Abstract
The present study evaluated brain development in persons with isolated cleft lip and/or cleft palate (iCL/P) compared to unaffected controls using an accelerated longitudinal design. A sample of 134 males and females, ages 7–27 years, with iCL/P (184 observations, total) was compared to 144 unaffected controls (208 evaluations, total) on Wechsler Index scores and volumetric data from structural MRI scans. Boys with isolated cleft palate had verbal IQ 15.5 points lower than perceptual IQ; a clinically significant difference. Participants with iCL/P had differential growth trajectories of regional cerebrum matter and consistently lower volumes of cerebellar gray matter and subcortical matter.
Introduction
Oral clefts are among the most common congenital anomalies, with population-based estimates at 10.25 per 10,000 live births (Mai et al., 2019). Of these cases, roughly 70% are considered “isolated” or not in connection with a known syndrome (Schutte & Murray, 1999). Isolated cleft lip and/or palate (iCL/P) can be further differentiated into isolated cleft lip only (iCL), isolated cleft palate only (iCP), or isolated cleft lip and palate (iCLP). Although isolated clefts are not associated with any known genetic syndromes or causes, decades of research have shown that iCL/P is associated with outcomes beyond facial structure. Research has found subtle, but meaningful, differences in neuropsychological functioning (Conrad, Nopoulos, et al., 2010; Nopoulos, Berg, VanDemark, et al., 2002; Nopoulos et al., 2007) as well as neuronal structure (Conrad et al., 2020; Nopoulos, Berg, Canady, et al., 2002; Nopoulos et al., 2007; Shriver et al., 2006) and activation (Conrad et al., 2015; Rao et al., 2020; Zhang et al., 2017) between persons with iCL/P compared to unaffected controls, from infancy to adulthood. These neuronal abnormalities have been related to differences in behavior (Nopoulos et al., 2010), socialization (Boes et al., 2007), cognition (Shriver et al., 2006), learning (Conrad et al., 2015), and speech (Conrad, Dailey, et al., 2010) suggesting a link between neuronal findings and functioning. While early exposure to anesthesia, obstructed airway, disrupted hearing and speech, and psychosocial stressors are likely related factors in these outcome findings, the presence of consistent sex and cleft type effects (i.e., males with palatal involvement tend to score lower than females and males with iCL; Collett et al., 2014; Conrad, 2018; Wehby et al., 2015) suggests an underlining biological link.
Etiological Theories for Neuronal Differences in Persons with iCL/P
There are 3 leading theories that have been discussed on why differences in brain structure exist for persons with iCL/P. First, authors have argued that early and frequent exposure to anesthesia may contribute to abnormal brain development and functional impairments (Laub & Williams, 2015). Animal studies have demonstrated neural cell death and associated learning problems following early exposure (Jevtovic-Todorovic et al., 2003) but human studies have shown minimal impact of short and single exposure (Conrad et al., 2021; Glatz et al., 2017; Hansen et al., 2011). No prospective research in iCL/P has been conducted to evaluate the impact of exposure to anesthesia, but preliminary work has presented possible neuronal differences in infants, before exposure to anesthesia (Conrad et al., 2020). The second theory suggests that obstructed airways and reduced oxygenation related to clefts contributes to cognitive deficits (Cielo, Konstantinopoulou, et al., 2016; Cielo, Montalva, et al., 2016; Cielo, Taylor, et al., 2016; Muntz, 2012). Only one study to date has been conducted to evaluate this possibility and found issues in sleep to be correlated to lower performance on cognitive tasks at 3 years of age (Smith et al., 2014). The third theory proposes that given the shared origination of the cells that make up the brain and face, abnormal migration of cells during facial development occurs concurrently with abnormal migration of neuronal cells (Kjaer, 1995; Sperber, 1992).
Key Studies Examining Brain Differences in Populations with iCL/P
The first imaging study in iCL/P was conducted by Nopoulos and colleagues (Nopoulos, Berg, Canady, et al., 2002) on 46 males with iCL/P and 46 unaffected males. Results demonstrated equal global brain volumes compared to unaffected controls, but regional analyses identified increased anterior cerebral gray matter (GM) and reduced posterior cerebral white matter (WM). Further analyses with this dataset found that for participants with iCL/P, enlarged cerebral spinal fluid was inversely correlated to cognitive functioning (Nopoulos et al., 2001; Nopoulos, Berg, VanDemark, et al., 2002), reduced orbitofrontal cortex was correlated to social dysfunction (Nopoulos et al., 2005), and disproportionately enlarged superior temporal plane was inversely related to IQ and language test scores but not to childhood hearing (Shriver et al., 2006).
This line of research was expanded with further cross sectional structural imaging studies on infant (Conrad et al., 2020; Yang et al., 2011) and child/adolescent (Adamson et al., 2014; Bodoni et al., 2020; Conrad et al., 2021; Conrad et al., 2015; Nopoulos et al., 2007) samples. The majority of these studies also reported equal global measures of intracranial volume (ICV) and whole brain volume (Bodoni et al., 2020; Conrad et al., 2021; Yang et al., 2011); only one study found decreased tissue in children/adolescents with iCL/P (Nopoulos et al., 2007). Within the cerebrum, decreased GM (Yang et al., 2011) and myelinated WM (Conrad et al., 2020) is noted in infancy. However, results are mixed for children/adolescents; Adamson and colleagues (2014) reported increased volume and cortical thickness across all cerebral lobes while Bodoni and colleagues (2020) found reductions in cortical thickness. Others have found differences regionally (i.e., differences only in the frontal and occipital lobe; Conrad et al., 2021; Nopoulos et al., 2007), between GM and WM (i.e., only increased GM; Adamson et al., 2014; Conrad et al., 2021; Nopoulos et al., 2007), and between sexes (i.e., patterns of increase vs decreased GM differing for males and females; Adamson et al., 2014; Nopoulos et al., 2007). Two findings that are consistent across the majority of these studies and across developmental stages include decreased subcortical volumes (Adamson et al., 2014; Bodoni et al., 2020; Nopoulos et al., 2007; Yang et al., 2011) and cerebellar volume (Conrad et al., 2020; Nopoulos, Berg, Canady, et al., 2002; Nopoulos et al., 2007).
