1 day | 4 days | 8 |
| Fremont [25] | U.S | 2010 | Retrospective | 192 | 39.0 | 131/61 | Traumatic injuries | ALI | PaO2/FiO2 | Blood | 3 days | 6–10 days | 7 |
| Determann [26] | Netherlands | 2009 | Retrospective | 22 | 65.0 | 17/5 | Ventilator-associated pneumonia | ALI/ARDS | AECC | BALF | 1 day | 8 days | 7 |
| Chi [27] | China | 2009 | Prospective | 27 | NA | NA | Orthotopic liver transplantation | ALI | PaO2/FiO2 | Blood | 1 day | 7 days | 6 |
| Kropski [28] | U.S | 2009 | Prospective | 32 | 43.0 | 15/17 | Mechanical ventilation | ARDS | AECC | Blood/BALF | 1 day | 2–14 days | 7 |
| Calfee [29] | U.S | 2009 | Prospective | 67 | 51.0 | 40/27 | Hydrostatic pulmonary edema | ALI | AECC | Blood/BALF | 4 h | 3 days | 8 |
| Van der Heijden [30] | Netherlands | 2008 | Prospective | 112 | 56.0 | NA | Critically ill | ALI/ARDS | AECC/LIS | Blood | 1 day | NA | 7 |
| Kurzius-Spencer [31] | U.S | 2008 | Prospective | 21 | NA | 20/1 | Smoke inhalation injury | ARDS | AECC/PaO2/FiO2 | BALF | 36 h | 72 h | 8 |
| Nathani [32] | U.K | 2008 | Prospective | 42 | 62.0 | 24/18 | ARDS risk population | ARDS/ALI | AECC | Blood/BALF | 1 day | 4 days | 7 |
| Ganter [33] | U.S | 2008 | Prospective | 208 | 41.0 | 155/53 | Traumatic injuries | ALI | AECC | Blood | 1 day | 28 days | 7 |
| Gallagher [34] | U.S | 2008 | Prospective | 63 | 67.0 | 35/28 | Critically ill | ALI/ARDS | AECC | Blood | 1 day | 2 months | 7 |
| Calfee [35] | U.S | 2007 | Prospective | 1451 | 52.0 | 609/839 | Trauma | ALI | Defined | Blood | 1 day | 180 days | 7 |
| Ware [36] | U.S | 2007 | Prospective | 878 | 52.0 | 514/364 | Acute cardiogenic pulmonary edema | ALI/ARDS | Defined | Blood | 1 day | 3 days | 7 |
| Perkins [37] | UK | 2007 | Prospective | 54 | NA | NA | ARDS risk population | ALI/ARDS | AECC | BALF | 1 day | 4 days | 6 |
| El Solh [38] | U.S | 2006 | Prospective | 51 | 36.6 | 22/29 | Aspiration pneumonitis | ALI | AECC | Blood/BALF | 1 day | 28 days | 8 |
| Parsons [39] | U.S | 2005 | Prospective | 49 | NA | NA | Critically ill | ALI | AECC | Blood | 1 day | 180 days | 8 |
| Bouros [40] | Greece | 2004 | Prospective | 59 | 51.7 | 43/16 | Critically ill | ALI | AECC | Blood/BALF | 1 day | NA | 7 |
| Nakae [41] | Japan | 2003 | Prospective | 21 | 62.0 | 15/6 | Sepsis | ARDS | Defined | Blood | NA | NA | 7 |
| Sato [42] | UK | 2004 | Prospective | 37 | 39.5 | 32/5 | Mechanical ventilation | ARDS | AECC | Blood | 1 day | 6.5 days | 8 |
| Nys [43] | Belgium | 2003 | Prospective | 67 | 54.0 | 43/24 | Pneumonia | ARDS | PaO2/FiO2 | BALF | 1–2 days | NA | 7 |
| Gessner [44] | Germany | 2003 | Prospective | 35 | 60.0 | 16/19 | Acute respiratory failure | ARDS | AECC | BALF | 1 day | 6 months | 7 |
| Ishizaka [45] | Japan | 2004 | Prospective | 35 | 68.0 | 27/8 | Cardiogenic pulmonary edema | ALI | AECC | BALF | 1 day | NA | 6 |
| Prabhakaran [46] | U.S | 2003 | Prospective | 51 | 50.0 | 29/22 | Hydrostatic edema | ARDS | AECC | Blood/BALF | 1 day | NA | 7 |
| Grissom [47] | U.S | 2003 | Prospective | 39 | 51.0 | 16/17 | ARDS risk population | ARDS | AECC | Blood/BALF | 96 h | 42 days | 8 |
| Agouridakis [48] | Greece | 2002 | Prospective | 65 | 44.0 | 40/25 | Mechanical ventilation | ARDS | AECC | Blood/BALF | 1 day | 15 days | 8 |
| Agouridakis [49] | Greece | 2002 | Prospective | 34 | 49.0 | 23/11 | Mechanical ventilation | ARDS | AECC | Blood/BALF | 1 day | 6 months | 8 |
| Thickett [50] | UK | 2002 | Prospective | 68 | 65.0 | 45/23 | ARDS risk population | ARDS | AECC | BALF | 1 day | 4 days | 6 |
| Hamacher [51] | France | 2002 | Prospective | 36 | 43.0 | 28/8 | ARDS risk population | ARDS | AECC | BALF | 1 day | 21 days | 7 |
| Takala [52] | Finland | 2002 | Prospective | 52 | 54.0 | 29/19 | Critically ill | ARDS | AECC | Blood | 1 day | 7 days | 8 |
| Park [53] | Switzerland | 2001 | Prospective | 69 | 43.8 | 41/28 | ARDS risk population | ARDS | AECC | BALF | 1 day | 21 days | 7 |
| Hirani [54] | UK | 2001 | Prospective | 56 | 48.0 | NA | Major trauma | ARDS | AECC | BALF | 1 day | 36 months | 8 |
| Geerts [55] | Belgium | 2001 | Prospective | 26 | 52.0 | 19/7 | ARDS risk population | ARDS | AECC | BALF | 1 day | NA | 7 |
| Siemiatkowski [56] | Poland | 2000 | Prospective | 36 | 44.3 | 27/9 | Major trauma | ARDS | LIS | Blood | 1 day | 10 days | 7 |
| Armstrong [57] | UK | 2000 | Prospective | 67 | 62.0 | 44/23 | Critically ill | ARDS/ALI | AECC | BALF | 48 h | NA | 7 |
| Bauer [58] | Spain | 2000 | Prospective | 66 | 57.2 | NA | Pneumonia | ARDS | AECC | Blood | 1 day | NA | 8 |
| Gando [59] | Japan | 1999 | Prospective | 58 | 58.0 | 37/21 | Critically ill | ARDS | Defined | Blood | 1 day | 4 days | 7 |
