Abstract
Introduction
Few studies have evaluated the economic burden of lupus nephritis (LN). The aim of this systematic literature review (SLR) was to assess the economic burden (direct and indirect costs, and healthcare resource utilization [HCRU]) associated with LN, with particular focus on the burden of renal flares and end-stage kidney disease (ESKD).
Methods
This SLR (GSK study 213531) was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Searches of the MEDLINE and Embase databases were conducted for English language publications reporting cost or HCRU data in patients with LN (regardless of age or LN histological class) until December 10, 2019. Handsearching of conference proceedings and keyword-based searches in PubMed, Google, and Google Scholar were also conducted.
Results
Twenty-two studies were identified from 28 publications reporting the cost (n = 19) and HCRU (n = 13) associated with LN. Most studies were from North America (n = 13) and many used administrative claims data (n = 9). LN was associated with substantially higher direct costs (e.g., total annual, hospitalization, and ESKD-related direct costs), total indirect costs, and HCRU (e.g., hospitalization, outpatient services, and medication use) compared with patients without systemic lupus erythematosus (SLE) or non-renal SLE controls. ESKD and dialysis were significant contributors to economic burden. No studies described the cost of renal flares.
Conclusions
The consensus across the 22 studies was that the economic burden of LN is substantial, particularly in active or severe disease, or if there is progression to ESKD. Total direct cost may be underestimated in claims data given the challenges of identifying patients with LN. Further studies are vital to ascertain the cost of renal flares; a renal flare is likely to result in a period of increased HCRU, which could be mitigated by treatments that extend renal remission.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00368-y.
Keywords: Cost, Economic burden, Lupus nephritis, Systematic literature review, Systemic lupus erythematosus
Key Summary Points
| The objective of this systematic literature review was to assess the economic burden (direct and indirect costs, and healthcare resource utilization [HCRU]) associated with lupus nephritis (LN), with a specific focus on the costs and HCRU associated with renal flares and end-stage kidney disease (ESKD). |
| LN was associated with substantially higher direct and indirect costs and HCRU compared with patients without systemic lupus erythematosus (SLE) or non-renal SLE control populations. |
| The largest gap in the literature is for HCRU and cost data characterizing a renal flare in patients with LN; a flare is likely to result in a period of increased HCRU and therefore optimal management and minimization of flares (i.e., maintaining renal remission) would reduce overall costs. |
| There are also limited cost and HCRU data on patients with LN and ESKD; presenting challenges for cost-effectiveness analysis where most data were derived from a non-SLE chronic kidney disease population. |
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystem autoimmune disease that predominantly affects women of child-bearing age [1]. It is characterized by abnormal B-cell and T-cell activation and the generation of pathogenic autoantibodies [2, 3]. The resulting inflammation and subsequent damage of tissue and organs underpin the diverse range of debilitating clinical manifestations associated with the disease [3].
Approximately 31–48% of patients with SLE develop lupus nephritis (LN), the most severe organ manifestation of SLE, with 7–31% of patients being diagnosed with LN at SLE diagnosis [4–7]. Patients with LN have a higher risk of death compared with the general population, a risk that increases further if LN progresses to end-stage kidney disease (ESKD) [8]. Overall, up to 28% of patients with LN will subsequently develop ESKD, with a cumulative incidence ranging from 6–19% over 10 years [4]. Thus, the spectrum of LN includes patients with a range of clinical severities and therefore economic burden.
A single LN renal flare can reduce the glomerular filtration rate by approximately 40% and usually results in irreversible nephron loss that shortens kidney lifespan by decades [7]; as such, prevention of renal flares, or put conversely, the maintenance of renal remission, is a critical long-term treatment goal. As well as the clinical impact of renal damage, chronic kidney disease (CKD; not specific to LN) has been shown to significantly increase all-cause costs compared with those in patients without CKD [9]. This highlights the additional economic importance of preventing deterioration of renal function in LN. Patients with LN are also more likely to develop cardiovascular comorbidities than patients with SLE who do not have LN [10], which has been shown to increase the annual total costs of SLE by a factor of 2.3 [11]. Finally, risk of infection in SLE is increased by both disease activity (including renal involvement) and immunosuppressive treatment [7, 12]. Serious infections in SLE were found to increase hospitalization rates by up to 24 times compared with those in patients without SLE [13], which would likely result in substantially higher direct costs.
There is a high economic burden on the healthcare system associated with the management of patients with SLE, with mean annual direct and indirect cost ranges of US$2214–16,875 and US$2239–35,540, respectively [14, 15]. In patients with SLE, pharmaceutical costs accounted for 19–30% of total expenditures; and inpatient and outpatient costs composed 16–50% and 24–56% of overall costs, respectively [14]. However, higher costs have been reported for patients with LN [14, 16], with a mean annual direct cost range of US$29,034–$62,651 [14]. Increased disease activity and organ damage have also been shown to increase costs in patients with SLE [17–20]. Despite this, few studies have evaluated the economic burden of the subgroup of patients with LN.
Given the emergence of new treatments for LN [21, 22], there is a renewed need to evaluate and summarize the direct (e.g., hospitalization, outpatient visits, diagnostics, and medications) and indirect (e.g., lost productivity and absenteeism) costs and healthcare resource utilization (HCRU) associated with LN to inform efficient resource allocation in future clinical practice.
Accordingly, the objective of this systematic literature review (SLR) was to assess the economic burden associated with LN, with a particular focus on the costs and HCRU related to renal flares and ESKD.
We aimed to answer two specific research questions:
What are the direct and indirect costs associated with LN?
How does LN impact HCRU?
Methods
Search strategy
In this study (GSK study 213531), a systematic literature search was conducted to identify publications reporting either cost or HCRU data in patients with LN (regardless of age, method of diagnosis, or LN histological class). Structured searches using indexed and free-text terms were conducted in MEDLINE and Embase from database inception to December 10, 2019. Both databases were searched via the Embase.com interface, using the specific disease and economic burden facets designed when developing the search strategy. The final search strategy is detailed in Supplementary Table 1. Handsearching of conferences proceedings (2017–2019) and keyword-based searches in PubMed, Google, and Google Scholar were also conducted to retrieve relevant evidence (Supplementary Methods).
Eligibility criteria and article selection
Screening of both title/abstract and full publication text was conducted by two independent reviewers in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23–25]; any disagreements were resolved by a third reviewer. Publications were included that met the pre-defined eligibility criteria summarized in Supplementary Table 2.
Data analysis and presentation
Direct and indirect costs associated with LN were stratified by disease stage and country, where possible. The impact of LN on HCRU was assessed by the frequency of hospitalizations and outpatient visits, and medication use. No quantitative synthesis was planned; the outcomes of this SLR are descriptive.
Ethics compliance
This article is an SLR of published articles and does not report a study conducted by the authors involving human participants or animals.