Moving Forward in Structural Imaging Work
Multiple studies have called for longitudinal evaluation of brain abnormalities in patients with iCL/P (Bodoni et al., 2020; Conrad et al., 2020; Nopoulos et al., 2007; Yang et al., 2011); providing the opportunity to evaluate changes across time within participants, rather than cross-sectionally. Without longitudinal data on brain structure in persons with iCL/P, it is difficult to draw conclusions on brain structure and development. Inconsistent findings in different age groups, sexes, and cleft subtypes point to the gap in the literature of a longitudinal study connecting brain structure and function from infancy to adulthood. The purpose of the study is to examine brain development in subjects with iCL/P ages 7 – 27 years-old when compared to unaffected controls using an accelerated longitudinal design. This permits evaluation of a wide age range as well as collection of longitudinal data through repeat visits (Galbraith et al., 2017).
Materials and Methods
Recruitment and Procedures
All participants were enrolled on brain structure and neuropsychological function in children and young adults with iCL/P. Recruitment was conducted via letters send from cleft clinic patient lists (iCL/P) and local advertisements (controls). Exclusion criteria for all participants included: presence of braces or other metal in the body that would be contra-indicatory to an MRI scan, history of brain injury, and presence of a major medical (aside from the cleft among cases), neurologic, or psychiatric illness. For participants with iCL/P, if there was suspicion of a genetic syndrome the case was reviewed by a clinical geneticist and only included if deemed “non-syndromic”. Finally, to ensure comparison to a typically developing group, unaffected participants were excluded if they had history of a learning or attention deficit (as reported by parents during the screening process).
Participants assessed during the first funding period (N = 232; November 2002 - June 2007) were 7 – 17 years old and have been described previously (Nopoulos et al., 2007). A total of 232 participants (130 unaffected and 102 with iCL/P) were assessed during this phase. The second funding period (March 2009 – February 2013) permitted return assessments for the original participants, recruited additional participants, extended the age range to 27 years old, and expanded the testing battery. During this phase, 77 new participants were enrolled (23 unaffected and 54 with iCL/P), 110 returned for a second visit, 21 had a third visit, and 1 participant had a 4th visit. Across both funding periods of this project, there were a total of 311 participants with 441 observations. After review of records, 7 participants (13 assessments) were removed for not meeting inclusion criteria (1 = did not have a cleft as coded, 1 = preterm birth, 1 = ADHD diagnosis, 2 = syndromic cleft, and 2 = discontinued due to illness). Of the remaining 428 observations, 16 were removed as they did not have MRI scans and 20 were removed because the obtained MRI scan did not pass quality control. The final sample included 278 participants with 392 usable observations for analysis.
We employed an accelerated longitudinal design (ALD), which is a commonly used approach for studying brain development (Galbraith et al., 2017). ALD encompasses cross-sectional and longitudinal components, which enables coverage of a wide age range in a relatively short study period.
All visits took place at the University of Iowa with completion of the neuropsychological battery and MRI scan on the same day. All procedures were reviewed and approved by the Intuitional Review Board (IRB). For participants under 18 years of age, parents signed an Informed Consent Document and participants provided written Assent. For participants 18 years and older, the participant signed an Informed Consent Document. Families were reimbursed for travel costs and participants were compensated monetarily for participation.
Materials
Intellectual Assessment
All participants were administered select subtests from the age-appropriate version of the Wechsler Intelligence battery. For those ages 7 – 16 years old, subtests from the Wechsler Intelligence Scale for Children, 3rd Edition (WISC-III; Wechsler, 1991) were given. For those 17 years and older, subtests from the Wechsler Adult Intelligence Scale, 3rd Edition (WAIS-III; Wechsler, 1997) were given. All assessments were repeated at each visit and return visits were never scheduled less than a year apart (to limit repeat testing effects). For both the WISC-III and WAIS-III, Vocabulary, Block Design, and Matrix Reasoning subtests were administered to all participants. The Similarities subtest was added in the second year of the first study phase; subsequently, 50 (13%) are missing this subtest. Pro-rated Verbal IQ (VIQ; Vocabulary and Similarities) and pro-rated Perceptual IQ (PIQ; Block Design and Matrix Reasoning) were calculated. Note that for those without the Similarities subtest, pro-rated VIQ was based on the Vocabulary subtest alone. To identify possible uneven cognitive development across these two indices, a difference score between VIQ and PIQ was calculated (VIQ/PIQ Difference), where a difference score approaching 0 indicates similar performance on both scales; and a negative score indicates poorer performance on PIQ relative to VIQ. Finally, pro-rated Full Scale IQ (FSIQ) was calculated using the pro-rated VIQ and PIQ values. VIQ, PIQ, VIQ/PIQ Difference, and FSIQ could not be calculated for 2 participants (both unaffected).
Structural Brain Imaging
Images were obtained on either a 1.5-Tesla GE Signa MR scanner (GE Medical Systems, Milwaukee, WI) or a 1.5 Siemens Avanto scanner (Siemens AG, Muenchen, Germany). From November of 2002 through November of 2005, 195 scans (78 iCL/P & 117 unaffected) were run on the GE scanner. After July of 2006, 197 scans (106 iCL/P & 91 unaffected) were run on the Siemens scanner. Similar acquisition sequences were used across the scanners. At the time of the switch in machine use, a study was done imaging subjects on both scanners. The two machines were found to produce comparable measures (Andreasen et al., 2011). To directly address potential impact of scanner, we harmonized the neuroimaging data using the Combat harmonization approach (Fortin et al., 2018). Harmonization effectively removed scanner-induced variation (Supplemental Table 1). Statistical analyses were conducted on the harmonized data. Scanner was added for the aforementioned regions to account for any residual variation due to scanner.
Three different sequences were acquired at each visit. T1-weighted images, using a spoiled gradient recalled sequence, were acquired with the following parameters: 1.5-mm coronal slices, 40° flip angle, 24-millisecond repetition time (TR), 5-millisecond echo time (TE), two excitations (NEX), 26-cm field of view (FOV), and a 256×192 matrix. The proton density (PD) and T2-weighted images were acquired with the following parameters: 3.0-mm coronal slices, 36-millisecond TE (for PD) or 96-millisecond TE (for T2), 3000-millisecond TR, one NEX, 26-cm FOV, 256×192 matrix, and one echo train length.