| Donnelly [60] | Scotland | 1999 | Prospective | 61 | NA | NA | Trauma | ARDS | Defined | BALF | NA | NA | 6 |
| Parsons [61] | U.S | 1997 | Prospective | 77 | 37.5 | 53/24 | ARDS risk population | ARDS | Defined | Blood | 1 day | 2 days | 7 |
| Schutte [62] | Germany | 1996 | Prospective | 56 | 54.5 | 45/11 | Pneumonia | ARDS | AECC | Blood/BALF | 2 days | 10 days | 7 |
| Chollet-Martin [63] | France | 1996 | Prospective | 14 | 61.0 | NA | Pneumonia | ARDS | LIS | Blood/BALF | 3 days | 7 days | 8 |
| Ricou [64] | U.S | 1996 | Prospective | 33 | 48.0 | 24/9 | Critically ill | ARDS | LIS | Blood/BALF | 3 days | 2 weeks | 8 |
| Schwartz [65] | U.S | 1996 | Prospective | 12 | 45.0 | 7/5 | Mechanical ventilation | ARDS | LIS | BALF | 1 day | NA | 7 |
| Jorens [66] | UK | 1995 | Prospective | 35 | 56.6 | 31/4 | Cardiopulmonary bypass | ARDS | Defined | BALF | 1 day | 3 days | 7 |
| Fuchs-buder [67] | Switzerland | 1996 | Prospective | 21 | NA | NA | Critically ill | ARDS | LIS | BALF | 2 days | 10 days | 8 |
| Leff [68] | U.S | 1993 | Prospective | 26 | NA | NA | Sepsis | ARDS | Defined | Blood | 1 day | 2 days | 7 |
| Sakamaki [69] | Japan | 1995 | Prospective | 48 | 49.0 | 29/19 | Sepsis | ARDS | Defined | Blood | 1 day | 15 days | 7 |
| Donnelly [70] | U.S | 1994 | Prospective | 82 | 49.5 | NA | ARDS risk population | ARDS | LIS | Blood | 1–3 days | NA | 8 |
| Moalli [71] | U.S | 1989 | Prospective | 47 | 66.0 | NA | ARDS risk population | ARDS | Defined | Blood | 1 day | 22 days | 7 |
| Rubin [72] | U.S | 1990 | Prospective | 45 | NA | NA | Sepsis | ARDS | LIS | Blood | 1 day | 3 days | 8 |
| Roten [73] | Switzerland | 1990 | Prospective | 50 | 49.0 | 31/19 | Critically ill | ARDS | Defined | Blood | 1 day | 5 days | 7 |
| Parsons [74] | U.S | 1992 | Prospective | 103 | 46.0 | 77/26 | ARDS risk population | ARDS | Defined | Blood | 1 day | 2 days | 7 |
AECC American-European Consensus Conference, BALF Bronchoalveolar Lavage Fluid, ALI Acute lung injury, ARDS Acute respiratory distress syndrome, NA not available
Inflammatory biomarkers and ARDS/ALI
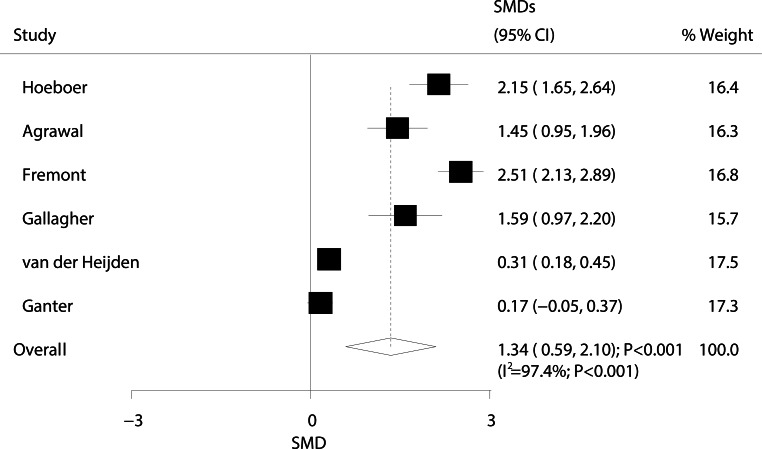
The relationship between ARDS/ALI and angiopoietin (ANG)-2 levels is presented in Fig. 2. The overall standard mean difference (SMD) from six studies showed that ARDS/ALI patients had higher ANG‑2 levels than those of unaffected individuals (SMD: 1.34; 95% CI: 0.59–2.10; P < 0.001); however, significant heterogeneity was detected (I2 = 97.4%; P < 0.001). Sensitivity analysis showed that the conclusion did not change after sequential removal of each study (Supplementary information Table S1).
Fig. 2.
Forest plot comparing ANG‑2 levels between ARDS/ALI patients and unaffected individuals
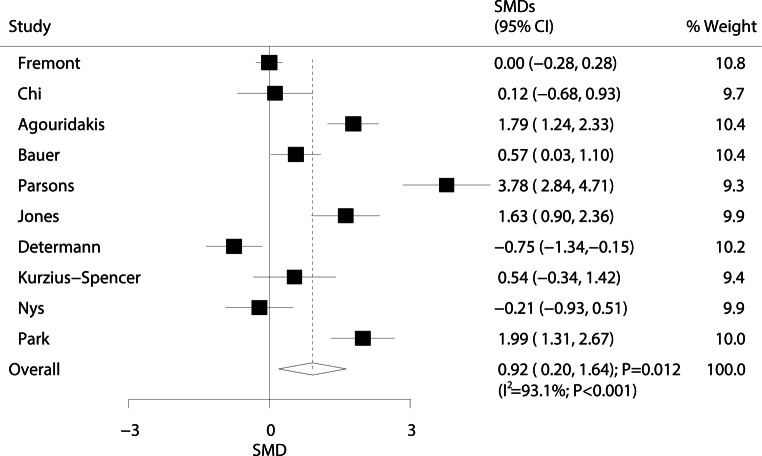
The relationship between ARDS/ALI and interleukin (IL)-1β levels is presented in Fig. 3. The pooled SMD from 10 studies indicated that ARDS/ALI patients exhibited significantly higher IL-1β levels than those of the individuals without ARDS/ALI (SMD: 0.92; 95% CI: 0.20–1.64; P = 0.012). Although substantial heterogeneity was observed across all studies (I2 = 93.1%; P < 0.001), the conclusion did not change after sequential exclusion of each study (Supplementary information Table S2).
Fig. 3.
Forest plot comparing IL-1β levels between ARDS/ALI patients and unaffected individuals
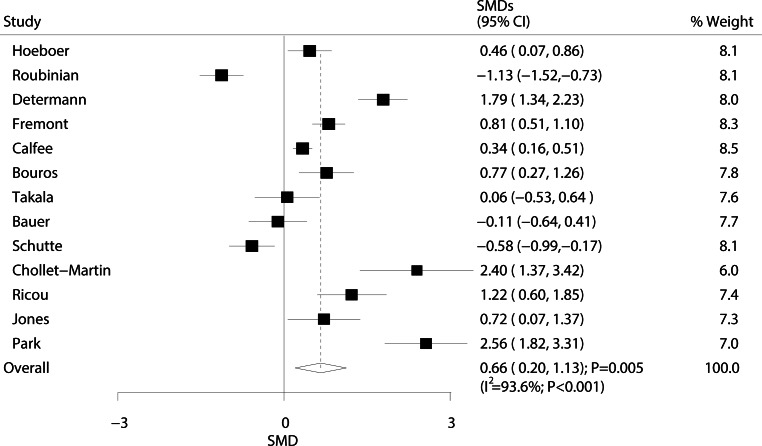
The relationship between ARDS/ALI and IL‑6 levels is shown in Fig. 4. Overall results showed that ARDS/ALI patients had higher IL‑6 levels than those of individuals in the population without ARDS/ALI (SMD: 0.66; 95% CI: 0.20 to 1.13; P = 0.005). Heterogeneity was observed at the same degree as the effect across the studies (I2 = 93.6%; P < 0.001). Sensitivity analysis showed that the conclusion was not affected by the exclusion of any specific study from the pooled analysis (Supplement information Table S3).
Fig. 4.
Forest plot comparing IL‑6 levels between ARDS/ALI patients and unaffected individuals
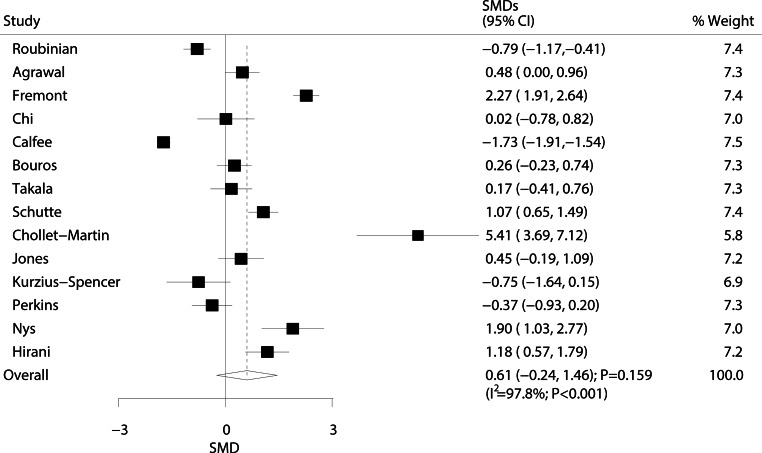
The relationship between ARDS/ALI and IL‑8 levels was analyzed in 14 studies, and results are shown in Fig. 5. No significant differences in IL‑8 levels were observed between ARDS/ALI patients and individuals of the population without ARDS/ALI (SMD: 0.61; 95% CI: −0.24–1.46; P = 0.159). Furthermore, substantial heterogeneity was detected (I2 = 97.8%; P < 0.001). Based on sensitivity analysis, we excluded the study conducted by Calfee et al. [57], which specifically included a large sample size of trauma patients. We concluded that ARDS/ALI were associated with higher IL‑8 levels (SMD: 0.76; 95% CI: 0.11–1.40; P = 0.021) (Supplement information Table S4).