Results
Study selection and characteristics
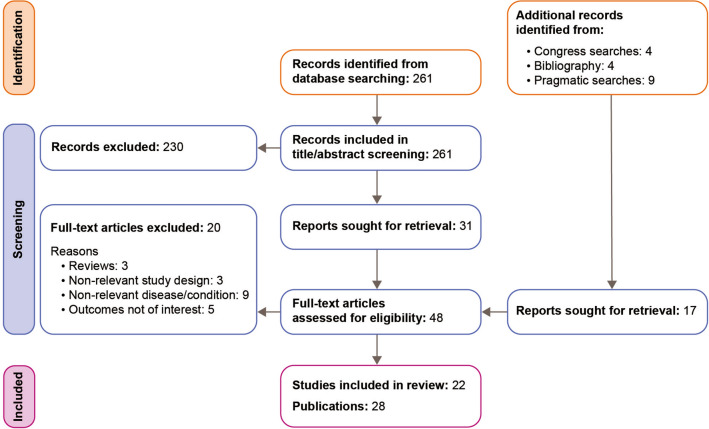
As shown in the PRISMA flow diagram (Fig. 1), 22 studies from 28 publications published between 2007 and 2019 were identified, which provided information on the cost (n = 19) and HCRU (n = 13) associated with LN [26–47].
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram
Study characteristics (excluding cost-utility analyses [CUAs]) are detailed in Table 1. Studies were conducted primarily in North America (n = 13) and Asia (n = 7), with two conducted in Europe. There were 17 retrospective studies, one prospective cohort study associated with a cost prediction model and four CUAs. The three most common sources of data were claims databases (n = 9), observational studies (n = 5), and CUAs (n = 4). The sources of data for the four CUAs were a national database [40] and information from the literature [35, 41, 46]. Diagnostic criteria differed between studies and were not always reported. Where reported, the American College of Rheumatology (ACR) classification criteria was used, as were combinations of diagnosis codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and pathological classification from renal biopsy [48–50].
Table 1.
Characteristics of included non-CUA studies
| First author, year | Study design | Country | Data collection period (analysis period/follow-up) | Patient population | LN diagnostic criteria | Populations compared |
|---|---|---|---|---|---|---|
| Carls et al. [29](2009) | Case–control claims database analysis | USA | 2000–2004 (1 year) | Patients with SLE (≥ 1 SLE inpatient claim or ≥ 2 SLE outpatient claims ≥ 30 days apart). Newly active patients with SLE selected | Nephritis ICD-9-CM codesa | LN vs. SLE without LN vs. matched control patients without SLE |
| Li et al. [38] (2009) | Retrospective claims database analysis | USA | 1999–2005 (5 years) | Patients with SLE (ICD-9-CM diagnosis code, or ≥ 2 SLE outpatient claims during office visit and/or ED visit ≥ 30 days apart; Medicaid population) | Nephritis ICD-9-CM codesa | LN vs. SLE without LN vs. matched patients without SLE |
| Pelletier et al. [42] (2009) | Retrospective claims database analysis | USA | 2007 (1 year) | Patients with SLE (2 continuous claims ICD-9-CM.710.0; including patients with Medicaid and Medicare) | ≥ 1 claim indicative of renal involvement, with ≥ 2 claims for SLE (ICD-9-CM codesa) | LN vs. SLE without LN |
| Tse et al. [44] (2009) | Retrospective observational study, cost source NR | Hong Kong | NR (2 years) | Patients with diffuse proliferative LN | Biopsy; Class IV (WHO) | CTX-AZA vs. MMF |
| Lateef et al. [36] (2010) | Retrospective observational study | Singapore | 2005–2008 (median: 28 months) | Patients with SLE (ACR criteria) who had received rituximab for treatment of severe, refractory disease | ACR; confirmed on histopathology | NA |
| Aghdassi et al. [26] (2011) | Patient survey, costs from various sources | Canada | 2004–2009 (NA) | Patients with SLE (at least 4/11 ACR criteria) attending tertiary specialist clinic | LN defined by histological findings on renal biopsies or by laboratory abnormalities; proteinuria > 0.5 g/24 h and/or presence of urinary cellular casts ever |
LN vs. SLE without LN Active LN vs. inactive LN |
| Hiraki et al. [33] (2012) | Retrospective claims database analysis | USA | 2000–2004 (NR) | Patients in the Medicaid Analytic eXtract (MAX) database aged 3 to < 18 years with SLE (≥ 3 ICD-9 codes of SLE [710.0], each > 30 days apart) | NR | NA |
| Furst et al. [31] (2013) | Case–control claims database analysis | USA | 2003–2008 (NR) | Patients with SLE (ICD-9-CM 710.0x, with evidence of ≥ 1 inpatient claim or ≥ 2 ED visits ≥ 30 days apart; Medicaid and Medicare population) | ICD-9-CM codesa | LN vs. matched controls without SLE |
| Yeh et al. [47] (2013) | Retrospective claims database analysis | USA | 2004–2011 (1 year) | Patients with SLE (ICD-9 code 710.0 from ≥ 2 outpatient or ≥ 1 inpatient claims) | NR | Cohorts defined by number of renal diagnoses |
| Jönsen et al. [34] (2016) | Retrospective registry analysis, costs from The Medicines Compendium | Sweden | 2003–2010 (8 years) | Patients with SLE (confirmed diagnosis and enrollment in a registry before or during study period) | ACR-SLICC-DI (manifestation of glomerulonephritis) | LN vs. SLE (total population including LN) |
| McCormick et al. [39] (2016) | Retrospective claims database analysis | Canada | 1996–2010 (19,139 patient years) | Patients with incident SLE from BC during 1996–2010 (no prior SLE diagnosis from 1990–1995) |
Primary (narrow) definition: > 2 renal-coded encounters AND > 2 nephrologist visits Secondary (broad) definition: > 2 renal encounters OR > 2 nephrologist visits Anytime from 12-months prior to SLE diagnosis to end of follow up |
Cohorts defined by number of renal encounters |
| Li [37] (2017) | Retrospective observational study, cost source NR | China | 2014–2015 (NR) | Patients with LN | Primary LN diagnosis from electronic medical records system | NA |
| Venegas et al. [45] (2017) | Patient survey, cost source NR | Philippines | 2016 (NA) | Patients with SLE > 18 years with a minimum 1-year follow up consecutively seen at Lupus Clinics | NR | LN vs. SLE without LN |
| Barber et al. [27] (2018) | Prospective cohort study and cost prediction model | US, Europe, Canada, Mexico, Korea | 1999–2013 (mean 6.3 years) | Patients with SLE from the SLICC network (fulfilling ACR revised classification criteria for SLE and enrolled into an inception cohort within 15 months of diagnosis) | Renal biopsy or fulfillment of the renal item on ACR criteria |
LN vs. patients with SLE without LN LN by level of eGFR LN by level of estimated proteinuria |
| Barbour et al. [28] (2018) | Retrospective observational study, costs from PharmaNet | Canada | 2000–2012 (mean 6.8 years) | Patients with LN | Renal biopsy | NA |
| Feldman et al. [30] (2018) | Retrospective claims database analysis | USA | 2000–2010 (mean 3.1 years) | Patients aged 5–65 years with SLE (≥ 3 SLE claims; Medicaid population) | ≥ 2 LN claims (ICD-9-CM codes: glomerulonephritis, renal failure, or nephrotic syndrome) | Male vs. female |
| Guerra et al. [32] (2018) | Retrospective observational study | USA | 2008–2017 (30 days) | Newly diagnosed pediatric patients with LN (including patients with Medicaid) | NR | Early readmitted to hospital group (< 30 days) vs. not early readmitted group |