Regions of interest (ROIs) were extracted using the BRAINSAutoWorkup pipeline which iteratively optimizes tissue classification producing robust brain parcellation in a multi-scanner setting (Young Kim & Johnson, 2013). BRAINSAutoWorkup labels regions using a multi-atlas, similarity-weighted, majority-vote procedure (i.e., joint label fusion; Wang et al., 2013) using expert-segmented templates adapted from the Desikan-Killiany atlas (Desikan et al., 2006).
It is important to account for intracranial volume (ICV) when comparing regions between groups, because regions scale with ICV. The goal of correction is to transform the region of interest (ROI) such that it is no longer related to ICV, which requires accounting for non-linear relationships. The power-proportion method (PPM) divides volume by β, where β is estimated from a non-linear regression model, ROI = αICVβ (34). We estimated β for each ROI, and divided each ROI by ICVβ. All ratios were subsequently standardized by subtracting out the grand mean, and dividing by the SD. The efficacy of detrending was checked by running linear regression models predicting the adjusted ROI from ICV. None of the estimates were statistically significant (see Supplementary Figure 1).
Statistical Analysis
Cognition
Mixed linear models were conducted to evaluate main effects of group and sex, as well as their interaction on FSIQ, VIQ, PIQ, and VIQ/PIQ Difference controlling for SES and random effect of participant. Models were also conducted to evaluate main and interaction effects of cleft type (i.e., unaffected, iCLO, iCPO, and iCLP) and sex.
Growth Trajectories of Brain Development in unaffected and iCL/P
To accommodate our study design, mixed linear models were conducted, which are designed to account for missing data. Across models, brain volume was predicted by the interaction between age and group. Additional predictor variables include sex and SES. Participant ID was included as a random effect to account for non-independency of observations. Because brain growth can be non-linear, we tested if adding non-linear components significantly improved the model fit. Age was expressed as a natural cubic spline with 2 degrees of freedom, which allows for 1 additional knot, plus the boundary knots. We also tested if additional knots significantly improved model fit. Trajectories for ICV, cerebrum (and each lobe), cerebellum, and subcortical regions (i.e., striatum, thalamus, amygdala, and hippocampus) were run separately. Main effects were considered significant at p < .05 and interaction effects were considered significant at p < .10. Correction for multiple comparisons was not conducted, therefore Cohen’s f2 were calculated to gauge the strength of the association of the reported effects (Selya et al., 2012). According to Cohen (1992), Cohen’s f2=.02 represents a small effect size; .15 a medium effect size and .35 a large effect size.
Growth Trajectories of Brain Development across Cleft Types
To identify potential differences in brain growth as a function of cleft type, mixed linear models were conducted within the iCL/P group only (i.e., iCLO, iCPO, and iCLP). Models were limited to regions where either a significant group main effect or age-by-group interaction effects were identified in the previous analyses. Given the smaller sample size, only main effects of cleft type, age, and sex were evaluated. As with previous models, SES was included as an additional predictor variable.
Results
Participants
An initial observation was obtained for 134 participants with iCL/P; 42 had a second visit and 8 had a third visit, for a total of 184 observations. Age at assessment ranged from 7 to 27 (mean = 15.59, SD = 5.07). One hundred and seventeen (64%) were male; consistent with the higher incidence of iCL/P in males. There were 43 participants with iCLO, 47 with iCPO, and 94 with iCLP. Consistent with the geographic location, 147 participants (80%) were White. The majority of participants (51%) were considered middle-class, based on a modified Hollingshead ordinal scale (Hollingshead, 1975).
An initial observation was obtained for 144 unaffected participants; 55 had a second visit and 9 had a third visit, for a total of 208 observations. Age at assessment ranged from 7 to 26 (mean = 14.90, SD = 4.32), which was not significantly different from participants with iCL/P (F (1, 390) = 2.124, p = .146). One hundred and three (48%) were male. The distribution of males and females differed significantly between groups (χ2 (1, 392) = 7.29, p = .007). Note that analyses were adjusted for sex. One hundred and ninety-seven participants (95%) were White. The distribution of White vs. Non-White was significantly different between groups (χ2 (1, 389) = 22.185, p < .001). The majority of unaffected participants (65%) were considered upper-middle class. The distribution of SES was significantly different between groups (χ2 (3, 392) = 29.12, p < .001; See Table 1 and Supplemental Table 2). It should be noted that statistical models included an SES predictor variable.
Table 1.
Demographics for all Observations.
| Unaffected | ICL/P | Overall | |
|---|---|---|---|
| Number of Unique Participants | 144 | 134 | 278 |
| Number of Observations | 208 | 184 | 392 |
| First Observation | 144 | 134 | 278 |
| Second Observation | 55 | 42 | 97 |
| Third Observation | 9 | 8 | 17 |
| Age at Observation | |||
| Mean (SD) | 14.9 (4.32) | 15.6 (5.07) | 15.2 (4.70) |
| Median [Min, Max] | 15.1 [7.08, 26.5] | 15.8 [7.00, 27.3] | 15.4 [7.00, 27.3] |
| Age Band at Observation | |||
| Number 7 – 11 years old | 45 | 41 | 86 |
| Number 12 – 14 years old | 54 | 40 | 94 |
| Number 15 – 18 years old | 70 | 57 | 127 |
| Number 19 – 22 years old | 33 | 29 | 62 |
| Number 23 – 27 years old | 6 | 17 | 23 |
| Sex | |||
| Females | 105 (50.5%) | 67 (36.4%) | 172 (43.9%) |
| Males | 103 (49.5%) | 117 (63.6%) | 220 (56.1%) |
| Race | |||
| White/Caucasian | 197 (94.7%) | 147 (79.9%) | 344 (87.8%) |
| Asian American | 2 (1%) | 19 (10.3%) | 21 (5.4%) |
| Black/African American | 1 (0.5%) | 2 (1.1%) | 3 (0.8%) |
| American Indian/Alaska Native | 0 (0%) | 3 (1.6%) | 3 (0.8%) |
| Native Hawaiian/Pacific Islander | 0 (0%) | 1 (0.5%) | 1 (0.3%) |
| Hispanic/Latino(a) | 3 (1.4%) | 5 (2.7%) | 8 (2%) |
| Multiracial | 3 (1.4%) | 6 (3.3%) | 9 (2.3%) |
| Not Provided | 2 (1%) | 1 (0.5%) | 3 (0.8%) |
| SES | |||
| High | 5 (2.4%) | 0 (0%) | 5 (1.3%) |
| Upper-Middle | 136 (65.4%) | 78 (42.4%) | 214 (54.6%) |
| Middle | 62 (29.8%) | 93 (50.5%) | 155 (39.5%) |
| Lower-Middle | 5 (2.4%) | 13 (7.1%) | 18 (4.6%) |
| Low | 0 (0%) | 0 (0%) | 0 (0%) |
Note. Demographics presented are for each observation; participants who had return visits are counted more than once. SES = Socioeconomic Status.
