Abstract
Background:
Cerebral edema is associated with worse outcome after acute stroke, however, the minimum clinically relevant threshold remains unknown. This study aimed to identify the minimal degree of midline shift (MLS) that predicts outcome in a cohort encompassing a broad range of acute stroke patients.
Methods:
Patient-level data from six acute stroke clinical trials were combined with endovascular thrombectomy registries from two academic referral centers, generating a combined cohort of 1,977 patients. MLS was extracted from the original trial data or was measured on computed tomography or magnetic resonance imaging a median of 47.0 hours (IQR 27.0, 75.1) after stroke onset. Logistic regression was performed to identify predictors of poor outcome and the minimal clinically relevant MLS threshold.
Results:
The presence of MLS was a predictor of poor outcome, independent of baseline clinical and demographic factors (adjusted OR 4.46, 95% CI 3.56 – 5.59, p < 0.001). Examining the full range of MLS values identified >3mm as the critical threshold that significantly predicted poor outcome (aOR 3.20 [1.31 – 7.82], p = 0.011).
Conclusion:
These results show that the presence of MLS predicts poor outcome and, specifically, MLS value >3mm is an important threshold across a variety of clinical settings. These findings may have relevance for the design and interpretation of future trials for anti-edema therapies.
Keywords: Brain edema, Ischemic stroke, Neuroimaging
Introduction
Following large hemispheric infarction, secondary injury from cerebral edema contributes to early neurological deterioration and worsens long-term clinical outcomes1. Whether cerebral edema negatively impacts outcome after mild or moderate stroke is controversial. One limitation is that midline shift (MLS) may be less sensitive to smaller infarctions. While this has led to the development of several additional novel techniques for quantifying edema 2–6, MLS remains the gold standard imaging marker7. However, there remains a need to define the minimum degree of MLS that is clinically significant, which could inform its use as a surrogate endpoint marker in ongoing and future trials of anti-edema therapy.
In the present analysis, we assembled a large cohort of ischemic stroke patients with patient-level data from multiple ischemic stroke trials and registries encompassing a broad range of stroke severity and across different periods of time. We sought to verify the link between MLS and outcome across this diverse cohort and to define the minimal clinically significant amount of MLS.
Subjects and methods
Patient characteristics
Patients were drawn from the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET, NCT00238537)8, the National Institute of Neurological Disorders and Stroke r-tPA Study9, the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy trial (MR RESCUE, NCT00389467)10, the Massachusetts General Hospital Specialized Program of Translational Research in Acute Stroke (SPOTRIAS) cohort, the Glyburide Advantage in Malignant Edema and Stroke trial (GAMES-RP, NCT01794182)11, and the Screening Technology and Outcomes Project in Stroke (StopStroke) cohort. These data were combined with acute stroke thrombectomy registry data from Brigham and Women’s Hospital (BWH; 2016–2020) and Yale New Haven Hospital (YNHH; 2018–2020). Patients were included if they had an anterior circulation stroke with outcome data available in the form of modified Rankin Scale (mRS) score and midline shift measurements or raw imaging data available. The study protocol was reviewed and approved by the Institutional Review Boards at Mass General Brigham and Yale University.
Image Analysis
MLS and stroke volume were previously measured in EPITHET, MR RESCUE and GAMES-RP trials. For the NINDS tPA trial, stroke volumes are available, and MLS was coded as present or absent. In SPOTRIAS, StopStroke and the stroke registry cohorts, MLS was measured on computed tomography or magnetic resonance imaging axial FLAIR sequence by first laying a straight line between the anterior and posterior attachment of the falx cerebri. MLS was quantified by drawing and measuring a second, perpendicular line to the septum pellucidum at the point of maximal deviation from the midline. Stroke volume was measured on diffusion-weighted MRI or follow-up computed tomography using a semi-automated seed-based method (AnalyzePro, Analyze Direct, Minneapolis, MN). Image analysis was performed while blinded to outcome data. When multiple scans were available, we recorded MLS from the scan (either MRI or CT) with the greatest degree of shift. Details on timing of scans used for volume and MLS measurement are available in Supplementary Table 1. We divided those patients with quantified MLS (i.e., all but the NINDS subjects) into strata based on the following levels of shift: 0mm (n = 944), >0–2mm (n=86), >2–4mm (n=123), >4–6 mm (n=77), >6–8 mm (n=43), >8–10mm (n=32) and >10 mm (n=64).
