Abstract
We report a patient with Japanese spotted fever caused by Rickettsia japonica who developed shock associated with hypercytokinemia. Elevated levels of cytokines (macrophage colony-stimulating factor, interleukin 1 beta, interleukin 10, and gamma interferon) decreased rapidly after a combination treatment using an antibiotic (minocycline hydrochloride [MINO]) and methylprednisolone; however, tumor necrosis factor alpha levels were increased. The patient's fever relapsed and was resolved only after the addition of ciprofloxacin hydrochloride. The administration of new quinolones alone may be another useful form of treatment to eradicate R. japonica even if the symptoms of hypercytokinemia appear to improve with the administration of MINO and methylprednisolone.
In 1984, the first case of Japanese spotted fever (JSF) caused by an infection with Rickettsia japonica was reported in Japan, and 144 cases of JSF diagnosed between 1984 and 1995 were summarized by the National Institute of Health in Japan (6). Areas where JSF is endemic are located along the coast of southwestern and central Japan in a warm climate (7). The clinical symptoms (high fever, skin eruption, and tick bite eschar) of JSF (7) are similar to those of tsutsugamushi disease (3, 4), which is caused by an infection with Orientia tsutsugamushi, another common rickettsial pathogen in Japan. However, JSF sometimes shows more severe clinical findings than tsutsugamushi disease and is a life-threatening illness. We report herein a case of active and severe JSF associated with hypercytokinemia.
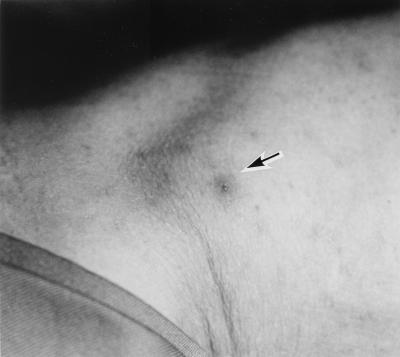
A 78-year-old Japanese woman was infected by R. japonica while collecting bamboo shoots in a wooded mountainous area in Tokushima Prefecture in April 1998. She was admitted to Mahara Hospital with a 3-day history of fever (38.0 to 40.5°C) and general malaise. Physical examination on admission revealed a high fever of 40.5°C, tonsillar swelling and exudate, fasciculation, and generalized maclopapular rash that included the palms and soles. The patient became confused and was in a state of shock, as indicated by low systolic blood pressure, 78 mm Hg. Furthermore, physical examination revealed an eschar, at the right side of the lumbar region, a characteristic site for a tick bite (Fig. 1). Laboratory examinations showed a white blood cell count of 6.3 × 109/liter (granulocytes, 92%; monocytes, 1%; lymphocytes, 6%; atypical lymphocytes, 1%), thrombocytopenia (platelet count, 74 × 109/liter), liver dysfunction (aspartate aminotransferase, 92 IU/liter; lactate dehydrogenase, 938 IU/liter) and raised levels of C-reactive protein (17.5 mg/dl) and fibrinogen degradation product (40 μg/ml). An indirect immunoperoxidase test revealed a strong serum reaction to R. japonica, and an increased level of anti-R. japonica immunoglobulin M (IgM) antibody was detected (1:1,280) in serum (Table 1). Rickettsiae were isolated by inoculating the acute-stage blood into L-929 cells (1) and were obtained by the same method from a tick, Haemaphysalis flava, taken from the bamboo plantation. This was the first isolation from the tick species at a restricted field in association with an outbreak. These pathogens were identified as R. japonica, based on reactivity to anti-R. japonica monoclonal antibody and phylogenetic analysis.
FIG. 1.
A tick bite eschar (arrow) on admission of the patient.
TABLE 1.
