Abstract
Ellagic acid (EA) is a polyphenolic bioactive with a wide range of pharmacological activities. Regrettably, it possesses poor solubility, stability and permeability (in the gastrointestinal tract); and first-pass metabolism. Therefore, to address these challenges, the present research was aimed to encapsulate EA in cyclodextrin nanosponges (CDNS). Herein, the melt method and microwave-assisted technique have been employed for crafting CDNS. EA was loaded in CDNS via freeze-drying, followed by appropriate characterization. EA-CDNS were also assessed for encapsulation, particle size, zeta potential, and polydispersity index, which presented satisfactory results. In vitro, antioxidant activity was conducted using the DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay. The solubilization efficacy of EA was analyzed in distilled water and compared with CDNS, which demonstrated ten folds augmentation for the selected batch. A remarkable improvement in the photostability of EA was also observed after its inclusion. In nutshell, the results demonstrated the superiority of the melt method in terms of solubility, entrapment, photostability, and antioxidant potential.
Keywords: β-cyclodextrin, Diphenylcarbonate, FE-SEM, Drug content, Solubility, DPPH scavenging activity
Introduction
In the past few years, there has been a renewed interest in natural antioxidants for their immense potential, role in several fields, like pharmaceuticals, cosmetics and the food industry. One such natural antioxidant is ellagic acid (EA), a natural polyphenol, obtained from variety of fruits and nuts including pomegranate, raspberries, strawberries, grapes, and walnuts (Mady and Ibrahim 2018). This widely used bioactive compound has been gaining popularity as a dietary supplement, nowadays (Usta et al. 2013). It is noteworthy that EA is also well recognized for its remarkable effects on human health (Kilic et al. 2014). It possesses strong anti-mutagenic and antioxidant potential that help in the management of several chronic disorders. This phenolic phytochemical is related to cellular antioxidant defense via activation of enzymes involved in this process, consequently, hindering the oxidative stress associated events (Usta et al. 2013). In addition, it possesses anti-inflammatory, anti-mutagenic, anti-allergic, anti-depressant, anti-diabetic, anti-nociceptive, anti-proliferative, anti-aging, hepatoprotective, cardioprotective, antifungal, antibacterial, and antiviral activities (Mansouri et al. 2015; Bulani et al. 2016). Howbeit, despite these promising actions, considerable limitations are correlated with this moiety that hinders its beneficial effects in the food, cosmetic, and pharmaceutical industry. To mention, EA possess poor aqueous solubility and stability in presence of light which pose formulation challenges for scientific community. Owing to this, poor absorption, followed by rapid elimination and extensive metabolisms, leading to ineffective systemic levels (Avachat and Patel 2015). Therefore, encapsulation would be an appropriate method to preserve EA and to circumvent above-mentioned drawbacks. The most appropriate nanoscale carriers for applications in the field of food include protein-, lipid—or carbohydrate-based, such as cyclodextrins (Garrido et al. 2019). For these reasons, β-cyclodextrin nanosponges seems to be a good choice, which may result in the improvement of the efficacy of this bioactive.
Earlier, some efforts have been made to incorporate ellagic acid into different carrier systems, including nanoparticles (Sonaje et al. 2007; Ratnam et al. 2009), niosomes (Junyaprasert et al. 2009), solid dispersions (Li et al. 2013), microparticles (Montes et al. 2016), inclusion complexes (Bulani et al. 2016), self-nano emulsifying agents (Wang et al. 2017), inclusion complex gel (Fan et al. 2017) and solid lipid nanoparticles (Aldawsari and Hosny 2018) to improve its therapeutic performance. Some of the merits of CDNS (proposed in the present investigation) are their particulate nature, easy to load in other vehicles (creams, lotions, and gels for topical application), easy and affordable to produce, and possess higher payload capacity in comparison to other formulation.
Nowadays, cyclodextrin nanosponges have been widely reported as promising drug delivery platform to enhance drug solubility, to improve drug permeability (Kumar et al. 2018, 2019), to prevent the degradation of drugs, and to control the drug release behavior (Gholibegloo et al. 2019). The CDNS are three-dimensional porous nanostructures crafted by cross-linking β-cyclodextrin polymers. Cyclodextrins represent viable polymers as they are non-toxic and some of these polymers are already approved as food excipient (Simionato et al. 2019). These cyclic oligosaccharides comprise of glucopyranose units attached via α-(1, 4) glucosidic bonds resulting in a toroidal shaped moiety. The most common types of cyclodextrins include: natural, corresponding to those available in nature, and modified form, corresponding to cyclodextrins obtained after chemical modifications of natural cyclodextrins to enhance their characteristic features (Garrido et al. 2019; Simionato et al. 2019). Hence, these can be employed to entrap both hydrophilic and hydrophobic therapeutic compounds (Rezaei et al. 2019). Among these, β-cyclodextrin is the most preferred one owing to the size of its cavity (approximately 8 Å), its availability as well as reasonable price (Garrido et al. 2019).
The commonly employed crosslinkers for CDNS includes carbonyldimiidazole, triphosgene, diphenyl carbonate, or organic dianhydrides. These crosslinkers form inclusion as well as non-inclusion complex with the drug moieties, allowing more interaction sites and a higher amount of drug entrapment in comparison to natural cyclodextrins (Trotta et al. 2012). CDNS have been reported for encapsulation of various bioactives such as camptothecin (Gigliotti et al. 2016), babchi oil (Kumar et al. 2018), cinnamon oil (Simionato et al. 2019), piperine (Garrido et al. 2019), curcumin (Gholibegloo et al. 2019) and azelaic acid (Kumar and Rao 2020). Previously, Mady and Ibrahim engineered cyclodextrin nanosponges employing dimethyl carbonate as a crosslinker loaded with ellagic acid (isolated from pomegranate) for oral delivery and reported higher solubility, improved bioavailability, and controlled release of the bioactive. EA was isolated from pomegranate (without purification) in this report reducing the authenticity of its use, thereby hindering its extrapolation for therapeutic utility. EA loaded CDNS reported in this study has been fabricated using a solvent evaporation technique employing dimethyl carbonate as crosslinker (Mady and Ibrahim 2018). However, in the current investigation, encapsulation of EA in cyclodextrin NS using melt and microwave techniques have been performed for the first time. Herein, diphenylcarbonate (DPC) was chosen as a crosslinker, owing to its good safety profile. Dimethylformamide (DMF), was selected as internal solvent as it is capable of dissolving β-cyclodextrin as well as diphenylcarbonate.
