Abstract
The objective of the study was to develop the oil blend with improved omega-6 to omega-3 fatty acid ratio (ω-6:ω-3) with good oxidative and thermal stability. Flaxseed oil and palm olein were selected for the blending. Flaxseed oil is rich in anti-inflammatory omega-3 fatty acid (ω-3 FA) but is oxidatively very unstable. Palm olein has low omega-6 fatty acid content and high thermal and oxidative stability. Blends containing various percent (v/v) of flaxseed oil and palm olein were prepared. Oxidative and thermal stability was determined by analyzing peroxide value, acid value, smoke point, % free fatty acids, para-Anisidine and TOTOX values. Nutritive quality was confirmed by determining fatty acid composition. Nine month storage stability of the blend containing highest flaxseed oil percentage was assessed in terms of peroxide and acid value and fatty acid composition. The biological effects were studied in THP-1 cell line as the effect on cell survival, FA uptake and inflammatory markers. The data indicates that, blending improved ω-6:ω-3 ratio. Oxidative and thermal stability study, and nine month storage stability study suggested that palm olein imparted stability to the blend. Fatty acid profiles of the cells treated with these blends showed uptake of Alpha Linolenic Acid (ω-3 FA). These blends lowered inflammatory TNFα level without affecting cell survival. Thus, blending of flaxseed oil with palm olein may result in better health benefits owing to their improved nutritional and stability properties.
Keywords: Flaxseed oil, Inflammation, Omega-3 fatty acid, Omega-6: Omega-3 ratio , Palm olein
Introduction
Non-communicable diseases (NCD) are major health problems worldwide. As per World Health Organization (WHO) report (2017), globally, almost 61% deaths are due to NCD. The excess of omega-6 (ω-6) fatty acid (FA) and very low omega-3 (ω-3) FA (i.e. high ω-6:ω-3 ratio) in the diet have resulted in the persistent low grade inflammation leading to the development and progression of NCD (Johnson and Fritsche 2012). Linoleic acid (LA; ω-6 FA, pro-inflammatory) and Alpha-linolenic acid (ALA; ω-3 FA, anti-inflammatory) are essential FA. Therefore, dietary intake of these polyunsaturated fatty acid (PUFA) is required (Kaur et al. 2014). Various studies and experimental evidences indicate that ω-6:ω-3 ratio in the range 1:1–5:1 are capable of preventing many NCD (Simopolous 2008).
Edible oils are major dietary source of FA and provide essential and non-essential FA in triacylglycerol form. As per the WHO recommendation (Choudhary et al. 2015), diet should consist of saturated FA (SFA), monounsaturated FA (MUFA) and PUFA in a ratio 1:1.5:1 with LA and ALA in a ratio 5–10:1 (Choudhary et al. 2015). But majority of vegetable oils are rich in ω-6 FA (LA). The vegetarian sources of ω-3 FA (ALA) are limited (Kaur et al. 2014). These PUFA are prone to the oxidative and thermal damage resulting in decreased nutritional value and formation of toxic compounds (Falade 2017). Unfortunately, no single oil offers ideal FA composition (nutritional quality) and oxidative and thermal stability (Koohikamali and Alam 2019). Therefore, developing the oil with appropriate ω-6:ω-3 ratio and good oxidative and thermal stability is required. Blending of the oils combines the potency of two/more edible oils. This approach is used to enhance nutritive quality, oxidative and thermal stability of the oils (Ghafoorunissa 2008).
Palm olein (PO) is increasingly being used as frying and cooking medium owing to its good thermal and oxidative stability. In the blends, it improves frying quality of PUFA rich vegetable oils (Farhoosh et al. 2009; Ismail 2005). PO contains ~ 42% oleic acid (OA) and ~ 10% LA. Though it is less saturated than palm oil, it still contains high percentage of SFA (palmitic acid; PA) and very negligible amount of ALA. On the other hand, flaxseed oil (FO, Linum usitatissimum, Linseed) is one of the richest plant sources of the ω-3 ALA with proven health benefits (Kaur et al. 2014). But due to high oxidative and thermal instability, FO is not suitable as cooking oil (Symoniuk 2016).
Therefore, the objective of present study was to develop the oil blends with balanced ω-6:ω-3 ratio with good oxidative and thermal stability. We selected to blend PO and FO. The choice is made on the basis of their unique fatty acid composition and stability properties. PO blends containing 20%, 10% and 5% (v/v) FO were prepared to achieve various ω-6:ω-3 ratios. Here, we report physico-chemical characterization of these blends to ensure their nutritive quality (FA composition), oxidative stability and thermal stability followed by evaluation of biological effects of these blends in THP-1 cell line. Finally nine month storage stability of blend containing 20% FO has been evaluated. By blending FO and PO, we could improve ω-6:ω-3 ratio in the blend without compromising oxidative stability, thermal stability and long term storage stability. Improved ω-6:ω-3 ratio was reflected in the lowered ω-6:ω-3 ratio in the cells treated with these blends resulting in lowering of inflammatory marker.
Materials and methods
PO was purchased from local market. Cold press FO was purchased from Real World Nutrition Laboratory Foundation, Pune (India). MTT and Lipo-polysaccharides (LPS) were procured from Sigma-Aldrich (Saint Louis, MO, USA), Human TNFα and IL-6 ELISA kits were supplied by eBiosciences (Vienna, Austria). RPMI 1640 medium, fetal bovine serum (FBS), cell culture grade DMSO and rest of the reagents essential for cell based studies were purchased from HiMedia (India). Analytical grade reagents were purchased from SRL Laboratories (India).
Preparation of oil blends
Three different percentages of FO (20, 10 and 5 v/v) were blended with PO. Individual oils (FO and PO) were mixed at defined proportion using mechanical homogenizer (REMI ELEKTROTECHNIK LTD, India) for 15–20 min. Base oils and blends were kept in air-tight glass bottle at room temperature and used for initial physico-chemical characterization studies and nine month storage stability study. The oils and their blends to be used for cell based studies were stored at −20 °C.