Titers of the indirect immunoperoxidase antibody test for R. japonica
| Clinical day | Titer of:
|
|
|---|---|---|
| IgM | IgG | |
| 1 | 1:1,280 | 1:320 |
| 7 | 1:10,240 | 1:2,560 |
| 14 | 1:10,240 | 1:20,480 |
| 23 | 1:5,120 | 1:20,480 |
| 30 | 1:2,560 | 1:20,480 |
| 37 | 1:1,280 | 1:10,240 |
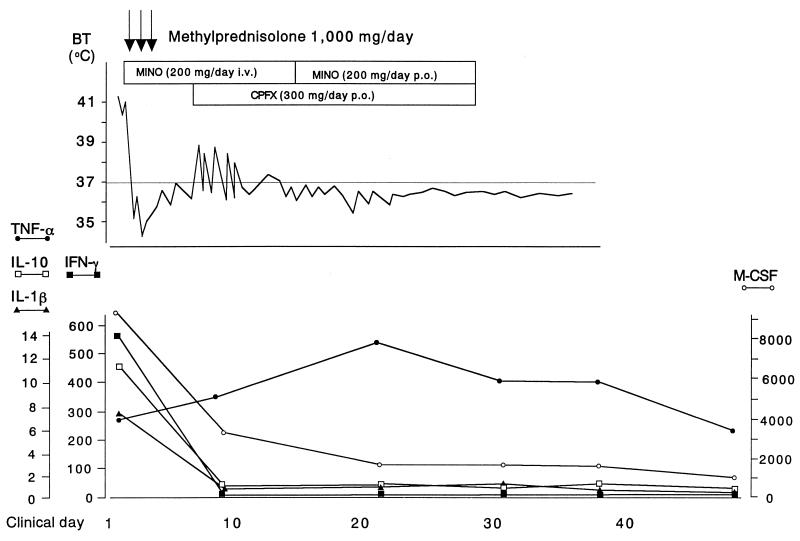
The patient showed a titer of antibody in the Weil-Felix reaction to Proteus OX-19 of 1:20, and the titers of antibodies to Proteus OX-2 and OX-K were negative. Minocycline hydrochloride (MINO) (200 mg/day) was administered to treat the rickettsial infection, and a high dose of a corticosteroid (methylprednisolone sodium succinate) (1,000 mg/day) was administered intravenously for treating shock. The patient's fever was reduced on day 2, and symptoms of shock improved on day 3, but after the end of high-dose corticosteroid therapy, her temperature increased to 39.0°C on day 6. Thereafter we added ciprofloxacin hydrochloride (300 mg/day) orally to eradicate R. japonica. Her confusion improved completely and her fever resolved again on the 12th day in hospital. The patient was doing well with no symptoms 1 month later.
Levels of cytokines in serum were assayed by a sandwich enzyme-linked immunosorbent assay kit (Cytoscreen or Quantikine; Biosource or R & D, respectively) at six different time points up to 7 weeks. Levels of macrophage colony-stimulating factor (8,740 pg/ml), interleukin 1 beta (IL-1β) (7.83 pg/ml), IL-10 (10.7 pg/ml), and gamma interferon (575 pg/ml) in serum were elevated above normal ranges in the acute phase, but these cytokine levels decreased rapidly after combined treatment with an antibiotic (MINO) and methylprednisolone. However, tumor necrosis factor alpha (TNF-α) increased from 7.58 pg/ml in the acute phase to the highest concentration of 13.2 pg/ml at day 23, and it decreased thereafter (Fig. 2).
FIG. 2.
Clinical course and changes in concentrations of five different cytokines in serum during antirickettsial treatment of a patient with JSF. All cytokine levels are expressed as pg/ml. The limits of assay sensitivity were as follows: 0.5 pg/ml for TNF-α, IL-1β, and gamma interferon (IFN-γ); 0.78 pg/ml for IL-10; and 31.3 pg/ml for macrophage colony-stimulating factor (M-CSF). BT, body temperature; CPFX, ciprofloxacin hydrochloride.
TNF-α is a decisive proinflammatory cytokine in the host defense to infection, whereas IL-10 is an anti-inflammatory cytokine and a potent inhibitor of TNF-α. The imbalance of these two cytokines, low levels of TNF-α and high levels of IL-10, may be important in the deterioration to a severe condition in patients with rickettsial infection, and after treatment by antibiotics, it is important that these cytokines be maintained at an appropriate level (11). We have already reported that fulminant tsutsugamushi disease is associated with hemophagocytic syndrome (4), and this syndrome may represent a hyperreaction of the immune system mediated by an accelerated cytokine network during the advanced stages of rickettsial infection. Furthermore, MINO affected the modulation of cytokine production in tsutsugamushi disease (5). In this JSF case, although cytokine modulation may be caused by the administration of MINO, the corticosteroid also may affect this phenomenon, and the new quinolone was required to eradicate the rickettsiae. The susceptibility of rickettsiae to new quinolones has already been studied, and the MICs were 0.125 to 0.25 μg/ml for R. rickettsii and 0.25 to 0.5 μg/ml for R. conorii (10).