Out of the 278 unique participants, 97 (35%) returned at least once. Those who returned for at least 1 other visit were younger than individuals who did have return visits (mean = 12.05 (3.59) years vs 14.82 (4.76) years; F (1, 278) = 25.256, p < .001). Given the age range of the study, younger participants would have more opportunities to return than older participants. There were no differences in patient type (χ2 (1, 278) = 1.434, p = .231), cleft type (χ2 (3, 278) = 2.466, p = .481), sex (χ2 (1, 278) = 1.574, p = .210), race (χ2 (1, 275) = 1.019, p = .313), ethnicity (χ2 (1, 276) = 1.879, p = .170), socioeconomic status (χ2 (3, 278) = 5.072, p = .167), or FSIQ (F (1, 275) = 0.026, p = .872).
Cognition
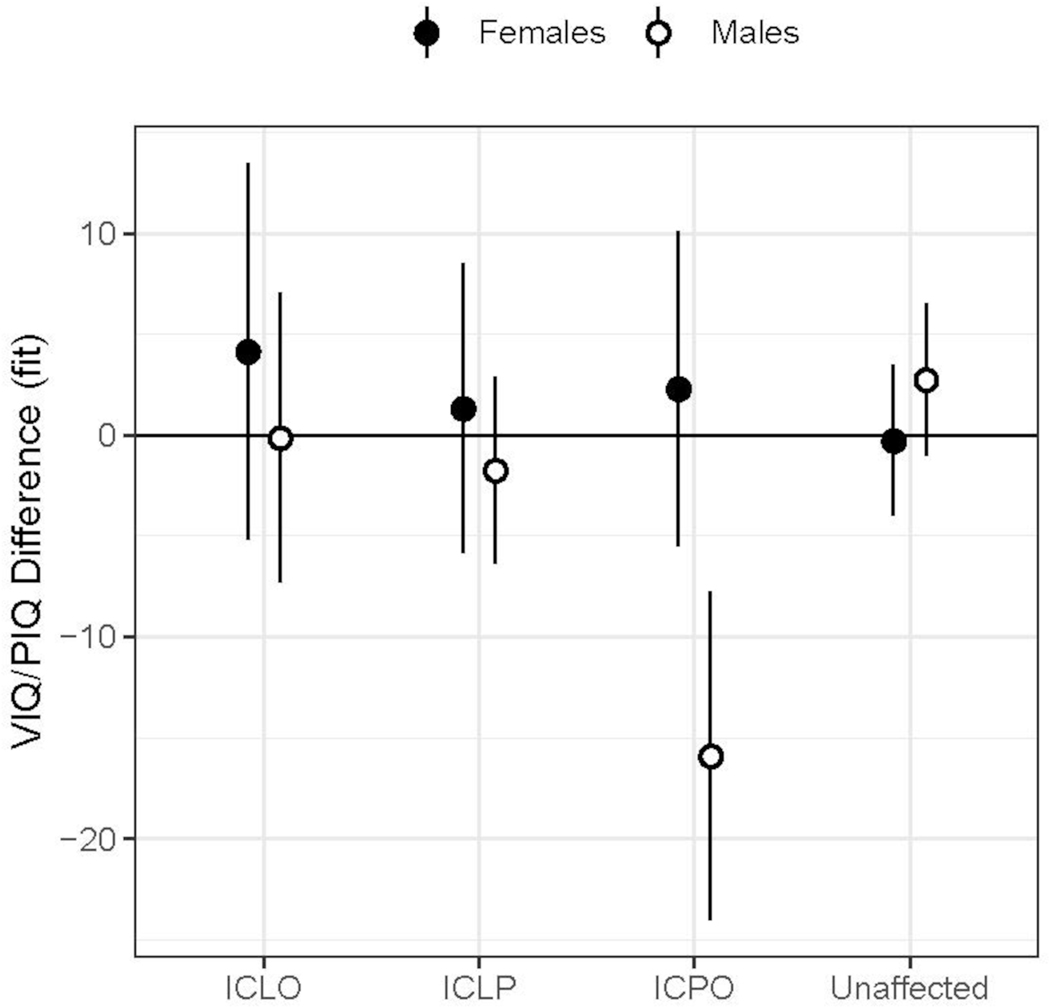
Results of the mixed linear models evaluating sex and group differences in cognitive index scores are presented in Supplemental Tables 3 and 4. Mean Index scores were in the average to above average range for all subgroups with significant main effects of group and sex. For VIQ/PIQ difference scores, there was a significant sex by cleft type interaction where boys with iCPO had VIQ an average of 15.5 points (SD = 14.2) below their PIQ (See Figure 1).
Figure 1.
VIQ-PIQ Difference Scores by Cleft Type and Sex.
Difference scores between VIQ and PIQ (y-axis) across groups (x-axis) for females (filled circles) and males (open circles) separately. Estimated marginal means (EMMs) are shown along with 95% confidence limits of EMMs. The EMMs were adjusted for sex, SES and random effects of participants.