Fig. 5.
Forest plot comparing IL‑8 levels between ARDS/ALI patients and unaffected individuals
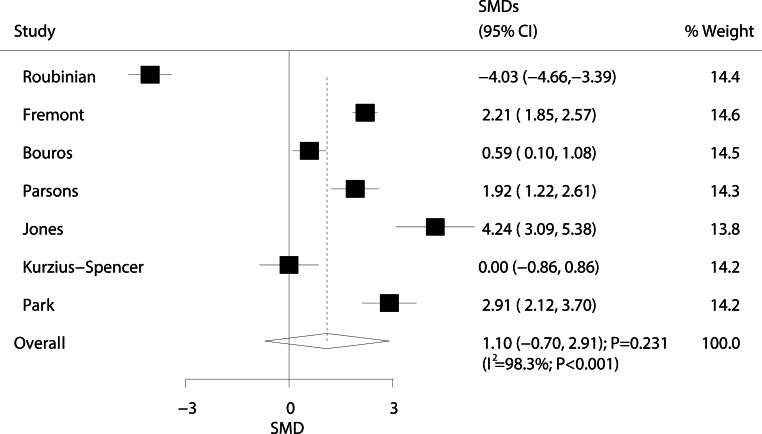
The relationship between ARDS/ALI and IL-10 levels was investigated in seven studies, and results are presented in Fig. 6. We detected no significant differences in IL-10 levels between ARDS/ALI and non-ARDS/ALI patients (SMD: 1.10; 95% CI: −0.70–2.91; P = 0.231). Substantial heterogeneity was observed (I2 = 98.3%; P < 0.001). Sensitivity analysis indicated that ARDS/ALI patients had higher IL-10 levels than those of non-ARDS/ALI patients when the study conducted by Roubinian et al. was excluded (Supplementary information Table S5) [82].
Fig. 6.
Forest plot comparing IL-10 levels between ARDS/ALI patients and unaffected individuals
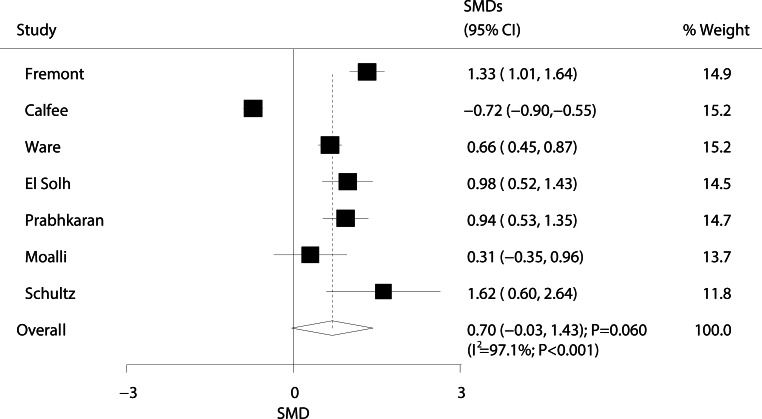
The relationship between ARDS/ALI and plasminogen activator inhibitor‑1 (PAI-1) levels was investigated in seven studies, and results are presented in Fig. 7. We detected no significant differences in PAI‑1 levels between ARDS/ALI patients and non-ARDS/ALI individuals (SMD: 0.70; 95% CI: −0.03–1.43; P = 0.060). Substantial heterogeneity was observed (I2 = 97.1%; P < 0.001). Sensitivity analysis showed that this result changed after excluding the study conducted by Calfee et al. (Supplementary information Table S6) [57].
Fig. 7.
Forest plot comparing PAI‑1 levels between ARDS/ALI patients and unaffected individuals
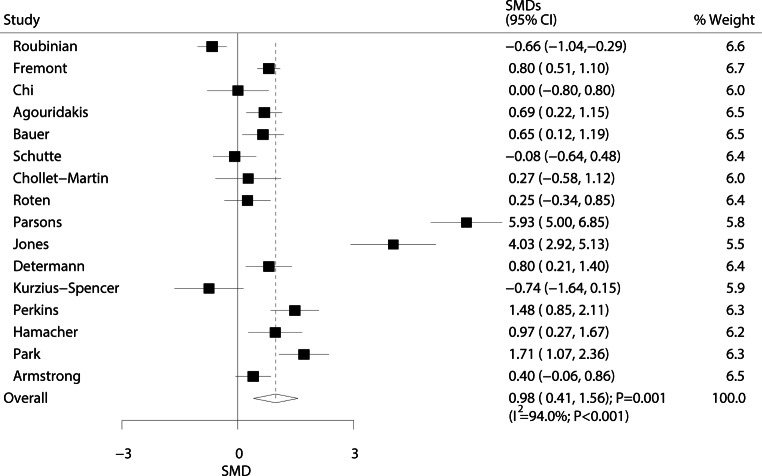
The relationship between ARDS/ALI and tumour necrosis factor (TNF)-α levels was investigated in 16 studies, and results are shown in Fig. 8. Pooled results showed that ARDS/ALI patients had significantly higher TNF‑α levels than those of individuals without ARDS/ALI (SMD: 0.98; 95% CI: 0.41–1.56; P = 0.001). Significant heterogeneity was detected across all included studies (I2 = 94.0%; P < 0.001). These results did not change after sequential exclusion of any specific study (Supplementary information Table S7).
Fig. 8.
Forest plot comparing TNF‑α levels between ARDS/ALI patients and unaffected individuals
The correlations between ARDS/ALI and other inflammatory factors based on sample origin are summarized in Table 2. Overall, ARDS/ALI patients showed higher levels of albumin (SMD: 2.15; P = 0.010), ANG‑1 (SMD: 4.60; P < 0.001), KL‑6 (SMD: 2.23; P = 0.044), myeloperoxidase (MPO) (SMD: 1.75; P < 0.001), transforming growth factor (TGF)-β1 (SMD: 0.83; P = 0.013), transfer factor (TF) (SMD: 5.57; P < 0.001), and TNF receptor‑1 (SMD: 5.40; P < 0.001). Moreover, ARDS/ALI patients had lower levels of IL-12 (SMD: −1.47; P < 0.001), surfactant protein D (SP-D) (SMD: −1.17; P = 0.012), and vascular endothelial growth factor (VEGF) (SMD: −4.52; P < 0.001) in the bronchial alveolar lavage fluid (BALF). In addition, ARDS/ALI patients had higher levels of KL‑6 (SMD: 3.36; P < 0.001), MPO (SMD: 2.58; P < 0.001), procalcitonin (PCT) (SMD: 0.41; P = 0.038), receptor for advanced glycation end products (RAGE) (SMD: 1.64; P = 0.031), sE-selectin (SMD: 0.55; P = 0.011), TF (SMD: 3.55; P < 0.001), and TNF receptor‑2 (SMD: 3.82; P < 0.001) than unaffected individuals. ARDS/ALI was associated with lower IL-12 (SMD: −0.80; P < 0.001) levels in the blood. No other significant differences were observed between ARDS/ALI and non-ARDS/ALI patients.
Table 2.
