Abstract
Ex-vivo lung perfusion (EVLP) is a novel lung preservation strategy that facilitates use of marginal allografts; however, it is more expensive than static cold storage (SCS). To understand how preservation method might affect postoperative costs, we compared outcomes and index hospitalization costs among matched EVLP and SCS preserved lung transplant (LTx) recipients at a single, high-volume institution. A total of 22 EVLP and 66 matched SCS LTx recipients were included; SCS grafts were further stratified as either standard-criteria (SCD) or extended-criteria donors (ECD). Median total preservation time was 857, 409, and 438 minutes for EVLP, SCD, and ECD lungs, respectively (p<0.0001). EVLP patients had similar perioperative outcomes and post-transplant survival compared to SCS SCD and ECD recipients. Excluding device-specific costs, total direct variable costs were similar among EVLP, SCD, and ECD recipients (median $200,404, vs $154,709 vs $168,334, p=0.11). The median direct contribution margin was positive for EVLP recipients, and similar to that for SCD and ECD graft recipients (all p>0.99). These findings demonstrate that use of EVLP was profitable at an institutional level; however, further investigation is needed to better understand the financial implications of EVLP in facilitating donor pool expansion in an era of broader lung sharing.
INTRODUCTION
Ex-vivo lung perfusion (EVLP) is a novel lung preservation strategy with numerous potential advantages over static cold storage (SCS). Most notably, EVLP supports broader use of allografts from extended-criteria donors (ECD),1–5 and extension of lung preservation time6,7 with comparable clinical outcomes.8,9 In recent years, EVLP has been used for only 5–7% of US lung transplants (LTx) annually,10,11 primarily in the context of clinical trials. As of 2019, however, at least two EVLP platforms have received US Food and Drug Administration pre-market approval for commercial distribution.12,13 It is therefore anticipated that EVLP will be utilized more frequently in the coming years.
Although EVLP has garnered considerable enthusiasm, the financial impact this expensive technology will have on individual transplant centers and organ procurement organizations (OPOs) remains unknown. Data from two international reports suggests that the net cost of EVLP is approximately £14,500 (~$20,000)14 to $61,70315 per patient transplanted. In addition to device hardware, these costs include disposable supplies, perfusate solutions, drugs, and human blood products in select devices. The potential financial advantages to EVLP include reduced lung discard rates,16 and consequently increased LTx volume.17 Given the encouraging early outcomes in recipients of EVLP lungs, some have hypothesized that these benefits may outweigh the upfront perfusion costs, and thus increase utilization of this promising technology.18
Cost is likely to play a pivotal role in wider adoption of EVLP, and dictate the utilization logistics coordinated between OPOs and transplant centers. Moreover, providers will face challenges when broadening adoption of EVLP technologies if the use of rehabilitated lungs leads to negative contribution margins for health systems. Accordingly, we sought to determine if upfront costs associated with use of EVLP might be offset by decreased costs of postoperative patient care. To test this hypothesis, we compared perioperative outcomes and index hospitalization costs among patients who received EVLP and non-EVLP lung allografts at a single high-volume US LTx center.
METHODS
Study population and design
We conducted a single-center retrospective cohort study using institutional and United Network for Organ Sharing (UNOS) data. Patients who underwent primary or redo isolated LTx at Duke University Hospital with donor lungs that underwent EVLP were eligible for inclusion. Patients for whom cost data was unavailable were excluded. A group of patients who underwent bilateral isolated LTx with donor lungs that did not undergo EVLP was matched 1:3 (nearest neighbor) based on age at LTx, disease group, lung allocation score (LAS), and history of prior LTx. Non-EVLP LTx recipients were further stratified as having received grafts from standard-criteria (SCD) or extended-criteria donors (ECD); ECDs were defined as those with age ≥55 years, PaO2/FiO2 (P/F) ratio ≤300, or donation after circulatory death (DCD) status. This study was approved by our Institutional Review Board (Pro00103325).
Recipient, operative, donor characteristics, and post-transplant outcomes were compared between EVLP and non-EVLP groups. Perioperative outcomes of interest included 30-day reintervention (surgical, bronchoscopic, or radiologic), 30-day hospital or intensive care unit (ICU) readmission, post-transplant ICU and hospital length of stay (LOS), grade 3 primary graft dysfunction (PGD3) at 72 hours post-transplant, need for postoperative extracorporeal membrane oxygenation (ECMO), postoperative date of extubation, tracheostomy within 7 days, reintubation or renal replacement therapy during the index hospitalization, 30-day biopsy-proven acute rejection, and 90-day mortality. Survival to death or graft failure, first biopsy-proven acute rejection episode, and onset of chronic lung allograft dysfunction (CLAD) were also explored. Bronchoscopic reintervention was defined by need for an intervention such as bronchial dilation or stenting beyond routine post-transplant surveillance. PGD3 and CLAD were defined according to International Society for Heart and Lung Transplantation guidelines.19,20
Cost analysis
All costs associated with the index hospitalization were provided by the Duke University Hospital Office of Finance for individual patient accounts. Total index hospitalization costs, and fixed and variable cost contributions were compared among EVLP, SCS-SCD, and SCS-ECD groups. To minimize the effect of outliers, costs were summarized using medians and interquartile ranges rather than means and standard deviations. Fixed costs were defined as those pertaining to hospital admission, building maintenance, utilities, equipment, employee salary, and overhead. Variable costs were defined as those incurred for individualized hospital services, supplies, diagnostics, and medications, and included direct variable costs (DVCs) related to intermediate care, intensive care, pharmacy, cardiology, surgery, respiratory care, physical/occupational/speech therapy, radiology, laboratory services, medical supplies, emergency room services, clinic services, blood bank, and other diagnostic testing. In addition to total index hospitalization costs, fixed costs, and variable costs, categorized DVCs related to the aforementioned services were compared between groups. Detailed financial definitions are provided in the Supplementary Materials and Methods.
Statistical analysis
Recipient, operative, and donor characteristics, perioperative outcomes, and index hospitalization costs were compared among EVLP, SCD, and ECD groups using nonparametric Kruskal-Wallis with Dunn’s multiple comparison tests for continuous variables and Chi-squared and Fisher’s exact tests for categorical variables. Unadjusted survival was estimated using the Kaplan-Meier method and compared among groups using log-rank tests. Associations between total index hospitalization cost and recipient LAS and donor pre-procurement P/F ratio were estimated separately for EVLP and non-EVLP LTx recipients using Spearman correlation coefficients.
To further investigate the association between EVLP and index hospitalization costs, a subgroup analysis was performed among recipients of donor lungs that underwent EVLP, stratified by EVLP device; during the study period, the following EVLP devices were used: Lung-Bioengineering (Lung-Bio), XVIVO Perfusion System (XPS), TransMedics Organ Care System (OCS). Index hospitalization costs were compared among device strata using nonparametric Kruskal-Wallis with Dunn’s multiple comparison tests.
A two-sided p-value less than 0.05 was considered statistically significant. All analyses were performed using R version 3.6.1 (Vienna, Austria) and GraphPad Prism version 9.1.1 (San Diego, CA).
RESULTS
Study population
A total of 88 LTx recipients met inclusion criteria, including 22 who received donor lungs that underwent EVLP and 66 matched non-EVLP controls. Among non-EVLP recipients, 45 received lungs from SCDs and 21 received lungs from ECDs. All transplants were performed between June 2014 and July 2020. Patient demographics and pre-transplant characteristics were similar between groups (Table 1).
Table 1.
