Abstract
The health benefits of cocoa depend on the flavan 3-ols, procyanidins, and methylxanthines, which decrease from the early stages of cocoa bean processing. The objective of this research was to obtain a cocoa extract high in these compounds with (−)-epicatechin as the primary reference. An evaluation of two pretreatments of cocoa beans with a control after harvesting was made: A (untreated/control), B (Frozen), and C (Polyphenol oxidase inhibition), all followed by dehydration at 45 °C until obtaining a cocoa powder. In terms of (−)-epicatechin content, the best pretreatment was put on to a hydroalcoholic extraction. Flavan 3-ols, procyanidins, methylxanthines, and total polyphenols content (TPC), were quantified in the cocoa powders and the hydroalcoholic extract. The results showed that the control (A), significantly conserves the (−)-epicatechin (24.964 ± 0.400 mg/g) ca. 7 times more than conventionally sun-dried and fermented beans (3.742 ± 1.977 mg/g) ca. The hydroalcoholic extraction increased the (−)-epicatechin ca. 3 times more based on pretreatment A (84.738 mg/g).
Keywords: Flavan 3-ols, Epicatechin, Hydroalcoholic extract, Cocoa
Introduction
Cocoa is a natural source of polyphenols, containing ca. 12–18% on a dry weight basis, which are into three groups: flavan 3-ols (catechins and epicatechins), anthocyanins and procyanidins; representing ca. 37, 4, and 58%, respectively, of the total antioxidants composition, reported in raw unfermented cocoa beans (Wang et al. 2019). Also, cocoa is rich in methylxanthines, containing ca. 3.2% on a dry weight basis, such as theobromine and caffeine, which represent ca. 3.7 and 0.2% (on a fat-free basis), respectively, and are considered as a central nervous system stimulant (Carrillo et al. 2014; Tan et al. 2019). It is important to consider that these contents of flavan 3-ols, methylxanthines, and procyanidins can be different between cocoa beans according to their origin, genetics, and culture conditions (Martin and Ramos 2016). Moreover, these substances are recognized for their beneficial effects and go beyond cardiovascular health, covering cognitive and other chronic diseases (Wang et al. 2019; Martin and Ramos 2021). Furthermore, different studies show that (−)-epicatechin is the first to be absorbed after oral consumption of cocoa; therefore much of the functional and nutritional benefits are attributed to this flavanol (EFSA Panel on Dietetic Products et al. 2012; Estrada et al. 2020; Garcia et al. 2021).
In the food industry, there is an interest in searching for technologies that allow the extraction and concentration of such compounds, to incorporate them into different products to contribute to the health and well-being of consumers. In the case of the cocoa industry, this is important because the traditional cocoa processing leads to losses of these biologically active compounds (Mariatti et al. 2021). Cocoa flavanols have a reduction of 60% c.a. during fermentation and drying (Gil 2012). For this reason, strategies should be seeking to improve the nutritional quality of cocoa, which are focused on making different pretreatments to cocoa beans after harvesting, in order to preserve as much as possible these compounds (Schinella et al. 2010; Toro-Uribe et al. 2018). These kinds of treatments are combined with extraction technologies (e.g., solid–liquid) using solvents such as water, ethanol, acetone, isopropanol, or mixtures of them, the most common solvents used (Steffan et al. 2012; Soares et al., 2020). Some regulations allow the use of these solvents. However, a minimum trace content should be in the final product. An example of this is reported by the U.S. Food and Drug Administration in the Code of Federal Regulations Title 21 about the secondary direct food additives permitted in food for human consumption (Food and Drug Administration (FDA) 2020).
In a regulatory context, the minimum quantities required for a nutritional declaration about the antioxidant content in food are not still established in most international laws. However, in 2012, EFSA approved the request of Barry Callebaut, who, with scientific evidence, showed that consuming 200 mg of cocoa flavanols per day helps cardiovascular health (EFSA Panel on Dietetic Products et al. 2012; Tanghe et al. 2021). With this in mind, this study aims to develop a solution that allows obtaining a functional cocoa extract high in bioactive compounds as (−)-epicatechin by solvent extraction for application in functional foods.
Materials and methods
Chemicals and reagents
All solvents (methanol, glacial acetic acid, ethanol, isopropanol, HCl, acetone) were analytical grade and supplied by Merk (Darmstadt, Germany). The water deionized in a Milli-Q water purification system (Millipore, Bedford, USA). Analytical standards of ( +)-catechin, (−)-epicatechin, caffeine, theobromine, procyanidin B2, and 4-dimethylaminocinnamaldehyde (DMAC) were purchased at Sigma Aldrich (Saint Louis, USA). Gallic acid from Merk (Hohenbrunn, Germany), n-hexane, Folin-Ciocalteu were supplied by Merk (Darmstadt, Germany). Finally, Sodium Carbonate Na2CO3 were supplied by Química Básica S.A. (Medellin, Colombia).
Pretreatments of cocoa beans
The cocoa samples were taken randomly from the experimental farm of Compañía Nacional de Chocolates S.A.S (CNCH), located in Antioquia, Colombia. The samples were undried and unfermented cocoa beans mixtures, with at least 70% of the IMC-67 (Iquitos Marañon Collection-67) variety and 30% of a mixture of CCN-51, ICS-95 and SSC-61. These cocoas were collected and undergone three pretreatments: A (untreated/control); B (Frozen at −20 °C [Gil, 2012]) using a freezer 3552A (Thermo scientific, Massachusetts, USA); C (Polyphenol oxidase [PPO] inhibition) this process consisted of submerging cocoa beans in water at 95 °C for 5 min and subsequently, a thermal shock was made, with water at 25 °C, as previously described (Schinella et al. 2010; Toro-Uribe et al. 2018).