| Tanaka et al. [43] (2018) | Retrospective claims database analysis | Japan | 2010–2012 (3 years) | Patients selected from the JMDC‐CDB, aged 15–65 years and a SLE-related visit (ICD-10-M32) with continuous inclusion eligibility for 6 months prior and 3 years post-index date | Combined elements of SLEDAI, SLAM, and BILAG criteria, with use of SLE medications and consensus clinical opinion | NP/LN vs. SLE without NP/LN |
aICD-9-CM codes for acute glomerulonephritis, nephrotic syndrome, chronic glomerulonephritis, nephritis not otherwise specified, acute renal failure, CKD, renal failure unspecified, kidney biopsy, hemodialysis, or peritoneal dialysis, kidney transplant
ACR American College of Rheumatology, ACR-SLICC-DI American College of Rheumatology-Systemic Lupus International Collaborating Clinics–Damage index, AZA azathioprine, BC British Columbia, BILAG British Isles Lupus Assessment Group index, CKD chronic kidney disease, CTX cyclophosphamide, CTX-AZA cyclophosphamide induction followed by azathioprine maintenance, CUA cost–utility analysis, ED emergency department, eGFR estimated glomerular filtration rate, HCRU healthcare resource utilization, ICD-9 International Classification of Diseases, Ninth Revision, ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-10-M32 International Classification of Diseases, Tenth Revision, systemic lupus erythematosus, JMDC-CDB Japanese Medical Data Center Claims Database, LN lupus nephritis, MMF mycophenolate mofetil, NA not applicable, NERG not early readmittance group, NP neuropsychiatric, NR not reported, SLAM Systemic Lupus Activity Measure, SLE systemic lupus erythematosus, SLEDAI Systemic Lupus Erythematosus Disease Activity Index, SLICC Systemic Lupus International Collaborating Clinics American College of Rheumatology Damage index, SLR systematic literature review, UK United Kingdom, USA United States of America, US United States, WHO World Health Organization
Characteristics of patients with LN
Patient characteristics were reported in 17 studies [26–34, 36, 38, 39, 42–45, 47]. The five studies not reporting patient characteristics were the four CUAs [35, 40, 41, 46] and one of the retrospective studies, which was only available as an abstract [37]. The included studies were primarily conducted in adults, with two studies conducted in children [32, 33], and three did not have an age restriction [29, 30, 34]. Patients were predominantly (> 70%) female.
Only four studies reported information on LN histological classification [28, 32, 36, 44]. Tse et al. [44] included only patients with Class IV, and Lateef et al. [36] included mainly patients with Class IV (57.1%). Most patients included in the study by Guerra et al. [32] had Class IV or V (73.7%) nephritis. Barbour et al. [28] stratified per-patient medication costs by LN class but did not report the proportions of patients in each class.
Only four studies reported LN activity [26, 29, 32, 46], one of which included only active patients [46]. One study included 37.6% active (defined as a SLE Disease Activity Index 2000 [SLEDAI-2 K] score > 6) and 18.4% inactive disease in patients with LN [26].
Few studies reported inclusion of patients with either CKD complications or ESKD. A Canadian study by Barbour et al. [28] and a US study by Li et al. [38] reported that 13.3% of patients with LN and 6.8% of patients with SLE had ESKD, respectively. A further US study by Feldman et al. [30] reported the 5-year cumulative incidence of ESKD to be 22.3% in males and 21.2% in females with LN.
Direct costs
Direct costs reported in the identified studies included the cost of hospitalization, outpatient visits/services, emergency department (ED) visits, diagnostic tests, medications, alternative treatments, assistive devices, and surgical procedures (Table 2).
Table 2.
Total direct medical costs by comparison type
| Author year, country (currency year, currency) | Period | Patients with LN, N | Comparison | Cost category | Cost results (USD) | p value |
|---|---|---|---|---|---|---|
| LN vs. no LN | ||||||
| Carls 2009 [29], USA (2005 USD) | 2000–2004 | 592 |
Patients with LN vs. Matched control patients without SLE |
Mean (SD) total medical expenditure in 12-month study period |
58,389 (99,483) vs 11,527 (21,935) |
< 0.001 |
| Li 2009 [38], USA (2006 USD) | 1999–2005 | 489 |
Patients with LN vs. Patients with SLE without LN Patients with LN without ESKD vs. Patients with ESKD |
Mean (median) annual medical costs per patient at year 5 Mean (median) annual medical costs per patient over time |
50,578 (21,500) vs. 16,638 (8496) |
NA |
| Year 1: | 18,002 (10,053) | |||||
| Year 2: | 15,953 (8706) | |||||
| Year 3: | 17,757 (9743) | |||||
| Year 4: | 30,899 (12,441) | |||||
| Year 5: | 38,434 (11,532) | |||||
| Year 1: | 47,660 (33,827) | |||||
| Year 2: | 43,614 (29,020) | |||||
| Year 3: | 58,357 (42,103) | |||||
| Year 4: | 83,232 (55,395) | |||||
| Year 5: | 106,982 (66,490) | |||||
| Pelletier 2009 [42], USA (2008 USD) | 2007 | 1068 |
Patients with LN vs. |
Total mean (SD) annual costs; SLE-related mean |
30,652 (51,749); 6991 (15,576) vs. |
N/A |
| Patients with SLE without LN | (SD) total annual costs | 12,029 (26,577); 2489 (11,194) | ||||
| Aghdassi 2011 [26], Canada (CAD)a | 2004–2009 | 79 |
Patients with LN vs. |
Mean (SD) total cost/4 weeks; Mean (SD) annual |
969 (765); 12,597 (9946) vs. |
|
| Patients with SLE without LN | cost | 814 (1011); 10,585 (13,149) | ||||
|
Patients with active LN vs. Patients with inactive LN |
1094 (790); 14,224 (10,265) vs. 703 (648); 9142 (8419) |
< 0.05 < 0.05 |
||||
| Furst 2013 [31], USA (2009 USD) | 2003–2008 | 907 |
Patients with LN vs. Matched patients without SLE |
Overall mean (95% CI) annual costs in 12-month post-index period |
33,472 (29,797–37,146) vs. 5347 (4719–5976) |
< 0.001 |
| Yeh 2013 [47], USA (USD) | 2004–2011 | 24,357 | Patients with differing numbers of renal diagnoses: | Annual medical costs | NA | |
|
≥ 1 renal diagnosis ≥ 2 renal diagnoses ≥ 3 renal diagnoses ≥ 3 renal diagnoses plus ≥ 3 nephrologist visits |
33,176 36,974 36,241 38,883 |
|||||
| Jönsen 2016 [34], Sweden (2011 USD) | 2003–2010 | 321 | Patients with LN |
Mean (SD) total direct cost; median (IQR) total |
14,190 (35,756); 4709 (1661–10,989) | NA |
| vs. | direct cost | vs. | ||||
| Patients with SLE without LN | 10,188 (28,352); 2860 (1153–7708) | |||||