Statistical analysis
Baseline characteristics were summarized as mean and standard deviation for normally distributed continuous variables, median and interquartile range for non-normal continuous or ordinal variables, and as percentages for dichotomous variables. Associations with outcome across the modified Rankin Scale (mRS) were tested using generalized ordinal regression with partial proportional odds models. Dichotomous outcomes, with poor outcome defined as mRS 4–6, were tested using univariate and multivariable logistic regression. Non-normal continuous variables were log-transformed prior to regression analysis. All analyses were performed using Stata 15 (StataCorp, College Station, TX).
Results
Study population
Of 2,255 subjects enrolled in EPITHET (n = 100), NINDS (n = 624), MR RESCUE (n = 118), SPOTRIAS (n = 461), GAMES-RP (n = 86), StopStroke (n = 136) and the BWH (n = 225) and YNHH (n = 505) stroke registries with anterior circulation strokes, 1,977 subjects were included in the present analysis. One hundred seventeen (117) patients were excluded because they did not include data on midline shift and imaging was not available to obtain these data. An additional 161 patients were excluded because they did not have mRS values at 3 months after stroke onset. Characteristics of the final cohort are summarized in Table 1. The mean patient age was 69±14 years, 50.5% were female. Median NIHSS was 14 [IQR 7, 19] and 24.3% of patients had any degree of midline shift. Median time since last seen well for measurement of MLS was 47.0 hours [27.0, 75.1] in the 1120 patients with detailed scan timing available. Those patients with shift had MLS measured at a later timepoint (63.0 hours [35.6, 92.0]) versus those without MLS (39.4 hours [24.8, 86.4], p < 0.0001). This difference was driven largely by data from MR RESCUE and GAMES-RP, which had high proportions of patients with shift and the latest median scan times at 157 and 79.7 hours, respectively (Supplementary Table 3). Among patients with measurable shift, there was no association between degree of shift and time-to-scan (r2 = 0.0025, p = 0.18).
Table 1:
Characteristics of the study cohort
| n = 1976 | |
|---|---|
| Age, years, mean±SD | 68±14 |
| Sex, female, n (%) | 997 (50.5) |
| Admission blood glucose (mg/dL), median [IQR] | 123 [106, 151] |
| Comorbidities, n (%) | |
| Diabetes mellitus | 425 (21.8) |
| Hypertension | 1367 (70.2) |
| Intervention | |
| Received IV tPA, n (%) | 734 (38.3) |
| Received mechanical thrombectomy, n (%) | 657 (34.2) |
| Baseline NIHSS, median [IQR] | 14 [7, 19] |
| Stroke volume, mL, median [IQR] | 18.9 [3.2, 77.5] |
| Midline shift, n (%) | 481 (24.3) |
Midline shift is independently associated with outcome
The presence of MLS was associated with an increased risk of worse outcome across the mRS (Figure 1; OR ranged from 3.3 to 13.8, p < 0.001 for all transitions, see Supplementary Table 3 for details). When examining mRS as a dichotomized outcome, MLS similarly predicted poor outcome (OR 4.46, 95% CI 3.56 – 5.59, p < 0.001). As a sensitivity analysis, we divided the cohort by thrombectomy status, finding that MLS was associated with outcome in both those who did (OR 2.69 [95% CI 1.88 – 3.84], p < 0.001) and did not (OR 5.66 [4.20 – 7.62], p < 0.001) undergo thrombectomy. Other univariate predictors of outcome in the complete cohort were age, admission NIHSS score, admission stroke volume, admission blood glucose, hypertension, diabetes and use of tPA (Table 2). Midline shift remained an independent predictor of poor outcome in a multivariable model adjusting for all univariate predictors with age, sex, history of diabetes, history of hypertension, admission NIHSS, stroke volume and tPA use remaining in the final model.
Figure 1. Neurologic outcome by presence of MLS.