After these pretreatments, cocoa beans were dehydrated under controlled conditions in an FBA_GFD1850 dehydrator (Gourmia, Brooklyn, NY) at 45 °C ± 1 to reach a humidity of 7–8% approximately (Tardzenyuy et al. 2020). Then, the cocoa beans were winnowing in a cocoa winnower 240–1–150 (Capco test equipment, Ipswich, UK), defatted in a screw press (CNCH, Medellín, Colombia), and milling in a processor (Capco test equipment, Ipswich, UK). The cocoa powders were stored at −20 °C until the hydroalcoholic extraction (Fig. 1).
Fig. 1.
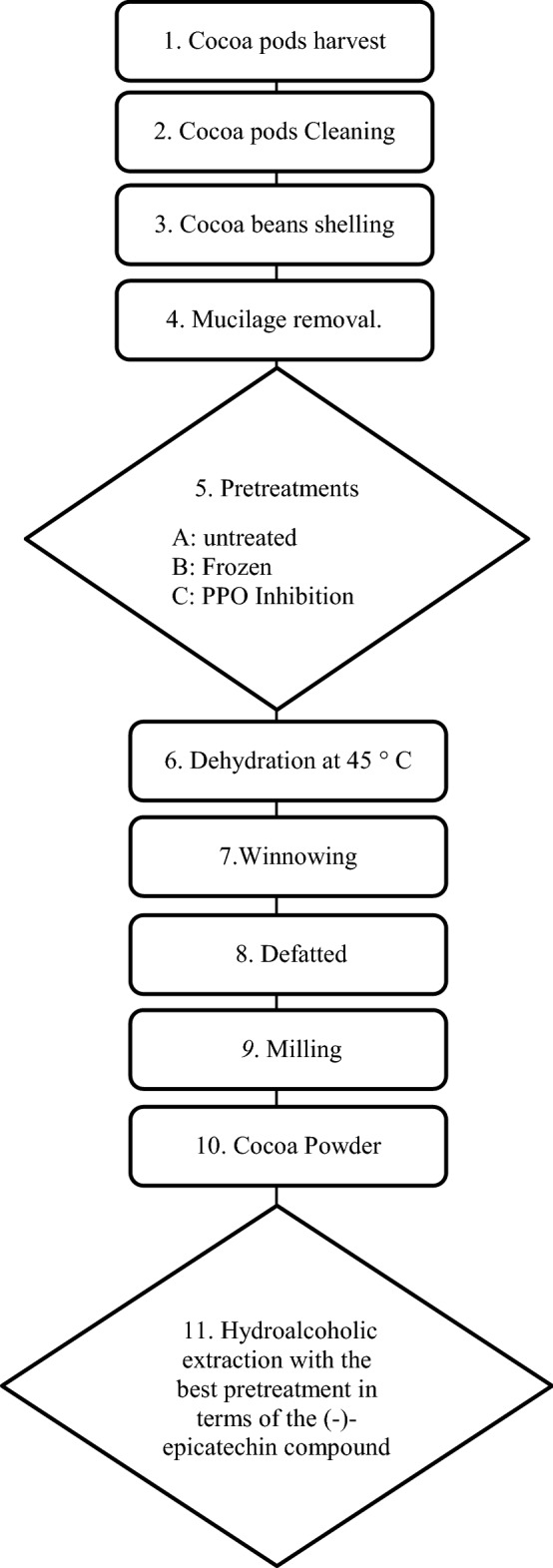
Pretreatment of cocoa beans and obtaining of cocoa powders with a high content of flavan 3-ols, procyanidins, and methylxanthines
Hydroalcoholic extraction of cocoa powder
A hydroalcoholic extraction was carried out on the cocoa powder that presented the best result in terms of (−)-epicatechin content. The cocoa powder extraction was carried out with a 70% v/v ethanol solution, in a ratio of 1:10 (cocoa: ethanol) at 60 °C for 60 min (Pasamar et al. 2006). Subsequently, the solvent was removed using a Buchi R-100 rotary evaporator® with a Buchi B-100 bath (50 °C), Buchi V-100vacuum pump (170–40 mbar which was decreasing as the concentration of EtOH was reducing). Buchi F-100 Chiller (10 °C) (Buchi, Mexico City, Mexico), the rotary evaporation time for 2L of the liquid extract was 8 h. After that, samples were freeze-dried in a Christ Alpha 1–2 LD Plus freeze dryer (Martin Christ, Osterode am Harz, Germany); in drying phase, the condensation temperature was −50 °C with 0.040 mbar vacuum for 5 h, and in the final drying phase the condensation temperature was −76 °C with 0.0010 mbar vacuum during 3 h. The cocoa extracts were stored in the dark at room temperature until the analyses.
HPLC analysis of flavan 3-ols and methylxanthines.