| McCormick 2016 [39], Canada (2010 CAD) | 1996–2010 | 303/632 |
> 2 renal AND > 2 nephrologist visits LN vs. |
Unadjusted 5-year mean per-patient-year costs |
85,292 vs. |
< 0.01 |
|
Patients with SLE without LN > 2 renal OR > 2 nephrologist visits LN vs. |
33,022 70,538 vs. |
|||||
| Patients with SLE without LN | 27,487 | |||||
| Venegas 2017 [45], Philippines (2016 Philippine peso) | 2016 | 166 |
Patients with SLE requiring dialysis vs. |
Annual cost |
595,400 vs. |
< 0.001 |
|
Patients with LN without dialysis vs. Patients with SLE without LN |
144,700 vs. 55,020 |
|||||
| Tanaka 2018 [43], Japan (USD) | 2010–2012 | 110 |
Patients with NP lupus/LN vs. Patients with SLE without NP lupus/LN |
Mean (SD) total costs over the 3-year study period |
39,976 (47,563) vs. 22,500 (36,128) |
0.0004 |
| Worsening eGFR | ||||||
| Barber 2018 [27], Multipleb (2015 CAD) | 1999–2013 | 609 | Patients stratified by LN status and state of eGFR: |
Predicted annual health costs, mean (95% CI) |
NA | |
|
State 1 (LN) State 2 (LN) State 3 (LN) ESKD vs. |
3858 (2858–4859) 4012 (2362–5662) 20,837 (3628–38,046) 51,313 vs. |
|||||
|
State 1 (no LN) State 2/3 (no LN) |
1813 (1034–2593) 2955 (37–5873) |
|||||
| Barbour 2018 [28], Canada (2016 CA$) | 2000–2012 | 362 |
Patients with LN class III or IV (± V) disease vs. Patients with LN class V disease |
Annual per-patient cost |
209 (year 2000) vs. 1592 (year 2013) vs. |
< 0.001 |
| 118 (year 200) vs. 1002 (year 2013) | 0.016 | |||||
aDisease activity was determined using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2 K). SLEDAI > 6 was considered an active disease. bUSA, n = 426; Europe, n = 405; Canada, n = 372; Mexico, n = 184; and Korea, n = 158
CAD Canadian dollars, CI confidence interval, eGFR estimated glomerular filtration rate, ESKD end-stage kidney disease, IQR interquartile range, IV intravenous, LN lupus nephritis, NA not applicable, NP neuropsychiatric, SD standard deviation, SLE systemic lupus erythematosus, USA United States of America, USD US dollars
Total costs for LN [29, 31, 34, 38, 42, 43] were consistently higher than comparator populations; both patients without SLE [29, 31] and patients with SLE without LN (Table 2) [34, 38, 42, 43]. In two studies, the increase in total annual costs observed in the LN population versus the matched controls without SLE was significant (p < 0.001) [29, 31]. The total costs reported by Carls et al. [29] were higher than other claims studies conducted in the US. The authors hypothesized that expenditure was based on actual experience of patients rather than relying on national- or country-specific estimates based on negotiated fee schedules, which is often the case in other claims database analyses.
Two cost-analyses conducted in the US and Canada compared different algorithms to identify LN from claims databases [39, 47]. In the US study [47], costs were higher with increased number of renal diagnoses (US$33,176 with ≥ 1 renal diagnosis, US$38,883 with ≥ 3 renal diagnoses plus ≥ 3 nephrologist visits). Similarly, in the Canadian study [39], the more stringent LN definition of > 2 renal-coded visits AND > 2 nephrologist visits resulted in higher unadjusted mean per-patient-year costs for patients with LN than when LN was defined as > 2 renal-coded visits OR > 2 nephrologist visits (CA$85,292 vs. CA$70,538, respectively). These data indicate that the stringency of codes used to identify a patient with LN has an impact on the reported costs.
Two other Canadian studies stratified costs by LN classification and severity [27, 28]. Barbour et al. [28] reported a significant increase in annual per-patient total costs in patients with Class III or IV (± V) LN disease (Year 2000: CA$209, Year 2013: CA$1592; 2016 CA$; p < 0.001). In patients with Class V LN alone, costs increased over the same time period, but this was not significant (CA$118, CA$1002; p = 0.016). Using prospectively collected data from the Systemic Lupus International Collaborating Clinics (SLICC) network inception cohort, Barber et al. [27] used a multistate Markov model to predict mean annual costs per patient in health states defined by the presence of LN and by either worsening estimated glomerular filtration rate (eGFR) or increasing estimated proteinuria. The model showed increasing costs in patients with LN and/or with worse renal function, versus those without LN or with eGFR > 60 ml/min. Conversely, when Aghdassi et al. [26] compared patients with SLE with or without LN, and active (defined as a SLEDAI-2 K score > 6) and inactive disease, there was no difference in annual costs between LN and patients with SLE without LN regardless of activity, but there was a significant difference in total annual costs between active and inactive LN (p < 0.05).
In several studies, an increase in annual hospitalization costs was observed between patients with LN and their matched control patients without SLE, the total SLE population or patients with SLE without LN [29, 34, 42]. However, in a Canadian study by Aghdassi et al. [26], hospitalization costs were slightly higher for patients with SLE without LN compared with patients with LN, and this difference was not significant.
No studies provided information about costs associated with renal flares specifically in patients with LN; however, Tanaka et al. [43] reported the cost of SLE flares (Table 2).
Costs for ESKD and its treatment were reported in six studies (Tables 2 and 3) [27, 35, 38, 40, 41, 45], three of which were CUAs [35, 40, 41]. In the US study by Li et al. [38], median annual medical costs for patients with SLE and ESKD increased by twofold between Year 1 and Year 5 (US$33,827–66,490), whereas costs for patients with LN without ESKD increased by approximately 1.2-fold in the same time period (US$10,053–11,532). Similarly, Venegas et al. [45] found that treatment costs were significantly increased in patients with SLE requiring dialysis, versus patients with LN who did not require dialysis versus patients with SLE without LN (2016 Philippine peso 595,400 vs. 144,700 vs. 55,020, respectively; p < 0.001); ESKD was also found to be a significant independent contributor to treatment costs (p < 0.001). In addition, Barber et al. [27] reported that predicted mean annual costs related to ESKD were more than 17-fold higher than health states reflecting good renal function.
Table 3.