Initial physicochemical characterization of the blends
Fatty acid analysis of the blends
Fatty acid profiles of the oils and blends were determined by a method described by Ichihara and Fukubayashi (2010) with some modifications. In brief, fatty acid methyl esters (FAME) from 50 mg oil/blend were prepared by adding methanolic HCl in the presence of BHT (50 mg/mL) at 80 °C for 2 h. FAME were extracted in hexane and dried under the nitrogen stream and reconstituted in hexane. Analysis of FAME was done by gas chromatography (GC) (7820A, Agilent, Santa Clara, CA, USA), coupled with a flame ionization detector (FID) with capillary column (HP88, i.d.0.25 mm, 30 m, thickness 0.2 µm). The GC conditions were as follows: the injector and FID temperature was 250 °C. Initial oven temperature (140 °C for 5 min) was raised to 230 °C (4 °C/min) and finally held at 230 °C for 12.5 min. Nitrogen was used as carrier gas (1.1 mL/min), with a split ratio of 1:25. The data is presented as Mean ± SD.
Determination of other physic-chemical parameters
Determination of the Acid Value (AV) was carried out as per AOAC official Method 940.28 and expressed as mg KOH/g oil. AOCS official method Ca 5a-40 was used to determine % Free Fatty Acid (%FFA) and expressed as %FFA (as oleic acid) (Jagtap et al. 2020). Peroxide Value (PV) was determined using AOCS official method Cd 8b-90 and expressed as milliequivalent O2/kg oil (De Boer et al. 2018). Method mentioned by Das et al. (2013) was used to determine smoke point of oils and blends. Data is represented as Mean ± SD.
Evaluation of effect of heating the blends on various chemical parameters
The oils and blends were heated at 180 °C for four hour. Samples were collected intermittently at every hour during heating. Samples were dissolved in iso-octane and absorbance was measured at 232 nm and 268 nm using UV/VIS spectrophotometer (UV 3000+ LABINDIA ANALYTICAL, INDIA). Using the formula mentioned by Malvis et al. (2019) values for K232 and K268 were calculated. PV, para-Anisidine Value (p-AV) and total oxidation value (TOTOX Value) were determined as per AOCS official methods (De Boer et al. 2018) for the oils/blends before and after four hour heating. The data is represented as Mean ± SD.
Determination of biological effects of these blends in THP-1 cell line
THP-1 (human monocytic leukemia cell line) was obtained from National Centre for Cell Sciences (Pune, India). The cells were cultured in RPMI 1640 medium containing 10% FBS. The cells were incubated at 37 °C in a humidified incubator containing 5% CO2.
Delivery of oils and blends in cell culture medium
DMSO stocks of the oils or blends (50 mg/mL) were diluted in 100% FBS and pre-incubated at 37 °C for one hour. The final concentration of FBS and DMSO in all the experiments was 10% and 0.5% respectively. DMSO alone pre-incubated with FBS served as the control (C).
Effect of the blends on cell viability
THP-1 cells were seeded in the 96W plate (1 × 105cells/well). Cells were treated with the oils or blends at 250 µg/mL, 100 µg/mL and 50 µg/mL for 24, 48 and 72 h. At the end of each incubation point, cell viability was determined by MTT assay. In brief, at the end of the incubation period, culture medium from each well was carefully removed and MTT stock (1 mg/mL in RPMI 1640) was added to each well (200 µL/well). Plate was incubated at 37 °C in a humidified incubator containing 5% CO2. After incubation for three hours, MTT solution was carefully removed from all the wells and DMSO was added to all the wells (100 µL/well). After 20 min incubation at room temperature, absorbance was read at 570 nm. The % viability was determined considering the viability of control cells as 100%. Data is represented as Mean ± SD.
Fatty acid analysis of THP-1 cells treated with the blends
THP-1 cells were treated with the oils or blends for 48 h. At the end of the treatment, cells were collected and washed twice with 1X PBS. The total lipid extraction was done as per Folch et al. (1957). The extracted cellular lipids were esterified as mentioned by Ichihara and Fukubayashi (2010). The ester derivatives were subjected to GC for determining cellular FA composition. The chromatographic conditions were as mentioned in “Fatty acid analysis of the blends” section. The data is presented as Mean ± SD of % FA of the total extracted lipids.
Effect of the blends on inflammatory markers
THP-1 cells were seeded in 24W plates (1 × 105 cells/well) and pre-treated with the oils or blends at 250 µg/mL for 48 h. Cells were stimulated with LPS (25 ng/mL). Supernatant was collected at 6 h and 24 h for TNFα and IL-6 estimation respectively. The cytokines were measured using ELISA microplate reader (BioTech, USA) following manufacturer’s protocol. The data is represented as fold change with respect to + LPS.
Determination of nine months storage stability of the blend
PO20 was selected for the long term storage (nine months) stability study as it had highest percentage of oxidatively susceptible FO. During this stability assessment, oils and blend were kept at room temperature. The stability was assessed periodically in terms of peroxide value, acid value and FA composition by using the methods mentioned in “Initial physicochemical characterization of the blends” section. Data is represented as fold change with respect to initial (0 month) readings.
Statistical analysis
Statistical analyses were done either by the one-way ANOVA or two-way ANOVA using GraphPad Prism (version 5.02) software. Significant differences were determined by Tukey's Multiple Comparison Test or Bonferroni posttests. For correlation analysis, Pearson’s correlation analysis was conducted.
Results and Discussion
Preparation of the blends
Blending of FO with PO was done with the aim to balance the ω-6:ω-3 ratio (LA:ALA) in the blend. Various percentages of the FO (5–52%) have been used in the blends with olive oil, canola oil and sesame oil (Mostafa et al. 2013; Hintze et al. 2016). A higher percentage of PUFA rich oil in the blend results in lower oxidative stability (Dean et al. 2011). We prepared the blends containing 20%, 10% and 5% FO (v/v) in PO to achieve ω-6:ω-3 ratios 5:1 or below and to ensure stability of the blends. Based on percentage of FO in the blend, these blends are symbolized as PO20, PO10 and PO5.