An Agilent 1260 high-resolution liquid chromatography (Agilent Technologies, CA, USA) with automatic autosampler, fluorescence, and diode array detectors (FLD and DAD) was used to HPLC analyzes. The signals were processed using the Agilent OpenLAB CDS software, ChemStation Edition. Samples were prepared following the methodology proposed by T-sanova-Savova. (2005). The extraction of the cocoa powders samples and hydroalcoholic extract (0.5 g) was done with 10 ml of a 60:40 pH 9.0 isopropanol/water solution during 60 min in an Elma E30H ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany), at room temperature. Subsequently, these samples were stirred in a vortex for 1 min and stored at −20 °C in a freezer for 60 min. They were centrifuged at 4000 rpm for 8 min, in a Rotofix 32A centrifuge (Andreas Hettich GmbH & Co.KG, Schwerin, Germany). The supernatant (1 ml) was filtered by a 0.45 µm Nylon membrane filter Millipore (Membrane Solutions, Auburn, EEUU), an aliquot of this filtrate was mixed with the mobile phase (0.1% acetic acid), with a dilution factor (DF) between 4 to 10 depending on the color solution. This solution was performed in a chromatography vial; next it was injected into the HPLC/DAD/FLD. A Zorbax bonus C18 HPLC Column was used (5 μm particle size, L × ID 25 cm × 4.6 mm), the detection was at 280 nm. The intensity of the acquired signals processed through the OpenLab CDS Chemstation Edition software. An external standard was applied for flavanols and methylxanthines quantification (a mixture of the four compounds ( +)-catechin, (−)-epicatechin, caffeine, and theobromine). The analyses were performed in triplicate for each sample and reported based on dry cocoa solids in mg/g.
Procyanidin analysis
In the samples of cocoa powders and hydroalcoholic extract were quantified the procyanidin content, following the methodology proposed by Payne et al. (2010). All samples were defatted with hexane to achieve a fat percent less than 5%. The DMAC solution was prepared with 0.030 g of this reagent in 3 ml HCl prepared in 27 mL of methanol. 0.500 g of each sample was taken and added in an AWAA solution (Acetone, water, and acetic acid) (70 + 29.5 + 0.5, v/v), with vortex stirring. A Centrifugation was done at 3000 rpm for 5 min, the solution was filtered using a 0.45 µm acrodisk. In the microwells of the reading plate were put 240 µl of DMAC solution and 40 µl of the sample. The used standard was the procyanidin B2, corresponding to the main dimers of flavanols in cocoa (Kothe et al. 2013). Finally, the measurement was with an absorbance of 650 nm in an ELx800 reader and the Ridasoft® software (Biotek, Winooski, USA).
Total polyphenol analysis (TPC)
The total polyphenol content was determined by UV–VIS spectroscopy using the micro-scale method of Folin-Ciocalteu (Merk, Darmstadt, Germany), described by Rover and Brown (2013). 300 µl of extract diluted (1/100), 150μL Folin-Ciocalteu reagent, 2250μL of distilled water and 300μL of Na2CO3 at 20% were mixed and, after 60 min incubation at room temperature, the absorbance of the color was at 760 nm against a blank sample on a GENESYS 10S UV–Vis spectrophotometer (Thermo Scientific, Waltham, Massachusetts). The TPC was expressed as equivalent milligrams of gallic acid per gram of dry sample material (mgGAE/g). All measurements were performed in triplicate and reported based on dry cocoa solids.
Statistical analysis
A completely randomized block model was performed, where the main factor was the pretreatment, which had three levels: A (untreated/control), B (Frozen at −20 °C), and C (PPO inhibition). The time was a block with two levels (t1 and t2), which correspond to the cocoa harvesting period (the difference between the two blocks was eight months) to eliminate the experimental noise associated with seasonal differences in the harvest. The assignment of the treatments to the experimental units brought in each event was random. An ANOVA analysis of variance and a Tukey test was done to establish significant differences between treatments. The statistical treatment of the data was using the R statistical language (R Development Core Team 2019).
Results
Flavan 3-ols
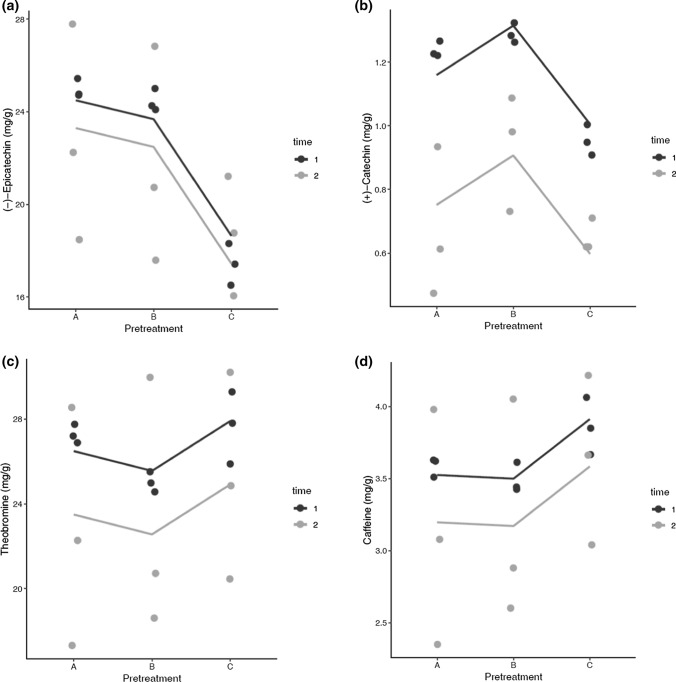
The average values of (−)-epicatechin were approximately 24.0 mg/g for A and B treatments without statistically significant differences (p < 0.05) between them. In contrast, an average value of 17.40 ± 0.903 mg/g was determined for pretreatment C, lower than the treatments mentioned above, and with a statistical difference (p > 0.05) (Fig. 2a, b, Table 1). These results have a similar behavior from the reported data by Kim and Kenney (1984), which found higher values of (−)-epicatechin in frozen beans (35–43 mg/g) and lower values in the treatment in which PPO was inhibited (21 mg/g). The differences between the (−)-epicatechin values reported by these authors and the values reported in this research, could be because the cocoa varieties evaluated in both studies were different (Trinidad-Jamaican hybrids, Catongo, Forastero, and Colombian Trinitario cocoa beans, respectively). Additionally, the process of PPO inhibition, because in their study, cocoa beans were heating at 105 °C into a microwave. This work used a thermal shock with water at 95 °C and 25 °C.