Medical costs and data sources from CUA studies
| Author year, country (currency year, currency) | Patient population and patients compared | Sources of data | Cost category | Cost results (USD) |
|---|---|---|---|---|
| Wilson 2007 [46], UK (2005 GBP) |
Patients with active LN requiring induction therapy MMF vs. IV CYC |
A SLR of the literature identified two studies comparing MMF and IV CYC, reporting results following induction therapy [59, 60] Further data was extracted from a Cochrane review of all treatments [61] |
Total mean costs per 12 weeks (including medication, secondary care activity, and other monitoring): | |
| MMF: | 843.25 | |||
| IV CYC: | 1754.54 | |||
| No immunosuppressive therapy: | 90.83 | |||
| Mohara 2014 [40], Thailand (2012 Thai Baht) |
Patients with newly diagnosed severe LN receiving induction and maintenance therapy Four treatment regimes |
The PubMed database was searched using the following keywords: (lupus nephritis [MeSH]) AND (cyclophosphamide [MeSH] OR azathioprine [MeSH] OR mycophenolic acid [MeSH]) Only articles published between January 2000 and July 2012 that were written in English, Spanish, or Thai were considered. Study types that were considered included controlled clinical trials, randomized controlled trials, clinical trials, and comparative studies Ten studies met the inclusion criteria by giving details of the dosage of the drugs under consideration and examined the treatment outcomes for any of the five defined health states Costs sourced from national databases HCRU estimated from a medical record review on LN treatment at four tertiary care hospitals (laboratory tests and drug administrative costs) |
Mean (SE) cost of dialysis for patients with end-stage renal failure (per year) | 497,019 (4998) |
| Nee 2015 [41], USA (2013 US$) |
Patients with proliferative LN receiving maintenance therapy Different treatment regimes |
A Cochrane meta-analysis of maintenance therapy with MMF vs. AZA was performed using data from three clinical trials (MAINTAIN, ALMS, and Contreras’s study) and Red Book, and was the foundation of this base-case model | Mean costs (range) over 1 year | |
| Remission (nonpharmaceuticala): | 3368.34 (1263.13–2105.21 | |||
| Relapse (nonpharmaceuticala): | 6486.85 (2432.57–4054.29) | |||
| ESKD/dialysis: | 86,608 | |||
| Kim 2019 [35], China (Chinese Yuan) |
Patients with moderate-to-severe LN requiring induction therapy Different treatment regimes |
Three CUAs were identified and assessed from the SLR: Four different treatment regimens combining IV CYC, AZA, and MMF for long-term therapy in Thailand [40] MMF and AZA as maintenance treatments from a US perspective [41] MMF and IV CYC as induction treatments from a UK perspective [46] |
Medical costs of all patients in the ESKD state (per year) | 80,188 |
aCare provided by specialists, nonspecialists, nonphysician healthcare professionals, laboratory studies, imaging studies, emergency room visits, outpatient surgery, and hospitalizations
ALMS Aspreva Lupus Management Study, AZA azathioprine, CYC cyclophosphamide, CUA cost–utility analysis, ESKD end-stage kidney disease, GBP pound sterling, HCRU healthcare resource utilization, IV intravenous, LN lupus nephritis, MeSH Medical Subject Headings, MMF mycophenolate mofetil, NR not reported, SE standard error, SLR systematic literature review, UK United Kingdom, USA United States of America, US United States, US$ US dollar
Indirect costs
Indirect costs reported in the identified studies included the cost of absenteeism (absence from work), disability, and other loss of productivity (for patient and caregiver) (Table 4).
Table 4.
Indirect costs
| Author, year, country (currency year, currency) | Study details | Indirect cost category | Results |
|---|---|---|---|
| Carls 2009 [29] USA (2005 USD) |
Case–control claims database analysis, 2000–2004 Age: LN vs. SLE: 44.4 vs. 47.1 years n = 592 patients with LN |
Mean (SD) costs during 12-month study period | |
| LN | |||
| Absenteeism (n = 10, 70.0% claimed): | 4781 (10,144), p = 0.946 | ||
|
Short-term disability (n = 20, 15.0% claimed): vs. |
1025 (2673), p = 0.375 | ||
| Matched control patients without SLE | |||
| Absenteeism (n = 10, 100% claimed): | 4552 (2878) | ||
| Short-term disability (n = 20, 5.0% claimed): | 386 (1728) | ||
| Mohara 2014 [40] Thailand (2012 Thai Baht) | CUA/SLR | Productivity lossa of patient and care giver per visit, mean (SE) | |
| LN | 176 (49) | ||
| Major infection per episode | 5739 (982) | ||
| Nee 2015 [41] USA (2013 USD) | CUA/SLR | 6-month/12-month mean costs of productivity loss (range) due to:b | |
| Remission: | 8033.19/16,066.38 (6024.89–10,041.49) | ||
| Relapse: | 8564.07/17,128.13 (6423.05–10,705.09) | ||
| Jönsen 2016 [34] Sweden (2011 USD) |
Retrospective registry analysis, 2003–2010 SLE mean age at diagnosis (range): 35.4 (3–85) years n = 321 patients with LN |
Mean (SD)/median (IQR) costsc | |
|
LN vs. |
23,181 (30,792)/0 (0–44,543) | ||
| SLE | 25,094 (31,387)/1255 (0–53,744) |
aDue to sick leave. bTime lost from labor and non-labor (i.e., household work) market activity, plus the time that a caregiver spent helping the patient receiving healthcare services and the time the caregiver spent doing housework. cBased on sickness leave and disability pensions
CI confidence interval, CUA cost–utility analysis, IQR interquartile range, LN lupus nephritis, SD standard deviation, SE,standard error, SLE systemic lupus erythematosus, SLR systematic literature review, USA United States of America, USD United States dollars
Four studies reported indirect costs, including cost of loss of productivity (for patient and caregiver) [40, 41], cost of absenteeism and short-term disability [29], and total indirect costs [34]. In the Swedish study by Jönsen et al. [34], although higher indirect costs were reported for the LN cohort compared with the total SLE cohort (mean [SD]: 2011 US$25,094 [31,387] vs. US$23,181 [30,792]), the difference was not significant. Overall, no significant differences in indirect costs between patients with LN and comparators were reported (Table 4).
In the Canadian study by Aghdassi et al. [26], 48.1% of patients with LN versus 45.2% of patients with SLE without LN were employed. Of these, more patients with LN (56.8%) missed work compared with patients with SLE without LN (42.9%) and had more days of missed work in the past month (8.5 vs. 4.1, respectively). Furthermore, caregivers of patients with LN missed more hours of work than those caring for patients with SLE without LN (p < 0.05).
HCRU
Overall, patients with LN required more inpatient, outpatient, and ED visits, were more likely to be hospitalized, spend longer in hospital, and need more medication than patients with SLE without LN or patients without SLE [26, 31, 38, 39, 42].
The mean annual numbers of inpatient, outpatient, and ED visits were higher for patients with LN (inpatient: 0.6–1.0, outpatient: 6.6–7.4, ED: 1.5–1.9) compared with the total SLE population (inpatient: 0.3–0.5, outpatient: 5.6–6.9, ED: 1.3–1.6) or patients without SLE (inpatient: 0.1–0.2, outpatient: 3.4–3.8, ED: 0.5–0.9) [31, 38, 42]. In addition, the mean annual numbers of inpatient, outpatient, and ED visits were higher in pediatric patients with LN (in the months prior to ESKD) (inpatient: 2.4, outpatient: 10.8, ED: 2.0) compared with adult patients with LN (inpatient: 0.6–1.0, outpatient: 6.6–7.4, ED: 1.5–1.9) [31, 33, 38]. Feldman et al. [30] reported that male patients with LN had fewer outpatient visits (incidence rate ratio [IRR], 95% confidence interval [95% CI] 0.88, 0.80–0.97) and fewer ED visits (IRR, 95% CI 0.75, 0.63–0.90) than female patients with LN.