Recipient characteristics.
| Characteristic | SCS SCD N = 45 |
SCS ECD N = 21 |
EVLP N = 22 |
P-value (KW or χ2) |
|---|---|---|---|---|
| Age (years) | 59.0 [43.5, 68.9] | 64.0 [49.5, 67.0) | 62.0 [47.0, 67.0] | 0.97 |
| Sex | 0.98 | |||
| Male | 27 (60.0%) | 13 (61.9%) | 13 (59.1%) | |
| Female | 18 (40.0%) | 8 (38.1%) | 9 (40.9%) | |
| Race | 0.06 | |||
| Caucasian/White | 36 (80.0%) | 21 (100%) | 20 (90.9%) | |
| Black or African American | 9 (20.0%) | 0 (0.0%) | 2 (9.1%) | |
| PRA at transplant (%) | ||||
| Class I | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.20 |
| Class II | 0.00 [0.00, 0.00] | 0.00 [0.00, 4.5] | 0.00 [0.00, 24.3] | 0.10 |
| Etiology of respiratory failure | 0.65 | |||
| ARDS/pneumonia | 1 (2.2%) | 0 (0.0%) | 0 (0%) | |
| Autoimmune interstitial lung disease | 3 (6.7%) | 0 (0.0%) | 1 (4.5%) | |
| COPD/emphysema | 3 (6.7%) | 1 (4.8%) | 2 (9.1%) | |
| Cystic fibrosis | 1 (2.2%) | 2 (9.5%) | 1 (4.5%) | |
| Idiopathic pulmonary fibrosis | 24 (53.3%) | 13 (61.9%) | 9 (40.9%) | |
| Pulmonary hypertension | 2 (4.4%) | 0 (0.0%) | 0 (0%) | |
| Re-transplant | 5 (11.1%) | 1 (4.8%) | 2 (9.1%) | |
| Other | 6 (13.3%) | 4 (19.0%) | 7 (31.8%) | |
| Disease group | 0.75 | |||
| A | 4 (8.9%) | 1 (4.8%) | 2 (9.1%) | |
| B | 3 (6.7%) | 0 (0.0%) | 1 (4.5%) | |
| C | 1 (2.2%) | 2 (9.5%) | 1 (4.5%) | |
| D | 37 (82.2%) | 18 (85.7%) | 18 (81.8%) | |
| Status at time of transplant | 0.82 | |||
| Inpatient/hospitalized | 1 (2.2%) | 1 (4.8%) | 1 (4.5%) | |
| Outpatient | 44 (97.8%) | 20 (95.2%) | 21 (95.5%) | |
| Interventions at time of transplant | ||||
| ECMO | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0.62 |
| Intubated | 1 (2.2% | 0 (0.0%) | 0 (0.0%) | 0.62 |
| History of prior lung transplant | 5 (11.1%) | 1 (4.8%) | 2 (9.1%) | 0.71 |
| History of prior thoracic surgery a | 15 (33.3%) | 7 (33.3%) | 5 (22.7%) | 0.65 |
| LAS at time of transplant | 40.1 [37.1, 45.0] | 42.3 [40.0, 44.9] | 42.4 [36.6, 43.7] | 0.69 |
| Last 6-minute walk distance (feet) | 1555 [1342, 1890] | 1599 [1347, 1828] | 1540 [1200, 1620] | 0.30 |
| Cardiac output (L/min) | 5.05 [4.45, 6.20] | 5.35 [4.45, 5.90] | 5.60 [5.10, 5.80] | 0.53 |
| Arterial pCO2 at transplant (mmHg) | 44.0 [39.5, 48.0] | 44.0 [39.5, 50.0] | 41.5 [38.3, 47.8] | 0.65 |
| Smoking history | 21 (46.7%) | 12 (57.1%) | 12 (54.5%) | 0.68 |
| BMI at transplant (kg/m 2 ) | 25.8 [22.1, 27.6] | 25.9 [20.9, 28.0] | 25.0 [23.3, 26.4] | 0.61 |
| Pre-transplant pulmonary rehabilitation | 45 (100%) | 21 (100%) | 22 (100%) | n/a |
| Pre-transplant pulmonary function | ||||
| FEV1 (% predicted) | 47.0 [31.5, 57.0] | 40.0 [28.5, 62.5] | 45.5 [25.0, 61.0] | 0.94 |
| FVC (% predicted) | 52.0 [41.5, 62.5] | 49.0 [37.0, 66.0] | 49.0 [41.0, 66.3] | 0.86 |
Median (interquartile range) for continuous variables and count (percent) for categorical variables.
ARDS, acute respiratory distress syndrome. BMI, body mass index. COPD, chronic obstructive pulmonary disease. ECD, extended-criteria donor. ECMO, extracorporeal membrane oxygenation. EVLP, ex-vivo lung perfusion. FEV1, forced expiratory volume in 1 second. FVC, forced vital capacity. KW, Kruskal-Wallis. LAS, lung allocation score. PRA, panel reactive antibody. SCD, standard-criteria donor. SCS, static cold storage.
Includes prior lung transplant.
Operative characteristics
Operative characteristics stratified by EVLP use are summarized in Table 2. SCS-SCD, SCS-ECD, and EVLP cases had similar operative durations (median 407 vs 403 vs 424 minutes, p=0.99), proportions of daytime cases (incision occurring between 06:00 and 17:59, 46.7% vs 47.6% vs 40.9%, p=0.88), and rates of intraoperative cardiopulmonary bypass use (28.9% vs 14.3% vs 22.7%, p=0.43).
Table 2.
Operative characteristics.
| A | B | C | P-value | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | SCS SCD N = 45 |
SCS ECD N = 21 |
EVLP N = 22 |
KW or χ2 | A vs B | A vs C | B vs C |
| Total preservation time (minutes) | 409 [373, 506] | 438 [383, 492] | 857 [760, 1103] | <0.0001 | >0.99 | <0.0001 | <0.0001 |
| Pre-EVLP preservation time | 100 [52, 232] | ||||||
| Post-EVLP preservation time | 304 [238, 406] | ||||||
| EVLP time (minutes) | 313 [239, 448] | ||||||
| Cold ischemic time (minutes) | 409 [373, 506] | 438 [383, 492] | 515 [305, 622] | 0.48 | |||
| EVLP device | |||||||
| Lung Bioengineering | 4 (18.2%) | ||||||
| XVIVO Perfusion System | 6 (27.3%) | ||||||
| TransMedics OCS | 12 (54.5%) | ||||||
| Operative duration (minutes) | 407 [368, 477] | 403 [350, 494] | 424 [349, 524] | 0.99 | |||
| Operation start time | 0.88 | ||||||
| 06:00–17:59 | 21 (46.7%) | 10 (47.6%) | 9 (40.9%) | ||||
| 18:00–05:59 | 24 (53.3%) | 11 (52.3%) | 13 (59.1%) | ||||
| Cardiopulmonary bypass used | 13 (28.9%) | 3 (14.3%) | 5 (22.7%) | 0.43 | |||
| Bypass time (minutes)a | 242 [169, 317] | 275 [243, 415] | 329 [290, 337] | 0.37 | |||
| Concomitant procedure | 3 (6.7%) | 0 (0.0%) | 3 (13.6%) | 0.21 | |||
| Atrial septal defect repair | 1 (2.2%) | 0 (0.0%) | 2 (9.1%) | 0.21 | |||
| Coronary artery bypass | 2 (4.4%) | 0 (0.0%) | 1 (4.5%) | 0.97 | |||
| Transfusion (units) | |||||||
| Packed red blood cells | 1.00 [0.00, 3.00] | 1.00 [0.00, 2.50] | 2.00 [0.75, 4.00] | 0.20 | |||
| Fresh frozen plasma | 0.00 [0.00, 1.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 1.75] | 0.61 | |||
| Cryoprecipitate | 0.00 [0.00, 1.00] | 0.00 [0.00, 0.50] | 0.00 [0.00, 1.25] | 0.44 | |||
| Platelets | 0.00 [0.00, 1.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 1.00] | 0.40 | |||
| Donor/recipient weight ratio | 1.02 [0.92, 1.23] | 1.08 [0.94, 1.21] | 1.07 [0.95, 1.61] | 0.55 | |||
| Serologies | |||||||
| Donor EBV positive | 41 (91.1%) | 20 (95.2%) | 20 (90.9%) | 0.83 | |||
| Donor CMV positive | 29 (64.4%) | 8 (38.1%) | 14 (63.6%) | 0.11 | |||
| Recipient CMV positive | 21 (46.7%) | 12 (57.1%) | 13 (59.1%) | 0.56 | |||
| CMV mismatch (D+/R−) | 16 (35.6%) | 5 (23.8%) | 6 (27.3%) | 0.58 | |||
| Transplant type (bilateral) | 45 (100%) | 21 (100%) | 22 (100%) | n/a | |||
Median (interquartile range) for continuous variables and count (percent) for categorical variables.