Initial Physico-chemical characterization of the blends
Fatty acid analysis of the blends by GC-FID
The altered ω-6:ω-3 ratios in the blends were confirmed by GC-FID. Table 1 represents FA analysis for the oils and their blends. ALA was the major FA in FO, followed by oleic acid (OA) and LA. For PO, PA was the major FA followed by OA. PO contained approximately 10% LA, but no ALA. As expected, as the percentage of FO increased in the blends, the percentage of ALA increased and that of PA decreased. These changes in the % of ALA and PA were statistically significant (p < 0.001) when compared with the % of ALA and PA in FO or PO respectively. Increase in ALA was reflected in a decreased LA:ALA ratio. The lowest LA:ALA ratio (0.98) was achieved in the blend containing 20% FO (i.e. PO20), while higher ratios of 1.79 and 3.85 were obtained for PO10 and PO5 respectively. The decrease in LA:ALA ratio were statistically significant when compared with PO5 (p < 0.001 PO10 and PO20 vs. PO5, g: p < 0.05 PO10 vs. PO20). As indicated in the table, FO was rich in PUFA and as the percentage of FO in the blends increased PUFA content of the blends also increased as seen in Saturated FA: Monounsaturated FA: Polyunsaturated (S:M:P) ratios of the blends. Thus, the data in Table 1 confirm that blending FO with PO can achieve desired ω-6:ω-3 ratios between 1:1(PO20) to 4:1 (PO5). It is important to note that even the blend containing 5% FO provides the acceptable ω-6:ω-3 ratio.
Table 1.
Fatty acid composition of the oils and their blends analyzed by GC-FID
| FA | FO | PO | PO20 | PO10 | PO5 |
|---|---|---|---|---|---|
| Palmitic Acid | 6.61 ± 0.08e | 41.36 ± 0.16a | 33.55 ± 0.05d | 36.73 ± 0.33c | 39.17 ± 0.08b |
| Stearic Acid | 6.59 ± 0.07b | 3.91 ± 0.06a | 3.83 ± 0.22a | 4.20 ± 0.05a | 3.44 ± 0.68a |
| Oleic Acid | 21.61 ± 0.01e | 43.83 ± 0.01a | 38.83 ± 0.07d | 41.31 ± 0.04c | 42.78 ± 0.25f |
| Linoleic Acid (LA) | 14.47 ± 0.10e | 10.89 ± 0.11a | 11.78 ± 0.04f | 11.39 ± 0.12a, f | 11.59 ± 0.81a, f |
| Alpha Linolenic Acid (ALA) | 50.72 ± 0.23e | – | 12.00 ± 0.16d | 6.37 ± 0.19c | 3.01 ± 0.2b |
| LA/ALA | 0.29 ± 0.00e, d | – | 0.98 ± 0.01d, g | 1.79 ± 0.03c | 3.85 ± 0.02b |
| S:M:P | 1.00:1.64:4.94 | 1.00:0.97:0.24 | 1.00:1.04:0.64 | 1.00:1.01:0.43 | 1.00:1.00:0.34 |
The FA composition of the oils and their blends was determined by GC−FID. Two Way ANOVA and Bonferroni Posttests were applied to determine statistically significant differences between and among different FA present in the oils and blends. S represents total saturated fatty acids, M represents total monounsaturated fatty acids and P represents total polyunsaturated fatty acids. Different letters in a row represent statistically significant difference between the means
a–ep < 0.001;fp < 0.01 and gp < 0.05 PO10 versus PO20
Other physico-chemical parameters of the blends
Table 2 represents AV, % FFA, PV and smoke point of the oils and blends determined immediately after the blend preparation. AV and FFA indicate quality of oil. AV for PO and the three blends were in the range of 0.60–0.70. FO had the lowest AV of 0.46. AV values of 0.4 for PO and 0.21–0.53 for FO have been reported (Ismail 2005; Symoniuk et al. 2016; Bhardwaj et al. 2015).
Table 2.
Characterization of the blends
| Oil | Parameters (immediately after blending) | |||
|---|---|---|---|---|
| AV (mg KOH/g oil) | % FFA (as oleic acid) | PV (milliequivalent O2/ kg oil) | Smoke Point (°C) | |
| FO | 0.46 ± 0.09 | 0.20 ± 0.01 | 0.40 ± 0.03 | 103 ± 1.41 |
| PO | 0.62 ± 0.11 | 0.26 ± 0.03 | 1.13 ± 0.11* | 212.5 ± 3.53$ |
| PO20 | 0.69 ± 0.07 | 0.26 ± 0.02 | 1.98 ± 0.28** | 203.5 ± 2.12$ |
| PO10 | 0.65 ± 0.06 | 0.26 ± 0.03 | 1.37 ± 0.23* | 203 ± 1.41$π |
| PO5 | 0.63 ± 0.03 | 0.26 ± 0.01 | 1.12 ± 0.13*# | 203.5 ± 2.12$ |
Acid Value (AV), % Free Fatty Acid (%FFA), Peroxide Value (PV) and Smoke Point(SP) of the blends were determined immediately after the blend preparation. One−way ANOVA and Tukey’s Multiple Comparison Test was applied to determine statistically significant differences among the oils and blends.
* and **p < 0.05 versus FO; #p < 0.05 versus PO20; $p < 0.01 versus FO; πp < 0.01 versus PO
Table 2 also shows that the % FFA for the oils and blends were not significantly different and ranged from 0.20 to 0.25. The % FFA for PO was reported in the range of 0.04–0.12 (Ismail 2005; Tarmizi and Ahmad 2015; Tarmizi and Ismail 2008) while Bhardwaj et al. (2015) have reported % FFA for FO as 0.32. The observed differences in these physico-chemical parameters are probably due to initial quality of raw material, oil extraction process, storage conditions and duration.
PV indicates primary oxidation levels of the oil. FO showed the much lower PV (0.40) than the reported values of 0.98–2.34 (Bhardwaj et al. 2015; Symoniuk et al. 2016). The lowest PV of FO indicates excellent quality of the FO used in our study. The literature indicates PV of 0.28–1.3 for PO (Ismail 2005; Tarmizi and Ahmad 2015; Tarmizi and Ismail 2008). PV for PO and the blends were significantly higher than that of FO (p < 0.05). PV for the blends raised as percentages of FO in the blends increased. Among the blends, PO20 showed highest PV. This may be because of oxidative products present in the PO, attacked the oxidatively susceptible components in the blends.