We previously reported that hypercytokinemia appeared to be responsible for the emergence of symptoms in several cases of tsutsugamushi disease (5). MINO is a very effective drug for treating tsutsugamushi disease or eradicating O. tsutsugamushi, and Murai et al. mentioned that the presence of O. tsutsugamushi DNA in peripheral blood mononuclear cells of the patients with tsutsugamushi disease was detectable in samples from the 3rd to the 8th day after the initiation of treatment with tetracyclines, despite the improvement of symptoms in the earlier days after the onset of illness (8). Thus, the improvement of symptoms, including defervescence, may not depend on the eradication of the pathogen. Since MINO is sometimes not effective enough in cases of JSF, as in this case, patients may need additional antirickettsial chemotherapy using new quinolones, even if the symptoms appear to have improved. Until now no fatal cases of JSF have been reported, although the death rates from other well-studied spotted fever group rickettsioses, such as 3 to 7% for Rocky Mountain spotted fever (2) and 2.5% for Mediterranean spotted fever (9), suggest that JSF may become a fatal illness when associated with hypercytokinemia unless it is carefully treated. In the future, it will be necessary to investigate the characteristics of JSF in regard to both cytokine modulation and chemotherapy for the eradication of the pathogen.
REFERENCES
- 1.Fujita H, Watanabe Y, Takada N, Tsuboi Y, Mahara F. Isolation and serological identification of causative rickettsiae from Japanese spotted fever patients. Asian Med J. 1993;36:660–665. [Google Scholar]
- 2.Hattwick M A, O'Brien R J, Hanson B F. Rocky Mountain spotted fever: epidemiology of an increasing problem. Ann Intern Med. 1976;84:732–739. doi: 10.7326/0003-4819-84-6-732. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki H, Ueda T, Uchida M, Nakamura T, Takada N, Mahara F. Atypical lymphocytes with a multilobated nucleus from a patient with tsutsugamushi disease (scrub typhus) in Japan. Am J Hematol. 1991;36:150–151. doi: 10.1002/ajh.2830360216. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki H, Hashimoto K, Takada N, Nakayama T, Ueda T, Nakamura T. Fulminant Rickettsia tsutsugamushi infection associated with haemophagocytic syndrome. Lancet. 1994;343:1236. doi: 10.1016/s0140-6736(94)92456-2. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki H, Takada N, Nakamura T, Ueda T. Increased levels of macrophage colony-stimulating factor, gamma interferon, and tumor necrosis factor alpha in sera of patients with Orientia tsutsugamushi infection. J Clin Microbiol. 1997;35:3320–3322. doi: 10.1128/jcm.35.12.3320-3322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahara F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis. 1997;3:105–111. doi: 10.3201/eid0302.970203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahara F. Rickettsioses in Japan. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millennium. New York, N.Y: American Society for Rickettsiology; 1999. pp. 233–239. [Google Scholar]
- 8.Murai K, Okayama A, Horinouchi H, Oshikawa T, Tachibana N, Tsubouchi H. Eradication of Rickettsia tsutsugamushi from patients' blood by chemotherapy, as assessed by the polymerase chain reaction. Am J Trop Med Hyg. 1995;52:325–327. doi: 10.4269/ajtmh.1995.52.325. [DOI] [PubMed] [Google Scholar]
- 9.Raoult D, Weiller P J, Chagnon A, Chaudet H, Gallais H, Casanova P. Mediterranean spotted fever: clinical, laboratory and epidemiological features of 199 cases. Am J Trop Med Hyg. 1986;35:845–850. doi: 10.4269/ajtmh.1986.35.845. [DOI] [PubMed] [Google Scholar]
- 10.Raoult D, Bres P, Drancourt M, Vestrls G. In vitro susceptibilities of Coxiella burnetii, Rickettsia rickettsii, and Rickettsia conorii to the fluoroquinolone sparfloxacin. Antimicrob Agents Chemother. 1991;35:88–91. doi: 10.1128/aac.35.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westendorp R G J, Langermans J A M, Huizinga T W J, Elouali A H, Verweij C L, Boomsma D I, Vandenbrouke J P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]