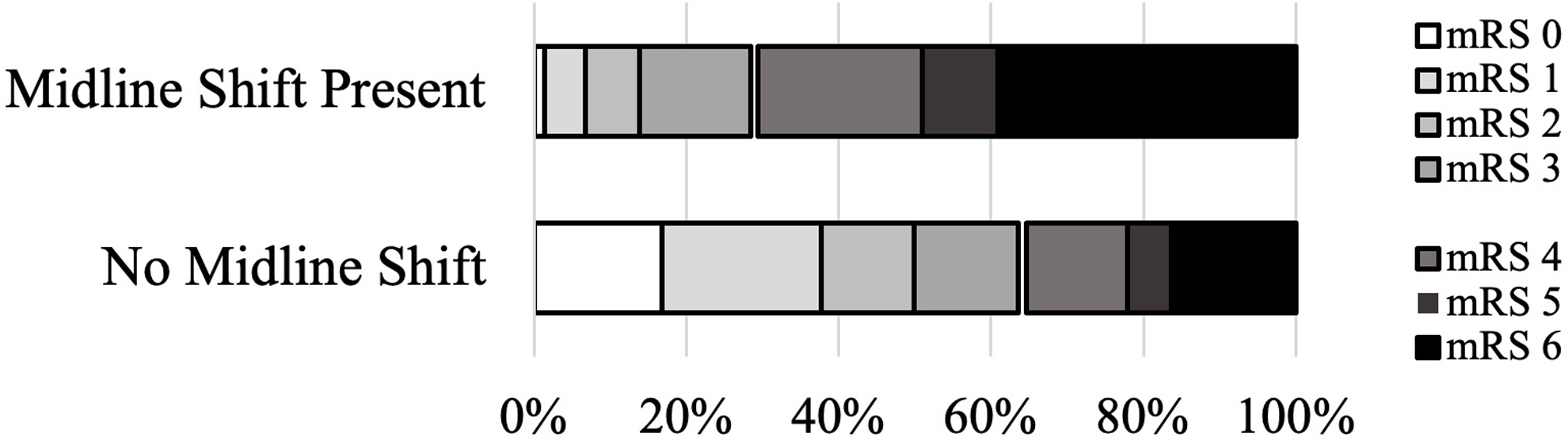
The presence of midline shift was associated with increased risk of poor outcome in ordinal analysis (OR range 3.3 to 13.8, p < 0.001 for all transitions, see Supplementary Table 1 for details).and when dichotomized to define poor outcome as mRS 4–6 (OR 4.46, 95% CI 3.56 – 5.59, p < 0.001).
Table 2:
Predictors of poor outcome
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| OR | 95% CI | p | Adjusted OR | [95% CI] | p | |
| Midline shift (yes or no) | 4.46 | [3.57 – 5.59] | < 0.001 | 2.33 | [1.69 – 3.22] | <0.001 |
| Age | 1.05 | [1.04 – 1.06] | < 0.001 | 1.07 | [1.06 – 1.08] | <0.001 |
| Sex (female) | 1.26 | [1.05 – 1.50] | 0.011 | 1.34 | [1.04 – 1.72] | 0.025 |
| History of diabetes | 1.64 | [1.32 – 2.04] | < 0.001 | 1.86 | [1.29 – 2.67] | 0.001 |
| History of hypertension | 1.92 | [1.57 – 2.35] | < 0.001 | 1.39 | [1.03 – 1.86] | 0.03 |
| Admission NIHSS Score | 1.17 | [1.15 – 1.19] | < 0.001 | 1.13 | [1.11 – 1.15] | <0.001 |
| Stroke volume (log) | 1.73 | [1.61 1.86] | < 0.001 | 1.46 | [1.33 – 1.60] | <0.001 |
| Admission glucose (log) | 1.83 | [1.40 – 2.40] | < 0.001 | 0.98 | [0.64 – 1.50] | 0.916 |
| Thrombectomy | 1.69 | [1.40 – 2.05] | < 0.001 | 0.8 | [0.16 – 1.05] | 0.109 |
| IV tPA (yes or no) | 0.80 | [0.66 – 0.96] | 0.016 | 0.65 | [0.50 – 0.85] | 0.001 |
A midline shift of >3mm is clinically relevant
To identify the degree of midline shift that impacts clinical outcome, we analyzed the subset of 1369 patients with categorized degree of shift in a model that adjusted for age, sex, history of diabetes, history of hypertension, admission NIHSS, stroke volume and tPA use. There were increasing odds of poor outcome with increasing degree of shift (Figure 2A and B), with shifts greater than 2mm associated with significantly increased risk (aOR 1.69 [1.00 – 2.85], p = 0.048). We then performed a further sensitivity analysis by subdividing the group of patients with MLS >2–4mm. We found no association between MLS >2–3mm and poor outcome (aOR 1.23 [0.67 – 2.30], p = 0.50) but MLS >3–4mm continued to have a significant association (aOR 3.20 [1.31 – 7.82], p = 0.011). Based on this identification of a threshold at 3mm, we collapsed the MLS bins into 0mm, >0–3mm, and >3mm finding that only in the latter group was shift associated with outcome (aOR 3.36 [2.08, 5.41], p < 0.001; Figure 2C).