Fig. 2.
Flavan 3-ols (a epicatechins and b catechins) and methylxanthines (c theobromine and d caffeine) in cocoa powers (1 and 2 correspond to cocoa harvesting time)
Table 1.
Content of flavan 3-ols and methylxanthines in cocoa powders in t1 and t2
| Flavan 3-ols | Methylxanthines | |||||||
|---|---|---|---|---|---|---|---|---|
| Time | t1* | t2* | t1* | t2* | ||||
| Pretreatment | (−) –epicatechin (mg/g) | ( +)-catechin (mg/g) | (−)-epicatechin (mg/g) | ( +)-catechin (mg/g) |
Theobromine (mg/g) |
Caffeine(mg/g) | Theobromine (mg/g) |
Caffeine(mg/g) |
| A | 24.965 ± 0.400a | 1.238 ± 0.025ac | 22.832 ± 4.678a | 0.674 ± 0.236ac | 27.279 ± 0.436abc | 3.586 ± 0.067abc | 22.712 ± 5.625abc | 3.1361 ± 0.816abc |
| B | 24.456 ± 0.489ab | 1.289 ± 0.031ab | 21.715 ± 4.701ab | 0.933 ± 0.183ab | 25.023 ± 0.471abc | 3.493 ± 0.104abc | 23.097 ± 6.048abc | 3.1778 ± 0.769abc |
| C | 17.403 ± 0.904c | 0.953 ± 0.048c | 18.681 ± 2.575c | 0.649 ± 0.052c | 27.658 ± 1.714abc | 3.859 ± 0.199abc | 23.168 ± 4.886abc | 3.6388 ± 0.588abc |
The values correspond to the mean and the standard deviation of the three analyses. Mean values with different letters in the same column show statistically significant differences (p < 0.05). *t1 and t2 correspond to cocoa harvesting time
Different studies have reported the PPO inhibition as one of the best treatments for the conservation of cocoa flavanols (Schinella et al. 2010), however, in this research, C was not the best pretreatment to the conservation of flavan 3-ols, especially the (−)-epicatechin. The lower concentration of (−)-epicatechin by enzymatical inhibition may occur by the exposure conditions of cocoa beans at temperatures above 60 °C, which can deteriorate it (Alañón et al. 2016). Hurst et al. (2011) presented comparative values of (−)-epicatechin at different stages of the industrialization process of cocoa beans. The highest contents were in unfermented raw ripe beans (13.35 mg/g) and the lowest in beans subjected to roasting (0.66 mg/g). Their results showed that the highest total polyphenol content was without high temperatures. A hypothesis about the results of A and B (higher (−)-epicatechin content than C), could be associate with dehydration at 45 °C. It is necessary to highlight that the PPO was not inhibited in these pretreatments, but this behavior is possible because the PPO is sensitive to drying; this does not mean that the enzyme loses its reaction power but this process is slower. Wollgast and Anklam (2000) mentioned that the PPO activity in dried beans is only 2% (the case of pretreatment A). For B, where the cocoa beans were frozen, the low temperatures reduce or inactivate the enzymatic activity, which delays the effect of degradation of bioactive compounds. This result allows mentioning that the traditional PPO inhibition (using temperatures of 95 °C) is not the best way to preserve the (−)-epicatechin content. On the other hand, this is possible using dehydration under controlled conditions or freezing of cocoa beans after harvesting.
There were no differences between t1 and t2 for (−)-epicatechin (p > 0.05), contrary to ( +)-catechins content in which statistically significant differences were found (p < 0.05), this may occur due to the variations that exist in cocoa beans baches between the analyzed times (eight months) since the climatic and stationary harvest conditions occurred at different times. However, these variabilities are acceptable in experiments of this type because during cocoa cultivation and harvest, many environmental variables are difficult to control (Carrillo et al. 2014). In that way, Quelal-Vásconez et al. (2020) quantified the flavanols and methylxanthines content of cocoa powders, highlighting the natural variability of this type of unprocessed samples due to their origins, which influences the dispersion of the values of these compounds.