The proportion of patients reporting inpatient hospitalizations increased by 1.3–2.2 times in patients with LN compared with patients with SLE without LN [26, 38, 39, 42] and by 3.7–5.3 times compared with matched control patients without SLE [31, 38]. Pelletier et al. [42] also reported that patients with LN had longer lengths of stay compared with patients with SLE without LN (16.52 vs. 9.69 days, p < 0.001). However, this was not that case in the Canadian analysis by Aghdassi et al. [26] (2.8 vs. 5.7 days; p ≥ 0.05). Patients with active LN were more likely to be hospitalized than those with inactive LN (7.8 vs. 3.8%), but patients with inactive LN spent longer in hospital than those with active LN (4.0 vs. 2.5 days). In a study conducted in Singapore, the length of hospitalization was significantly longer before versus after treatment with rituximab (p = 0.027) [36], while in an analysis conducted in Hong Kong, the duration of hospitalization was longer in patients with LN treated with sequential cyclophosphamide (CYC) induction followed by azathioprine maintenance compared with patients treated with mycophenolate mofetil (MMF) (mean [SD] 6.2 [18.2] vs. 1.1 [2.8] days) [44].
Over the 13-year period analyzed in Barbour et al. [28], the use of rituximab (from 0 to 3.5%), calcineurin inhibitor (from 0 to 4.5%), and MMF (from 3.3 to 55.3%) for treatment of LN all increased from the year 2000 to 2013. Patients with LN also averaged 128.6 more dispensed prescriptions than patients with SLE without LN over 5 years [39].
Discussion
This SLR included 22 studies from 28 articles published between 2007 and 2019 that provided information on the cost and HCRU associated with LN.
LN was associated with substantially higher direct costs compared with patients without SLE or patients with SLE without LN [26–29, 31, 34, 37–39, 42, 43, 45, 47]. Direct healthcare costs were 1.2–3.0 times greater in patients with LN versus patients with SLE without LN [26, 34, 38, 42, 43, 45]. As expected, differences were greater (5.1–6.3 times) when comparisons were made between patients with LN and matched control patients without SLE [29, 31].
Costs for patients with ESKD were higher than for patients with LN who had not progressed to ESKD [38]. The need for dialysis significantly increased the cost of treatment (4.1 times) compared with patients with LN not requiring dialysis, and ESKD was a significant independent contributor to treatment costs [45]. In addition, Barber et al. [27] reported that increased costs were associated with worsening eGFR, with a marked increase among patients with LN reaching < 30 ml/min eGFR without ESKD. This trend is observed in studies of CKD due to other causes [9, 51], and reflects the importance of preventing any deterioration of renal function including prior to reaching kidney failure.
Barbour et al. [28] previously reported that costs of immunosuppressive treatments for glomerulonephritis were increasing over time due to changing patterns in clinical practice. In particular, the Aspreva Lupus Management Study (ALMS) reported pivotal data for LN treatment in 2009 and 2011, which likely consolidated the use of MMF as standard therapy for LN, especially in the US and Europe [52, 53]. These changes in clinical practice over the study period (2007–2019) may have influenced the direct costs of LN and make it more difficult to compare costs between studies.
Indirect costs were infrequently reported and no significant differences were observed between patients with LN and comparators [29, 34, 40, 41]. Although SLE tends to affect patients during their most productive years of life, in terms of professional and familial achievement [1], there was limited information on the degree of productivity lost among patients with LN. Further research is also needed to understand the impact of LN on health-related quality of life and activities of daily living, which in turn may impact productivity. Only one of the studies included in this analysis reported limited data on absenteeism/presenteeism in LN [29], a substantial contributor to lost productivity among patients with SLE. A full understanding of indirect costs (notably productivity) is a particular gap in the literature and a future area of research.
In most of the included studies, patients with LN were more likely to be hospitalized and spend longer in hospital than their comparators [26, 31, 32, 39, 42, 43]. However, Aghdassi et al. [26] found that patients with SLE alone spent longer in hospitals than patients with active LN. This observation could be due to the more intensive treatment required for SLE, when extrarenal manifestations are severe, compared with LN. In addition, pediatric patients with LN (in the months prior to ESKD) were found to have more inpatient, outpatient, and ED visits than adult patients with LN [31, 33, 38]. This could suggest that pediatric patients would have higher HCRU costs than adult patients with LN.
Patients with LN were also more likely to require outpatient visits and a greater quantity of medication than their comparators [26, 28, 31, 38, 42], particularly immunosuppressants and corticosteroids [42, 43]. As new treatments for LN emerge, it is important to understand the relationship between medication use and HCRU for cost-effectiveness studies. Evidence suggests that there is a cost-saving potential of earlier aggressive therapy to prevent disease progression. Despite the higher initial costs of biologics compared with standard therapies, rituximab has been found to be cost saving in the treatment of LN, as cost and number of hospitalizations are decreased after treatment [36].
This SLR has several limitations. As the search was performed in 2019 relevant recent publications could have been missed. For example, Padiyar et al. [54] has recently reported a comparison of the costs of oral CYC compared with intravenous CYC and Bell et al. [55] recently published an abstract reporting the burden of illness in LN; it was reported that patients with LN have significantly higher ambulatory visits, ED visits, hospitalizations, and costs than patients without SLE. Miyazaki et al. [56] also recently reported HCRU of patients with LN compared with patients without central nervous system (CNS) lupus or LN; a higher proportion of patients with LN had ≥ 1 hospitalization compared with patients without CNS lupus or LN.
The method of LN case ascertainment also differed between the included studies. Nine of the 22 studies included in this SLR derived data from claims databases, which are inherently reliant on accurate coding of medical conditions. The identification of patients from administrative claims data, particularly if a disease does not have a specific ICD diagnostic code, necessitates the use of proxies for diagnosis. For example, diagnosis of LN was assumed if patients had concurrent codes for SLE and renal disease. Notably, two studies included in this review demonstrated that the increasing stringency of diagnosis code algorithms used to identify patients with LN resulted in an increase in the reported costs [39, 47].
In future studies, increased reporting of potential prognostic factors such as LN histological class and activity status would be useful, since a limited number of studies included in this analysis reported such information [26, 28, 32, 36, 43, 44, 46]. This highlights a gap in the literature as stratification of cost and HCRU by disease classification and/or severity would allow for a more comprehensive assessment of heterogeneity across studies, generalizability of conclusions, and quantitative synthesis.
In some studies, data were taken from claims databases of employed individuals, meaning patients not in work were not captured. This can introduce bias as analyses are consequently conducted on a “healthier” population with milder SLE who are able to work, rather than the general SLE population; this may particularly affect estimates generated for patients with LN given that it is the severe form of the disease. However, several studies using Medicare and Medicaid databases were also included in this study. Therefore, the potential bias introduced by claims databases may not have such an effect on this analysis.
In the US healthcare system, the cost of care for patients with ESKD is funded almost entirely by Medicare [38, 57]; hence, the costs associated with dialysis, kidney transplant, and associated medications will be underestimated by claims analyses that do not include all claims paid by Medicare. Given the high per-patient cost of dialysis and kidney transplant, this may have an important impact on the estimation of economic burden of LN.