Figure 2. Relationship between degree of MLS and neurologic outcome.
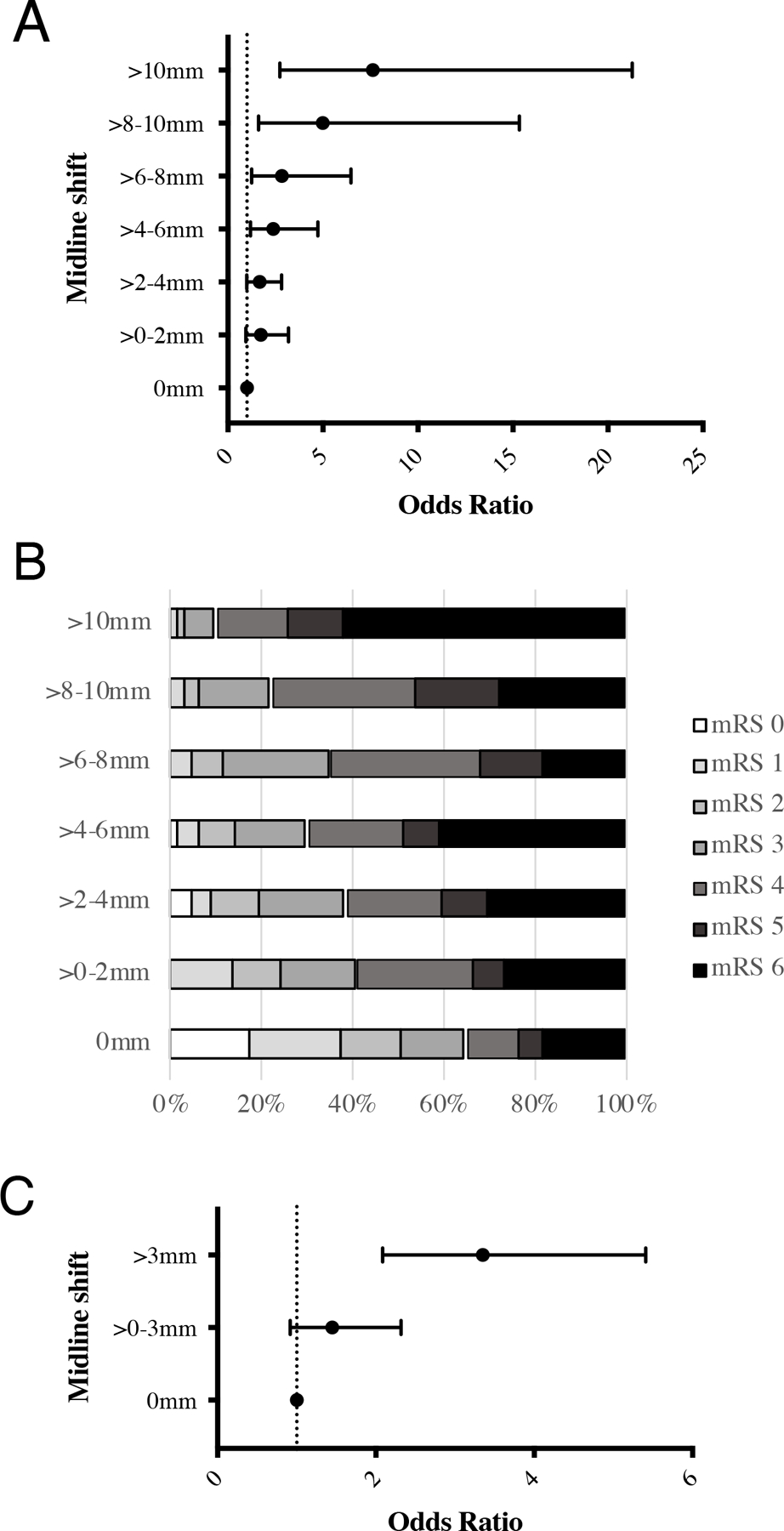
A) Odds of poor outcome increase with increasing degree of MLS. MLS above 2mm was independently associated with a significant risk of poor outcome (aOR 1.69 [1.00 – 2.85], p = 0.04). B) Stacked bar chart of mRS values for each MLS bin. C) Odds of poor outcome increase with increasing degree of MLS, MLS above 3mm is independently associated with a significant risk of poor outcome.