Growth Trajectories of Brain Development in unaffected and iCL/P
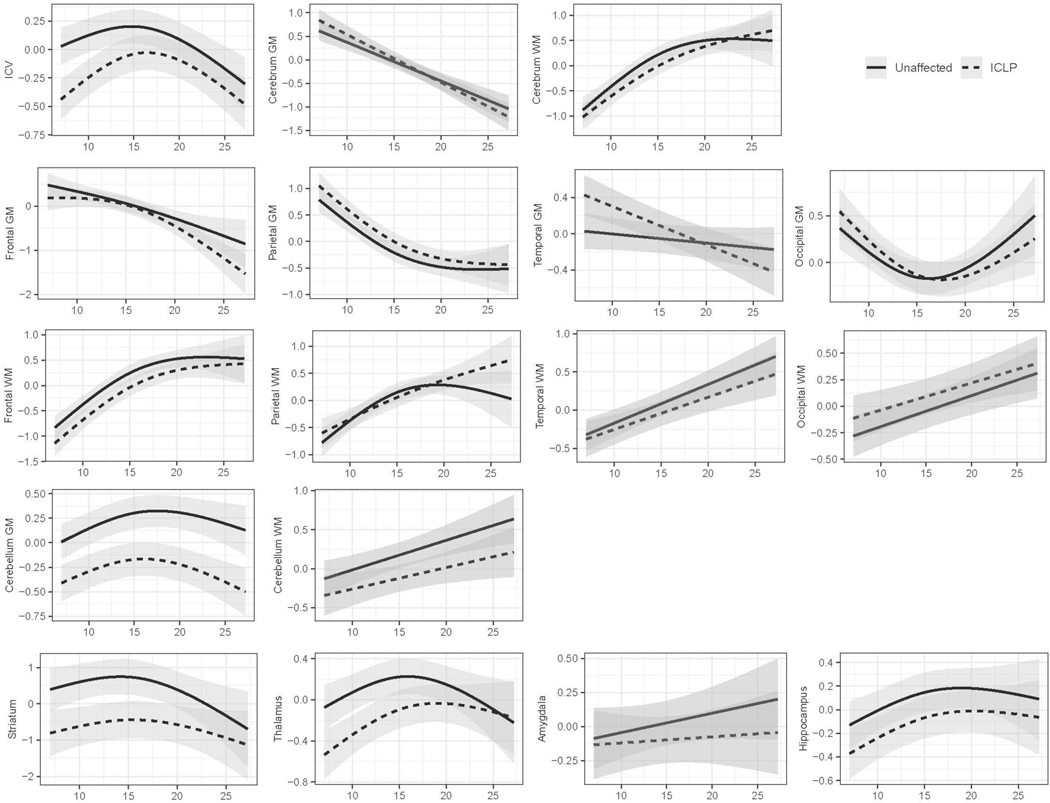
Statistics for main effects (age, sex, and group) and interaction effects (age*group) across all models are presented in Table 2 and visually depicted in Figure 2. Cerebrum WM, temporal WM, occipital WM and cerebellum WM were fitted using a linear model. Addition of an additional knot was determined to be a better fit for all other regions included in the analysis.
Table 2.
Summary Statistics for Growth Trajectories of Brain Development (Unaffected vs iCL/P).
| Age * Group | Age | Group | Sex | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X2 | p | f2 | X2 | p | f2 | X2 | p | f2 | X2 | p | f2 | |
| ICV | 12.70 | .002 | 0.319 | 23.24 | < .001 | 0.764 | 15.00 | < .001 | 0.198 | 87.40 | < .001 | 0.562 |
| Cerebrum GM* | 1.84 | .175 | 0.089 | 68.00 | < .001 | 0.851 | 2.22 | .136 | 0.086 | 2.87 | .090 | 0.108 |
| Frontal GM | 2.95 | .229 | 0.120 | 36.80 | < .001 | 0.630 | 2.10 | .147 | 0.084 | 0.05 | .820 | 0.014 |
| Parietal GM | 0.48 | .787 | 0.052 | 109.87 | < .001 | 1.090 | 2.38 | .123 | 0.088 | 0.19 | .665 | 0.027 |
| Temporal GM* | 7.69 | .006 | 0.220 | 1.76 | .185 | 0.366 | 8.73 | .003 | 0.177 | 15.14 | < .001 | 0.238 |
| Occipital GM | 3.15 | .207 | 0.143 | 23.93 | < .001 | 0.627 | 1.12 | .290 | 0.057 | 0.09 | .764 | 0.018 |
| Cerebrum WM | 1.40 | .497 | 0.081 | 106.10 | < .001 | 0.973 | 0.57 | .452 | 0.043 | 5.69 | .017 | 0.144 |
| Frontal WM | 0.48 | .789 | 0.050 | 123.83 | < .001 | 1.110 | 3.12 | .077 | 0.100 | 6.02 | .014 | 0.148 |
| Parietal WM | 4.73 | .094 | 0.149 | 56.30 | < .001 | 0.675 | 0.86 | .355 | 0.053 | 3.81 | .051 | 0.117 |
| Temporal WM* | 0.43 | .514 | 0.047 | 34.07 | < .001 | 0.523 | <0.01 | .992 | 0.001 | 0.13 | .715 | 0.022 |
| Occipital WM* | 0.11 | .738 | 0.026 | 17.01 | < .001 | 0.403 | 0.89 | .347 | 0.055 | 2.88 | .089 | 0.102 |
| Cerebellum GM | 2.47 | .291 | 0.141 | 34.06 | < .001 | 0.634 | 10.22 | .001 | 0.164 | 10.97 | .001 | 0.199 |
| Cerebellum WM* | 0.44 | .507 | 0.044 | 12.75 | < .001 | 0.282 | 0.28 | .597 | 0.031 | 0.14 | .709 | 0.023 |
| Striatum | 1.35 | .509 | 0.097 | 7.14 | .028 | 0.251 | 7.23 | .007 | 0.139 | 0.31 | .581 | 0.033 |
| Thalamus | 4.72 | .095 | 0.180 | 9.58 | .008 | 0.435 | 7.84 | .005 | 0.148 | 0.04 | .836 | 0.013 |
| Amygdala* | 0.42 | .519 | 0.043 | 1.94 | .163 | 0.085 | 0.01 | .932 | 0.005 | 0.15 | .702 | 0.023 |
| Hippocampus | 0.19 | .908 | 0.037 | 14.17 | .001 | 0.448 | 2.56 | .110 | 0.082 | 0.19 | .666 | 0.026 |
Denotes linear models. Bold text denotes significance (p < .01 for interaction effects and p < .05 for main effects).
Figure 2.
Models of Brain Growth Trajectories by Group (unaffected vs iCL/P).
Mixed linear models for each region of interest (y-axis), with the effects of age (x-axis) and group (iCL/P = dashed line and unaffected = solid line) demonstrated. Models also controlled for sex and socioeconomic status.