Summary of results of the association of other inflammatory factors with ARDS/ALI based on specimen source
| Factors | No. of studies | Groups | SMD | 95% CI | P value | Heterogeneity (%) | P for heterogeneity |
|---|---|---|---|---|---|---|---|
| Albumin | 1 | Blood | −0.82 | −1.79 to 0.14 | 0.095 | – | – |
| 2 | BALF | 2.15 | 0.51 to 3.79 | 0.010 | 82.2 | 0.018 | |
| ANG‑1 | 1 | Blood | 0.80 | 0.28 to 2.30 | 0.676 | – | – |
| 1 | BALF | 4.60 | 3.09 to 6.12 | < 0.001 | – | – | |
| CC16 | 3 | Blood | −0.31 | −2.7 to 2.08 | 0.799 | 96.8 | < 0.001 |
| 4 | BALF | −0.44 | −3.06 to 2.18 | 0.742 | 96.1 | < 0.001 | |
| CRP | 3 | Blood | 1.64 | −0.31 to 3.59 | 0.100 | 92.2 | < 0.001 |
| Endotoxin | 2 | BALF | 0.30 | −0.15 to 0.75 | 0.191 | 0.0 | 0.887 |
| G‑CSF | 2 | Blood | −0.49 | −1.47 to 0.49 | 0.326 | 93.9 | < 0.001 |
| ICAM | 4 | Blood | −0.14 | −2.47 to 2.20 | 0.909 | 99.4 | < 0.001 |
| 2 | BALF | 0.79 | −1.61 to 3.18 | 0.520 | 96.3 | < 0.001 | |
| IL‑2 | 2 | Blood | 0.01 | −0.26 to 0.28 | 0.934 | 0.0 | 0.817 |
| 1 | BALF | −0.36 | −1.16 to 0.44 | 0.380 | – | – | |
| IL‑4 | 2 | Blood | 0.69 | −0.16 to 1.54 | 0.111 | 81.1 | 0.022 |
| 1 | BALF | 0.30 | −0.38 to 0.99 | 0.387 | – | – | |
| IL-12 | 1 | Blood | −0.8 | −1.09 to −0.50 | < 0.001 | – | – |
| 1 | BALF | −1.47 | −2.18 to −0.75 | < 0.001 | – | – | |
| KL‑6 | 3 | Blood | 3.36 | 2.50 to 4.21 | < 0.001 | 49.5 | 0.138 |
| 3 | BALF | 2.23 | 0.06 to 4.41 | 0.044 | 93.0 | < 0.001 | |
| LDH | 2 | Blood | 1.82 | −0.23 to 3.87 | 0.082 | 85.9 | < 0.001 |
| MPO | 1 | Blood | 2.58 | 2.20 to 2.97 | < 0.001 | – | – |
| 1 | BALF | 1.75 | 0.90 to 2.60 | < 0.001 | – | – | |
| NF-κβ | 2 | BALF | 0.86 | −0.45 to 2.17 | 0.198 | 67.1 | 0.081 |
| PCT | 2 | Blood | 0.41 | 0.02 to 0.80 | 0.038 | 23.7 | 0.252 |
| Protein C | 2 | Blood | −2.00 | −7.15 to 3.16 | 0.447 | 99.9 | < 0.001 |
| RAGE | 4 | Blood | 1.64 | 0.15 to 3.14 | 0.031 | 96.3 | < 0.001 |
| 1 | BALF | 0.16 | −0.68 to 1.00 | 0.704 | – | – | |
| sE-selectin | 3 | Blood | 0.55 | 0.13 to 0.97 | 0.011 | 15.2 | 0.307 |
| SP‑D | 4 | Blood | −0.05 | −1.65 to 1.55 | 0.950 | 98.5 | < 0.001 |
| 1 | BALF | −1.17 | −2.08 to −0.25 | 0.012 | – | – | |
| TGF-β1 | 4 | BALF | 0.83 | 0.17 to 1.49 | 0.013 | 81.2 | < 0.001 |
| TF | 1 | Blood | 3.55 | 2.71 to 4.39 | < 0.001 | – | – |
| 1 | BALF | 5.57 | 3.55 to 7.60 | < 0.001 | – | – | |
| TNFR‑1 | 2 | Blood | 1.61 | −4.42 to 7.64 | 0.601 | 98.9 | < 0.001 |
| 1 | BALF | 5.40 | 4.22 to 6.58 | < 0.001 | – | – | |
| TNFR‑2 | 1 | Blood | 3.82 | 2.80 to 4.83 | < 0.001 | – | – |
| 2 | BALF | 3.22 | −2.62 to 9.05 | 0.280 | 98.4 | < 0.001 | |
| VEGF | 1 | Blood | 0.49 | −0.23 to 1.04 | 0.063 | – | – |
| 1 | BALF | −4.52 | −5.43 to −3.61 | < 0.001 | – | – | |
| vWF | 6 | Blood | 0.81 | −0.94 to 2.54 | 0.365 | 99.2 | < 0.001 |
ANG‑1 Angiopoietin‑1, CC16 Clara cell secretory protein, CRP C-reactive protein, G‑CSF granulocyte colony-stimulating factor, ICAM intercellular cell adhesion molecule, IL‑2 interleukin‑2, LDH Lactate dehydrogenase, MPO myeloperoxidase, NF-κβ nuclear factor-κβ, PCT procalcitonin, RAGE receptor for advanced glycation end products, SP‑D surfactant protein, TGF-β1 transforming growth factor-β1, TF tissue factor, TNFR‑1 Tumor necrosis factor receptor‑1, TNFR‑2 Tumor necrosis factor receptor‑2, VEGF vascular endothelial growth factor, vWF von Willebrand factor
Meta-regression and subgroup analyses
A relatively large heterogeneity was observed among the studies included in our meta-analysis. We therefore performed a meta-regression analysis for ANG‑2, IL-1β, IL‑6, IL‑8, IL-10, PAI‑1, and TNF‑α; results are presented in Supplementary information Figures S1–S14. Overall, sample size was determined to influence the association between PAI‑1 levels and ARDS/ALI (P = 0.025); no other significant associations were observed. Subgroup analyses were also conducted based on sample size, mean age, patient status, and sample source (Table 3). First, ARDS/ALI did not show a significant influence on ANG‑2 levels when the mean age was < 60.0 years, and patients with ALI. Second, ARDS/ALI was not associated with IL-1β levels if the study sample size was ≥ 100, patients with ALI, or samples were collected from the BALF. Third, no significant associations were detected between ARDS/ALI and IL‑6 levels when the study sample size was ≥ 100, the mean age was < 60.0 years, patients with ALI, and samples were collected from the blood. Fourth, ARDS/ALI were associated with higher IL‑8 levels if the study sample size was < 100, patients had ARDS, or samples were collected from BALF. Fifth, ARDS/ALI were significantly associated with higher IL-10 levels when the study sample size was < 100, patients with ARDS, and samples were collected from the BALF. Sixth, ARDS/ALI patients showed significantly higher PAI‑1 levels when the study sample size was < 100, patients with ARDS or ARDS/ALI, and samples were collected from the BALF. Finally, ARDS/ALI were not associated with TNF‑α levels if the study sample size was ≥ 100, patients with ALI or ARDS/ALI.
Table 3.