Smoke point (SP) represents the temperature at which the oil components break down and are detectable as smoke. Under most cooking conditions like frying, a temperature of 180 °C is achieved (Fan et al. 2013). PO had the highest SP (212 °C), which is consistent with reports stating SP of 212–220 °C (Tarmizi and Ahmad 2015; Fan et al. 2013). The high SP of PO indicates thermal stability of PO. Definitely, FO with SP of 103 °C is not suitable for cooking involving heat. At all percentages of FO used for the blending, SP dropped from 212 °C to 203 °C. There were no significant differences in SP among the blends with different percentages of FO but they were significantly higher than that of FO (p < 0.01) signifying that blends can be used as cooking oil. Therefore, it can be stated that upto 20% FO can be safely accommodated in PO without disturbing the suitability of PO as a cooking oil. Thus, Table 2 indicates that blending FO with PO did not adversely affect the studied physico-chemical properties of acid value, rancidity, primary oxidation level and smoke point.
Evaluation of effect of heating the blends on various chemical parameters
Heating deteriorates quality of the oil as it results in the formation of primary and secondary oxidation products. K232 represents oil deterioration in terms of hydroperoxides, conjugated dienes and carboxylic compounds while K268 represents additional oxidation of the products from K232 (Malvis et al. 2019). Tables 3 and 4 show changes in K232 and K268 values respectively upon heating the oils and blends at 180 °C upto 240 min. As shown in Table 3, initial K232 value for FO was significantly lower than that for PO. However, heating FO at all the four time points showed the significant increase in K232 values when compared with the unheated (0 min) FO (p < 0.001). In contrast, there was no statistically significant increase in K232 values across all the time points for PO. PO5 showed statistically significant increase in K232 only when heated for the longest duration of 240 min (p < 0.001), whereas PO10 and PO20 blends showed significant increase in K232 when heated for a shorter duration of 120 min.
Table 3.
Effect of continuous heating of the oils and blends at 180 °C on K232
| Oil/Blend | 0 min | 60 min | 120 min | 180 min | 240 min |
|---|---|---|---|---|---|
| FO | 1.71 ± 0.01@ | 2.73 ± 0.05$ | 3.52 ± 0.04$ | 3.68 ± 0.02$ | 3.76 ± 0.03$ |
| PO | 3.36 ± 0.02 | 2.63 ± 0.02* | 3.65 ± 0.02 | 3.98 ± 0.02 | 3.46 ± 0.02 |
| PO20 | 2.77 ± 0.02 | 3.24 ± 0.01 | 3.73 ± 0.02# | 4.65 ± 0.02$ | 5.18 ± 0.03$ |
| PO10 | 2.42 ± 0.03% | 2.67 ± 0.02 | 4.01 ± 0.03$ | 3.63 ± 0.02$ | 4.16 ± 0.02$ |
| PO5 | 2.95 ± 0.01 | 2.71 ± 0.02 | 3.43 ± 0.06 | 3.56 ± 0.02 | 4.65 ± 0.01$ |
The K232 values were determined for oils/blends before and after heating at 180 °C for above mentioned time points. Two Way ANOVA and Bonferroni Posttests were applied to determine statistically significant differences. Different letters represent statistically significant difference between the means
$p < 0.001 versus 0 min; @p < 0.001 versus PO and *p < 0.05 versus 0 min; #p < 0.01 versus 0 min and %p < 0.01 versus PO
Table 4.
Effect of continuous heating of the oils and blends at 180 °C on K268
| 0 min | 60 min | 120 min | 180 min | 240 min | |
|---|---|---|---|---|---|
| FO | 0.20 ± 0.01@ | 1.00 ± 0.03$ | 1.73 ± 0.05$ | 2.43 ± 0.02$ | 2.79 ± 0.04$ |
| PO | 0.70 ± 0.02 | 1.01 ± 0.02$ | 1.47 ± 0.08$ | 1.62 ± 0.05$ | 1.41 ± 0.12$ |
| PO20 | 0.49 ± 0.04 | 0.95 ± 0.09$ | 1.11 ± 0.08$ | 1.39 ± 0.04$ | 1.59 ± 0.08$# |
| PO10 | 0.51 ± 0.03 | 0.87 ± 0.06$ | 1.29 ± 0.09$ | 1.19 ± 0.05$ | 1.37 ± 0.08$ |
| PO5 | 0.59 ± 0.01 | 0.78 ± 0.06$ | 0.96 ± 0.04$ | 1.29 ± 0.05$ | 1.64 ± 0.08$ |
The K268 values were determined for oils/blends before and after heating at 180 °C for above mentioned time points. Two Way ANOVA and Bonferroni Posttests were applied to determine statistically significant differences. Different letters represent statistically significant difference between the means
$p < 0.001 versus 0 min; @p < 0.001 versus PO and #p < 0.01 versus 0 min
As seen in the Table 4, FO had significantly low K268 compared to PO and blends (@: p < 0.001). All oils/blends showed statistically significant rise in the K268 when compared with 0 min value ($: p < 0.001) but percent change was different for individual oil/blend. Highest percent change (increase) was seen for FO (0 and 240 min). Percent change in the K268 values were in order FO > PO20 > PO10 > PO5 > PO when compared with initial value (0 min) for the respective oils or blends. When compare with PO, percent changes were not significantly different for PO10 and PO5 but PO20 showed statistically significant percent change (#: p < 0.01) at 240 min. Percent rise in PO never reached to this significance level.