CMV, cytomegalovirus. EBV, Epstein-Barr virus. ECD, extended-criteria donor. EVLP, ex-vivo lung perfusion. KW, Kruskal-Wallis. OCS, Organ Care System. SCD, standard-criteria donor. SCS, static cold storage.
Among those who underwent lung transplantation on full cardiopulmonary bypass.
Total preservation time (defined as the time from donor cross clamp to recipient reperfusion) was significantly longer for EVLP versus SCD and ECD lungs (median 857 vs 409 vs 438 minutes, p<0.0001); for SCD and ECD lungs, total preservation time was defined as the total ischemic time of the second implanted lung. Among EVLP lungs, the preservation period included median 313 minutes of EVLP and median 515 minutes of cold ischemic time (CIT); there was no difference in CIT among SCD, ECD, or EVLP lungs (median 409 vs 438 vs 515 minutes, p=0.48). Median pre-EVLP preservation time (defined as the time between donor cross clamp and start of EVLP) was 100 minutes and median post-EVLP preservation time (defined as the time between end of EVLP and recipient reperfusion) was 304 minutes (Table 2).
Donor characteristics
Compared to SCDs, ECDs and EVLP donors had lower pre-procurement P/F ratios (median 456 vs 431 vs 334, p<0.0001; P/F ratio ≤300: 0% vs 43.9% vs 45.5%, p<0.0001) and were more likely to be DCD donors (0% vs 33.3% vs 27.3%, p=0.0004). Additional donor characteristics including age, cause of death, and smoking history ≥20 pack-years were similar between groups. EVLP lungs were recovered from donors located farther from the transplant center, but this difference was not statistically significant (EVLP vs SCD vs ECD: median 413 vs 203 vs 299 nautical miles, p=0.38) (Table 3).
Table 3.
Donor characteristics.
| A | B | C | P-value | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | SCS SCD N = 45 |
SCS ECD N = 21 |
EVLP N = 22 |
KW or χ2 | A vs B | A vs C | B vs C |
| Age (years) | 34.0 [27.0, 40.5] | 38.0 [28.5, 55.0] | 34.0 [26.5, 44.5] | 0.22 | |||
| PHS increased risk | 21 (46.7%) | 3 (14.3%) | 6 (27.3%) | 0.03 | 0.01 | 0.19 | 0.46 |
| Smoking history ≥20 pack-years | 6 (13.3%) | 4 (19.0%) | 1 (4.5%) | 0.34 | |||
| PaO2/FiO2 (P/F) ratio | 456 [407, 521] | 431 [234, 468] | 334 [213, 421] | <0.0001 | 0.01 | 0.0001 | 0.83 |
| P/F ratio ≤300 | 0 (0.0%) | 9 (43.9%) | 10 (45.5%) | <0.0001 | <0.0001 | <0.0001 | >0.99 |
| Donor type | 0.0004 | 0.0002 | 0.0007 | >0.99 | |||
| DBD donor | 45 (100%) | 15 (66.7%) | 16 (72.7%) | ||||
| DCD donor | 0 (0.0%) | 7 (33.3%) | 6 (27.3%) | ||||
| Cause of death | 0.21 | ||||||
| Anoxia | 19 (42.2%) | 4 (19.0%) | 6 (27.3%) | ||||
| CVA/stroke | 8 (17.8%) | 7 (33.3%) | 8 (36.4%) | ||||
| Head trauma | 17 (37.8%) | 8 (38.1%) | 8 (36.4%) | ||||
| Other | 1 (2.2%) | 2 (9.5%) | 0 (0.0%) | ||||
| Blood infection | 2 (4.4%) | 2 (9.5%) | 1 (4.5%) | 0.68 | |||
| Pulmonary infection | 26 (57.8%) | 15 (71.4%) | 14 (63.6%) | 0.56 | |||
| Distance (nautical miles) a | 203 [105, 451] | 299 [126, 496] | 413 [83, 614] | 0.38 | |||
Median (interquartile range) for continuous variables and count (percent) for categorical variables.
CVA, cerebrovascular accident. DBD, donation after brain death. DCD, donation after circulatory death. ECD, extended-criteria donor. EVLP, ex-vivo lung perfusion. KW, Kruskal-Wallis. PHS, US Public Health Service. SCD, standard-criteria donor. SCS, static cold storage.
Distance from transplant center.
Post-transplant outcomes
At 72 hours, grade 3 PGD was present in 22.0% of SCD recipients, 16.7% of ECD recipients, and 28.6% of EVLP recipients (p=0.67). Additional perioperative outcomes including rates of postoperative ECMO use, dialysis, tracheostomy within 7 days, and ICU and hospital LOS were similar among SCD, ECD, and EVLP recipients (Table 4). There were no differences in patient or CLAD-free survival among groups; however, there was a trend toward improved rejection-free survival in EVLP recipients compared to ECD recipients (median 1.0 vs 0.25 years, log-rank p=0.057) (Figure 1).
Table 4.
Post-transplant perioperative complications.
| Characteristic | SCS SCD N = 45 |
SCS ECD N = 21 |
EVLP N = 22 |
P-value (KW or χ2) |
|---|---|---|---|---|
| Reintervention a | 7 (15.6%) | 3 (14.3%) | 5 (22.7%) | 0.71 |
| Surgical | 6 (13.3%) | 3 (14.3%) | 5 (22.7%) | 0.60 |
| Radiologic | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0.62 |
| Grade 3 PGD at 72 hours b | 9/41 (22.0%) | 3/18 (16.7%) | 6/21 (28.6%) | 0.67 |
| N Excluded (Ungradablec) | 1 (2.2%) | 1 (4.8%) | 0 (0.0%) | |
| N Excluded (Data Unavailable) | 3 (6.7%) | 2 (9.5%) | 1 (4.5%) | |
| Post-operative ECMO | 7 (15.6%) | 3 (14.3%) | 4 (18.2%) | 0.94 |
| Extubated in >48 hours | 15 (33.3%) | 3 (14.3%) | 10 (45.5%) | 0.09 |
| Tracheostomy within 7 days | 8 (17.8%) | 2 (9.5%) | 5 (22.7%) | 0.51 |
| Reintubated d | 4 (8.9%) | 5 (23.8%) | 6 (27.3%) | 0.11 |
| Renal replacement therapy d | 5 (11.1%) | 3 (14.3%) | 5 (22.7%) | 0.45 |
| ICU readmission a | 5 (11.1%) | 3 (14.3%) | 4 (18.2%) | 0.72 |
| Hospital readmission a | 11 (24.4%) | 8 (38.1%) | 5 (22.7%) | 0.40 |
| Post-transplant ICU LOS (days) | 3 [2, 8] | 5 [2, 11.5] | 6 [3.8, 20.5] | 0.10 |
| Post-transplant hospital LOS (days) | 20 [14.5, 33.5] | 24 [15, 30] | 27 [18, 56.8] | 0.26 |
| Acute rejection a | 11 (24.4%) | 6 (28.6%) | 3 (13.6%) | 0.47 |
| Mortality within 90 days | 1 (2.2%) | 1 (4.8%) | 1 (4.5%) | 0.82 |
Median (interquartile range) for continuous variables and count (percent) for categorical variables.
ECD, extended-criteria donor. ECMO, extracorporeal membrane oxygenation. EVLP, ex-vivo lung perfusion. ICU, intensive care unit. LOS, length of stay. PGD, primary graft dysfunction. SCD, standard-criteria donor. SCS, static cold storage.
Within 30 days.
A total of 82/88 patients were included in the analysis of grade 3 primary graft dysfunction at 72 hours, excluding 6 patients for whom primary graft dysfunction could not be graded either due to ECMO status (see footnote c) or data missingness. Patients who were on extracorporeal membrane oxygenation support and had radiographic evidence of pulmonary edema at 72 hours post-transplant were classified as having grade 3 primary graft dysfunction per International Society for Heart and Lung Transplantation guidelines.
In accordance with International Society for Heart and Lung Transplantation guidelines, patients who were on extracorporeal membrane oxygenation support and had no radiographic evidence of pulmonary edema at 72 hours post-transplant were designated as “ungradable” and excluded from the analysis of 72-hour primary graft dysfunction.