Recently, Rezaei and their research group reported improved solubility of poorly water-soluble natural antioxidant ferulic acid using β-cyclodextrin based nanosponges prepared by the melt method. In this study, fabricated nanosponges encapsulating ferulic acid were characterized and evaluated appropriately. Results revealed the formation of stable inclusion complexes of this moiety with augmented anti-proliferative activity and anti-cancer potential (Rezaei et al. 2019).
Cyclodextrin nanosponges prepared using microwave-assisted technique enhanced the loading capacity of quercetin twice in comparison to conventionally prepared nanoparticles. This group reported microwave technique as a very efficient, simple, reproducible, scalable, and economic method to synthesize cyclodextrin nanosponges, within a short period. In the following year, Zainuddin et al. synthesized cyclodextrin nanosponges via microwave mediated method for bioavailability enhancement of rilpivirine hydrochloride. This group employed DPC as a crosslinker and optimized various parameters such as polymer to watt power, crosslinker ratio, and solvent volume. Paracrystalline CDNS obtained in the study led to two-fold increase in the dissolution of rilpivirine hydrochloride with remarkably enhanced oral bioavailability of this drug in fasted sprawly rats (Zainuddin et al. 2017).
The current investigation was aimed to study the influence of the preparation technique used for ellagic acid nanosponges fabrication using β-cyclodextrin as polymer and diphenyl carbonate as the crosslinker. The EA loaded CDNS has been engineered via melt method and microwave-assisted synthesis and evaluated for their influence on solubility, photostability, and antioxidant property. This is the first report to deal with the encapsulation of EA in cyclodextrin NS using DPC as crosslinker via the above-mentioned techniques. Further, to the best of our knowledge, no studies are reporting the comparison between melt and microwave techniques of nanosponge upto now. The solubility, photostability, and antioxidant properties were investigated and compared for EA nanosponges obtained from both techniques.
Materials and methods
Materials
The ellagic acid was purchased from Himedia, India. β-cyclodextrin was supplied from Roquette (France). Diphenyl carbonate was procured from Sigma Aldrich (India) and dimethylformamide was obtained from Finar (Gujarat, India). All other chemicals and reagents used were of an analytical grade. Double distilled water was employed in all the experimental studies.
Preparation of cyclodextrin nanosponges
Synthesis of blank nanosponges by melt method
β-cyclodextrin based NS were prepared using a previously reported procedure (Rezaei et al. 2019). CDNSs were fabricated by changing ratios of DPC and β-CD (4:1 and 6:1). For synthesis, anhydrous β-CD and DPC (which were finely homogenized at 90–100 °C), were gradually heated for 5 h under magnetic stirring. Phenol crystals were formed at the flask’s neck, which was removed carefully. After cooling the reaction mixture at room temperature, powdered product was washed repeatedly using Milli Q water to remove uncomplexed β-CD. Subsequently, uncomplexed DPC and phenol were removed using acetone. Prepared nanoformulations were stored at 25 °C for further characterization and evaluation (Rao and Shirsath, 2017). Nanosponges were named as NS1 (1:4) and NS2 (1:6).
Synthesis of blank nanosponges by microwave method
Cyclodextrin nanosponges were prepared using a cross-linking agent (DPC) and polymer (β-CD), in the same ratios as mentioned in melt method (Zainuddin et al. 2017). Briefly, a solution of DPC and β-CD in DMF was taken in a vial (20 ml) and proceeded for irradiation under the microwave (power -18 W) (Anatoparr monowave 200, Austria) upto 3.5 min approximately. The obtained product was repeatedly washed using distilled water and subsequently, purified via soxhlet extraction [with acetone (80 to 90 ml) in 100 ml round bottom flask] upto four hours. Dried the obtained white color powder overnight at 60 °C in an oven and then, crushed in a mortar (Anandam and Selvamuthukumar 2014; Zainuddin et al. 2017). Obtained nanosponges were denoted as NS3 (1:4) and NS4 (1:6).
Fabrication of ellagic acid loaded β-cyclodextrin based nanosponges
Ellagic acid nanosponges (EANS) were fabricated by freeze-drying method. Weighed amounts of CDNS (1 g) were properly suspended in distilled water (200 ml) using a magnetic stirrer and enough quantity of EA (ratio 1: 4 w.r.t. CDNS) was added to it. The resulting suspension was sonicated for 10 min, followed by stirring for 24 h. The suspension was centrifuged for 10 min (at 2,000 rpm) to remove the unreacted residues of the bioactive, under colloidal supernatant. The supernatant was lyophilized using Milli rock lyophilizer (Alpha 2–4 LD Plus CHRIST, Germany), at −20 °C, operating pressure 13.33 mbar (temperature corresponding to 98.68% vacuum; vapor pressure − 20 °C: 960 mt) to get bioactive loaded CDNS. At this stage, formulations were identified as EANS1 (1:4), EANS2 (1:6), EANS3 (1:4), and EANS4 (1:6), on behalf of the ratio of β-CD: DPC and the technique of preparation. The dried NS powder was sieved using sieve no. #60, and subsequently stored in a desiccator till further use (Zainuddin et al. 2017).