Discussion
In this study, we sought to identify the minimally clinically important degree of midline shift using a large cohort of ischemic stroke patients. We generated this cohort to include a wide range of stroke severity and included patients who did and did not receive reperfusion therapy to achieve the broadest possible applicability of our findings. We identified an association between midline shift and functional outcome, independent of other clinical predictors. We were then able to quantify the degree of shift associated with a significant change in clinical outcome, identifying 3mm as a key threshold level.
Most prior studies of mass effect from ischemic cerebral edema have focused on its impact in large hemispheric infarction3,11–16. Furthermore, the bulk of these studies were conducted prior to the widespread use of thrombectomy. While we have previously shown an effect of edema in small stroke2 and following revascularization1,17,18, these studies relied on more complex measures of edema volume that are not easily used at the bedside. The present analysis improves on these studies by verifying the relevance of MLS in a broad range of stroke severity and in the context of endovascular thrombectomy. Our results suggest that midline shift remains a relevant measure of edema-related mass effect in current clinical stroke care.
We identified a threshold level of shift of 3mm to predict poor outcome after stroke. In prior studies, 5mm was identified as a threshold that led to alterations in level of consciousness7 and indicates need for surgical intervention19. Our findings suggest that a lesser amount of shift may be clinically relevant and could be important when designing trials and identifying intermediate outcomes in future trials of anti-edema therapy. While there have been animal studies correlating the degree of post-stroke MLS with functional outcome20, such quantification has not been reported in human stroke.
There are several limitations to this study. First, timing of stroke volume and MLS measurement varied between the individual cohorts due to differences in the way individual trials were designed and registry data was collected. It is possible that measurement on later scans may have resulted in greater degree of shift. Conversely, those subjects with larger strokes and at greater risk for mass effect may have been more likely to receive more frequent scans, therefore making later scans available for analysis. Reassuringly, we did not find any relationship between scan time and degree of shift. Subjects from the NINDS tPA trial (n = 608) only had data indicating the presence of shift, but not the numerical value of that shift. These patients could not be included in the quantitative analysis of shift. This quantitative analysis was also limited by skewness in the distribution of values, with approximately 75% of patients having no measurable MLS. This precluded linear regression analysis, however, we addressed by binning MLS into 2mm increments with additional sensitivity analysis through further classification within the >2–4mm bin.
Summary
Taken together, our findings suggest that midline shift is an independent predictor of clinical outcomes after acute stroke, irrespective of reperfusion and/or thrombectomy therapy. Midline shift greater than 3mm was identified as a critical threshold with clinical relevance. Our data support the continued relevance of midline shift as a clinical tool to quantify mass effect from post-stroke edema.
Supplementary Material
Funding/Support
This work was funded by NIH K23NS112474 (M.B.B.), AAN CRTS AI18–0000000062 (M.B.B.), NIH R01 NS099209 (W.T.K.), and AHA 20SRG35540018 (W.T.K.).
Footnotes
Details
Disclosure of Potential Conflict of Interests (COI)
Dr. Bevers reports current grants from NIH K23NS112474 and AAN CRTS AI18–0000000062; in addition, Dr. Bevers reports prior grants from Andrew David Heitman Neurovascular fund, and prior grants and personal fees from Biogen, personal fees from Atlas Ventures, and personal fees from Dynamed.