Effect of heating was confirmed by evaluating PV, pAV, TOTOX value for 0 min and 240 min (four hour) heated oils/blends. Further, Pearson’s correlation coefficient was used for correlation analysis. While pAV is an indicator for secondary oxidation in the oil, TOTOX value represents oxidative state of the oil (primary and secondary oxidation) (Tarmizi and Ahmad 2015). As shown in the Table 5, when PV values for oil/blend were compared before and after heating, only FO showed statistically significant rise (#: p < 0.01) but statistically significant rise was seen in all oils/blends when pAV and TOTOX values were compared before and after heating ($; p < 0.001). Before heating, there was no statistically significant difference for the blends and PO for all the parameters. After heating, when compared with PO, pAV and TOTOX values were significantly higher for the blends (@: p < 0.001 vs. PO after heating) but all these parameters were statistically lower than the values for FO except PV for PO20 (%: p < 0.05 and &: p < 0.001 vs. FO after heating). The degree of deterioration due to heating for FO, PO and blends was also calculated in terms of % change. The trend of rise in PV, pAV and TOTOX value after heating the oil was also reported by Ismail (2005) and Tarmizi and Ahmad (2015). Thus, the data indicates that the studied parameters were significantly altered after four hour heating which is similar to observations for K232 and K268.
Table 5.
Chemical parameters of oils and blends before and after heating at 180 °C for four hour
| Oil/Blend | PV before heating (meq O2/kg oil) | PV after heating | pAV before heating | pAV after heating | TOTOX value before heating | TOTOX value after heating | |||
|---|---|---|---|---|---|---|---|---|---|
| (meq O2/kg oil) | % Change | % Change | % Change | ||||||
| FO | 0.40 ± 0.02 | 12.73 ± 0.75# | 3183 | 0.11 ± 0.05 | 227.97 ± 16.3$ | 207,245 | 0.91 ± 0.06 | 253.44 ± 17.53$ | 27,851 |
| PO | 1.13 ± 0.08 | 2.57 ± 0.12 | 227 | 5.57 ± 0.93 | 51.57 ± 2.84$ | 926 | 7.82 ± 0.1 | 56.71 ± 3.06$ | 725 |
| P20 | 1.98 ± 0.2 | 3.37 ± 0.06% | 170 | 4.72 ± 0.57 | 87.15 ± 4.13$@& | 1846 | 8.68 ± 1.13 | 93.88 ± 4.15$@& | 1082 |
| P10 | 1.37 ± 0.16 | 3.22 ± 0.03% | 235 | 4.85 ± 0.18 | 74.91 ± 1.83$@& | 1545 | 7.59 ± 0.37 | 81.35 ± 1.86$@& | 1072 |
| P5 | 1.12 ± 0.09 | 2.67 ± 0.06% | 238 | 6.11 ± 1.5 | 67.12 ± 2.46$@& | 1099 | 8.35 ± 1.52 | 72.45 ± 2.43$@& | 868 |
The chemical parameters were determined for oils/blends before and after heating at 180 °C for 240 min (four hour). Two Way ANOVA and Bonferroni Posttests were applied to determine statistically significant differences. Different letters represent statistically significant difference between the means
$p < 0.001 versus before heating; @p < 0.001 versus PO after heating; #p < 0.01 versus before heating; %p < 0.05 and &p < 0.001 versus FO
From the Table 6, it is very clear that, PV is well correlated with pAV, TOTOX value and K268. TOTOX value is strongly and positively correlated with PV and pAV. Similar correlation was observed by Giuffrè et al. (2018) when palm oil was heated at 180/220 °C for 120 min. pAV and K268 both indicate secondary lipid oxidation in the oil. These two parameters are very well correlated. Such strong correlation has been also reported by Jaswir et al. (2005) where they have used flax seed oil for frying. Thus, oxidation state of the oil (TOTOX value) is well correlated with PV (primary oxidation) and pAV (secondary oxidation) parameters of oil/blends. The parameters indicating secondary oxidation of oil/blend are also well correlated.
Table 6.
Correlation analysis for various parameters studied
| PV | pAV | TOTOX value | K232 | K268 | |
|---|---|---|---|---|---|
| PV | 0.966 | 0.972 | 0.343 | 0.885 | |
| pAV | 0.97 | 1.00 | 0.526 | 0.96 | |
| TOTOX value | 0.97 | 1.00 | 0.511 | 0.956 | |
| K232 | 0.34 | 0.526 | 0.511 | 0.69 | |
| K268 | 0.89 | 0.96 | 0.956 | 0.69 |
Pearson’s correlation coefficients were determined for various chemical parameters studied for oils/blends before and after heating at 180 °C for four hour
Thus, all these chemical parameters highlight thermal instability of FO. PO with initial high chemical parameter values, did not show significant rise as seen for FO, indicating robust nature of PO. Notably, different blends tolerated different durations of the heating before showing significant deterioration in the form of K232 and K268. The thermal deterioration of the oils/blends was confirmed by determining pAV and TOTOX value. Though the blends showed significant changes, these values were always lower than FO. It is important to note that, the thermal deterioration accelerated as the percentage of FO in the blend increased. It is reflected as level of significance and time required to achieve that significance level. Our data show that PO imparts some thermal stability to the FO containing blends.
Biological effects of these blends studied in THP-1 cell line
Effect of the blends on viability
Tables 1–6 showed that the physico-chemical characteristics of the blends confirm to the good cooking oil characterization. Therefore, we further evaluated the biological effects of these blends using THP-1 cell line. This cell line is widely used for studies evaluating lipo-toxicity and inflammation. Although there is data on the effects of PA and ALA on differentiated THP-1 cells (Wang et al. 2009; Salehipour et al. 2010), there are no reports on the effects of palm olein and flaxseed oil blends on viability and inflammation of THP-1 cells.
Figure 1 shows that adding FO, PO and the blends (250 µg/mL) for 24–48 h did not significantly affect THP-1cell viability. Similar data was obtained when lower concentrations of the oils and blends were added to THP-1 cells (100 and 50 µg/mL; data not shown). Interestingly, THP-1 cells treated with FO for 72 h, showed a small but significant increase in viability when compared with PO (p < 0.05).
Fig. 1.