Subgroup analysis of inflammatory related factors and incidence of ARDS/ALI
| Inflammatory factors | Groups | No. of studies | SMD | 95%CI | P value | Heterogeneity (%) | P for heterogeneity |
|---|---|---|---|---|---|---|---|
| ANG‑2 | Sample size | ||||||
| ≥ 100 | 5 | 1.30 | 0.47 to 2.13 | 0.002 | 97.8 | < 0.001 | |
| < 100 | 1 | 1.59 | 0.97 to 2.20 | < 0.001 | – | – | |
| Mean age (years) | |||||||
| ≥ 60.0 | 3 | 1.74 | 1.30 to 2.18 | < 0.001 | 51.1 | 0.129 | |
| < 60.0 | 3 | 0.98 | −0.02 to 1.98 | 0.055 | 98.4 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 1 | 2.15 | 1.65 to 2.64 | < 0.001 | – | – | |
| ALI | 3 | 1.38 | −0.19 to 2.93 | 0.085 | 98.3 | < 0.001 | |
| ARDS/ALI | 2 | 0.91 | −0.34 to 2.16 | 0.152 | 93.7 | < 0.001 | |
| Specimen source | |||||||
| Blood | 6 | 1.34 | 0.59 to 2.10 | < 0.001 | 97.4 | < 0.001 | |
| BALF | 0 | – | – | – | – | – | |
| IL-1β | Sample size | ||||||
| ≥ 100 | 2 | −0.33 | −1.05 to 0.40 | 0.379 | 79.7 | 0.026 | |
| < 100 | 8 | 1.26 | 0.48 to 2.04 | 0.002 | 89.9 | < 0.001 | |
| Mean age (years) | |||||||
| ≥ 60.0 | 1 | −0.75 | −1.34 to −0.15 | 0.014 | – | – | |
| < 60.0 | 7 | 1.33 | 0.43 to 2.23 | 0.004 | 94.4 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 6 | 1.39 | 0.41 to 2.37 | 0.005 | 91.8 | < 0.001 | |
| ALI | 4 | 0.22 | −0.59 to 1.04 | 0.590 | 88.0 | < 0.001 | |
| Specimen source | |||||||
| Blood | 5 | 1.39 | 0.15 to 2.62 | 0.028 | 95.3 | < 0.001 | |
| BALF | 6 | 0.73 | −0.19 to 1.66 | 0.121 | 90.2 | < 0.001 | |
| IL‑6 | Sample size | ||||||
| ≥ 100 | 5 | 0.45 | −0.26 to 1.16 | 0.215 | 96.1 | < 0.001 | |
| < 100 | 8 | 0.83 | 0.11 to 1.55 | 0.024 | 91.6 | < 0.001 | |
| Mean age (years) | |||||||
| ≥ 60.0 | 3 | 1.49 | 0.37 to 2.61 | 0.009 | 92.1 | < 0.001 | |
| < 60.0 | 10 | 0.43 | −0.06 to 0.92 | 0.088 | 93.1 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 7 | 0.80 | 0.03 to 1.57 | 0.043 | 92.6 | < 0.001 | |
| ALI | 6 | 0.54 | −0.12 to 1.20 | 0.108 | 95.3 | < 0.001 | |
| Specimen source | |||||||
| Blood | 11 | 0.37 | −0.04 to 0.77 | 0.074 | 88.8 | < 0.001 | |
| BALF | 7 | 1.33 | 0.24 to 2.42 | 0.016 | 93.2 | < 0.001 | |
| IL‑8 | Sample size | ||||||
| ≥ 100 | 4 | 0.06 | −1.84 to 1.95 | 0.953 | 99.2 | < 0.001 | |
| < 100 | 10 | 0.74 | 0.15 to 1.33 | 0.014 | 87.7 | < 0.001 | |
| Mean age (years) | |||||||
| ≥ 60.0 | 2 | 2.87 | −1.95 to 7.70 | 0.243 | 96.6 | < 0.001 | |
| < 60.0 | 9 | 0.52 | −0.61 to 1.65 | 0.364 | 98.5 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 6 | 1.28 | 0.34 to 2.21 | 0.007 | 90.4 | < 0.001 | |
| ALI | 7 | 0.13 | −1.13 to 1.40 | 0.834 | 98.6 | < 0.001 | |
| ARDS/ALI | 1 | −0.37 | −0.93 to 0.20 | 0.201 | – | – | |
| Specimen source | |||||||
| Blood | 9 | 0.67 | −0.50 to 1.85 | 0.262 | 98.3 | < 0.001 | |
| BALF | 8 | 0.78 | 0.08 to 1.48 | 0.029 | 86.1 | < 0.001 | |
| IL-10 | Sample size | ||||||
| ≥ 100 | 2 | −0.90 | −7.01 to 5.21 | 0.772 | 99.6 | < 0.001 | |
| < 100 | 5 | 1.89 | 0.59 to 3.19 | 0.004 | 93.4 | < 0.001 | |
| Mean age (years) | |||||||
| ≥ 60.0 | 0 | – | – | – | – | – | |
| < 60.0 | 6 | 1.29 | −0.75 to 3.32 | 0.216 | 98.6 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 3 | 1.62 | 0.05 to 3.19 | 0.043 | 91.7 | < 0.001 | |
| ALI | 4 | 0.73 | −2.15 to 3.61 | 0.618 | 99.0 | < 0.001 | |
| Specimen source | |||||||
| Blood | 4 | 0.18 | −2.64 to 3.00 | 0.899 | 99.0 | < 0.001 | |
| BALF | 4 | 1.90 | 0.12 to 3.68 | 0.037 | 94.3 | < 0.001 | |
| PAI‑1 | Sample size | ||||||
| ≥ 100 | 3 | 0.42 | −0.78 to 1.61 | 0.497 | 98.8 | < 0.001 | |
| < 100 | 4 | 0.89 | 0.52 to 1.27 | < 0.001 | 42.1 | 0.159 | |
| Mean age (years) | |||||||
| ≥ 60.0 | 1 | 0.31 | −0.35 to 0.96 | 0.363 | – | – | |
| < 60.0 | 6 | 0.77 | −0.04 to 1.57 | 0.063 | 97.6 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 2 | 0.68 | 0.06 to 1.29 | 0.030 | 61.1 | 0.109 | |
| ALI | 4 | 0.77 | −0.54 to 2.08 | 0.250 | 98.1 | < 0.001 | |
| ARDS/ALI | 1 | 0.66 | 0.45 to 0.87 | < 0.001 | – | – | |
| Specimen source | |||||||
| Blood | 6 | 0.44 | −0.34 to 1.22 | 0.267 | 97.2 | < 0.001 | |
| BALF | 3 | 1.57 | 0.77 to 2.36 | < 0.001 | 69.6 | 0.037 | |
| TNF‑α | Sample size | ||||||
| ≥ 100 | 4 | 1.66 | −0.10 to 3.43 | 0.065 | 98.3 | < 0.001 | |
| < 100 | 12 | 0.76 | 0.27 to 1.24 | 0.002 | 85.5 | < 0.001 | |
| Mean age (years) | |||||||
| ≥ 60.0 | 3 | 0.51 | 0.17 to 0.84 | 0.003 | 0.0 | 0.482 | |
| < 60.0 | 10 | 1.36 | 0.52 to 2.20 | 0.002 | 96.0 | < 0.001 | |
| Patients’ status | |||||||
| ARDS | 9 | 1.05 | 0.11 to 1.99 | 0.028 | 94.7 | < 0.001 | |
| ALI | 5 | 0.90 | −0.14 to 1.95 | 0.090 | 95.1 | < 0.001 | |
| ARDS/ALI | 2 | 0.92 | −0.14 to 1.97 | 0.089 | 86.5 | 0.007 | |
| Specimen source | |||||||
| Blood | 9 | 1.00 | 0.12 to 1.89 | 0.026 | 95.7 | < 0.001 | |
| BALF | 10 | 0.91 | 0.26 to 1.57 | 0.006 | 87.6 | < 0.001 | |
Publication bias
Funnel plots of inflammatory factors and ARDS/ALI incidence are presented in Supplementary information Figures S15–S21. No significant publication biases were detected between ARDS/ALI and IL-1β (P-value for Egger’s test (PEgger): 0.148; P-value for Begg’s test (PBegg): 0.283), IL‑6 (PEgger: 0.330; PBegg: 0.161), IL-10 (PEgger: 0.874; PEgger: 1.000), PAI‑1 (PEgger: 0.184; PBegg: 0.548), and TNF‑α (PEgger: 0.111; PBegg: 0.224). Although results of the Begg’s tests showed no evidence of publication bias for ANG‑2 (P = 0.707) and IL‑8 (P = 0.827), results of Egger’s test showed potential publication bias (P-value for ANG-2: 0.048; P-value for IL-8: 0.013). Conclusions did not change after correction using the trim and fill method [83].
Discussion
In our study, ARDS/ALI were found to be associated with higher levels of ANG‑2, IL-1β, IL‑6, and TNF‑α, whereas no significant associations were detected between ARDS/ALI and IL‑8, IL-10, and PAI‑1 levels. Furthermore, serum levels of KL‑6, MPO, RAGE, sE-selectin, TF, and TNF receptor‑2 were significantly higher in ARDS/ALI patients than in unaffected individuals; however, ARDS/ALI patients had lower IL-12 levels. The BALF concentrations of albumin, ANG‑1, KL‑6, MPO, TGF-β1, TF, and TNF receptor‑1, were significantly higher in ARDS/ALI patients than in individuals without ARDS/ALI. In addition, ARDS/ALI were associated with lower levels of IL-12 and VEGF; however, heterogeneity among studies was substantial, and the amount of data available was insufficient. Therefore, more research is needed to verify the results of our meta-analysis.