Solubilization efficiency of nanosponges
The solubilization efficiency of all batches of nanosponges was investigated and compared with β-CD. An excess amount of EA (50 mg) was taken with a fixed quantity of blank nanosponges (20 mg) (NS1, NS2, NS3, NS4) using Milli Q water (20 ml) in a volumetric flask and were allowed to shake at room temperature. After equilibrium (24 h), the nanosuspensions were centrifuged (12,298×g) for 10 min and methanol (10 ml) was added to separate free EA and collect the colloidal supernatant. To extract the encapsulated EA from nanocolloidal systems, after keeping it for 2 h, the solution was estimated for EA, using UV spectrophotometer (λmax 372 nm) (UV-1800, Shimadzu UV-Spectrophotometry, Japan) (using the calibration curve of the bioactive prepared previously) (Anandam and Selvamuthukumar 2014).
2.4 Physicochemical characterization of ellagic acid-loaded nanosponges
Fourier transform infrared spectroscopy
Infra-red spectra were recorded for EA, polymer mix, blank CDNS and EA loaded CDNS using Perkin Elmer Spectrum BX II USA Spectrophotometer for evaluation of the interaction between EA and cyclodextrin NS. Sample (Approx. 1–2 mg) was appropriately mixed with dry potassium bromide (50 mg) and the obtained samples were checked over a range of 4000–400 cm−1, under transmission mode (Kumar et al. 2021).
Field emission scanning electron microscopy (FE-SEM)
The surface morphology of plain NS1, NS3, and EA loaded nanosponges (EANS1 and EANS3) were observed using field emission scanning electron microscope (FEI, model: Nova nano SEM 450). An electron beam was produced by a field emission gun with a higher density of the current, which is produced by the application of an intense electric field using a needle-shaped tip to a tungsten single crystal. This requires an ultra-high vacuum (10−100 Torr). The samples were suitably sprinkled over a double-sided adhesive tape attached with an aluminum stub. Then, stubs were coated with platinum upto thickness (10 Å) in an argon atmosphere with the help of a gold sputter module, under high vacuum. The samples placed in stub were kept in the chamber of FE-SEM (Rao and Shirsath 2017).
Determination of ellagic acid content in cyclodextrin nanosponges
Ellagic acid-loaded nanosponges were weighed accurately and dispersed in methanol. To break the complex, the dispersion was sonicated for 10 min, suitably diluted, and subsequently analyzed by UV spectrophotometer (at 264 nm) to evaluate the amount of ellagic acid in the formulations. Using the following formula, the ‘percent drug association’ was evaluated (Anandam and Selvamuthukumar 2014).
| 1 |
Particle size, polydispersity index, and zeta potential
Employing a 90 plus particle sizer (Malvern Instruments Ltd, Worcestershire, UK), the polydispersity index of fabricated nanosponges and their sizes were checked using a dynamic light scattering, (MAS Option particle sizing software fixed angel of 90°). The samples were appropriately diluted (with water), before observation. Measurements of Zeta potential were also performed by using an additional electrode in that instrument. The particle polydispersity index (PI) and mean hydrodynamic diameter (Dh) were obtained (Ansari et al. 2011).
Photostability studies
Photodegradation studies of EA were performed using a short wavelength range UV lamp. Aqueous solutions of ellagic acid formulations (EANS1 and EANS3) and pure ellagic acid were studied by this system. A suitable aliquot (10 ml) of each sample was kept 10 cm away from the lamp and while constantly stirring, samples were irradiated. At 10 min of scheduled time (for 1 h of total irradiation), aliquots of every sample were taken out. EANS1 and EANS3 were appropriately diluted (using methanol) for spectroscopical analysis (Anandam and Selvamuthukumar 2014).
Antioxidant activity/scavenging activity by DPPH free radical
As previously reported, some modifications were done to assess the scavenging capacity of EA using DPPH-free radicals. Absorption of DPPH radical is generally declined upon the absorption of this radical (at 517 nm). Further, prepared solution of DPPH (0.1 mM) in methyl alcohol and added this suspension (0.5 ml) to EA solution (using methyl alcohol) (1.5 ml), at various concentrations (5–100 µg/ml). These suspensions were vigorously vortexed for 30 min and finally, measured the absorbance (at 517 nm), taking DPPH-methanol solution as a blank sample. Calculated the % effect of DPPH scavenging, with help of the following equation:
| 2 |
where A1 represents the absorbance in the presence of EA and A0 stands for the absorbance (Kilic et al. 2014).
Statistical analysis
All the experiments were performed in triplicate and the results were reported as mean ± standard deviation (± SD). Statistical measurements were analyzed out by employing GraphPad Prism (version 5.01 software); (GraphPad Software, San Diego, CA, USA). One-way ANOVA was chosen to appraise the significant difference between the findings.
Results and discussion
Numerous methods of preparation have been devoted to β-cyclodextrin nanosponges over the last decade, namely microwave-assisted synthesis, solvent evaporation technique, emulsion solvent diffusion method, ultrasound-assisted synthesis, and melt method. Amongst these, the melt method and microwave-assisted synthesis have been chosen for crafting CDNS for present investigation and comparison purpose. Both these techniques have been found promising and have attracted significant interest in the research community. The melt method is very simple and reproducible. Another advantage of this technique is that it produces crystalline nanostructures, omitting the use of organic solvents. On the other hand, concerning microwave-assisted synthesis, it causes a faster reaction, with uniform heating throughput capacities and resulting in crystalline products. Hence, both these techniques were compared for ellagic acid loaded nanosponges, and solubility, photostability, and antioxidant activity of these were also performed.
Fabrication of ellagic acid loaded β-cyclodextrin based nanosponges
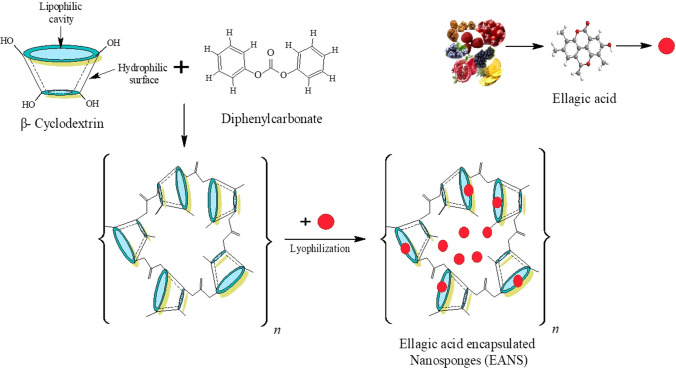
Nanosponges have been successfully obtained using both selected techniques melt method and microwave-assisted synthesis, followed by EA loading through lyophilization. The crafted EA nanosponges (Fig. 1) were observed skin in color for all batches. It is speculated that the incorporation of ellagic acid in CDNS may enhance solubility and photostability of this bioactive compound. Hence, cyclodextrin nanosponges may prove an appropriate carrier system for enhancing the physicochemical properties of EA while retaining its therapeutic efficacy.