Dr. Kimberly reports grants from NIH R01 NS099209 and grants AHA 20SRG35540018 related to the current work; in addition, Dr. Kimberly reports grants and personal fees from Biogen, and grants and personal fees from NControl Therapeutics outside the submitted work; in addition, Dr. Kimberly has a patent to PCT/US2018/018537 pending and licensed.
Dr. Sheth reports grants from Biogen, AHA, Hyperfine, NIH, Novartis and Bard. He reports consulting fees from NControl Therapeutics and Zoll, outside the submitted work. He is on a data safety monitoring board for Zoll. He has patents for wearables and nanoparticles, unrelated to the submitted work. Dr. Sheth reports stock options in Alva Health.
Dr. Lev reports grants from GE Heathcare and Siemens Healthcare. He reports consulting fees from GE Healthcare and Takea/Roche-Genentech outside the submitted work. He reports patents pending in Electrical Impedance Spectroscopy and machine learning for CT scan lesion detection.
All other authors report no COI disclosures.
References
- 1.Kimberly WT, Dutra BG, Boers AMM, et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: A secondary analysis of the MR CLEAN Trial. JAMA Neurol 2018;75(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battey TWK, Karki M, Singhal AB, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke 2014;45(12):3643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vorasayan P, Bevers MB, Beslow LA, et al. Intravenous Glibenclamide Reduces Lesional Water Uptake in Large Hemispheric Infarction. Stroke 2019;50(11):3021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minnerup J, Broocks G, Kalkoffen J, et al. Computed tomography–based quantification of lesion water uptake identifies patients within 4.5 hours of stroke onset: A multicenter observational study. Ann Neurol 2016;80(6):924–34. [DOI] [PubMed] [Google Scholar]
- 5.Dhar R, Yuan K, Kulik T, et al. CSF Volumetric Analysis for Quantification of Cerebral Edema After Hemispheric Infarction. Neurocrit Care 2016;24(3):420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harston GWJ, Carone D, Sheerin F, Jenkinson M, Kennedy J. Quantifying Infarct Growth and Secondary Injury Volumes: Comparing Multimodal Image Registration Measures. Stroke 2018;49(7):1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med 1986;314(15):953–8. [DOI] [PubMed] [Google Scholar]
- 8.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008;7(4):299–309. [DOI] [PubMed] [Google Scholar]
- 9.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333(24):1581–7. [DOI] [PubMed] [Google Scholar]
- 10.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368(10):914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016;15(11):1160–9. [DOI] [PubMed] [Google Scholar]
- 12.Kimberly WT, Bevers MB, Demchuk AM, et al. Effect of IV glyburide on adjudicated edema endpoints in the GAMES-RP Trial. Neurology 2018;91:e2163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torbey MT, Bösel J, Rhoney DH, et al. Evidence-Based Guidelines for the Management of Large Hemispheric Infarction: A Statement for Health Care Professionals from the Neurocritical Care Society and the German Society for Neuro-Intensive Care and Emergency Medicine. Neurocrit Care 2015;22(1):146–64. [DOI] [PubMed] [Google Scholar]
- 14.Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee JM. Enhanced Detection of Edema in Malignant Anterior Circulation Stroke (EDEMA) Score. Stroke 2017;48(7):1969–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung C, Murek M, Z’Graggen WJ, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke 2012;43(12):3207–11. [DOI] [PubMed] [Google Scholar]
- 16.Thomalla G, Hartmann F, Juettler E, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol 2010;68(4):435–45. [DOI] [PubMed] [Google Scholar]
- 17.Irvine HJ, Ostwaldt A-C, Bevers MB, et al. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cereb Blood Flow Metab 2017;0271678X1772055. [DOI] [PMC free article] [PubMed]
- 18.Bevers MB, Battey TWK, Ostwaldt A-C, et al. Apparent Diffusion Coefficient Signal Intensity Ratio Predicts the Effect of Revascularization on Ischemic Cerebral Edema. Cerebrovasc Dis 2018; [DOI] [PMC free article] [PubMed]
- 19.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80(1):6–15. [DOI] [PubMed] [Google Scholar]
- 20.Scheulin KM, Jurgielewicz BJ, Spellicy SE, et al. Exploring the predictive value of lesion topology on motor function outcomes in a porcine ischemic stroke model. Sci Rep 2021;11(1):3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