Current treatment for ARDS/ALI consists of respiratory support and immunological treatment. Evidence suggests that the dynamic balance between proinflammatory and anti-inflammatory factors plays a key role in the pathogenesis and prognosis of ARDS/ALI [84]; however, cytokine interactions are highly complex and difficult to study. When proinflammatory and anti-inflammatory factors are unbalanced, excess inflammatory cytokines are released, which in turn damage the lung tissues or even whole body tissues. Therefore, studies that investigate inflammatory factors present during the onset of ARDS/ALI can help elucidate the mechanisms underlying ARDS/ALI pathogenesis and serve as the basis for the development of new treatment approaches for ARDS/ALI.
ANG‑2 is a proinflammatory cytokine and a member of the vascular growth factor family. ANG‑2 mainly promotes cell apoptosis and disrupts vascularization and can also act in conjunction with VEGF to promote neovascularization [85, 86]. The findings of the present study indicated that serum levels of ANG‑2 were significantly higher in ARDS/ALI patients than in unaffected individuals. Similar to our current findings, serum levels of ANG‑2 have been associated with other diseases, such as sepsis and pulmonary hypertension [87]. In particular, ANG‑2 serum levels were associated with the onset of septic shock, and ANG‑2 blood concentrations have been observed to increase during endothelial cell inflammation. Furthermore, elevated ANG‑2 levels in the blood are known to promote vascular permeability and leakage; however, only a small number of studies have explored the relationship between ANG‑2 and ARDS/ALI incidence, and the overall results might have been altered by more recent findings from subsequent studies.
Our current results demonstrated that ARDS/ALI patients had significantly higher serum levels of IL-1β than individuals without ARDS/ALI. IL-1β is synthesized and released by mononuclear macrophages. It is recognized as the primary proinflammatory cytokine that triggers inflammation and is known to exert multiple biological functions, such as promoting the activity of natural killer cells, increasing chemotaxis of macrophages and neutrophils, and regulating the immune response as an endogenous heat source [88, 89]. During infection or sepsis, IL-1β can destroy the blood-brain barrier and increase the risk of patient mortality. IL-1β has an inherent antagonist in the human body, IL-1ra, which can inhibit IL-1β activity by competitively binding to its receptor. One study included in this analysis showed that IL-1ra is significantly upregulated in ARDS patients [41]. Anakinra is an IL-1β antagonist approved by the U.S. Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis and other autoimmune diseases to reduce clinical symptoms and suppress joint destruction [90]. Determining whether this antagonistic effect can also be observed in ARDS patients is an interesting topic for future research.
IL‑6 is an acute inflammatory mediator that is released by various cell types. IL‑6 is not expressed under normal physiological conditions but is secreted upon stimulation by inflammatory factors [91]. Our study showed that IL‑6 concentrations in the BALF were significantly higher in ARDS/ALI patients than in unaffected individuals. Consistent with these results, IL‑6 is recognized as a reliable and objective indicator of local lung tissue damage.
IL‑8 is a member of the C-X-C subfamily of chemokines and is produced by various cell types [92]. IL‑8 plays a significant role in neutrophil chemotaxis and also inhibits neutrophil apoptosis. In response to local lung tissue injury, IL‑8 specifically binds to its receptor, which in turn induces neutrophil aggregation and triggers the release of proteolytic enzymes that mediate inflammation and severe tissue damage. Our findings suggested that IL‑8 concentrations are associated with ARDS/ALI incidence.
IL-10 is an anti-inflammatory cytokine that inhibits the secretion of TNF‑α, IL‑1, and IL‑6 [93]. IL-10 can also suppress NF-κβ activity and regulate the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway. Our analysis suggested that IL-10 levels are correlated with ARDS/ALI incidence; however, the elevated IL-10 levels could have been caused by inflammation in the lungs.
PAI‑1 is mainly secreted by vascular endothelial cells, and its production is a risk factor for thrombosis and atherosclerosis [94]. During ARDS pathogenesis, the coagulation fibrinolysis system is impaired, leading to disseminated intravascular coagulation. Increased PAI‑1 levels may lead to local fibrin deposition in lungs. Our results support the idea that PAI‑1 plays an important role in the development of ARDS. In addition, PAI‑1 has been demonstrated to promote local formation of diseased connective tissue and has been used as an indicator of prognosis of ARDS patients.
The results of our study suggested a strong correlation between TNF‑α concentrations in the BALF and ARDS incidence. TNF‑α is considered as one of the most important proinflammatory factors in ARDS/ALI [95]. TNF‑α is a multifunctional proinflammatory factor that stimulates the secretion of endothelin and nitric oxide by endothelial cells, promotes the expression of adhesion molecules by endothelial cells and leukocytes, and contributes to the progression of severe microcirculatory disorder. Therefore, TNF‑α inhibition can potentially serve as an important approach for ARDS prevention and treatment.
The general objective of this study was to identify inflammatory factors that can serve as drug targets to reduce the incidence of ARDS/ALI. Multiple lines of evidence have suggested that proinflammatory cytokines participate in or trigger the inflammatory response in the lungs; however, currently available clinical data are insufficient to verify the correlations between the levels of proinflammatory factors and ARDS/ALI incidence.
Our current meta-analysis has several limitations First, results were based on other studies but not at the individual level. Second, the included studies showed significant heterogeneity, making it difficult to eliminate alternative explanations for the results, such as differences in the definition of ARDS/ALI, severity of disease, underlying diseases, sample collection times, and treatment strategies. Third, unpublished articles and articles written in other languages were not searched, which could have skewed the obtained results.
Conclusion
The results of our study indicated that ARDS/ALI are associated with elevated levels of ANG‑2, IL-1β, IL‑6, and TNF‑α, but do not significantly affect IL‑8, IL-10, and PAI‑1 levels. Furthermore, ARDS/ALI incidence was also determined to be significantly associated with several other inflammatory factors; however, further studies using large sample sizes are required to verify our conclusions. Future studies should also measure the levels of inflammatory factors over time. Log transformation of the measures of inflammatory factors is recommended to obtain a normally distributed data, especially in studies with small sample sizes.
Supplementary Information
Supplementary information Figures S1–S21. Meta-regression and publication bias of inflammatory biomarkers with acute respiratory distress syndrome or acute lung injury
Acknowledgments
Acknowledgements
This work was supported by grant of a special fund project of science and technology cooperation of Guizhou province (Provinces division [2015] 39) and the health of the family planning commission of Guizhou province (Gzwjki2016-1-019).
Funding
This publication was supported by grant of a special fund project of science and technology cooperation of Guizhou province (Provinces division [2015] 39) and the health of the family planning commission of Guizhou province (Gzwjki2016-1-019).
Abbreviations
- ALI
Acute lung injury
- ANG
Angiopoietin
- ARDS
Acute respiratory distress syndrome
- IL
Interleukin
- KL
Krebs von den Lungen
- LDH
Lactate dehydrogenase
- PAI
Plasminogen activator inhibitor
- RAGE
Receptor for advanced glycation end products
- SMD
Standard mean difference
- TNF
Tumor necrosis factor
- vWF
von Willebrand factor
Author Contribution
Daishun Liu conceived and designed the experiments. Zhenfeng Liu, Guoqi Zhou, Yugang Zou, Haixia Wang, Xiao Li and DeliangZheng performed the experiments. Zhenfeng Liu analyzed the data and wrote the paper. Daishun Liu contributed reagents/materials/analysis tools. All authors have read and approved the final version of this manuscript.
Declarations
Conflict of interest
Z. Liu, D. Liu, Z. Wang, Y. Zou, H. Wang, X. Li, D. Zheng and G. Zhou declare that they have no competing interests.
Ethical standards
For this article no studies with human participants or animals were performed by any of the authors. All studies cited were in accordance with the ethical standards indicated in each case. No ethical approval was required. Consent for publication: not applicable.