In previous studies, Compañía Nacional de Chocolates S.A.S. performed an analysis of (−)-epicatechin content in fermented and dried Colombian cocoa; the results showed that the samples evaluated did not exceed 8 mg/g of (−)-epicatechin (unpublished results). Nazaruddin et al. (2006) reported values of (−)-epicatechin in fermented and dried Malaysian cocoa beans of 4.61 mg/g. Samaniego et al. (2020) showed values of (−)-epicatechin content in fermented and dried Ecuadorian cacao beans, between 3.45–13.16 mg/g. This behavior occurs because of about 60% of the polyphenol content decrease in cocoa pre-industrialization processes (Gil 2012). Montoya et al. (2021) reported values of (−)-epicatechin of different unfermented and dried cocoa beans around 10.26–16.71 mg/g. The comparison of the average values presented in these studies and the results of A and B (24.0 mg/g) and C (17 mg/g), allows mentioning that these processes (untreated and freezing respectively, with dehydration at 45 °C in both), could be a more appropriate way to preserve (−)-epicatechin content. Also, it is worth mentioning that it is necessary to control the drying process because that affects the content of these compounds. Additionally, it is relevant to highlight that Colombian cocoa compared to other cocoas, seems to have great potential to obtain cocoa extracts with a high content of flavan 3-ols.
According to the results obtained, it is possible to conclude that the better pretreatments to preserve the (−)-epicatechin content in cocoa beans are A and B. However, freezing can become a treatment that generates higher processing costs. Therefore, there were no statistical differences between both pretreatments; thus, it is possible to select treatment A as better scalability. Subsequently, the hydroalcoholic extract was obtained, applying this extraction to the cocoa powder of pretreatment A, as mentioned before. For the cocoa extract, the average values of ( +)-catechins and (−)-epicatechin were 3.699 ± 1.297 mg/g and 84.738 ± 4.805 mg/g, respectively. These results represent an increase of approximately 30% of (−)-epicatechin compared to the obtained in the cocoa powder A, which is relevant to future applications in the production of functional foods. This concentration occurs due to the high polarity of the flavanols; therefore, they are more soluble in solvents such as ethanol, methanol, and water (Okiyama et al. 2018).
Other emerging technologies were previously explored by CNCH for the extraction and concentration of these compounds, as presented in Table 3. However, the best (−)-epicatechin content was achieved from hydroalcoholic extraction, as discussed previously. Technologies such as supercritical fluids in cocoa are an alternative for defatting the bean, which is expensive, but the (−)-epicatechin obtained was 14.620 mg/g. Moreover, technologies such as extraction by hydrothermal treatments also were tested (Mazza Innovations LTD 2014), but the (−)-epicatechin content was 29,430 mg/g. On the other hand, the (−)-epicatechin value reported in this study in the hydroalcoholic extract (84,738 ± 4.805 mg/g) is higher than the reported in a commercial cocoa extract (50 mg/g), this may be due to the contribution of the pretreatments A and B to preserve the content of flavan 3-ols. Additionally, as was stated above, Colombian cocoa has a high content of these compounds compared to other cocoa beans from other regions (Urbanska and Kowalska 2019).
Table 3.
Obtaining cocoa extracts from unfermented beans using different technologies (unpublished Results)
| Technology/Commercial Extract | (−)-epicatechin (mg/g) |
TPC (mgGAE/g) |
|---|---|---|
| Unfermented cocoa powder | 24.960 | 153.067 |
| Extraction by supercritical fluids | 14.620 | – |
| Chromatography extraction for compound purification | 41.500 | – |
| Extraction by hydrothermal treatments | 29.430 | 188.400 |
| Extract obtained by biotechnological methods | 5.187 | 15.172 |
| Ultrasound-Assisted Extraction | 3.371 | – |
| Commercial extract | 50.000 | 197.220 |
Methylxanthines
The theobromine values in t1 were between 25 and 27 mg/g (B, A, and C), and in the caffeine was between 3.5 and 3.8 mg/g (A, B, and C). In t2 were between 22 and 23 mg/g (A, B, and C) and 3.1–3.6 mg/g (A and B, C), theobromine and caffeine, respectively (Fig. 2c, d, Table 1). There were no statistically significant differences among pretreatments and between harvest time (p > 0.05). The theobromine was higher than the catechines; this is common in cocoas and does not correspond to the effect of any treatments performed in this study. Carrillo et al. (2014) also reported that in different Colombian cocoas, finding that methylxanthines are present in a higher proportion than catechines, being the theobromine the predominant compound. Brunetto et al. (2007) described a similar result. In the case of the hydroalcoholic extract, theobromine and caffeine values were 83.418 ± 5.354 mg/g and 12.681 ± 0.437 mg/g, respectively, which represented an increase of 25% approximately.
On the other hand, cocoa has classification into three genetic varieties: Forastero, Trinitario, and Criollo, and it is possible to validate this classification through the theobromine/caffeine ratio (Carrillo et al. 2014). The theobromine/caffeine ratio of the cocoa mixtures used in these experiments showed average values between 7.22 ± 0.123; it is in the range of Trinitario cocoa variety (5–10) with average amounts of xanthines.
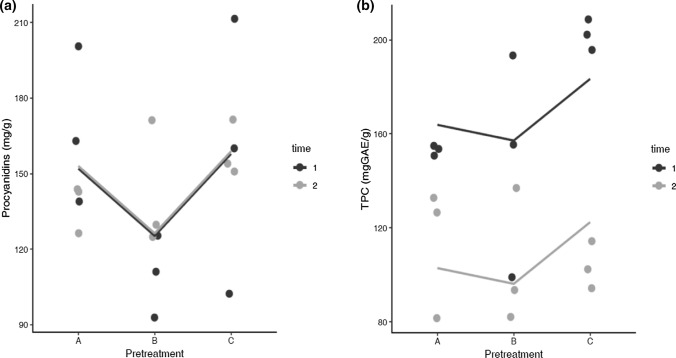
Procyanidins content
According to the statistical analysis, it was possible to establish that there are no differences between A, B, and C pretreatments or t1 and t2 (p > 0.05), indicating that the procyanidin content is not affected by any of the pretreatments made to cocoa beans. Table 2 and Fig. 3a present the results obtained for the content of procyanidins of the pretreated cocoa beans. The results had a high variation, Payne et al. (2010) mentioned that this variability might occur due to the colorimetric method used for the measurement of this variable.