Fig. 1.
Schematic representation of the two-step preparation of a carbonated β-cyclodextrin ellagic acid impregnated nanosponges
Solubilization efficiency of nanosponges
The solubilization capacity of all the four preparation batches of CDNS was investigated for EA (hydrophobic moiety). These nanosponges solubilized EA variably, as illustrated in Table 1 and Fig. 4a (One way ANOVA statistics: ***p < 0.0001). Among all, NS1 (1:4) fabricated by the melt method has shown maximum solubility (721 μg/ml) in comparison with free EA (162.5 μg/ml). NS3 (1:4) fabricated via microwave synthesis showed lesser solubility (607 μg/ml) than NS1, however, it was higher than plain EA. A similar trend was observed for NS2 and NS4. The results indicated that the encapsulation of EA in CDNS may have resulted in the enhancement of solubility of this bioactive. Further, differences in solubilization efficiency of 1:4 and 1:6 ratios of CDNS in both cases may be due to the formation of a lesser number of nanochannels (varying the amount of crosslinker in both ratios). The difference in solubilization capacity of the CDNS obtained via different synthetic techniques could be ascribed to the differences in crystal nature or morphology and shape of nanosponges (nearly spherical for microwave synthesized and undefined for melt method). To evaluate this differential behavior of the nanosponges, preliminary characterization of blank CDNS was carried out employing FTIR, FE-SEM, and PSA. In a previous investigation in our lab, a similar trend was observed for the solubilization of babchi oil nanosponges (1:4 and 1:6), prepared via melt method employing DPC (crosslinker) (Kumar et al. 2018). Similar results have been reported for rilpivirine hydrochloride cyclodextrin nanosponges, using DPC as crosslinker by Zainuddin and his research team (Zainuddin et al., 2017).
Table 1.
Formulation parameters, particle sizes, zeta potential, poly dispersity index, and percentage encapsulation efficiency of the ellagic acid nanosponges (n = 3, mean ± SD)
| Techniques | Variables (β-CD and DPC ratio) | Bioactive loaded CDNS | Average diameter | Zeta potential | Polydispersity index | % Encapsulation efficiency ± SD |
|---|---|---|---|---|---|---|
| Melt method | 1:4 | EANS1 | 202 | -21.6 | 0.751 | 73 ± 15.39* |
| 1:6 | EANS2 | 406 | -14.5 | 0.648 | 53 ± 8.178 | |
| Microwave assisted synthesis | 1:4 | EANS3 | 353 | -15.4 | 0.587 | 58 ± 3.789 |
| 1:6 | EANS4 | 406 | -20.8 | 0.225 | 46 ± 1 |
Statistical analysis from the one-way ANOVA (and nonparametric) Kruskal–Wallis test (encapsulation efficiency: Kruskal–Wallis statistics 8.167) followed by Dunn's Multiple Comparison Test with *p < 0.05
SD, Standard deviation; EANS1, EANS2 and EANS3, EANS4 represent drug loaded nanosponges prepared at molar ratios 1:4, 1:6 of β-CD: DPC via melt method and microwave assisted synthesis, respectively
Fig. 4.
a Solubilization of ellagic acid (EA) by nanosponges (having different cross-linking density), fabricated via different methods and β-CD. Statistical data analysis from the one-way ANOVA was followed by Bonferroni's Multiple Comparison Test: (***p < 0.0001). c indicates p < 0.001 vs. NS1, e indicates p < 0.001 vs. NS3, d indicates p < 0.0001 vs. NS2, f indicates p < 0.05 vs. NS4. b Encapsulation efficiency of all the batches of nanosponges, ellagic acid loaded nanosponge fabricated via melt method (EANS1 and EANS 2) and ellagic acid loaded nanosponges fabricated via microwave synthesis (EANS3 and EANS4)
Physicochemical characterization of ellagic acid-loaded nanosponges
Fourier transform infrared spectroscopy
The FTIR spectra of EA, plain CDNS, physical mixture, and EA loaded CDNS were displayed in Fig. 2. The FTIR spectrum of EA (Fig. 2a) showed characteristic peaks at 3471 (O–H), 1714 (C=O), 1614, 1581, 1508 (aromatic nucleus), 1320–1000 (C–O), 756 (aromatic C–H) cm−1. Plain NS1 formulations (prepared by melt method) exhibited a characteristic absorbance band around at 1776 cm−1 (carbonate bond), emphasizing the formation of β-cyclodextrin nanosponge (Fig. 2b). Additionally, the other characteristic peaks of NS1 were observed at 3233 cm−1 (C–H stretching) 1162 cm−1 (C–H bending), and 1027 cm−1 (C–O stretching). Similarly, for blank NS3 (prepared by microwave-assisted synthesis), characteristic spectrum around 1758 cm−1 (carbonate bond) confirmed nanosponge formation and other prominent peaks discussed earlier were also displayed in Fig. 2c Therefore, FTIR spectrums of both nanosponges ascertained the formation of carbonate bond as a result of interaction between β-CD and DPC (crosslinker). However, the FTIR spectrum of the physical mixture (β-CD and EA) displayed no peak around 1700 cm−1. The comparison of FTIR peaks of EA, NS, and EANS (fabricated by melt and microwave-assisted synthesis) evidenced that the prominent peaks of EA shifted or broadened in nanoformulations, suggesting the interactions between bioactive and nanosponges (Fig. 2a, c). Our data corroborate well with ellagic acid nanosponges prepared earlier, using dimethyl carbonate as a crosslinker, as reported by Mady and Ibrahim (Mady and Ibrahim 2018).