Footnotes
Availability of data and material
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011;27:719–733. doi: 10.1016/j.ccc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 4.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morisawa K, Fujitani S, Taira Y, Kushimoto S, Kitazawa Y, Okuchi K. Difference in pulmonary permeability between indirect and direct acute respiratory distress syndrome assessed by the transpulmonary thermodilution technique: a prospective, observational, multi-institutional study. J Intensive Care. 2014;2:24. doi: 10.1186/2052-0492-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan SM. Postperfusion lung syndrome: respiratory mechanics, respiratory indices and biomarkers. Ann Thorac Med. 2015;10:151–157. doi: 10.4103/1817-1737.150736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreoli SP, McAteer JA. Reactive oxygen molecule-mediated injury in endothelial and renal tubular epithelial cells in vitro. Kidney Int. 1990;38:785–794. doi: 10.1038/ki.1990.272. [DOI] [PubMed] [Google Scholar]
- 8.Rockx B, Baas T, Zornetzer GA, Haagmans B, Sheahan T, Frieman M. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groeneveld AB. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascul Pharmacol. 2002;39:247–256. doi: 10.1016/s1537-1891(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 10.Bhargava M, Wendt CH. Biomarkers in acute lung injury. Transl Res. 2012;159:205–217. doi: 10.1016/j.trsl.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld AB. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2014;42:691–700. doi: 10.1097/01.ccm.0000435669.60811.24. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 15.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25:646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 16.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. London: The Cochrane Collaboration; 2008. [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 20.Moalli R, Doyle JM, Tahhan HR, Hasan FM, Braman SS, Saldeen T. Fibrinolysis in critically ill patients. Am Rev Respir Dis. 1989;140:287–293. doi: 10.1164/ajrccm/140.2.287. [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roten R, Markert M, Feihl F, Schaller MD, Tagan MC, Perret C. Plasma levels of tumor necrosis factor in the adult respiratory distress syndrome. Am Rev Respir Dis. 1991;143:590–592. doi: 10.1164/ajrccm/143.3.590. [DOI] [PubMed] [Google Scholar]
- 23.Parsons PE, Moore FA, Moore EE, Ikle DN, Henson PM, Worthen GS. Studies on the role of tumor necrosis factor in adult respiratory distress syndrome. Am Rev Respir Dis. 1992;146:694–700. doi: 10.1164/ajrccm/146.3.694. [DOI] [PubMed] [Google Scholar]
- 24.Leff JA, Parsons PE, Day CE, Taniguchi N, Jochum M, Fritz H. Serum antioxidants as predictors of adult respiratory distress syndrome in patients with sepsis. Lancet. 1993;341:777–780. doi: 10.1016/0140-6736(93)90558-x. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly SC, Haslett C, Dransfield I, Robertson CE, Carter DC, Ross JA. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994;344:215–219. doi: 10.1016/s0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- 26.Jorens PG, Sibille Y, Goulding NJ, van Overveld FJ, Herman AG, Bossaert L. Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. Eur Respir J. 1995;8:1647–1653. doi: 10.1183/09031936.95.08101647. [DOI] [PubMed] [Google Scholar]
- 27.Sakamaki F, Ishizaka A, Handa M, Fujishima S, Urano T, Sayama K. Soluble form of P-selectin in plasma is elevated in acute lung injury. Am J Respir Crit Care Med. 1995;151:1821–1826. doi: 10.1164/ajrccm.151.6.7539327. [DOI] [PubMed] [Google Scholar]
- 28.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996;154:594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs-Buder T, de Moerloose P, Ricou B, Reber G, Vifian C, Nicod L. Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:163–167. doi: 10.1164/ajrccm.153.1.8542111. [DOI] [PubMed] [Google Scholar]
- 30.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 31.Schütte H, Lohmeyer J, Rosseau S, Ziegler S, Siebert C, Kielisch H. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J. 1996;9:1858–1867. doi: 10.1183/09031936.96.09091858. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med. 1996;24:1285–1292. doi: 10.1097/00003246-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Parsons PE, Moss M, Vannice JL, Moore EE, Moore FA, Repine JE. Circulating IL-1ra and IL-10 levels are increased but do not predict the development of acute respiratory distress syndrome in at-risk patients. Am J Respir Crit Care Med. 1997;155:1469–1473. doi: 10.1164/ajrccm.155.4.9105096. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly SC, Bucala R, Metz CN, Grant IS, Robertson CR, Haslett C. Macrophage migration inhibitory factor and acute lung injury. Chest. 1999;116:111S. doi: 10.1378/chest.116.suppl_1.111s. [DOI] [PubMed] [Google Scholar]
- 35.Gando S, Nanzaki S, Morimoto Y, Kobayashi S, Kemmotsu O. Systemic activation of tissue-factor dependent coagulation pathway in evolving acute respiratory distress syndrome in patients with trauma and sepsis. J Trauma. 1999;47:719–723. doi: 10.1097/00005373-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong L, Thickett DR, Christie SJ, Kendall H, Millar AB. Increased expression of functionally active membrane-associated tumor necrosis factor in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2000;22:68–74. doi: 10.1165/ajrcmb.22.1.3728. [DOI] [PubMed] [Google Scholar]
- 37.Bauer TT, Monton C, Torres A, Cabello H, Fillela X, Maldonado A. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax. 2000;55:46–52. doi: 10.1136/thorax.55.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siemiatkowski A, Kloczko J, Galar M, Czaban S. von Willebrand factor antigen as a prognostic marker in posttraumatic acute lung injury. Haemostasis. 2000;30:189–195. doi: 10.1159/000054134. [DOI] [PubMed] [Google Scholar]
- 39.Geerts L, Jorens PG, Willems J, De Ley M, Slegers H. Natural inhibitors of neutrophil function in acute respiratory distress syndrome. Crit Care Med. 2001;29:1920–1924. doi: 10.1097/00003246-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Hirani N, Antonicelli F, Strieter RM, Wiesener MS, Ratcliffe PJ, Haslett C. The regulation of interleukin-8 by hypoxia in human macrophages—a potential role in the pathogenesis of the acute respiratory distress syndrome (ARDS) Mol Med. 2001;7:685–697. [PMC free article] [PubMed] [Google Scholar]
- 41.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, 2nd, Park DR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 42.Agouridakis P, Kyriakou D, Alexandrakis MG, Perisinakis K, Karkavitsas N, Bouros D. Association between increased levels of IL-2 and IL-15 and outcome in patients with early acute respiratory distress syndrome. Eur J Clin Invest. 2002;32:862–867. doi: 10.1046/j.1365-2362.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- 43.Agouridakis P, Kyriakou D, Alexandrakis MG, Prekates A, Perisinakis K, Karkavitsas N. The predictive role of serum and bronchoalveolar lavage cytokines and adhesion molecules for acute respiratory distress syndrome development and outcome. Respir Res. 2002;3:25. doi: 10.1186/rr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Endo S, Sato N, Nakae H, Yamada Y, Makabe H, Abe H. Surfactant protein A and D (SP-A, AP-D) levels in patients with septic ARDS. Res Commun Mol Pathol Pharmacol. 2002;111:245–251. [PubMed] [Google Scholar]
- 45.Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A. Tumor necrosis factor-alpha and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:651–656. doi: 10.1164/rccm.2109004. [DOI] [PubMed] [Google Scholar]
- 46.Takala A, Jousela I, Takkunen O, Kautiainen H, Jansson SE, Orpana A. A prospective study of inflammation markers in patients at risk of indirect acute lung injury. Shock. 2002;17:252–257. doi: 10.1097/00024382-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Thickett DR, Armstrong L, Millar AB. A role for vascular endothelial growth factor in acute and resolving lung injury. Am J Respir Crit Care Med. 2002;166:1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 48.Gessner C, Hammerschmidt S, Kuhn H, Lange T, Engelmann L, Schauer J. Exhaled breath condensate nitrite and its relation to tidal volume in acute lung injury. Chest. 2003;124:1046–1052. doi: 10.1378/chest.124.3.1046. [DOI] [PubMed] [Google Scholar]
- 49.Grissom CK, Orme JF, Jr, Richer LD, McIntyre TM, Zimmerman GA, Elstad MR. Platelet-activating factor acetylhydrolase is increased in lung lavage fluid from patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:770–775. doi: 10.1097/01.CCM.0000053647.82608.29. [DOI] [PubMed] [Google Scholar]