During index transplant hospitalization.
Figure 1. Kaplan-Meier survival analysis stratified by use of ex-vivo lung perfusion (EVLP).
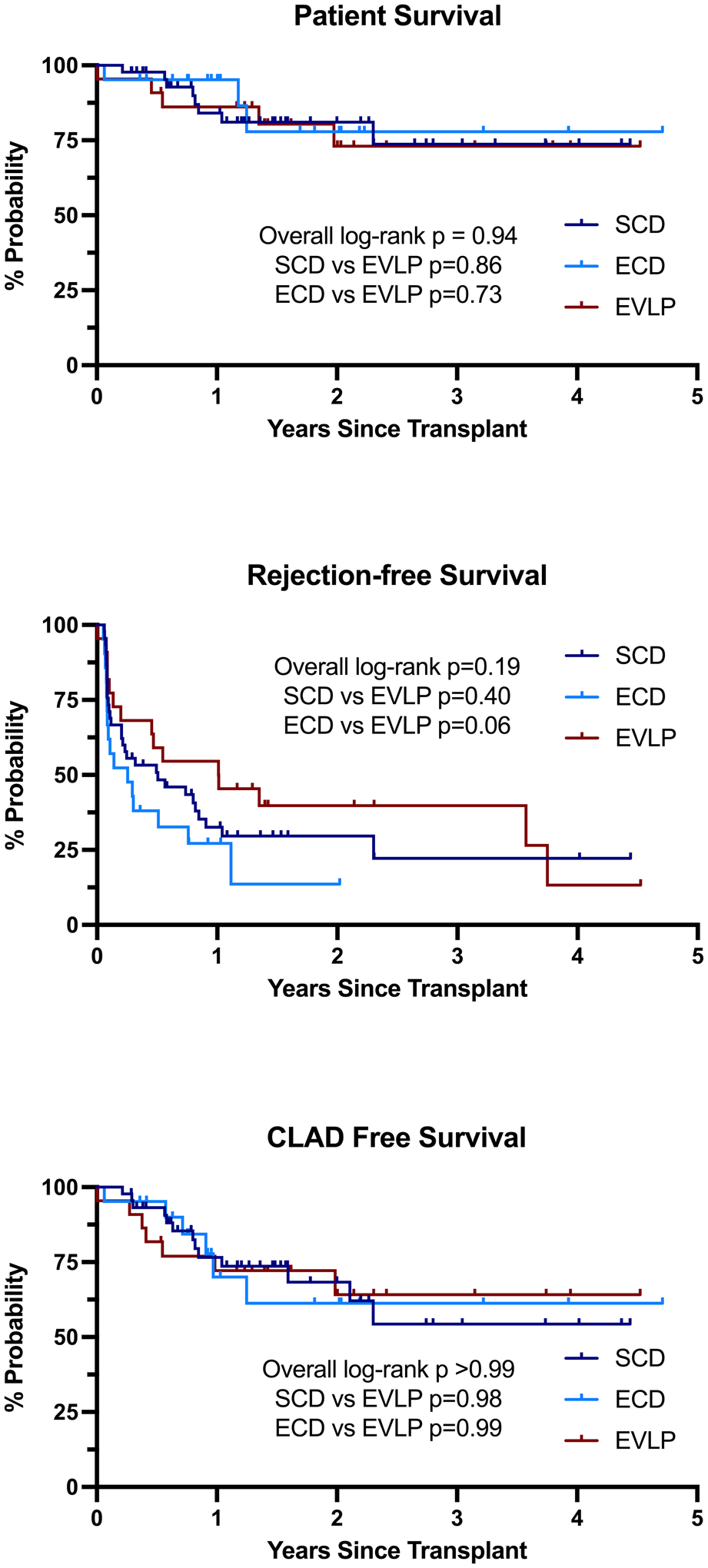
(A) Patient or graft survival. (B) Rejection-free survival. (C) Chronic lung allograft dysfunction-free survival.
Cost analysis
Direct variable costs
Index hospitalization DVCs stratified by EVLP use are summarized in Table 5. Total DVCs were similar among SCD, ECD, and EVLP groups (median $154,709 vs $168,334 vs $200,404, p=0.11). When DVCs were separated by category, EVLP patients incurred significantly higher diagnostic testing costs compared to SCD recipients (median $1,386 vs $428 p=0.003), primarily representing costs related to bronchoscopies. There was also a trend toward higher inpatient pharmacy costs in the EVLP group compared to the SCD group (median $40,900 vs $20,989, p=0.06), but not the ECD group (median $40,900 vs $24,989, p=0.63).
Table 5.
Direct variable cost of index hospitalization.
| A | B | C | P-value | ||||
|---|---|---|---|---|---|---|---|
| Service | SCS SCD N = 45 |
SCS ECD N = 21 |
EVLP N = 22 |
KW | A vs B | A vs C | B vs C |
| A. Direct variable costs | |||||||
| Intermediate Care | 11798 (8489, 16841), [0, 124796] | 14452 (9928, 19944), [7467, 79968] | 12673 (9297, 22165), [0, 96356] | 0.47 | |||
| Intensive Care | 7914 (4290, 22739), [2008, 281655] | 6436 (4554, 21840), [3792, 85179] | 12980 (7294, 51059), [2097, 409759] | 0.26 | |||
| Pharmacy | 20989 (15668, 40948), [11251, 364563] | 24989 (18666, 44614), [15534, 80685] | 40900 (21564, 75751), [6849, 279335] | 0.07 | |||
| Surgical | 15852 (13454, 21244), [10017, 136856] | 16420 (11961, 25419), [9798, 42443] | 19170 (13101, 33029), [10488, 97692] | 0.60 | |||
| Cardiology | 258 (225, 461), [37, 6031] | 301 (261, 479), [202, 1543] | 355 (228, 881), [60, 3042] | 0.43 | |||
| Respiratory Care | 5437 (3001, 18170), [863, 193249] | 6407 (2774, 12186), [1228, 42835] | 10237 (3580, 27779), [912, 114383] | 0.48 | |||
| PT/OT/Speech | 1453 (1042, 3134), [690, 33274] | 1758 (1446, 2538), [1167, 13246] | 2482 (1470, 6192), [656, 22548] | 0.11 | |||
| Laboratory | 4278 (3548, 7345), [2204, 40553] | 4797 (3799, 6882), [2835, 17243] | 5626 (3523, 10084), [2980, 33630] | 0.40 | |||
| Radiology | 1178 (541, 2709), [230, 22389] | 1188 (683, 1568), [214, 7385] | 1709 (1003, 4430), [84, 15435] | 0.20 | |||
| Blood Bank | 1139 (359, 5082), [35, 55621] | 1146 (843, 3748), [66, 8976] | 2331 (1443, 3936), [284, 42432] | 0.16 | |||
| Clinic Services | 0 (0, 0), [0, 765] | 0 (0, 0), [0, 129] | 0 (0, 0), [0, 407] | 0.96 | |||
| Medical Supplies | 752 (522, 1317), [187, 4443] | 795 (511, 1705), [95, 3083] | 1246 (710, 2861), [223, 15172] | 0.10 | |||
| Emergency Room | 0 (0, 0), [0, 381] | 0 (0, 0), [0, 381] | 0 (0, 0), [0, 279] | >0.99 | |||
| Other Diagnostics | 428 (207, 998), [0, 44819] | 857 (428, 1396), [0, 12877] | 1386 (701, 4362), [0, 46226] | 0.003 | 0.20 | 0.003 | 0.69 |
| TOTALa | 154709 (137890, 218180), [104071, 1372733] | 168334 (151102, 209383), [129978, 418510] | 200404 (161966, 362143), [104032, 940844] | 0.11 | |||
| B. Organ acquisition costs | |||||||
| Organ Acquisitionb | 79135 (78915, 81547), [56030, 83370] | 79135 (79135, 83370), [71130, 83370] | 81547 (78915, 83370), [64048, 89955] | 0.38 | |||
| Adjusted Organ Acquisitionc | 77201 (76981, 79613), [54096, 81436] | 77201 (77201, 81436), [69196, 81436] | 132091 (98955, 134613), [82114, 143021] | <0.0001 | >0.99 | <0.0001 | <0.0001 |
Median (interquartile range), [full range].