As the search included in this study focused on the subgroup of patients with LN, relevant aspects of economic burden borne by the broader SLE population, such as productivity losses, may not be reflected. For example, the claims data analysis by Garris et al. [58] was not included in the present SLR as cost data specific to an LN population was required to be included in the study.
The clear absence of data on the cost associated with a flare in SLE generally, but particularly with a renal flare, is a notable knowledge gap in the current literature. Although it is likely that the cost of flare is incorporated into other costs reported, without explicit data describing flare costs it is difficult to determine the immediate economic impact if these important clinical events can be avoided. Similarly, there are limited data available describing patients with LN with ESKD, with data used in published cost-effectiveness analyses coming from the general ESKD population rather than LN-related ESKD. As new interventions emerge for the treatment of active LN, greater delineation of these costs at the patient level will be critical to demonstrating their economic value.
Conclusions
There is consensus across the studies included in this SLR that LN is expensive to manage. Specifically, LN was associated with higher direct costs (including total annual costs and costs of hospitalization and ESKD), total indirect costs, and HCRU (including hospitalization, outpatient services, and medication use) compared with those of either patients without SLE or patients with SLE without LN. However, limitations of current studies mean that it is difficult to determine the true cost of illness associated with LN. The greatest gap in the literature, which should be prioritized as a future research priority, is the absence of specific data for the cost of renal flare in patients with LN, despite it being a clinically important and frequently occurring medical emergency. As a disease flare is likely to result in a period of intense resource use for a patient with SLE, minimization of flare recurrence should reduce overall costs associated with LN disease control.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The study (GSK study 213531) was funded by GlaxoSmithKline (GSK). GSK commissioned Bridge Medical Consulting Ltd to conduct this SLR. The journal’s Rapid Service was also funded by GSK.
Medical Writing and Editorial Assistance
Editorial support was provided by Helen Taylor, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
JCT, AM, and DAS were involved in the conception of the study, data acquisition, and analysis/interpretation. KG was involved in the conception of the study and data analysis/interpretation.
Disclosures
Juliette C. Thompson, Anadi Mahajan and David A. Scott are employees of Bridge Medical Consulting Ltd. Juliette C. Thompson and David A. Scott, are also employees of Visible Analytics Ltd. Kerry Gairy is an employee of GSK and holds stocks and shares in the company.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are available in this published article or as supplementary information files.
References
- 1.Nusbaum JS, Mirza I, Shum J, et al. Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin Proc. 2020;95:384–394. doi: 10.1016/j.mayocp.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Grammer AC, Lipsky PE. B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther. 2003;5:S22–S27. doi: 10.1186/ar1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis. 2006;1:6. doi: 10.1186/1750-1172-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahajan A, Amelio J, Gairy K, et al. Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression. Lupus. 2020;29:1011–1020. doi: 10.1177/0961203320932219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galindo-Izquierdo M, Rodriguez-Almaraz E, Pego-Reigosa JM, et al. Characterization of patients with lupus nephritis included in a large cohort from the Spanish Society of Rheumatology Registry of Patients with Systemic Lupus Erythematosus (RELESSER) Medicine (Baltimore) 2016;95:e2891. doi: 10.1097/MD.0000000000002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanly JG, O'Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–262. doi: 10.1093/rheumatology/kev311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6:7. doi: 10.1038/s41572-019-0141-9. [DOI] [PubMed] [Google Scholar]
- 8.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27:3248–3254. doi: 10.1093/ndt/gfs073. [DOI] [PubMed] [Google Scholar]
- 9.Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23:S163–S172. [PubMed] [Google Scholar]
- 10.Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford) 2017;56:709–715. doi: 10.1093/rheumatology/kew475. [DOI] [PubMed] [Google Scholar]
- 11.Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Systemic lupus erythematosus with neuropsychiatric manifestation incurs high disease costs: a cost-of-illness study in Hong Kong. Rheumatology (Oxford) 2009;48:564–568. doi: 10.1093/rheumatology/kep031. [DOI] [PubMed] [Google Scholar]
- 12.Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–723. doi: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 13.Tektonidou MG, Wang Z, Dasgupta A, Ward MM. Burden of Serious infections in adults with systemic lupus erythematosus: a national population-based study, 1996–2011. Arthritis Care Res (Hoboken) 2015;67:1078–1085. doi: 10.1002/acr.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slawsky KA, Fernandes AW, Fusfeld L, Manzi S, Goss TF. A structured literature review of the direct costs of adult systemic lupus erythematosus in the US. Arthritis Care Res (Hoboken) 2011;63:1224–1232. doi: 10.1002/acr.20502. [DOI] [PubMed] [Google Scholar]
- 15.Meacock R, Dale N, Harrison MJ. The humanistic and economic burden of systemic lupus erythematosus: a systematic review. Pharmacoeconomics. 2013;31:49–61. doi: 10.1007/s40273-012-0007-4. [DOI] [PubMed] [Google Scholar]
- 16.Turchetti G, Yazdany J, Palla I, Yelin E, Mosca M. Systemic lupus erythematosus and the economic perspective: a systematic literature review and points to consider. Clin Exp Rheumatol. 2012;30:S116–S122. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arthritis Rheum. 2009;61:1159–1167. doi: 10.1002/art.24725. [DOI] [PubMed] [Google Scholar]
- 18.Yeo AL, Koelmeyer R, Kandane-Rathnayake R, et al. Lupus low disease activity state and reduced direct health care costs in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2020;72:1289–1295. doi: 10.1002/acr.24023. [DOI] [PubMed] [Google Scholar]
- 19.Kan H, Guerin A, Kaminsky MS, et al. A longitudinal analysis of costs associated with change in disease activity in systemic lupus erythematosus. J Med Econ. 2013;16:793–800. doi: 10.3111/13696998.2013.802241. [DOI] [PubMed] [Google Scholar]
- 20.Panopalis P, Petri M, Manzi S, et al. The systemic lupus erythematosus Tri-Nation study: cumulative indirect costs. Arthritis Rheum. 2007;57:64–70. doi: 10.1002/art.22470. [DOI] [PubMed] [Google Scholar]
- 21.Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–1128. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 22.Arriens C, Polyakova S, Adzerikho I, Randhawa S, Solomons N. OP0277 AURORA phase 3 study demonstrates voclosporin statistical superiority over standard of care in lupus nephritis (LN) Ann Rheum Dis. 2020;79(172):2–3. [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochrane Handbook for Systematic Reviews of Interventions. 2020. Available from: https://training.cochrane.org/handbook/current. Accessed 8 Oct 2020