Fig. 2.
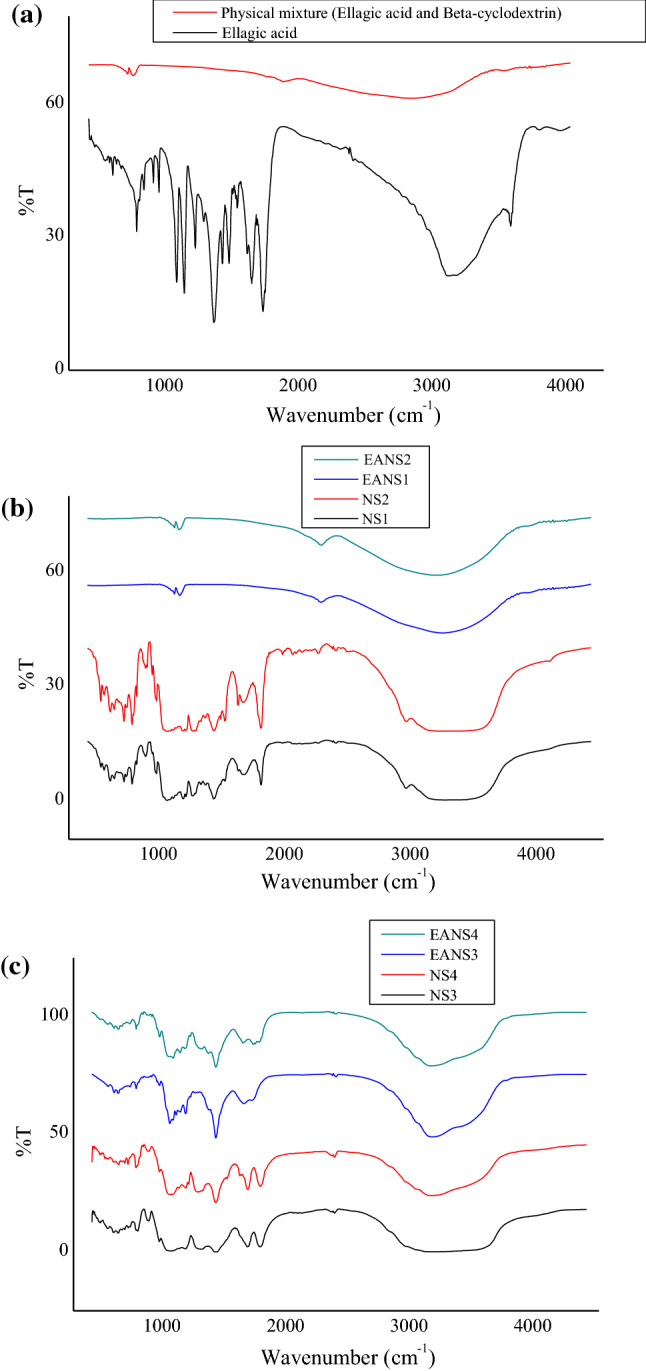
a FTIR spectra of ellagic acid and bioactive polymer physical mixture. b FTIR spectra of blank nanosponges (NS1, NS2) and ellagic acid loaded nanosponges (EANS1, EANS2) prepared by melt method. c FTIR spectra of blank nanosponges (NS3, NS4) and ellagic acid loaded nanosponges (EANS3, EANS4) prepared by microwave-assisted synthesis
Field emission scanning electron microscopy (FE-SEM)
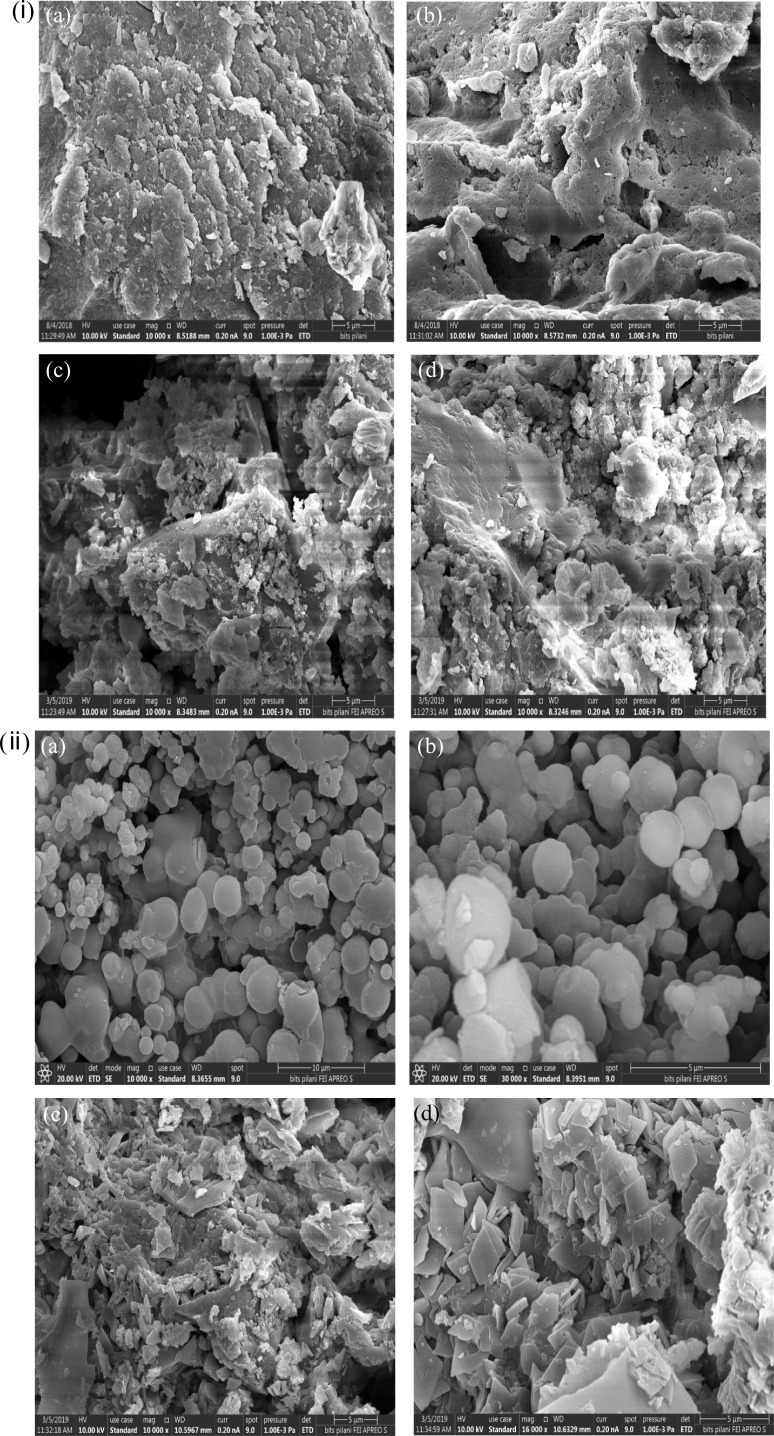
Field emission scanning electron microscopy facilitates more clearer and detailed images, with regard to SEM, therefore, it was chosen for morphological investigation in current work. FE-SEM of blank cyclodextrin nanosponges (1:4) and their EA loaded counterparts, fabricated by melt and microwave-assisted synthesis have been illustrated in Fig. 3(i) and (ii), respectively. All the formulations exhibited a porous nature with clear internal cavities. The NS prepared by the melt method were not uniform in size; however, microwave synthesized nanosponges displayed controlled synthesis. Further, it was observed NS prepared by microwave technique were roughly spherical. Additionally, it was observed that EA partially filled the pores of CDNS in API (active pharmaceutical ingredient) loaded formulations, as depicted in Fig. 3(i) c, d, and (ii) c, d respectively. Previously, similar results have been reported for efavirenz CDNS, using DPC as a crosslinker (Rao and Shirsath 2017).
Fig. 3.
(i) Field emission electron microscopy at magnification × 10,000, showing the nanoporous structure of optimized formulation (1:4) fabricated by melt method; blank nanosponges (NS1) (a, b) and ellagic acid loaded nanosponges (EANS1) (c, d). (ii) Field emission electron microscopy image at different magnifications showing the nanoporous structure of optimized formulation (1:4) fabricated by microwave-assisted synthesis; blank nanosponges (NS3) (a, b) and ellagic acid loaded nanosponges (EANS3) (c, d)
Determination of ellagic acid content in cyclodextrin nanosponges
In the present investigation, the encapsulation efficiency of all EA loaded CDNS was calculated and compared thereof. Formulation NS1 (employing 1:4 polymer, crosslinker ratio) exhibit maximum bioactive entrapment of 73 ± 0.5% (Table 1; Fig. 4b). All the prepared batches showed EA incorporation in the order NS1 > NS3 > NS2 > NS4. This might be ascribed variation in the interaction between the aromatic ring structure and the inner portion of β-CD. However, results