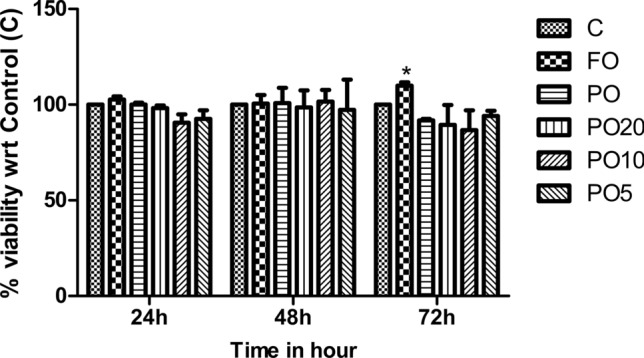
Viability of THP-1 cells treated with the oils and blends. THP-1 cells were treated with and blended oils (250 µg/mL) for 24, 48 and 72 h. At the end of the incubation periods, MTT assay was done to determine the viability. Data is represented as % viability wrt Control (C). Two-way ANOVA and Bonferroni Posttests were used for statistical analysis. *p < 0.05 versus PO
A majority of other studies have examined effects of the free FA on cell viability. Thus, Song et al. (2013) found that macrophages derived from THP-1 monocytes showed decreased viability when treated with PA, OA, LA or eicosapentaenoic acid (EPA) (200 µM for 72 h). However, the final viability of these macrophages was still above 75%. The observed differences in the viability could be because of type of lipid used for the treatment (i.e. free FA vs. triacylglycerol). Overall, our results show that FO, PO and the blends have no toxic effect on THP-1 cells.
Fatty acid composition of THP-1 cells treated with the blends
It is known that treatment of monocytes with a FA results in enrichment of that particular FA within the cells. Therefore, FA uptake by THP-1 cells treated with oils or the blends was evaluated by FA analysis of the cells by GC-FID. Results in Table 7 show FA levels expressed as a % of the total lipid extracted from THP-1 cells treated with the oils or blends. Here, we have identified FA, present in the oils/blends and major FA acids derived from ALA and LA. Rest all the FA are collectively termed as ‘Others’. Both Control (C) and PO treated cells were devoid of ALA. However, treatment with ALA rich FO resulted in significant incorporation of ALA in THP-1 cells compared to C. Increased percentages of FO in the blends resulted in a dose dependent increase in ALA incorporation (0.3, 0.62 and 1.33% for PO5, PO10 and PO20 respectively), though these changes were not statistically significant. On the other hand, treatment of cells with PO or the blends caused a statistically significant increase in incorporation of OA in THP-1 cells, while percentages of LA, AA and docosahexaenoic acid (DHA) were not significantly altered. These observations of significant enrichment of ALA in FO and OA in PO treated cells, are consistent with published reports (Zhao et al. 2005; Galella et al. 1993).
Table 7.
Fatty acid composition of THP-1 cells treated with the oils and blends
| FA | C | FO | PO | PO20 | PO10 | PO5 |
|---|---|---|---|---|---|---|
| Palmitic acid | 19.90 ± 4.76 | 16.14 ± 3.74 | 18.90 ± 0.37 | 17.18 ± 0.85 | 16.72 ± 2.74 | 16.63 ± 2.61 |
| Stearic acid | 10.50 ± 2.86 | 13.62 ± 7.37 | 8.92 ± 1.43 | 9.68 ± 0.48 | 9.77 ± 1.47 | 8.72 ± 0.07 |
| Oleic acid | 5.91 ± 0.40 | 9.01 ± 1.41 | 15.18 ± 3.06*** | 14.91 ± 0.03*** | 10.39 ± 2.70 | 11.61 ± 5.13** |
| Linoleic acid (LA) | 1.76 ± 1.21 | 3.68 ± 1.25 | 3.30 ± 1.82 | 4.04 ± 0.72 | 2.42 ± 0.68 | 3.00 ± 0.24 |
| Alpha linolenic acid (ALA) | – | 7.02 ± .38*** | – | 1.33 ± 0.45 | 0.62 ± 0.02 | 0.30 ± 0.01 |
| Arachidonic acid (AA) | 4.42 ± 1.51 | 2.61 ± 1.94 | 3.81 ± 1.19 | 3.98 ± 0.24 | 4.16 ± 0.97 | 3.67 ± 0.24 |
| Docosahexanoic acid (DHA) | 1.66 ± 0.09 | 1.31 ± 0.43 | 1.59 ± 0.47 | 2.73 ± 1.46 | 1.87 ± 0.06 | 1.66 ± 0.04 |
| Others | 55.86 ± 7.60 | 46.60 ± 8.51 | 48.32 ± 2.16 | 46.15 ± 2.79 | 54.05 ± 7.28 | 54.41 ± 8.17 |
| LA/ALA | – | 0.52 ± 0.15 | – | 3.32 ± 1.67# | 3.95 ± 1.23# | 10.03 ± 1.28 |
| (LA + AA)/(ALA + DHA) | 3.73 ± 0.02 | 0.74 ± 0.31 | 4.74 ± 1.80 | 2.25 ± 1.18 | 2.65 ± 0.03 | 3.41 ± 0.19 |
THP−1 cells were treated with the oils and blends for 48 h. FAME were prepared after total lipid extraction from the cells and were subjected to FA analysis by GC−FID. FA present in the oils/blends and major FA acids derived from ALA and LA have been identified. Rest all the FA are collectively termed as ‘Others’. Data is represented as Mean ± SD of %FA of the total extracted lipids. Two−way ANOVA and Bonferroni Posttests were applied to determine statistical significance
***p < 0.001 versus C; **p < 0.01 versus C and #p < 0.01 versus PO5
Our primary interest was to determine whether the blends of FO and PO added to the cells, can balance the intracellular ω-6:ω-3 ratio (LA:ALA). If we consider the LA:ALA ratio, we observed that, ratio was decreased from 10.03 (PO5) to 3.32 (PO20) (p < 0.01 PO10 and PO20 vs. PO5). Due to dose dependent increase in %ALA within the cells, the ratio of LA:ALA lowered as the percentage of FO in the blend increased. Notably, the ratio of total ω-6 FA (LA + AA) to total ω-3 FA (ALA + DHA), also showed a decreasing trend from 3.41(PO5) to 2.25 (PO20).