Main Effects
Age was consistently associated with ROIs, except for the amygdala. Main effects of age (in the absence of a significant age*group interaction) reflected decreases in regional cerebral GM and increases in regional cerebral WM over time. Within the cerebellum, both GM and WM increased, with GM reaching a peak just before age 20 years. The striatum and hippocampus mirrored the trajectory of cerebellar GM, with volumes gradually increasing over time with a peak in later adolescence.
Some residual impact of sex after application of the power proportion was identified for several regions, including: ICV, temporal and cerebellar GM, and cerebral WM (particularly in the frontal lobe). For ICV and GM in the temporal lobe and cerebellum, males had a higher volume than did females. For cerebral/frontal WM, females had higher volumes than males.
Main effects of group (in the absence of a significant age*group interaction) were identified for cerebellar GM and the striatum. Both regions demonstrated smaller volumes for participants with iCL/P (See Table 2 and Figure 2).
Age-by-Group Interactions
Significant age*group interactions were identified for ICV, temporal GM, parietal WM, and the thalamus. Specifically, ICV was lower in the iCL/P group relative to the unaffected group throughout childhood and adolescence, but no differences were observed in early adulthood. Temporal GM volume decreased with age and at a higher rate for participants with iCL/P. Parietal WM volume peaked in the unaffected group around age 20, but kept increasing after age 20 years for participants with iCL/P. Finally, the thalamus grew for both groups with a leveling off at age 15 for unaffected participants and around age 20 for those with iCL/P. (See Supplementary Table 5 for full model statistics)
Growth Trajectories of Brain Development across Cleft Types
Significant group, or age*group interaction effects were observed for ICV, temporal GM, parietal WM, cerebellar GM, striatum, and thalamus volume. Subsequent mixed linear models evaluating the impact of cleft type were limited to these regions. No significant impact of cleft type was observed (See Supplemental Table 6).
Discussion
The current study is the first to report global and regional structural growth trajectories in participants with iCL/P compared to unaffected controls. Significant differences in cognitive profile as well as global and regional growth trajectory differences were identified.
Cognition
Consistent with previous research, participants with iCL/P had IQ scores within the average range, but significantly lower than unaffected controls. Of particular relevance is the finding that boys with iCPO had a significantly larger split between their VIQ and PIQ Wechsler scores. All other cleft types and unaffected controls had a mean split at or below 3.1 IQ points; boys with iCPO had a mean split of 15.5 IQ points (with VIQ less than PIQ). This difference is both statistically and clinically significant; where a discrepancy this large is usually indicative of a language disorder. Previous work has identified sex and cleft type differences where boys, particularly those with iCPO, are at the highest risk for language and reading disorders (Collett et al., 2014; Conrad, 2018; Conrad et al., 2014; Wehby et al., 2015). This finding provides additional support for differential roles of sex and cleft type and suggests that biological processes, rather than psychosocial factors, are primary drivers in observed differences in brain structure and development.
ICV Differences
The current analysis identified an interaction between age and presence of a cleft for ICV. As reported in the baseline findings from this sample, in childhood, those with iCL/P started with significantly lower volumes (Nopoulos et al., 2007). The growth models in the current study identified a steeper growth trajectory through adolescence compared to unaffected participants. After adolescence, there was a trajectory of decline in volume which was similar between the two groups. However, caution is warranted in interpreting findings from the later age ranges, as there were fewer participants ≥20 years old. The predicted decline in volume after age 20 is likely an artifact of the spline models that were utilized. Interpretation should be limited to childhood and early adolescence, where participants with iCL/P had significantly lower ICV in childhood with some catchup through adolescence. Further studies in young adults with iCL/P is required to understand later ICV trajectories.
Cerebrum Differences
Historically, there are mixed findings when the cerebrum is evaluated; decreased GM has been reported in infancy (Conrad et al., 2020; Yang et al., 2011) and increased GM (particularly with males) has been reported in childhood/adolescence (Adamson et al., 2014; Nopoulos et al., 2007) and adulthood (Nopoulos, Berg, Canady, et al., 2002). Evaluation of WM is conflicting across studies and age ranges. In the current study, no group differences were identified for cerebral GM or WM. However, when specific lobes were evaluated, differences in temporal GM and parietal WM were identified. The current study found significant differences in the growth trajectory of temporal GM, where participants with iCL/P started with a higher volume and there was a steep linear decline through the assessed age range. This resulted in significantly higher volume until adolescence and then significantly lower volume by young adulthood. This is consistent with decreased temporal GM that Nopoulos found in a separate sample of adult males with iCL/P (Nopoulos, Berg, Canady, et al., 2002). However, this is somewhat contradictory to the decreased GM volume in the left auditory cortex identified in infancy by Yang and colleagues (2011). This variance may be due to the small sample size of the Yang (2011) study (n = 27 iCL/P and 27 controls; whereas the current sample is approximately 6 times larger) or differences in scan acquisition or processing. Further collection of data with standardized image collection and processing is needed. For parietal WM, participants with iCL/P were fairly consistent with controls in volume throughout the assessed age range, until about 20 years-old, when the growth trajectory for unaffected controls leveled out but volumes continued to increase for those with iCL/P. This may be related to an artifact with the spline models given the smaller number of participants older than 20 years old and this finding should be interpreted with caution.
Cerebellum
The main effect of participants with iCL/P having lower cerebellar volume (specific to GM) across all ages is consistent with initial reports from this sample (Nopoulos et al., 2007). Additionally, it fits with preliminary reports of cerebellar volume in infants (Conrad et al., 2020) and findings from a separate adult male sample (Nopoulos, Berg, Canady, et al., 2002). Reduced cerebellar GM in persons with iCL/P is the most robust finding across the developmental trajectory and across different samples. Could this reduction be due to underlining genetic or biological factors associated with the cleft and a less visible part of the phenotype? Future research needs to ensure that measurement of the cerebellum (including tissue segmentation) is included and evaluated in relation to cleft type, sex, treatment factors, and outcome measures to establish the potential etiology and functional impact of this finding.
Subcortical
Finally, participants with iCL/P had lower volumes in the striatum (which includes the caudate, putamen, and accumbens) and thalamus, with some catch-up by adulthood. This is consistent with initial reports from this sample (Nopoulos et al., 2007) and replicates findings from infancy (Yang et al., 2011) and childhood/adolescence (Adamson et al., 2014; Bodoni et al., 2020). Evaluation of the growth trajectory provided insight into reported lack of differences in subcortical structures in adulthood (Nopoulos, Berg, Canady, et al., 2002).