depicted that the bioactive entrapment efficiency of formulations prepared by the melt method (NS1, NS2) was found higher in both the ratios (1:4 and 1:6) when compared to nanosponges prepared by microwave-assisted technique. This may be ascribed to crystalline nanostructures in comparison to paracrystalline ones and more porous nature of nanostructures obtained via the melt method. It has been well documented in literature reports that drug loading is higher in crystalline nanosponges in comparison to paracrystalline nanostructures (Kumar et al. 2018).
Particle size, polydispersity, and zeta potential
Table 1 represented the average particle size and polydispersity index of CDNS prepared by melt and microwave-assisted techniques. All the prepared EA loaded formulations displayed size in the nano range. The particle size of EA loaded CDNS was found satisfactory ranging from 120 to 630 nm. Zeta potential of various EA nanosponges was also assessed, as a measurement of surface charge. The findings of zeta potential observed are reported in Table 1. Higher values of zeta potential showed that prepared porous CDNS would be stable, due to high repulsive forces, resulting in a decrease in aggregation tendency. Reduced values of PDI (in a narrow range) demonstrated that the prepared nanosponge suspensions were stable as well as homogenous. All the fabricated batches of nanosponges were found as free-flowing fine powders.
Photostability studies
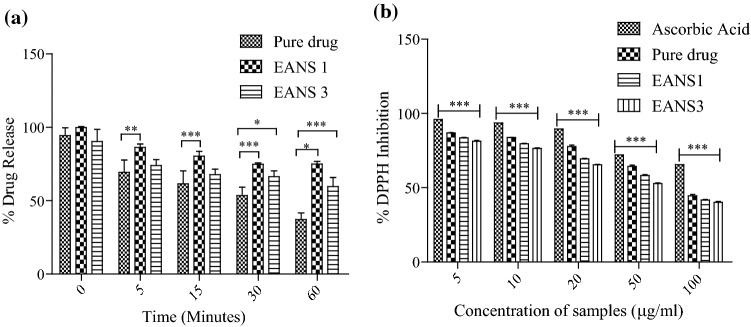
Ellagic acid absorbs rays in the UV region, exhibiting a peak around 264 nm, whose intensity retarded upon UV-A irradiation, evidencing photolysis of the bioactive. From the photostability findings, it was ascertained that EANS were more photostable in comparison to free EA (Fig. 5a). This is attributed to the encapsulation of drugs within cyclodextrin nanosponges, which resulted in enhanced photostability. However, while comparing EANS1, the batch was found more photostable than EANS3. Thus, results showed that CDNS prepared by the melt method were more photostable than formulations prepared by the microwave synthesis technique. Cyclodextrin nanosponges provided EA with a physical barrier against UV –induced degradation. It was also observed that the degradation of EA from nanoformulations was faster in the first 10 min. This can be due to the presence of free EA on the surface of CDNS. Similar results have been presented by Ansari et al. (2011) for resveratrol nanosponges.