Therefore, the data clearly show that exogenously added blends of PO and FO can result in a significant, dose dependent decrease in the ω-6:ω-3 (LA:ALA) ratio in THP-1 cells. To our knowledge, this is the first report to show that cellular ω-6:ω-3 ratio can be favorably altered by addition of blend containing FO and PO.
Effect of the blends on inflammatory markers
A high ω-6:ω-3 ratio results in persistent low grade inflammation and development of NCD (Johnson and Fritsche 2012). FO is rich in the ω-3 FA; ALA, which gets converted into EPA and DHA. All these three FA possess anti-inflammatory properties (Zhao et al. 2005, 2007). As the data from Table 7 indicated that exogenously added oils and the blends can alter the LA:ALA ratio in THP-1 cells, we studied the effect of these blends on the secretion of inflammatory markers TNFα and IL-6 by these cells.
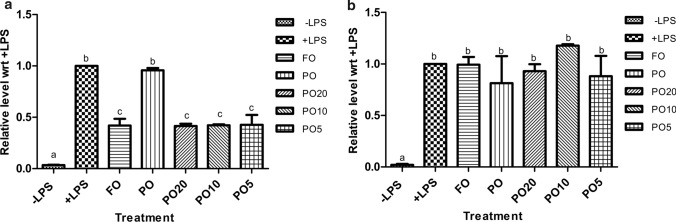
Here, Fig. 2a shows that LPS stimulation of THP-1 cells resulted in increased levels of TNFα. The pre-treatment of THP-1 cells with FO or the three blends prior to LPS addition, resulted in the significant decrease in TNFα levels. Notably, Fig. 2a clearly shows that pre-treatment of THP-1 cells with PO did not alter TNFαlevel. Figure 2b shows that LPS stimulation resulted in the increased level of IL-6 in the supernatant (+ LPS) but pre-treatment of THP-1 cells with either of the oils or blends, did not significantly alter IL-6 levels in the supernatant. We did not see down-regulation of IL-6 by ALA rich FO pre-treatment.
Fig. 2.
Effect of the oils and blends on TNFα (a) and IL-6 (b) in THP-1 cells. THP-1 cells were pretreated with the oils or blends (250 µg/mL) for 48 h followed by co-incubation with LPS (25 ng/mL) for 6 h (TNFα) and 24 h (IL-6). Quantitation of TNFα and IL-6 was done in the cell supernatants by ELISA. Data are presented as fold change wrt + LPS. One-way ANOVA and Tukey’s Multiple Comparison Test was applied to determine statistically significant differences among the oils and blends. Bars labeled with different letters have statistically significant difference (p < 0.01)
Zhao et al. (2005) have reported that PA (major FA in PO) had no effect on the inflammatory cytokines but ALA treatment lowers them (TNFα and IL-6). Thus, our data is consistent with this published report for effect of PO on TNFα and IL-6. It is also in line with TNFα and ALA pre-treatment. However, we did not see down-regulation of IL-6 by ALA rich FO pre-treatment as reported by the authors.
The data is also consistent with human and animal studies determining effect of FO on inflammatory markers either in serum or peripheral blood mononuclear cells (PBMC) obtained from participants. Caughey et al. (1996) have shown the beneficial effects of ALA, EPA and DHA in human and that their anti-inflammatory effects are dose dependent. Similar results are reported by Zhao et al. (2007). In their study, PBMC isolated from participants in ALA rich diet group and LA rich diet group were stimulated by LPS. PBMC from ALA rich group showed significant down-regulation of TNFα and IL-1β when compared PBMC from LA rich diet group.
The effect of PUFA on the cytokine production may be regulated not only by the ω-6:ω-3 ratio but also by their concentrations. Indeed, Hintze et al. (2016) have shown that in case of LPS induced inflammation in mice, certain cytokines including TNFα were affected by ω-6:ω-3 ratio while certain cytokines like IL-6 were affected by the concentrations of FA. It is important to note that, pro-inflammatory LA and anti-inflammatory ALA compete for the same set of enzymes for elongation and desaturation. Thus, in our study possibly the various ratios of ω-6:ω-3 achieved by PO and FO blending and lipid mediators derived from ALA (due to higher affinity towards the enzymes) are adequate for down-regulating TNFα level but their concentrations are not sufficient to show beneficial effect on IL-6. But we need to confirm role of ALA as major anti-inflammatory player by studying lipid mediators of this ω-3 FA in this experimental system.
Nine months storage stability of PO20
The physico-chemical characterization and studies on THP-1 cells suggest that all the blends were fairly stable at room temperature as well as elevated temperature. They had TNFα lowering capacity without any cytotoxic effect irrespective of percentage of FO present. Therefore, we conducted nine months storage stability study for the blend PO20 which had highest percent of oxidatively susceptible FO. We have analyzed FA composition of PO and PO20 along with determination of PV and AV.
Here, Table 8 represents FA composition of PO and PO20 stored upto nine months. As seen in the table, there were marginal changes in FA analyzed including ALA and LA. There are contradictory results for changes in the FA composition during storage. Semwal and Arya (2001) have reported no changes in the FA composition but Gulla and Waghray (2011) have reported increase in the SFA and MUFA with simultaneous decrease in PUFA content on storage. Here, we have seen no change in the FA composition. This may be because initial quality of the oils used for the blending especially their moisture content.
Table 8.