- 50.Nys M, Deby-Dupont G, Habraken Y, Legrand-Poels S, Kohnen S, Ledoux D. Bronchoalveolar lavage fluids of ventilated patients with acute lung injury activate NF-kappaB in alveolar epithelial cell line: role of reactive oxygen/nitrogen species and cytokines. Nitric Oxide. 2003;9:33–43. doi: 10.1016/j.niox.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 52.Bouros D, Alexandrakis MG, Antoniou KM, Agouridakis P, Pneumatikos I, Anevlavis S. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for acute respiratory distress syndrome. BMC Pulm Med. 2004;4:6. doi: 10.1186/1471-2466-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 54.Sato H, Callister ME, Mumby S, Quinlan GJ, Welsh KI, duBois RM. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J. 2004;23:142–145. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- 55.Parsons PE, Matthay MA, Ware LB, Eisner MD, National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 56.El Solh AA, Bhora M, Pineda L, Aquilina A, Abbetessa L, Berbary E. Alveolar plasminogen activator inhibitor-1 predicts ARDS in aspiration pneumonitis. Intensive Care Med. 2006;32:110–115. doi: 10.1007/s00134-005-2847-2. [DOI] [PubMed] [Google Scholar]
- 57.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax. 2007;62:36–42. doi: 10.1136/thx.2006.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29:656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 62.Kurzius-Spencer M, Foster K, Littau S, Richey KJ, Clark BM, Sherrill D. Tracheobronchial markers of lung injury in smoke inhalation victims. J Burn Care Res. 2008;29:311–318. doi: 10.1097/BCR.0b013e3181667991. [DOI] [PubMed] [Google Scholar]
- 63.Nathani N, Perkins GD, Tunnicliffe W, Murphy N, Manji M, Thickett DR. Kerbs von Lungren 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care. 2008;12:R12. doi: 10.1186/cc6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63:903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 65.Calfee CS, Eisner MD, Parsons PE, Thompson BT, Conner ER, Jr, Matthay MA. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009;35:248–257. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chi XJ, Cai J, Luo CF, Cheng N, Hei ZQ, Li SR. Relationship between the expression of Toll-like receptor 2 and 4 in mononuclear cells and postoperative acute lung injury in orthotopic liver transplantation. Chin Med J (Engl) 2009;122:895–899. [PubMed] [Google Scholar]
- 67.Determann RM, Millo JL, Waddy S, Lutter R, Garrard CS, Schultz MJ. Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study. BMC Pulm Med. 2009;9:49. doi: 10.1186/1471-2466-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135:1440–1447. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Determann RM, Royakkers AA, Haitsma JJ, Zhang H, Slutsky AS, Ranieri VM. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med. 2010;10:6. doi: 10.1186/1471-2466-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68:1121–1127. doi: 10.1097/TA.0b013e3181c40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aman J, van der Heijden M, van Lingen A, Girbes AR, van Nieuw Amerongen GP, van Hinsbergh VW. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:89–97. doi: 10.1097/CCM.0b013e3181feb46a. [DOI] [PubMed] [Google Scholar]
- 73.Guervilly C, Lacroix R, Forel JM, Roch A, Camoin-Jau L, Papazian L. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit Care. 2011;15:R31. doi: 10.1186/cc9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jabaudon M, Futier E, Roszyk L, Chalus E, Guerin R, Petit A. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39:480–488. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 75.Kohno T, Anzai T, Shimizu H, Kaneko H, Sugano Y, Yamada S. Impact of serum high-mobility group box 1 protein elevation on oxygenation impairment after thoracic aortic aneurysm repair. Heart Vessels. 2011;26:306–312. doi: 10.1007/s00380-010-0056-6. [DOI] [PubMed] [Google Scholar]
- 76.Osaka D, Shibata Y, Kanouchi K, Nishiwaki M, Kimura T, Kishi H. Soluble endothelial selectin in acute lung injury complicated by severe pneumonia. Int J Med Sci. 2011;8:302–308. doi: 10.7150/ijms.8.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quesnel C, Piednoir P, Gelly J, Nardelli L, Garnier M, Lecon V. Alveolar fibrocyte percentage is an independent predictor of poor outcome in patients with acute lung injury. Crit Care Med. 2012;40:21–28. doi: 10.1097/CCM.0b013e31822d718b. [DOI] [PubMed] [Google Scholar]
- 78.Schultz MJ, Determann RM, Royakkers AA, Wolthuis EK, Korevaar JC, Levi MM. Bronchoalveolar activation of coagulation and inhibition of fibrinolysis during ventilator-associated lung injury. Crit Care Res Pract. 2012;2012:961784. doi: 10.1155/2012/961784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187:736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones SW, Zhou H, Ortiz-Pujols SM, Maile R, Herbst M, Joyner BL., Jr Bronchoscopy-derived correlates of lung injury following inhalational injuries: a prospective observational study. PLoS ONE. 2013;8:e64250. doi: 10.1371/journal.pone.0064250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoeboer SH, Groeneveld AB, van der Heijden M, Oudemans-van Straaten HM. Serial inflammatory biomarkers of the severity, course and outcome of late onset acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new-onset fever. Biomark Med. 2015;9:605–616. doi: 10.2217/bmm.15.15. [DOI] [PubMed] [Google Scholar]
- 82.Roubinian NH, Looney MR, Kor DJ, Lowell CA, Gajic O, Hubmayr RD. Cytokines and clinical predictors in distinguishing pulmonary transfusion reactions. Transfusion. 2015;55:1838–1846. doi: 10.1111/trf.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 84.Meduri GU, Yates CR. Systemic inflammation-associated glucocorticoid resistance and outcome of ARDS. Ann N Y Acad Sci. 2004;1024:24–53. doi: 10.1196/annals.1321.004. [DOI] [PubMed] [Google Scholar]
- 85.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA, Network NA. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med. 2012;40:1731–1737. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhong M, Zhang L, Wang F, Peng S, Zhang J, Xuan G. The levels of angiopoietin-2 in patients with acute respiratory distress syndrome and its value on prognosis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:804–809. doi: 10.3760/cma.j.issn.2095-4352.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Stiehl T, Thamm K, Kaufmann J, Schaeper U, Kirsch T, Haller H. Lung-targeted RNA interference against angiopoietin-2 ameliorates multiple organ dysfunction and death in sepsis. Crit Care Med. 2014;42:e654–e662. doi: 10.1097/CCM.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 88.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 89.Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 90.Baskar S, Klein AL, Zeft A. The use of IL-1 receptor antagonist (anakinra) in idiopathic recurrent pericarditis: a narrative review. Cardiol Res Pract. 2016;2016:7840724. doi: 10.1155/2016/7840724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galani V, Tatsaki E, Bai M, Kitsoulis P, Lekka M, Nakos G. The role of apoptosis in the pathophysiology of acute respiratory distress syndrome (ARDS): an up-to-date cell-specific review. Pathol Res Pract. 2010;206:145–150. doi: 10.1016/j.prp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Gu Y, Hu X, Liu C, Qv X, Xu C. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br J Haematol. 2008;142:109–114. doi: 10.1111/j.1365-2141.2008.07161.x. [DOI] [PubMed] [Google Scholar]
- 93.Strle K, McCusker RH, Tran L, King A, Johnson RW, Freund GG. Novel activity of an anti-inflammatory cytokine: IL-10 prevents TNFalpha-induced resistance to IGF-I in myoblasts. J Neuroimmunol. 2007;188:48–55. doi: 10.1016/j.jneuroim.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daniel AE, Timmerman I, Kovacevic I, Hordijk PL, Adriaanse L, Paatero I. Plasminogen activator inhibitor-1 controls vascular integrity by regulating VE-cadherin trafficking. PLoS ONE. 2015;10:e0145684. doi: 10.1371/journal.pone.0145684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azevedo ZM, Moore DB, Lima FC, Cardoso CC, Bougleux R, Matos GI. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) single nucleotide polymorphisms: importance in ARDS in septic pediatric critically ill patients. Hum Immunol. 2012;73:661–667. doi: 10.1016/j.humimm.2012.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information Figures S1–S21. Meta-regression and publication bias of inflammatory biomarkers with acute respiratory distress syndrome or acute lung injury