Fig. 5.
a Photostability studies of pure ellagic acid, ellagic acid (EA) loaded nanosponge fabricated via melt method (EANS1) and ellagic acid loaded nanosponges fabricated via microwave synthesis (EANS3). The data were statistically analyzed by two-way ANOVA followed by Bonferroni posttests. Each value represents (mean ± SD) from triplicate measurements (n = 3). *p < 0.05, **p < 0.01 and *** p < 0.001 represents significant difference between pure drug vs. EANS at same time. b DPPH inhibition activity of ascorbic acid, ellagic acid, (EANS1) ellagic acid loaded nanosponge fabricated via melt method and (EANS3) ellagic acid loaded nanosponges fabricated via microwave synthesis. Each value represents (mean ± SD) from triplicate measurements (n = 3). ***p < 0.0001 indicates a significant difference between pure bioactive vs. EANS at the same concentrations, two-way ANOVA, followed Bonferroni posttests
Antioxidant activity/scavenging activity by DPPH free radical
The reaction of the sample with DPPH is commonly used for evaluation of the ability of samples to transfer labile atom to the radical, a widely used mechanism for antioxidant production (Sapino et al. 2013). This evaluation is based on the reduction of DPPH, which resulted in a decrease in absorbance at 517 nm, after accepting hydrogen radical or an electron, from an antioxidant moiety. In the present study, the antioxidant potential of EA (pure bioactive), EANS1 (1:4 prepared by melt method), EANS2 (1:4 prepared by microwave method), and ascorbic acid (standard) were evaluated in the range of 5-100 µg/ml (Fig. 5b). From Fig. 5b, it was illustrated that the percentage inhibition of all the samples increased with increasing the sample concentration. Ascorbic acid exhibited maximum free radical scavenging potential, followed by EA > EANS1 > EANS3 in a dose-dependent manner. The anti-radical activity of EANS slightly differed from pure EA. Further, the results indicated a slight difference in the antioxidant potential of nanosponges prepared by the melt method (EANS1) from that of nanoparticles prepared by microwave-assisted technique (EANS3). This trend was similar in all concentrations of the tested samples. In comparison to ascorbic acid, antioxidant activity % inhibition of EA towards DPPH was found slightly lower. In a previous study, similar results have been reported by Killic et al. (2014) for ellagic acid. Sapino et al. (2013) evaluated the antioxidant property of gamma-oryzanol in cyclodextrin loaded nanosponges in an earlier report.
Conclusion
In this work, β-cyclodextrin nanosponges were successfully fabricated using diphenyl carbonate (crosslinker) by two techniques; namely melt method and microwave-assisted synthesis. Ellagic acid was loaded in the prefabricated blank nanocarriers, prepared (by both these techniques), employing lyophilization. All the four batches of cyclodextrin nanosponges augmented the solubility of EA in comparison to pure ellagic acid (in distilled water). Amongst these, NS1 exhibited maximum solubilization, i.e. more than ten folds, advocating the efficacy of the melt method. The prepared ellagic acid loaded nanoformulations were characterized employing FTIR, which confirmed bioactive encapsulation in the cyclodextrin nanosponges. FE-SEM illustrated the porous nature of prepared EA nanosponges. Further, entrapment efficiency was found maximum for ellagic acid loaded nanosponges (molar ratio 1:4), synthesized by melt method. Results also suggested that cyclodextrin nanosponges have increased the photostability of ellagic acid, particularly those prepared via the melt method. Additionally, antioxidant activity performed on optimized batches of EA loaded nanosponges revealed that the functionality of EA have been preserved after encapsulation. Hence, it was concluded from the findings of the present investigation that the melt method is superior for the synthesis of nanosponges in comparison to the microwave-assisted technique. Ellagic acid loaded nanosponges can be further explored for oral and topical delivery, for various ailments, in which efficacy of EA is well documented.
Availability of data and material
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Authors' contributions
RR and KS designed the research work, KS performed the study as the first author. KS and VK drafted the manuscript. VK and AK helped in interpretation of results. SM helped in editing English of manuscript. RR directed the research and reviewed the manuscript.
Funding
None.