Table 2.
Content of procyanidins and total polyphenol content (TPC) cocoa powders in t1 and t2
| Procyanidins | TPC | |||
|---|---|---|---|---|
| Time | t1* | t2* | t1* | t2* |
| Pretreatment | Procyanidin B2 (mg/g) | Procyanidin B2 (mg/g) | TPC (mgGAE/g) | TPC (mgGAE/g) |
| A | 167.429 ± 31.080abc | 137.583 ± 9.703abc | 153.0678 ± 2.034abc | 113.6442 ± 27.883abc |
| B | 109.750 ± 16.181abc | 141.870 ± 25.472abc | 149.1456 ± 47.499abc | 104.1996 ± 28.945abc |
| C | 157.929 ± 54.522abc | 158.745 ± 11.169abc | 202.1441 ± 6.491abc | 103.6454 ± 10.105abc |
The values correspond to the mean and the standard deviation of the three analyses. Mean values with different letters in the same column show statistically significant differences (p < 0.05). *t1 and t2 correspond to cocoa harvesting time
Fig. 3.
(a) Procyanidins and (b) TPC in cocoa powders (1 and 2 correspond to cocoa harvesting time)
Gültekin-Ozgüven et al. (2016) mention that cocoa and its derivatives contain a variable content of procyanidin B2 between 0.144 mg/g to 0.457 mg/g. CNCH sampled different post-harvested cocoa varieties in a conventional way (fermented and dried), where procyanidin values were between 10.9–43.1 mg/g (unpublished results). Kramer et al. (2019) also reported procyanidin B2 value for a cocoa extract of 21.39 mg/g. That allows mentioning that the pretreatments evaluated in this study contribute to the conservation of the phenolic compounds present in the cocoa bean. In the case of the hydroalcoholic extract, the procyanidin value was of 823.6 ± 26.715 mg/g. Compared to pretreatment A, it was possible an increase in the procyanidin content of 20%. That is relevant to applying this extract in the development of functional food; since consuming cocoa-based products with high procyanidin content can contribute to health benefits.
Total polyphenol content (TPC)
The average values of total phenols were between 103 and 202mgGAE/g, and there were no statistically significant differences (p > 0.05) between the treatments evaluated (Fig. 3b, Table 2). On the other hand, significant differences occurred when the two evaluated times were analyzed (p < 0.05) since t2 had lower values of TPC (t1: 153–202 mg/g; t2:103–113 mg/g), which is due to the variability of cocoa. Additionally, it is possible to observe that the TPC is slightly higher in treatment C in t1 (202.144 ± 6.49 mg/g). Gil (2012) reported that the PPO has a direct relationship with the reduction of the ( +)-catechin and (−)-epicatechin content. However, not all the antioxidants present in cocoa are a substrate of the PPO, that is the reason because the TPC is not significantly affected. Statistical analysis shows that there are no differences between the three treatments. The Colombian cocoa has a TPC average of 37.81mgGAE/g (fermented and dried cocoa beans), reported by Urbanska and Kowalska (2019). That allows concluding that it is possible to conserve approximately 90% of the TPC present in unprocessed cocoa beans through the pretreatments evaluated in this research. In the cocoa extract, the average TPC was 348,967 ± 6,653mgGAE/g, which doubles the content of compounds present in pre-treated cocoa beans (A: 153.067 ± 2.034 mg/g) and exceeding those reported by some commercial extracts and obtained by other extraction technologies (Table 3).
Todorovic et al. (2015) conducted a study on the content of methylxanthines and polyphenols in cocoa products. The authors found that the average TPC was 35.35mgGAE/g for powdered chocolates and dark chocolates between 7.21 and 12.65mgGAE/g; these contents are below the regulatory limits to make a nutritional claim. Different clinical studies have reported that daily consumption above 100 mg/day of these compounds is required to obtain such benefits (EFSA Panel on Dietetic Products et al. 2012). That demonstrates the need for enriching a cocoa-based product since the TPCs at the end of the transformation processes are below the recommended daily doses required.
Conclusions
Antioxidant-rich cocoa powder was obtained using unfermented beans and immediately dehydrated at 45 °C contributing to cocoa polyphenols preservation.
The extraction of cocoa antioxidants using hydroalcoholic treatments significantly concentrates them, even obtaining better yields comparing to some emerging technologies such as supercritical fluids, hydrothermal treatments, and ultrasound.
The polyphenol oxidase inhibition at 95 °C significantly affects the content of epicatechin.
In future works are recommended, scaling up and optimization of control (A) and hydroalcoholic extraction. Additionally, it is relevant to evaluate some aspects, such as the antioxidant capacity and functionality of the cocoa extract obtained and its application in a cocoa-based product, to establish its applicability in functional products.
Acknowledgements
This study was financed and developed by personnel from R&D and Quality department of Compañía Nacional de Chocolates S.A.S (Medellín, Colombia).