This study does have some limitations that warrant mentioning. The sample was over-represented by White participants (87.8%) and there was a higher ratio of males in the iCL/P group (63.6%). Generalizing of findings should be made with caution. Most importantly, there were fewer participants that were ≥ 20 years of age, which lead to over-interpolation with natural splines. This was not problematic for most ROI, but did result in unusual findings for ICV (unexpected drop in volume after 20) and occipital GM (unexpected increase in volume after 20).
Conclusion
This study presents the largest evaluation of brain structure and function in persons with isolated oral clefts; including males and females from 7 through 27 years old and compared to a unaffected controls. Findings continue to reflect differential patterns of cerebral growth with consistently reduced cerebellar and subcortical volumes across the age span. Further research needs to extend this trajectory down and evaluate the earliest markers of brain structure and function in relation to genetics and medical factors. New imaging methods and protocols (e.g., Silent imaging; Alibek et al., 2014) increase the likelihood of success in scanning at younger ages and scanning very early (prior to surgery) can help determine the potential impact of anesthesia and airway obstruction on development.
Supplementary Material
Supplemental Figure 1. Non-Significant Linear Regression Models Predicting Adjusted ROI from ICV.
Linear regression models for each region of interest (y-axis) demonstrating the lack of relationship to ICV (x-axis) following power-proportion correction.
Acknowledgements:
We would like to thank Dr. Peg Nopoulos for permitting us to use data from her grant to conduct this study. We would also like to thank Dr. Ian DeVolder for his initial work on this project and continued support in finalizing the dataset and analyses.
Funding Details: Research reported in this publication was supported by the National Institutes of Health [grant numbers DE024511 (Dr. Conrad, PI), UL1TR002537, and R01 DEO14399-05 (Dr. Peg Nopoulos, PI)]. This work was conducted on an MRI instrument funded by 1S10OD025025-01.
Footnotes
Declaration of Interest Statement: The authors have no relevant financial or non-financial competing interests to report.
References
- Adamson CL, Anderson VA, Nopoulos P, Seal ML, & Da Costa AC (2014). Regional brain morphometric characteristics of nonsyndromic cleft lip and palate. Dev Neurosci, 36(6), 490–498. doi: 10.1159/000365389 [DOI] [PubMed] [Google Scholar]
- Alibek S, Vogel M, Sun W, Winkler D, Baker CA, Burke M, & Gloger H. (2014). Acoustic noise reduction in MRI using Silent Scan: an initial experience. Diagnostic and Interventional Radiology, 20(4), 360–363. doi: 10.5152/dir.2014.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, & Ho BC (2011). Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biological psychiatry, 70(7), 672–679. doi: 10.1016/j.biopsych.2011.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoni PSB, Leoni RF, do Vale AB, da Silva PHR, Meira Junior SG, Richieri Costa A, & Tabaquim MLM (2020). Neuropsychological functioning and its relationship with brain anatomical measures of children and adolescents with non-syndromic cleft lip and palate. Child Neuropsychology, 1–15. doi: 10.1080/09297049.2020.1776240 [DOI] [PubMed] [Google Scholar]
- Boes AD, Murko V, Wood JL, Langbehn DR, Canady J, Richman L, & Nopoulos P. (2007). Social function in boys with cleft lip and palate: relationship to ventral frontal cortex morphology. Behavioural brain research, 181(2), 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cielo CM, Konstantinopoulou S, & Hoque R. (2016). OSAS in Specific Pediatric Populations. Curr Probl Pediatr Adolesc Health Care, 46(1), 11–18. doi: 10.1016/j.cppeds.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Cielo CM, Montalva FM, & Taylor JA (2016). Craniofacial disorders associated with airway obstruction in the neonate. Semin Fetal Neonatal Med, 21(4), 254–262. doi: 10.1016/j.siny.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cielo CM, Taylor JA, Vossough A, Radcliffe J, Thomas A, Bradford R, . . . Marcus CL (2016). Evolution of Obstructive Sleep Apnea in Infants with Cleft Palate and Micrognathia. J Clin Sleep Med, 12(7), 979–987. doi: 10.5664/jcsm.5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen. (1992). Statistical Power Anaysis. Current Directions in Psychological Science, 1(3), 98–101. [Google Scholar]
- Collett BR, Wehby GL, Barron S, Romitti PA, Ansley TN, & Speltz ML (2014). Academic achievement in children with oral clefts versus unaffected siblings. J Pediatr Psychol, 39(7), 743–751. doi: 10.1093/jpepsy/jsu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL (2018). Are predictors of reading impairment in isolated cleft similar to those in idiopathic dyslexia? Ann Dyslexia. doi: 10.1007/s11881-018-00166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, Dailey S, Richman L, Canady J, Karnell MP, Axelson E, & Nopoulos P. (2010). Cerebellum Structure Differences and Relationship to Speech in Boys and Girls With Nonsyndromic Cleft of the Lip and/or Palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association, 47(5), 469–475. doi: 10.1597/08-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, Kuhlmann E, van der Plas E, & Axelson E. (2021). Brain Structure and Neural Activity Related to Reading in Boys with Isolated Oral Clefts. Child Neuropsychology. doi: 10.1080/09297049.2021.1879765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, McCoy TE, DeVolder I, Richman LC, & Nopoulos P. (2014). Reading in subjects with an oral cleft: speech, hearing and neuropsychological skills. Neuropsychology, 28(3), 415–422. doi: 10.1037/neu0000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, Nopoulos P, & Richman L. (2010). Brain Structure Correlates of Neurological Soft Signs in Children with Isolated CL/P. Paper presented at the American Cleft Palate-Craniofacial Association, Fort Worth, TX. [Google Scholar]
- Conrad AL, Richman L, & Nopoulos P. (2015). Reading Achievement in Boys With Non-Syndromic Cleft Palate Only: Relationship to Neuropsychological Skill and Neurocircuitry. Dev Neuropsychol, 40(7–8), 395–406. doi: 10.1080/87565641.2016.1142991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad AL, Wermke K, Eisenmann M, Kuhlmann E, Benavides A, Koscik T, & Magnotta V. (2020). Preliminary Evaluation of Pre-Speech and Neurodevelopmental Measures in 7–11 week Old Infants with Isolated Oral Clefts. Pediatric Research, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, . . . Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, . . . Shinohara RT (2018). Harmonization of cortical thickness measurements across scanners and sites. Neuroimage, 167, 104–120. doi: 10.1016/j.neuroimage.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith S, Bowden J, & Mander A. (2017). Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Stat Methods Med Res, 26(1), 374–398. doi: 10.1177/0962280214547150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, & Granath F. (2017). Association of Anesthesia and Surgery During Childhood With Long-term Academic Performance. JAMA Pediatr, 171(1), e163470. doi: 10.1001/jamapediatrics.2016.3470 [DOI] [PubMed] [Google Scholar]
- Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, & Christensen K. (2011). Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology, 114(5), 1076–1085. doi: 10.1097/ALN.0b013e31820e77a0 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four Factor Index of Social Status. New Haven: Department of Sociology, Yale University. [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, . . . Wozniak DF (2003). Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience, 23(3), 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer I. (1995). Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta odontologica Scandinavica, 53(3), 135–143. [DOI] [PubMed] [Google Scholar]
- Laub DR Jr., & Williams RK (2015). Neonatal Anesthesia Neurotoxicity: A Review for Cleft and Craniofacial Surgeons. Cleft Palate Craniofac J, 52(4), 494–498. doi: 10.1597/14-126 [DOI] [PubMed] [Google Scholar]
- Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, . . . National Birth Defects Prevention, N. (2019). National population-based estimates for major birth defects, 2010–2014. Birth Defects Res, 111(18), 1420–1435. doi: 10.1002/bdr2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz HR (2012). Management of sleep apnea in the cleft population. Curr Opin Otolaryngol Head Neck Surg, 20(6), 518–521. doi: 10.1097/MOO.0b013e3283585685 [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, & Andreasen NC (2002). Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genetics in Medicine, 4(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, & Andreasen NC (2001). Increased incidence of a midline brain anomaly in patients with nonsyndromic clefts of the lip and/or palate. J Neuroimaging, 11(4), 418–424. doi: 10.1111/j.1552-6569.2001.tb00072.x [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, & Andreasen NC (2002). Cognitive dysfunction in adult males with non-syndromic clefts of the lip and/or palate. Neuropsychologia, 40(12), 2178–2184. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Boes AD, Jabines A, Conrad AL, Canady J, Richman L, & Dawson JD (2010). Hyperactivity, impulsivity, and inattention in boys with cleft lip and palate: relationship to ventromedial prefrontal cortex morphology. Journal of neurodevelopmental disorders, 2(4), 235–242. doi: 10.1007/s11689-010-9060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Choe I, Berg S, Van Demark D, Canady J, & Richman L. (2005). Ventral frontal cortex morphology in adult males with isolated orofacial clefts: relationship to abnormalities in social function. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association, 42(2), 138–144. doi: 10.1597/03-112.1 [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Langbehn DR, Canady J, Magnotta V, & Richman L. (2007). Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med, 161(8), 753–758. [DOI] [PubMed] [Google Scholar]
- Rao B, Cheng H, Fan Y, Zhang W, Chen R, & Peng Y. (2020). Topological properties of the resting-state functional network in nonsyndromic cleft lip and palate children after speech rehabilitation. J Integr Neurosci, 19(2), 285–293. doi: 10.31083/j.jin.2020.02.19 [DOI] [PubMed] [Google Scholar]
- Schutte BC, & Murray JC (1999). The many faces and factors of orofacial clefts. Hum Mol Genet, 8(10), 1853–1859. doi: 10.1093/hmg/8.10.1853 [DOI] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, & Mermelstein RJ (2012). A Practical Guide to Calculating Cohen’s f(2), a Measure of Local Effect Size, from PROC MIXED. Front Psychol, 3, 111. doi: 10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver AS, Canady J, Richman L, Andreasen NC, & Nopoulos P. (2006). Structure and function of the superior temporal plane in adult males with cleft lip and palate: pathologic enlargement with no relationship to childhood hearing deficits. Journal of Child Psychology and Psychiatry, 47(10), 994–1002. [DOI] [PubMed] [Google Scholar]
- Smith CB, Walker K, Badawi N, Waters KA, & MacLean JE (2014). Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. Sleep, 37(5), 919–925. doi: 10.5665/sleep.3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber GH (1992). First year of life: prenatal craniofacial development. Cleft Palate Craniofacial Journal, 29(2), 109–111. [DOI] [PubMed] [Google Scholar]
- Wang H, Suh JW, Das SR, Pluta JB, Craige C, & Yushkevich PA (2013). Multi-Atlas Segmentation with Joint Label Fusion. IEEE Trans Pattern Anal Mach Intell, 35(3), 611–623. doi: 10.1109/TPAMI.2012.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler. (1991). Wechsler Intelligence Scale for Children, 3rd Edition, manual. Washington, DC: The Psychological Corporation. [Google Scholar]
- Wechsler. (1997). Wechsler Adult Intelligence Scale, 3rd Edition, manual. Washington, DC: The Psychological Corporation. [Google Scholar]
- Wehby GL, Collett BR, Barron S, Romitti P, & Ansley T. (2015). Children with oral clefts are at greater risk for persistent low achievement in school than classmates. Arch Dis Child, 100(12), 1148–1154. doi: 10.1136/archdischild-2015-308358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FF, McPherson B, Shu H, Xie N, & Xiang K. (2011). Structural abnormalities of the central auditory pathway in infants with non-syndromic cleft lip and/or palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. doi: 10.1597/11-014 [DOI] [PubMed] [Google Scholar]
- Young Kim E, & Johnson HJ (2013). Robust multi-site MR data processing: iterative optimization of bias correction, tissue classification, and registration. Front Neuroinform, 7, 29. doi: 10.3389/fninf.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Li C, Chen L, Xing X, Li X, Yang Z, . . . Chen R. (2017). Increased activation of the hippocampus during a Chinese character subvocalization task in adults with cleft lip and palate palatoplasty and speech therapy. Neuroreport, 28(12), 739–744. doi: 10.1097/WNR.0000000000000832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Non-Significant Linear Regression Models Predicting Adjusted ROI from ICV.
Linear regression models for each region of interest (y-axis) demonstrating the lack of relationship to ICV (x-axis) following power-proportion correction.