Declaration
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aldawsari HM, Hosny KM. Solid lipid nanoparticles of vancomycin loaded with ellagic acid as a tool for overcoming nephrotoxic side effects: preparation, characterization, and nephrotoxicity evaluation. J Drug Deliv Sci Technol. 2018;45:76–80. doi: 10.1016/j.jddst.2018.02.016. [DOI] [Google Scholar]
- Anandam S, Selvamuthukumar S. Fabrication of cyclodextrin nanosponges for quercetin delivery: physicochemical characterization, photostability, and antioxidant effects. J Mater Sci. 2014;49:8140–8153. doi: 10.1007/s10853-014-8523-6. [DOI] [Google Scholar]
- Ansari KA, Vavia PR, Trotta F, Cavalli R. Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech. 2011;12:279–286. doi: 10.1208/s12249-011-9584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avachat AM, Patel VG. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm J. 2015;23:276–289. doi: 10.1016/j.jsps.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulani VD, Kothavade PS, Kundaikar HS, et al. Inclusion complex of ellagic acid with β-cyclodextrin: characterization and in vitro anti-inflammatory evaluation. J Mol Struct. 2016;1105:308–315. doi: 10.1016/j.molstruc.2015.08.054. [DOI] [Google Scholar]
- Fan G, Xu Z, Liu X. Preparation of pomegranate Ellagic acid inclusion complex gel and its transdermal permeation in vitro. Procedia Eng. 2017;174:724–731. doi: 10.1016/j.proeng.2017.01.212. [DOI] [Google Scholar]
- Garrido B, González S, Hermosilla J, et al. Carbonate-β-cyclodextrin-based nanosponge as a nanoencapsulation system for piperine: physicochemical characterization. J Soil Sci Plant Nutr. 2019;19:620–630. doi: 10.1007/s42729-019-00062-7. [DOI] [Google Scholar]
- Gholibegloo E, Mortezazadeh T, Salehian F, et al. Improved curcumin loading, release, solubility and toxicity by tuning the molar ratio of cross-linker to β-cyclodextrin. Carbohyd Polym. 2019;213:70–78. doi: 10.1016/j.carbpol.2019.02.075. [DOI] [PubMed] [Google Scholar]
- Gigliotti CL, Minelli R, Cavalli R, et al. In vitro and in vivo therapeutic evaluation of camptothecin-encapsulated β-cyclodextrin nanosponges in prostate cancer. J Biomed Nanotechnol. 2016;12:114–127. doi: 10.1166/jbn.2016.2144. [DOI] [PubMed] [Google Scholar]
- Junyaprasert VB, Teeranachaideekul V, Souto EB, et al. Q10-loaded NLC versus nanoemulsions: stability, rheology and in vitro skin permeation. Int J Pharm. 2009;377:207–214. doi: 10.1016/j.ijpharm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Kilic I, Yeşiloğlu Y, Bayrak Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;130:447–452. doi: 10.1016/j.saa.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rao R. Enhancing efficacy and safety of azelaic acid via encapsulation in cyclodextrin nanosponges: development, characterization and evaluation. Polym Bull. 2020 doi: 10.1007/s00289-020-03366-2. [DOI] [Google Scholar]
- Kumar S, Trotta F, Rao R. Encapsulation of Babchi oil in cyclodextrin-based nanosponges: physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics. 2018;10:169. doi: 10.3390/pharmaceutics10040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Dalal P, Rao R (2019) Cyclodextrin nanosponges: a promising approach for modulating drug delivery. In: Colloid science in pharmaceutical nanotechnology. IntechOpen
- Kumar S, Prasad M, Rao R. Topical delivery of clobetasol propionate loaded nanosponge hydrogel for effective treatment of psoriasis: formulation, physicochemical characterization, antipsoriatic potential and biochemical estimation. Mater Sci Eng C Mater Biol Appl. 2021;119:111605. doi: 10.1016/j.msec.2020.111605. [DOI] [PubMed] [Google Scholar]
- Li B, Harich K, Wegiel L, et al. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohyd Polym. 2013;92:1443–1450. doi: 10.1016/j.carbpol.2012.10.051. [DOI] [PubMed] [Google Scholar]
- Mady FM, Ibrahim SR-M. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of ellagic acid. Pak J Pharm Sci. 2018;31:2069–2076. [PubMed] [Google Scholar]
- Mansouri MT, Naghizadeh B, Ghorbanzadeh B. Ellagic acid enhances the antinociceptive action of venlafaxine in mouse acetic acid-induced pain: an isobolographic analysis. Pharmacol Rep. 2015;67:473–477. doi: 10.1016/j.pharep.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Montes A, Wehner L, Pereyra C, De La Ossa EM. Generation of microparticles of ellagic acid by supercritical antisolvent process. J Supercrit Fluids. 2016;116:101–110. doi: 10.1016/j.supflu.2016.05.019. [DOI] [Google Scholar]
- Rao MR, Shirsath C. Enhancement of bioavailability of non-nucleoside reverse transciptase inhibitor using nanosponges. AAPS PharmSciTech. 2017;18:1728–1738. doi: 10.1208/s12249-016-0636-6. [DOI] [PubMed] [Google Scholar]
- Rao M, Bajaj A, Khole I, et al. In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J Incl Phenom Macrocycl Chem. 2013;77:135–145. doi: 10.1007/s10847-012-0224-7. [DOI] [Google Scholar]
- Ratnam DV, Chandraiah G, Meena AK, et al. The co-encapsulated antioxidant nanoparticles of ellagic acid and coenzyme Q10 ameliorates hyperlipidemia in high fat diet fed rats. J Nanosci Nanotechnol. 2009;9:6741–6746. doi: 10.1166/jnn.2009.1461. [DOI] [PubMed] [Google Scholar]
- Rezaei A, Varshosaz J, Fesharaki M, et al. Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges. Int J Nanomed. 2019;14:4589–4599. doi: 10.2147/IJN.S206350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapino S, Carlotti ME, Cavalli R, et al. Photochemical and antioxidant properties of gamma-oryzanol in beta-cyclodextrin-based nanosponges. J Incl Phenom Macrocycl Chem. 2013;75:69–76. doi: 10.1007/s10847-012-0147-3. [DOI] [Google Scholar]
- Simionato I, Domingues FC, Nerín C, Silva F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem Toxicol. 2019;132:110647. doi: 10.1016/j.fct.2019.110647. [DOI] [PubMed] [Google Scholar]
- Sonaje K, Italia JL, Sharma G, et al. Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine A-induced nephrotoxicity in rats. Pharm Res. 2007;24:899–908. doi: 10.1007/s11095-006-9207-y. [DOI] [PubMed] [Google Scholar]
- Trotta F, Zanetti M, Cavalli R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J Org Chem. 2012;8:2091–2099. doi: 10.3762/bjoc.8.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usta C, Ozdemir S, Schiariti M, Puddu PE. The pharmacological use of ellagic acid-rich pomegranate fruit. Int J Food Sci Nutr. 2013;64:907–913. doi: 10.3109/09637486.2013.798268. [DOI] [PubMed] [Google Scholar]
- Wang S-T, Chou C-T, Su N-W. A food-grade self-nanoemulsifying delivery system for enhancing oral bioavailability of ellagic acid. J Funct Foods. 2017;34:207–215. doi: 10.1016/j.jff.2017.04.033. [DOI] [Google Scholar]
- Zainuddin R, Zaheer Z, Sangshetti JN, Momin M. Enhancement of oral bioavailability of anti-HIV drug rilpivirine HCl through nanosponge formulation. Drug Dev Ind Pharm. 2017;43:2076–2084. doi: 10.1080/03639045.2017.1371732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.