ECD, extended-criteria donor. EVLP, ex-vivo lung perfusion. KW, Kruskal-Wallis. OT, occupational therapy. PT, physical therapy. SCD, standard-criteria donor. SCS, static cold storage.
Total direct variable costs did not include organ acquisition costs (see footnote b).
Organ acquisition was defined by our institution as a variable cost, however these values represented a standardized fee that varied annually; accordingly, reported organ acquisition costs did not include disposable and device-related costs specific to EVLP and were not included in the calculation of total direct variable costs.
To understand how use of EVLP actually impacts the cost of organ acquisition, reported organ acquisition costs were adjusted to allocate all disposable and device-related costs specific to EVLP entirely to recipients of EVLP lungs. Cost adjustments for individual patients were based on the specific EVLP platform used (Lung-Bioengineering, XVIVO Perfusion System, TransMedics Organ Care System).
To understand underlying differences in inpatient pharmacy costs among EVLP versus non-EVLP LTx recipients, itemized pharmacy DVCs were evaluated in both groups; the top 10 pharmacy expenses among EVLP patients are shown in Table S1. Among EVLP patients, intravenous immunoglobulin G (IVIG) was the most significant pharmacy expense. IVIG was administered to 14/22 (63.6%) EVLP patients compared to 14/45 (31.1%) SCD patients (p=0.02) and 7/21 (33.3%) ECD patients (p=0.07). The median cost of IVIG was $6,406 among EVLP patients compared to $0 among both SCD and ECD patients (p=0.02 and p=0.07, respectively). Basiliximab was the second-highest pharmacy expense among EVLP patients (used in 22/22 [100%] cases, median $7,510) and the highest pharmacy expense among non-EVLP patients (used in 44/45 [97.8%] SCD cases, median $7,787, p=0.55, and 21/21 [100%] ECD cases, median $7,787, p=0.07). Among EVLP patients, there were also notable outliers in the cost of amphotericin B, dialysis solutions, and posaconazole, however costs related to these drugs were not significantly different among EVLP, SCD, and ECD groups.
Total index hospitalization costs
Compared to non-EVLP LTx recipients, EVLP LTx recipients incurred higher total index hospitalization costs (median $336,290 vs $271,306, p=0.03). Total index hospitalization costs were comparable between EVLP and ECD LTx recipients (median $336,290 vs $289,591, p=0.71); however, EVLP recipients tended to incur higher index hospitalization costs than SCD recipients (median $336,290 vs $260,357, p=0.06). This trend persisted when the total cost was subdivided into total fixed and total variable costs (Figure 2). There was no correlation between recipient LAS or donor pre-procurement P/F ratio and total index hospitalization cost in either group (Figure 3).
Figure 2. Total hospitalization cost stratified by use of ex-vivo lung perfusion (EVLP).
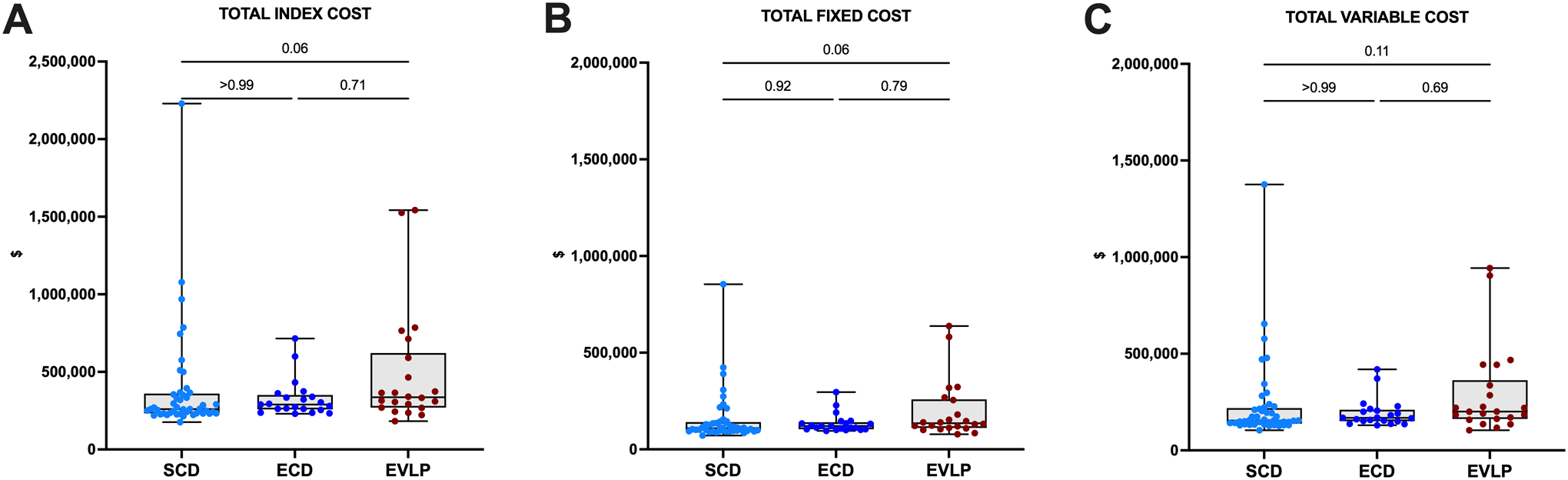
(A) Total index hospitalization costs. B) Total fixed costs only. C) Total variable costs only. Data presented as median (line), interquartile range (box), and minimum-maximum (error bar). Points represent individual patients in each group.
Figure 3.
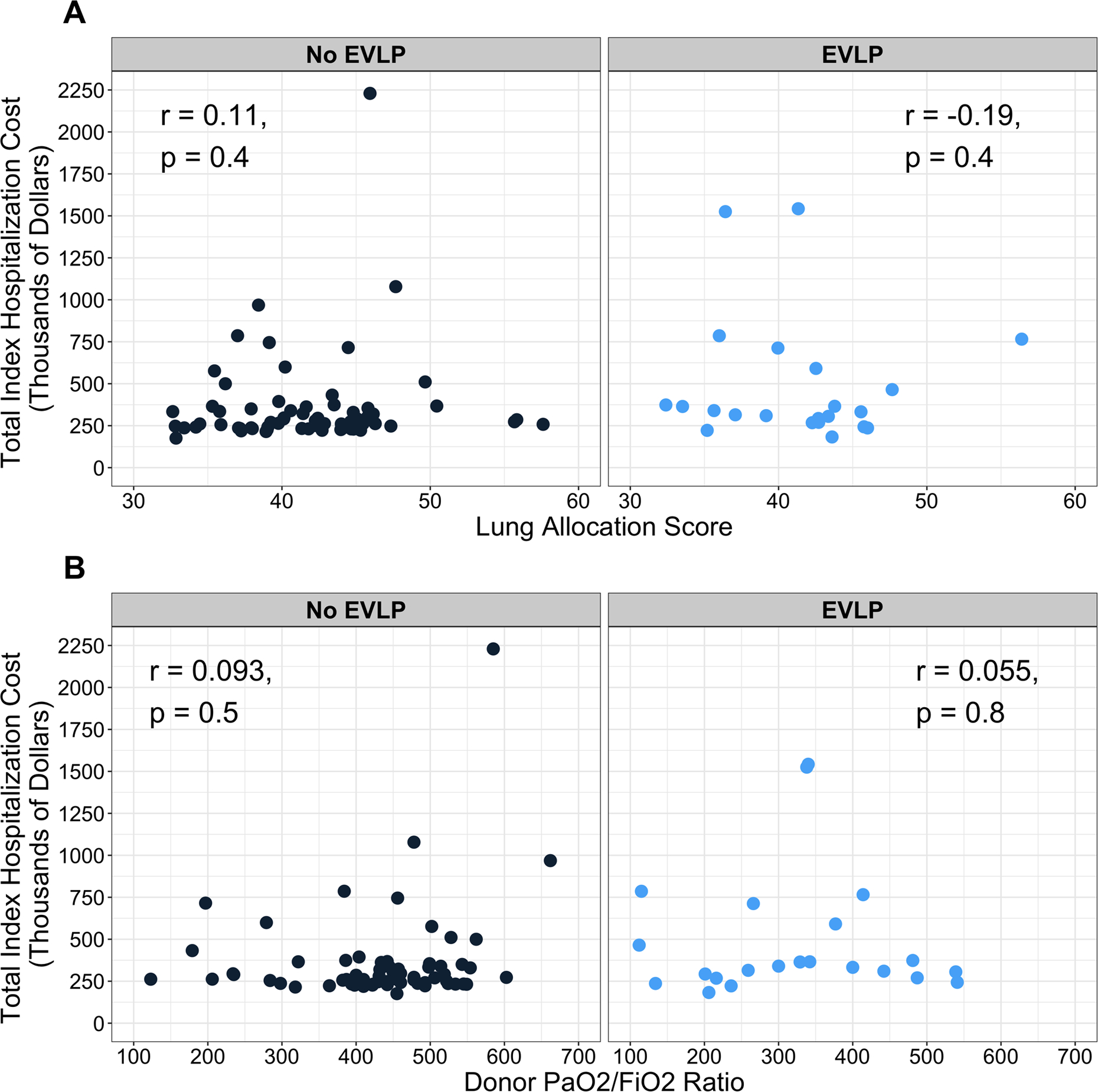
Association between total index hospitalization cost and A) lung allocation score at transplantation and B) donor pre-procurement PaO2/FiO2 ratio stratified by use of ex-vivo lung perfusion (EVLP).