- 26.Aghdassi E, Zhang W, St-Pierre Y, et al. Healthcare cost and loss of productivity in a Canadian population of patients with and without lupus nephritis. J Rheumatol. 2011;38:658–666. doi: 10.3899/jrheum.100482. [DOI] [PubMed] [Google Scholar]
- 27.Barber MRW, Hanly JG, Su L, et al. Economic evaluation of lupus nephritis in the Systemic Lupus International Collaborating Clinics Inception cohort using a multistate model approach. Arthritis Care Res (Hoboken) 2018;70:1294–1302. doi: 10.1002/acr.23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbour S, Lo C, Espino-Hernandez G, et al. The population-level costs of immunosuppression medications for the treatment of glomerulonephritis are increasing over time due to changing patterns of practice. Nephrol Dial Transplant. 2018;33:626–634. doi: 10.1093/ndt/gfx185. [DOI] [PubMed] [Google Scholar]
- 29.Carls G, Li T, Panopalis P, et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med. 2009;51:66–79. doi: 10.1097/JOM.0b013e31818a405a. [DOI] [PubMed] [Google Scholar]
- 30.Feldman CH, Broder A, Guan H, Yazdany J, Costenbader KH. Sex differences in health care utilization, end-stage renal disease, and mortality among Medicaid beneficiaries with incident lupus nephritis. Arthritis Rheumatol. 2018;70:417–426. doi: 10.1002/art.40392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furst DE, Clarke A, Fernandes AW, et al. Medical costs and healthcare resource use in patients with lupus nephritis and neuropsychiatric lupus in an insured population. J Med Econ. 2013;16:500–509. doi: 10.3111/13696998.2013.772058. [DOI] [PubMed] [Google Scholar]
- 32.Guerra AAH, Prahalad S, Rouster-Stevens KA, Garro R, Bryan L, Hong Y. Readmission rate within 30 days of hospitalization due to new onset lupus nephritis and associated risk factors: The importance of intravenous pulse methylprednisolone therapy. Arthritis Rheumatol. 2018;70:abstract 459. [Google Scholar]
- 33.Hiraki LT, Feldman CH, Alarcon GS, et al. Variation in healthcare utilization by region and number of rheumatologists per state among pediatric Medicaid patients with lupus nephritis prior to end-stage renal disease in the United States, 2000–2004. Arthritis Rheum. 2012;64:S125. [Google Scholar]
- 34.Jönsen A, Hjalte F, Willim M, et al. Direct and indirect costs for systemic lupus erythematosus in Sweden. A nationwide health economic study based on five defined cohorts. Semin Arthritis Rheum. 2016;45:684–690. doi: 10.1016/j.semarthrit.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Reen Ooi AY, Stephens T, Jiang H. Cost-effectiveness of tacrolimus for the treatment of moderate-to-severe lupus nephritis in China. J Comp Eff Res. 2019;8:1125–1141. doi: 10.2217/cer-2018-0111. [DOI] [PubMed] [Google Scholar]
- 36.Lateef A, Lahiri M, Teng GG, Vasoo S. Use of rituximab in the treatment of refractory systemic lupus erythematosus: Singapore experience. Lupus. 2010;19:765–770. doi: 10.1177/0961203309358599. [DOI] [PubMed] [Google Scholar]
- 37.Li T. The pharmacotherapeutic pattern of lupus nephritis patients and its effect on the hospitalisation cost in China. Lupus Sci Med. 2017;4:A81. [Google Scholar]
- 38.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large Medicaid population. Arthritis Rheum. 2009;61:755–763. doi: 10.1002/art.24545. [DOI] [PubMed] [Google Scholar]
- 39.McCormick N, Marra C, Avina-Zubieta A. 220 Longitudinal, incremental direct medical costs of lupus nephritis amongst a general population-based cohort of systemic lupus erythematosus. J Rheumatol. 2016;43:1234. [Google Scholar]
- 40.Mohara A, Perez Velasco R, Praditsitthikorn N, Avihingsanon Y, Teerawattananon Y. A cost-utility analysis of alternative drug regimens for newly diagnosed severe lupus nephritis patients in Thailand. Rheumatology (Oxford) 2014;53:138–144. doi: 10.1093/rheumatology/ket304. [DOI] [PubMed] [Google Scholar]
- 41.Nee R, Rivera I, Little DJ, Yuan CM, Abbott KC. Cost-Utility Analysis of Mycophenolate Mofetil versus Azathioprine Based Regimens for Maintenance Therapy of Proliferative Lupus Nephritis. Int J Nephrol. 2015;2015:917567. doi: 10.1155/2015/917567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelletier EM, Ogale S, Yu E, Brunetta P, Garg J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin Ther. 2009;31:2653–2664. doi: 10.1016/j.clinthera.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Mizukami A, Kobayashi A, Ito C, Matsuki T. Disease severity and economic burden in Japanese patients with systemic lupus erythematosus: a retrospective, observational study. Int J Rheum Dis. 2018;21:1609–1618. doi: 10.1111/1756-185X.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tse KC, Tang CS, Lam MF, Yap DY, Chan TM. Cost comparison between mycophenolate mofetil and cyclophosphamide-azathioprine in the treatment of lupus nephritis. J Rheumatol. 2009;36:76–81. doi: 10.3899/jrheum.080517. [DOI] [PubMed] [Google Scholar]
- 45.Venegas E, Geslani K, Navarra S. 426 Renal activity and damage incur highest medical costs among Filipino patients with systemic lupus erythematosus. Lupus Sci Med. 2017;4:A203–A204. [Google Scholar]
- 46.Wilson EC, Jayne DR, Dellow E, Fordham RJ. The cost-effectiveness of mycophenolate mofetil as firstline therapy in active lupus nephritis. Rheumatology (Oxford) 2007;46:1096–1101. doi: 10.1093/rheumatology/kem054. [DOI] [PubMed] [Google Scholar]
- 47.Yeh WS, Clark DW, McCarty KM. Health resource utilization of lupus nephritis patients-a comparison of result across case identification algorithms. Value Health. 2013;16:A21. [Google Scholar]
- 48.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2015. Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed 8 Apr 2021
- 50.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 51.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24:1478–1483. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dooley MA, Jayne D, Ginzler EM, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365:1886–1895. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 54.Padiyar S, Arya S, Surin A, Viswanath V, Danda D. Comparison of safety, efficacy and cost between oral pulse cyclophosphamide versus intravenous cyclophosphamide pulse therapy in severe systemic lupus erythematosus. Int J Rheum Dis. 2020;23:800–804. doi: 10.1111/1756-185X.13823. [DOI] [PubMed] [Google Scholar]
- 55.Bell CF, Wu B, Xie B, et al. Burden of illness of lupus nephritis in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2020;31:553. [Google Scholar]
- 56.Miyazaki C, Sruamsiri R, Mahlich J, Jung W. Treatment patterns and medical cost of systemic lupus erythematosus patients in Japan: a retrospective claims database study. J Med Econ. 2020;23:786–799. doi: 10.1080/13696998.2020.1740236. [DOI] [PubMed] [Google Scholar]
- 57.Eggers PW. Medicare’s end stage renal disease program. Health Care Financ Rev. 2000;22:55–60. [PMC free article] [PubMed] [Google Scholar]
- 58.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16:667–677. doi: 10.3111/13696998.2013.778270. [DOI] [PubMed] [Google Scholar]
- 59.Ong LM, Hooi LS, Lim TO, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton) 2005;10:504–510. doi: 10.1111/j.1440-1797.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 60.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 61.Flanc RS, Roberts MA, Strippoli GF, Chadban SJ, Kerr PG, Atkins RC. Treatment for lupus nephritis. Cochrane Database Syst Rev. 2004;2004:002922. doi: 10.1002/14651858.CD002922.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available in this published article or as supplementary information files.