Abbreviations
- CNCH
Compañía Nacional de Chocolates S.A.S.;
- IMC-67
Iquitos Marañon Collection-67
- CCN-51
Colección Castro Naranjal-51
- ICS-95
Imperial college selection-95
- SSC-61
Selección Colombia Corpoica-61
- PPO
Polyphenol oxidase;
- TPC
Total polyphenol content
Authors' contributions
Elly Acosta-Otálvaro performed experiments, conducted data analysis, and interpreting results. She wrote most of the manuscript. Wilmar Valencia-Gallego has made substantial contributions in the conception, design of the work, acquisition, analysis, and interpretation of field and laboratory data. Juan Camilo Mazo Rivas has contributed to the construction, conceptualization, and direction of the project. He is the person who follows up on all aspects related to the project, resolving doubts and advising on methodology, contributing with his experience and knowledge in the consolidation of guiding elements of the research. Cristina García-Viguera has contributed to the writing, interpretation, and analysis of the results. She has revised the paper and approved the final version of the manuscript before publication. In addition to having an advisory and guiding role in all aspects of the work.
Funding
This study was financed by Compañía Nacional de Chocolates S.A.S (Medellín, Colombia).
Availability of data and material
The data obtained in this study are available.
Code availability
Not Applicable.
Declarations
Conflicts of interest
There is no conflict of interest
Ethics approval
Not Applicable.
Consent to participate
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Consent for publication
All data and images included are our own and can be published without any difficulty.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elly Acosta-Otálvaro, Email: eacosta@chocolates.com.co.
Wilmar Valencia-Gallego, Email: wgvalencia@chocolates.com.co.
Juan Camilo Mazo-Rivas, Email: jcmazo@chocolates.com.co.
Cristina García-Viguera, Email: cgviguera@cebas.csic.es.
Reference s
- Alañón ME, Castle SM, Siswanto PJ, Spencer JPE. Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates. Food Chem. 2016;208:177–184. doi: 10.1016/j.foodchem.2016.03.116. [DOI] [PubMed] [Google Scholar]
- Brunetto R, Gutie L, Ramos G, et al. Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem. 2007;100:459–467. doi: 10.1016/j.foodchem.2005.10.007. [DOI] [Google Scholar]
- Carrillo LC, Londoño-londoño J, Gil A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res Int. 2014;60:273–280. doi: 10.1016/j.foodres.2013.06.019. [DOI] [Google Scholar]
- Garcia LB, Pires GA, Oliveira DAJ, et al. Industrial Crops & Products Incorporation of glycolic extract of cocoa beans (Theobroma cacao L.) into microemulsions and emulgels for skincare. Ind Crop Prod. 2021;161:1–10. doi: 10.1016/j.indcrop.2020.113181. [DOI] [Google Scholar]
- Okiyama DC, Soares ID, Cuevas MS. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res Int. 2018;114:20–29. doi: 10.1016/j.foodres.2018.07.055. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, & Allergies (2012) Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13 (5) of Regulation (EC) No 1924/2006. EFSA Journal 10(7) 2809:1–20
- Estrada RA, Restrepo UL, Noriega EM, Muñoz DK, Alvarez CM, Mazo JC, Penagos L (2020) Dietary supplement derived from natural products by hot melt extrusion (HME) processing. US 10.555.986 B2
- Food and Drug Administration (FDA) (2020) Secondary direct food additives permitted in food for human consumption. Code of Federal Regulations Title 21(3). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=173&showFR=1&subpartNode=21:3.0.1.1.4.3
- Gil JA (2012) Estabilidad y actividad antioxidante de catequinas presentes en cacaos colombianos durante los procesos de pre industrialización. Repositorio Universidad de Antioquia sede Medellín. http://bibliotecadigital.udea.edu.co/bitstream/10495/1621/1/TESIS%20Jorge%20Andres%20Gil%20FINAL.pdf. Accesed January 2019
- Gültekin-Ozgüven M, Berktas I¸ Ozçelik B, Change in stability of procyanidins, antioxidantcapacity and in-vitro bioaccessibilityduringprocessing of cocoapowderfromcocoabeans. Lwt-FoodSciTechnol. 2016;72:559–565. doi: 10.1016/j.lwt.2016.04.065. [DOI] [Google Scholar]
- Hurst WJ, Krake SH, Bergmeier SC, et al. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem Cent J. 2011;5(1):1–8. doi: 10.1186/1752-153X-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim & Kenney PG. Content in fermented and unfermented. J Food Sci. 1984;49:1090–1092. doi: 10.1111/j.1365-2621.1984.tb10400.x. [DOI] [Google Scholar]
- Kothe L, Zimmermann BF, Galensa R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013;141:3656–3663. doi: 10.1016/j.foodchem.2013.06.049. [DOI] [PubMed] [Google Scholar]
- Mariatti F, Gunjević V, Boffa L, et al. Process intensification technologies for the recovery of valuable compounds from cocoa by-products. Innov Food Sci Emerg Technol. 2021;68:1–14. doi: 10.1016/j.ifset.2021.102601. [DOI] [Google Scholar]
- Martin MA, Ramos S. Cocoa polyphenols in oxidative stress: Potential health implications. J Funct Foods. 2016;27:570–588. doi: 10.1016/j.jff.2016.10.008. [DOI] [Google Scholar]
- Martin MA, Ramos S. Impact of cocoa flavanols on human health. Food Chem Toxicol. 2021;151:1–19. doi: 10.1016/j.fct.2021.112121. [DOI] [PubMed] [Google Scholar]
- Mazza Innovations LTD (2014) Pressurized low polarity water extraction apparatus and methods of use. Canadian Patent: CA2836200C.