Subgroup analysis
Among 22 EVLP LTx, EVLP was performed using the Lung-Bio, XPS, and OCS devices in 4 (18.2%), 6 (27.3%), and 12 (54.5%) cases, respectively. In a subgroup analysis of patients who received donor lungs that underwent EVLP, total DVCs were statistically similar among EVLP devices (Lung-Bio vs XPS vs OCS: median $170,687 vs $93,430 vs $112,590, overall p=0.3) (Table S2); however, total DVCs were notably higher for the Lung-Bio device compared to the XPS and OCS. There were no statistical differences in total index hospitalization costs across EVLP devices (Figure 4).
Figure 4. Total index hospitalization cost among patients who received donor lungs that underwent ex-vivo lung perfusion (EVLP) stratified by EVLP device.
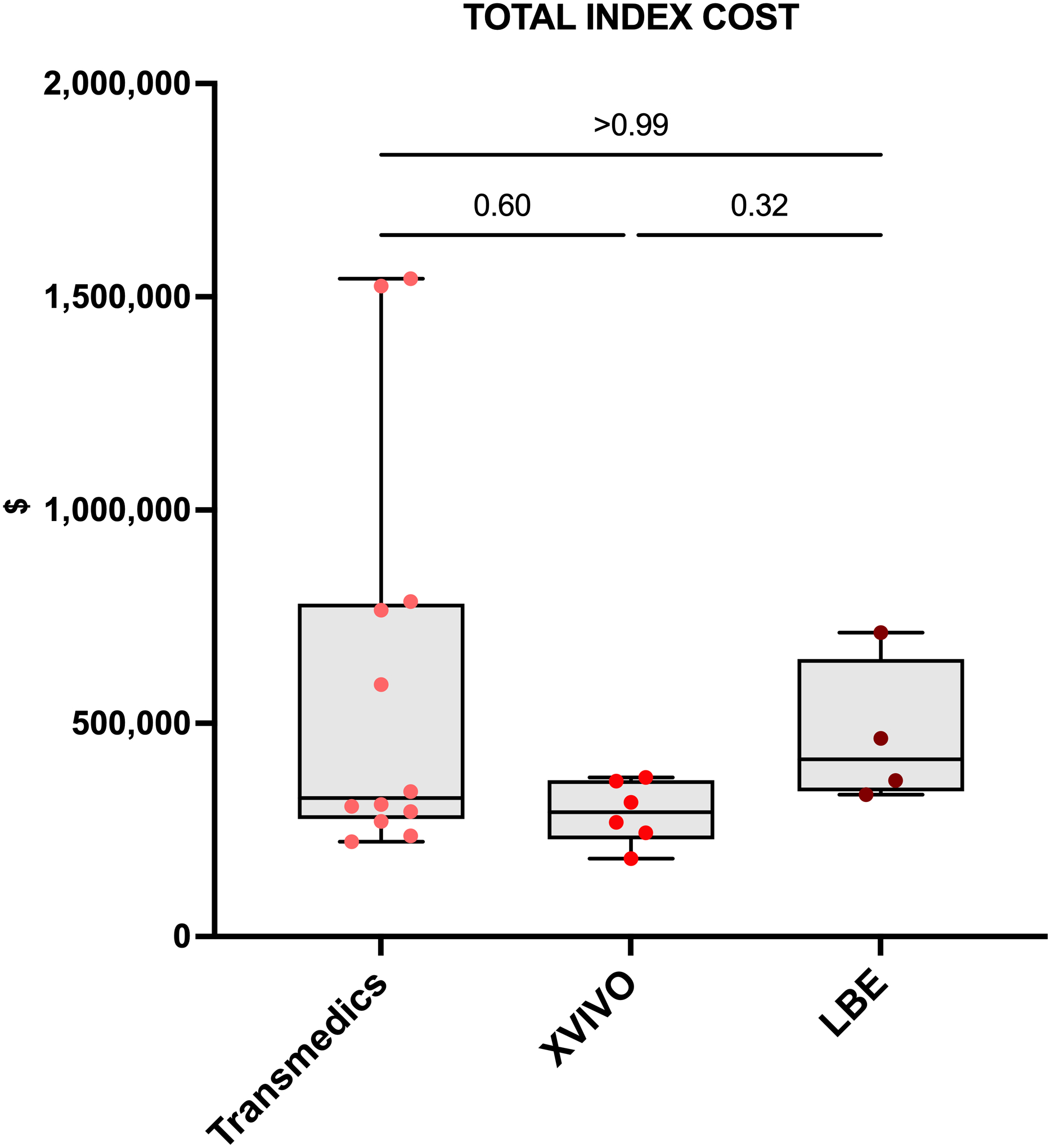
Data presented as median (line), interquartile range (box), and minimum-maximum (error bar). Points represent individual patients in each group.
Potential institution-level profitability of EVLP
To understand the potential profitability of EVLP in an institution-level payment structure, we examined the direct contribution margin (DCM) for EVLP LTx, and compared them to the DCM for non-EVLP LTx. For each LTx performed, the DCM represents the difference between the net revenue generated and the cost of patient care; accordingly, positive values correspond to financial gains and negative values correspond to financial losses for the institution for individual transplant episodes, excluding organ acquisition costs (OACs). In this analysis, 3 (3.4%) patients with non-zero account balances at the time of data collection were excluded as the net revenue for these cases had not yet been determined. Among 85 included patients, the median DCM was positive in the SCD, ECD, and EVLP groups; this suggests that overall, use of EVLP was profitable for our institution. Although the median EVLP DCM was overall positive, it was approximately 44.4% lower than SCD LTx and 50.9% lower than ECD LTx, though these differences were not statistically significant (both p>0.99).