- Montoya CC, Valencia WG, Sierra JA, et al. Enhanced pink-red hues in processed powders from unfermented cacao beans. Lwt-Food Sci Technol. 2021;138:1–7. doi: 10.1016/j.lwt.2020.110671. [DOI] [Google Scholar]
- Nazaruddin R, Seng LK, Hassan O, Said M. Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma Cacao) during fermentation. Ind Crops Prod. 2006;24:87–94. doi: 10.1016/j.indcrop.2006.03.013. [DOI] [Google Scholar]
- Pasamar MA, Ibarra A, Cienfuegos-Jovellanos E, Laghi S (2006) Natraceutical S.A. Proceso de obtención de extractos de cacao con elevado contenido de polifenoles. WO2007138118 A1
- Payne MJ, Jeffrey HW, Stuart DA. Determination of total procyanidins in selected chocolate and confectionery products using DMAC. J AOAC Int. 2010;93(1):89–96. doi: 10.1093/jaoac/93.1.89. [DOI] [PubMed] [Google Scholar]
- Quelal-vásconez MA, Lerma-garcía MJ, Pérez-esteve É, et al. Changes in methylxanthines and fl avanols during cocoa powder processing and their quantification by near-infrared spectroscopy. LWT-Food Sci Technol. 2020;117:1–8. doi: 10.1016/j.lwt.2019.108598. [DOI] [Google Scholar]
- Rover MR, Brown RC. Quantification of total phenols in bio-oil using the Folin-Ciocalteu method. J Anal Appl Pyrolysis. 2013;104:366–371. doi: 10.1016/j.jaap.2013.06.011. [DOI] [Google Scholar]
- Samaniego I, Espín S, Quiroz J, et al. Effect of the growing area on the methylxanthines and flavan-3-ols content in cocoa beans from Ecuador. J Food Compos Anal. 2020;88:1–9. doi: 10.1016/j.jfca.2020.103448. [DOI] [Google Scholar]
- Schinella G, Mosca S, Cienfuegos-Jovellanos E, et al. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res Int. 2010;43:1614–1623. doi: 10.1016/j.foodres.2010.04.032. [DOI] [Google Scholar]
- Soares ID, Okiyama DCG, da Rodrigues CE, C, Simultaneous green extraction of fat and bioactive compounds of cocoa shell and protein fraction functionalities evaluation. Food Res Int. 2020;137:1–10. doi: 10.1016/j.foodres.2020.109622. [DOI] [PubMed] [Google Scholar]
- Steffan W, Duffin-Maxwell K, Bradbury A. Polyphenol-rich extract from plant material US. 2012;20120035252:A1. [Google Scholar]
- T-sanova-Savova R, MG, (+)-Catechin and (−)-epicatechin in Bulgarian Fruits. J food Compos Anal. 2005;18:691–698. doi: 10.1016/j.jfca.2004.06.008. [DOI] [Google Scholar]
- Tan J, Li R, Jiang ZT, Tang SH, Wang Y. Rapid and non-destructive prediction of methylxanthine and cocoa solid contents in dark chocolate by synchronous front-face fluorescence spectroscopy and PLSR. J Food Compost Anal. 2019;77:20–27. doi: 10.1016/j.jfca.2019.01.001. [DOI] [Google Scholar]
- Tanghe A, Heyman E, Vanden Wyngaert K, et al. Evaluation of blood pressure lowering effects of cocoa flavanols in diabetes mellitus: a systematic review and meta-analysis. J Funct Foods. 2021;79:1–12. doi: 10.1016/j.jff.2021.104399. [DOI] [Google Scholar]
- Tardzenyuy ME, Jianguo Z, Akyene T, et al. Improving cocoa beans value chain using a local convection dryer: a case study of Fako division Cameroon. Scientific African. 2020;8:1–10. doi: 10.1016/j.sciaf.2020.e00343. [DOI] [Google Scholar]
- Todorovic V, Redovnikovic IR, Todorovic Z, et al. Polyphenols, methylxanthines, and antioxidant capacity of chocolates produced in Serbia. J Food Compos Anal. 2015;41:137–143. doi: 10.1016/j.jfca.2015.01.018. [DOI] [Google Scholar]
- Toro-Uribe S, Montero L, López-Giraldo L, et al. Characterization of secondary metabolites from green cocoa beans using focusing-modulated comprehensive two-dimensional liquid chromatography coupled to tandem mass spectrometry. Anal Chim Acta. 2018;1036:204–213. doi: 10.1016/j.aca.2018.06.068. [DOI] [PubMed] [Google Scholar]
- Urbanska B, Kowalska J. Comparison of the total polyphenol content and antioxidant activity of chocolate obtained from roasted and unroasted cocoa beans from different regions of the world. Antioxidants. 2019;8(283):1–13. doi: 10.3390/antiox8080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Feltham BA, Suh M, Jones PJH. Trends in food science & technology Cocoa flavanols and blood pressure reduction: is there enough evidence to support a health claim in the United States. Trends Food Sci Technol. 2019;83:203–210. doi: 10.1016/j.tifs.2018.11.023. [DOI] [Google Scholar]
- Wollgast J, Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int. 2000;33:423–447. doi: 10.1016/S0963-9969(00)00068-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data obtained in this study are available.
Not Applicable.