DISCUSSION
In this single-institution study, we compared post-transplant outcomes and index hospitalization costs among patients who underwent LTx with or without the use of EVLP. In well-matched patient cohorts, we found that recipients of EVLP lungs had comparable overall, rejection-free, and CLAD-free survival to recipients of SCS-SCD lungs, despite a higher proportion of DCD donor lungs, lower pre-procurement P/F ratios, and significantly longer preservation times in the EVLP group. These encouraging findings are consistent with larger reported series.9
In our cohort, patients who received EVLP lungs had similar rates of postoperative complications, hospital LOS, and readmission to those who received non-EVLP lungs. Furthermore, early allograft function was similar among SCD, ECD, and EVLP LTx recipients, as evidenced by comparable rates of PGD3 at 72 hours and need for postoperative ECMO support among groups. Despite few statistically significant differences among groups, it is probable that the difference in total index hospitalization cost between EVLP and SCD groups was influenced by outliers in ICU and pharmacy costs among EVLP recipients. On average, EVLP recipients incurred approximately $25,378 (+105%) more in intensive care DVCs compared to all non-EVLP recipients. While this difference was not statistically significant, intensive care DVCs were the most variable and most expensive DVC category, with one EVLP recipient incurring $409,759 in ICU costs alone. In this instance, the patient developed PGD requiring venovenous ECMO and acquired resistant Pseudomonas infection, ultimately requiring multiple trips to the operating room, tracheostomy, and dialysis over a period of 98 ICU and 200 hospital days. Differences in pharmacy-related costs also played a significant role in overall cost differences, particularly those related to increased use of IVIG among EVLP patients. This reflects routine pre-transplant IVIG administration to highly-sensitized recipients with high pre-transplant class II panel reactive antibody levels in this group, and is consistent with our experience in which EVLP is often used to maximize donor opportunities for candidates with donor allocation challenges such as short stature or HLA sensitization. In addition, the proportion of EVLP patients requiring dialysis was more than twice that of the SCD group (22.7% vs 11.1%, p=0.21), and the range of dialysis solution costs was greater for the EVLP patients (range $0–10,514 vs $0–37,274). Lastly, EVLP recipients also incurred significantly higher DVCs for diagnostics such as bronchoscopies, though these DVCs accounted for an overall small fraction of the total index hospitalization cost in both groups and were unlikely a major contributor to the difference in total costs.
Regarding postoperative index hospitalization costs, we found that recipients of EVLP lungs trended toward higher total, fixed, and variable costs compared to recipients of SCS-SCD lungs, likely related to the presence of two outliers in the EVLP group with significantly higher index hospitalization costs than most EVLP patients in our study; importantly, however, these costs were not different compared to SCS-ECD recipients, suggesting that EVLP was not significantly more expensive than ECD graft use, regardless of EVLP device-specific costs. This is unsurprising given that most allografts our series underwent EVLP under clinical trial protocols such as the OCS EXPAND,1,2 XPS NOVEL,21,22 and Lung-Bioengineering trials that sought to evaluate the utility of EVLP as a means to expand the donor pool for LTx through increased use of ECD lung allografts.
It is important to discuss why unadjusted OACs were similar among SCD, ECD, and EVLP LTx in our study. At our institution, OACs are determined on an annual basis by aggregating all organ procurement, surgeon, and air and ground transportation fees incurred for lung procurements and dry runs, and distributing the total cost evenly across all LTx performed over the course of the year. Accordingly, while organ acquisition was defined by our institution as a variable cost, these values actually represented a standardized fee that varied annually, but was not affected by EVLP versus non-EVLP status. Alternatively, adjusted OACs allocating all disposable and device-related costs specific to EVLP entirely to recipients of EVLP lungs were significantly higher among EVLP versus non-EVLP LTx, highlighting the additional upfront costs associated with use of EVLP compared to SCS. As EVLP utilization increases in the coming years, OACs will increase accordingly as disposable and device-related expenses associated with EVLP become more prevalent factors. Regardless, within our current billing framework, these additional costs are distributed among recipients of both EVLP and non-EVLP lungs, and we anticipate that OACs for LTx patients at our institution will increase universally, with minimal contribution to differential patient care costs between EVLP and non-EVLP recipients.
Additionally, it will be particularly important for future studies to clarify indications for EVLP utilization, and clearly delineate cases in which EVLP was employed for routine storage of standard lung allografts versus salvage therapy for otherwise unusable organs. The interpretation of any analysis of the cost of this technology changes considerably if otherwise unacceptable organs can now be used for human transplants and more waitlisted patients receive organs for which they would not otherwise have been eligible. Understanding of long-term outcomes among recipients of EVLP lungs also remains an active area of research and the long-term cost-benefit of this technology remains to be determined. For example, although our results indicate that there may be superior rejection-free survival in our predominantly ECD EVLP cohort compared to SCS-ECD recipients (which in turn, would affect treatment costs following index hospitalization) this potential financial impact is not captured in our current analysis. In the coming years, continued follow-up with attention to long-term graft survival, quality of life, and healthcare utilization will be critical to characterize the overall financial impact of EVLP.
Our study provides promising preliminary evidence to suggest that use of EVLP can increase the donor pool without adverse financial consequences for LTx institutions, despite portending higher index hospitalization costs than SCS-SCD grafts. While the contribution margin, or potential profit associated with EVLP is highly dependent on the revenue earned per patient transplanted, the median DCM was positive in our series, suggesting that at least a subset of US LTx programs like ours could be financially incentivized to increase transplant volume through wider use of EVLP. In particular, EVLP could be leveraged to increase LTx volume through increased use of ECD lungs without adversely impacting patient outcomes. Indeed, only 22% of available donor lungs were used for transplantation in 2019; of those that were discarded, poor organ function, donor medical history, and suboptimal P/F ratios were commonly cited reasons, suggesting that the utilization rate for ECD lungs was likely considerably lower.23 In contrast, utilization rates for ECD lungs in the OCS EXPAND trial were calculated between 81–87%.1,3 While this highlights the immense potential for EVLP to expand the donor pool, preservation of higher-risk grafts could introduce additional OACs in the event of increased discard rates following EVLP. A favorable solution in our current institutional experience is that following “dry-runs” in which an organ is perfused but ultimately discarded, the device disposables are replaced by the manufacturer. We believe this is an attractive model for transplant centers, as the manufacturer bears the upfront financial risk of perfusing a lung allograft, and could incentivize hospitals to use EVLP to pursue and evaluate marginal organs they may otherwise decline. This model would also benefit less-experienced institutions as they overcome the technical learning curve in adopting EVLP. Regardless, in the current US model, the increased OAC does not impact a health system’s DCM from each transplant.
Importantly, variable LTx revenue across centers could still lead to high center-level variation in EVLP use due to associated unpredictability in the profitability of using this technology. To increase and standardize EVLP use, some have proposed to employ shared devices among centers within OPOs. In this scenario, the OPO would bear the upfront costs of EVLP and the average device costs would be pooled between transplant centers such that all patients in a given region may benefit equally from expansion of the donor pool. While this scenario emulates the current utilization structure for hypothermic kidney perfusion devices, a recent American Society of Transplant Surgeons Standards Committee paper discussing liver perfusion devices noted that this strategy would likely increase OACs for individual transplant centers.24 From the transplant center perspective, practice varies based on how OACs are determined. As previously described, our institution determines OACs by aggregating all expenses related to lung acquisition, including those specific to EVLP, and averaging the cost across all LTx performed in a given year. This standardized OAC is included as a separate line-item charge on the inpatient claim. Regardless, further investigation is required to identify an EVLP utilization structure that is financially sustainable for the greatest number of transplant programs and maximizes transplant opportunities for a growing number of LTx patients.
Our study carries several limitations. As we examined a series of LTx performed at a single, large, academic institution with a high annual LTx volume, our experience may not generalize to other programs. However, use of institutional data allowed for inclusion of granular perioperative outcome and cost data that are unavailable in national datasets. Additionally, use of institutional data allowed us to evaluate survival beyond 4 years post-transplant for some patients; available national data precludes such longitudinal investigation as UNOS did not begin collecting confirmed donor EVLP status until 2018. As the UNOS registry accrues data on EVLP utilization and outcomes among corresponding recipients, future studies should investigate long-term outcomes among recipients of EVLP and non-EVLP lungs nationally, and regional variations in EVLP utilization to further elucidate implications of this novel technology for LTx recipients and transplant centers of variable size and location. As our series included only 22 patients who received EVLP donor lungs, we are likely underpowered to detect differences in perioperative outcomes and costs between EVLP, SCD, and ECD recipients. However, the most important determination and goal of the study remains important and valid in that the index hospitalization remains profitable for the health system when using EVLP to rehabilitate otherwise unusable lungs. The small number of EVLP recipients also precluded rigorous multivariable analysis to understand the independent effect of EVLP on post-transplant outcomes and costs; future multi-institutional and national studies should make provisions for appropriate risk adjustment to better understand how EVLP impacts outcomes and costs of postoperative patient care among transplant centers with variable recipient and donor populations. During the study period, three different EVLP devices were employed, which may have introduced unmeasured variability into lung perfusion and perioperative care processes that may affect cost differences noted in our study. Finally, our findings demonstrate that EVLP is profitable for LTx institutions, however this conclusion was based on our institutional experience, and may not apply broadly to other institutions or healthcare systems. Collaborative efforts among institutions of variable size, location, and experience are required to facilitate multi-institutional examination of the financial implications of EVLP, and determine the overall cost-effectiveness of EVLP in the US.
CONCLUSIONS
EVLP is a promising lung preservation strategy that is instrumental in expanding the donor pool for LTx through increased utilization of historically unacceptable donor lungs. Compared to SCS, EVLP adds significant cost to organ acquisition, which varies across EVLP platforms. At our institution, index hospitalization costs were also higher among recipients of EVLP donor lungs compared to SCS-preserved SCD lungs, but not SCS-preserved ECD lungs. Overall, the additional hospitalization costs appear reasonable considering that a patient who received EVLP lungs may not have not been transplanted otherwise. Nonetheless, for EVLP to be fiscally solvent, each institution must understand its contribution margin for each transplant performed. As EVLP becomes increasingly adopted in the coming years, we anticipate that multi-institutional studies with longer follow-up will expand upon our findings and better define the cost-benefit of this promising technology. This information will be critical in guiding future policy and validating specific indications for EVLP utilization over conventional cold storage.
Supplementary Material
Acknowledgments
SEH is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR002555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CIT
cold ischemic time
- CLAD
chronic lung allograft dysfunction
- DCD
donation after circulatory death
- DCM
direct contribution margin
- DVC
direct variable cost
- ECD
extended-criteria donor
- ECMO
extracorporeal membrane oxygenation
- EVLP
ex-vivo lung perfusion
- ICU
intensive care unit
- IVIG
intravenous immunoglobulin G
- Lung-Bio
Lung-Bioengineering
- LAS
lung allocation score
- LOS
length of stay
- LTx
lung transplantation
- OAC
organ acquisition cost
- OCS
Organ Care System
- OPO
organ procurement organization
- P/F ratio
PaO2/FiO2 ratio
- PGD3
grade 3 primary graft dysfunction
- SCD
standard-criteria donor
- SCS
static cold storage
- UNOS
United Network for Organ Sharing
- XPS
XVIVO Perfusion System
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Ceulemans L, Neyrinck A, Loor G, et al. Lung Transplantation from Donation after Circulatory Death Donors Following Portable Ex-Vivo Lung Perfusion: Post-Hoc Analysis of OCS Lung EXPAND Trial. The Journal of Heart and Lung Transplantation. 2019;38(4):S320. [Google Scholar]
- 2.Loor G, Warnecke G, Smith M, et al. The OCS lung EXPAND international trial interim results. The Journal of Heart and Lung Transplantation. 2016;35(4):S68–S69. [Google Scholar]
- 3.Loor G, Warnecke G, Villavicencio M, et al. Results of the OCS Lung EXPAND International Trial using portable normothermic OCS lung perfusion system (OCS) to recruit and evaluate extended criteria donor (ECD) lungs. The Journal of Heart and Lung Transplantation. 2018;37(4):S147. [Google Scholar]
- 4.Loor G, Warnecke G, Villavicencio MA, et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. The Lancet Respiratory Medicine. 2019;7(11):975–984. [DOI] [PubMed] [Google Scholar]
- 5.Luc J, Jackson K, Weinkauf J, Freed D, Nagendran J. Feasibility of lung transplantation from donation after circulatory death donors following portable ex vivo lung perfusion: a pilot study. Paper presented at: Transplantation proceedings 2017. [DOI] [PubMed] [Google Scholar]
- 6.Spratt JR, Mattison LM, Kerns NK, et al. Prolonged extracorporeal preservation and evaluation of human lungs with portable normothermic ex vivo perfusion. Clinical Transplantation. 2020;34(3):e13801. [DOI] [PubMed] [Google Scholar]
- 7.Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: a prospective randomized clinical trial. The Journal of Heart and Lung Transplantation. 2017;36(7):744–753. [DOI] [PubMed] [Google Scholar]
- 8.Tian D, Wang Y, Shiiya H, et al. Outcomes of marginal donors for lung transplantation after ex vivo lung perfusion: A systematic review and meta-analysis. The Journal of Thoracic and Cardiovascular Surgery. 2020;159(2):720–730. e726. [DOI] [PubMed] [Google Scholar]
- 9.Divithotawela C, Cypel M, Martinu T, et al. Long-term outcomes of lung transplant with ex vivo lung perfusion. JAMA surgery. 2019;154(12):1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jawitz OK, Raman V, Becerra D, et al. Lung Transplantation After Ex Vivo Lung Perfusion: Early Outcomes From a US National Registry. Annals of Surgery. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff R, Daly R, Lease E. Ex Vivo Lung Perfusion on Donor Lungs in the United States: National Trends and Post-Transplant Outcomes. The Journal of Heart and Lung Transplantation. 2020;39(4):S217. [Google Scholar]
- 12.Benjamin R Fisher PD. Approval Letter - TransMedics OCS Lung System - P160013/S002. In: Administration UFaD, ed. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160013S002A.pdf2019. [Google Scholar]
- 13.Benjamin R Fisher PD. Approval Letter - XVIVO Perfusion System (XPS™) with STEEN Solution™ Perfusate - P180014. In: Administration UFaD, ed. FDA.gov: US Food and Drug Administration; 2019. [Google Scholar]
- 14.McMeekin N, Chrysos A, Vale L, Fisher A. Incorporating ex-vivo lung perfusion into the UK adult lung transplant service: an economic evaluation and decision analytic model. BMC health services research. 2019;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins P, Chambers D, Smith I, et al. Cost Effectiveness of Ex Vivo Lung Perfusion Warrants Analysis of Long Term Recipient Outcome and Donor Organ Utilization Rate. The Journal of Heart and Lung Transplantation. 2015;34(4):S174. [Google Scholar]
- 16.Zhang ZL, van Suylen V, van Zanden JE, et al. First experience with ex vivo lung perfusion for initially discarded donor lungs in the Netherlands: a single-centre study. European Journal of Cardio-Thoracic Surgery. 2019;55(5):920–926. [DOI] [PubMed] [Google Scholar]
- 17.Cypel M, Yeung JC, Donahoe L, et al. Normothermic ex vivo lung perfusion: does the indication impact organ utilization and patient outcomes after transplantation? The Journal of thoracic and cardiovascular surgery. 2020;159(1):346–355. e341. [DOI] [PubMed] [Google Scholar]
- 18.Tian D, Wang Y, Deng S-Y. Should All Donors Be Treated by Ex Vivo Lung Perfusion? JAMA surgery. 2020;155(6):535–535. [DOI] [PubMed] [Google Scholar]
- 19.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on primary lung graft dysfunction, part I: definition and grading—a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2017;36(10):1097–1103. [DOI] [PubMed] [Google Scholar]
- 20.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment―A consensus report from the Pulmonary Council of the ISHLT. The Journal of Heart and Lung Transplantation. 2019;38(5):493–503. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez P, Davis R, D’Ovidio F, et al. The NOVEL lung trial one-year outcomes. The Journal of Heart and Lung Transplantation. 2014;33(4):S71–S72. [Google Scholar]
- 22.Sanchez P, Davis R, D’ovidio F, et al. Normothermic ex vivo lung perfusion as an assessment of marginal donor lungs–the NOVEL Lung Trial. The Journal of Heart and Lung Transplantation. 2013;32(4):S16–S17. [Google Scholar]
- 23.Israni A, Zaun D, Rosendale J, Schaffhausen C, McKinney W, Snyder J. OPTN/SRTR 2019 Annual Data Report: Deceased Organ Donors. American Journal of Transplantation. 2021;21:521–558. [DOI] [PubMed] [Google Scholar]
- 24.Quintini C, Martins PN, Shah S, et al. Implementing an innovated preservation technology: The American Society of Transplant Surgeons’(ASTS) Standards Committee white paper on ex situ liver machine perfusion. American Journal of Transplantation. 2018;18(8):1865–1874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