Fatty acid composition of PO and PO20 stored upto nine months at room temperature
| FA | Oil/Blend | 0 month | 3rd month | 6 month | 9th month |
|---|---|---|---|---|---|
| ALA | PO | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| PO20 | 11.25 ± 0.07 | 11.30 ± 0.13 | 11.13 ± 0.11 | 11.08 ± 0.04 | |
| LA | PO | 11.08 ± 0.04 | 10.49 ± 0.06 | 11.11 ± 0.13 | 11.11 ± 0.13 |
| PO20 | 11.15 ± 0.13 | 11.71 ± 0.17 | 11.52 ± 0.1 | 11.53 ± 0.08 | |
| MUFA | PO | 44.60 ± 0.07 | 43.645 ± 0.06 | 44.15 ± 0.14 | 44.12 ± 0.1 |
| PO20 | 39.30 ± 0.06 | 38.90 ± 0.07 | 38.93 ± 0.07 | 38.88 ± 0.14 | |
| SFA | PO | 44.21 ± 0.09 | 45.43 ± 0.04 | 44.85 ± 0.07 | 44.85 ± 0.07 |
| PO20 | 38.17 ± 0.1 | 37.78 ± 0.04 | 38.35 ± 0.00 | 38.33 ± 0.04 |
PO and PO20 were stored at room temperature upto nine months. FAME were prepared and subjected to FA analysis by GC−FID at mentioned time intervals. Data is represented as Mean±SD of % FA of the total lipids. Two−way ANOVA and Bonferroni Posttests were applied to determine statistical significance
Table 9 represents PV and AV at indicated time points. Table 9 clearly indicates that PV for individual oils and PO20 increased significantly throughout study period (p < 0.001 vs. 0 month). But rise in the PV for FO was very high (≈21 fold) as compared to PO and PO20 (≈4.5 fold rise) by nine months. Fold rise in the PV for PO and PO20 showed very similar trend indicating addition of FO to the blend has not significantly accelerated peroxidation when blend was stored at room temperature.
Table 9.
Nine months storage stability study—effect on peroxide value and acid value
| Oil/Blend | 0 month | 3rd month | 6th month | 9th month |
|---|---|---|---|---|
| Peroxide value (meq O2/kg oil) | ||||
| PO | 1.98 ± 0.0 | 3.63 ± 0.057*** | 5.3 ± 0.0*** | 9.26 ± 0.06*** |
| FO | 0.6 ± 0.05 | 3.3 ± 0.00*** | 5.4 ± 0.1*** | 12.73 ± 0.05*** |
| PO20 | 2.5 ± 0.0 | 4.33 ± 0.057*** | 7.2 ± 0.0*** | 11.43 ± 0.11*** |
| Acid value (mg KOH/g oil) | ||||
| PO | 0.89 ± 0.0 | 0.89 ± 0.0 | 0.89 ± 0.0 | 1.34 ± 0.0*** |
| FO | 0.48 ± 0.00 | 0.75 ± 0.02*** | 1.59 ± 0.00*** | 2.01 ± 0.03*** |
| PO20 | 0.89 ± 0.0 | 0.89 ± 0.0 | 0.89 ± 0.0 | 1.79 ± 0.0*** |
PO, FO and PO20 were stored at room temperature upto nine months. Peroxide value and acid value were determined at mentioned time intervals. Data is represented as Mean±SD of three experiments. Two−way ANOVA and Bonferroni Posttests were applied to determine statistical significance
***p< 0.001 versus 0 month
Table 9 also represents AV. Similar to PV, FO showed gradual and significant rise in the AV (p < 0.001) while PO and PO20 did not show significant change in the values until nine month. As seen in case of PV, PO and PO20 followed similar trends but fold rise in AV for PO20 at nine months was slightly higher than PO. It has been shown that, keeping oils at room temperature, exposed to light accelerates the rise in the PV and % FFA (Almeida et al. 2019). Our data is in agreement with this reported data. Taken together, it might be concluded that, PO offers storage stability to FO containing blend.
Conclusion
The objective of balancing ω-6 to ω-3 ratio in blend was accomplished by selecting oil with low ω-6 like PO and blending with FO which is rich in ω-3 FA, so as to get a ω-6 to ω-3 ratio below 5:1. Physico-chemical characterization of edible oil or its blend is important to evaluate their health value and suitability as good cooking oil. The characterization of the blends indicated that, with blends containing 20% or lower FO, desired FA profile could be achieved, especially the LA:ALA ratio. The AV, PV and % FFA determination confirmed that these values did not vary drastically from the base oil (PO) used. Smoke Point and chemical parameters (K232 and K268, pAV and TOTOX value) determination assured that these blends were thermally stable up-to certain time during heating. Biological effects of these blends especially in terms of inflammation were evaluated in THP-1 cells. Treatment of these blends resulted into improved LA:ALA ratio in THP-1 cells without affecting cell viability. Improvement in LA:ALA ratio was reflected in lower TNFα levels compared to LPS stimulated THP-1 cells.
The study indicates that even the ω6:ω3 ratio and concentration of ALA achieved by lower percentage of FO (5%) had favorable effect on inflammation. But the mechanisms for these observed biological effects need to be elucidated further along with the expansion of the studies in the cell lines derived from organs involved in lipid homeostasis. Finally nine months storage stability study indicates that PO could provide oxidative stability to the blend containing oxidatively vulnerable FO.
Acknowledgements
Authors are thankful to Mr. Yogesh S. Badhe and Mr. Pramod D. Farde for assistance in carrying out initial physico-chemical characterization and nine months storage stability study.
Abbreviations
- AA
Arachidonic acid
- ALA
Alpha-linolenic acid
- AOCS
American oil chemists' society
- AV
Acid Value
- BHT
Butylated hydroxytoluene
- DHA
Docosahexaenoic acid
- FO
Flaxseed oil
- DMSO
Dimethyl sulfoxide
- EPA
Eicosapentaenoic acid
- FBS
Fetal Bovine Serum
- IL-6
Interleukin-6
- LA
Linoleic acid
- LPS
Lipopolysaccharide
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- OA
Oleic acid
- PA
Palmitic acid
- p-AV
Para-anisidine value
- PO
Palm olein
- PV
Peroxide value
- SP
Smoke point
- TNFα
Tumor necrosis factor alpha
- TOTOX value
Total oxidation value
- % FFA
% Free fatty acid
Author contributions
AJ—Conducted experiments, analyzed data and drafted of the manuscript, MH—Conceptualization and supervision, AZ—Project monitoring, data curation, review, Resources management and editing of manuscript.
Funding
Authors are thankful to Indian Council of Agriculture Research, New Delhi and Bharati Vidyapeeth (Deemed to be University), Pune, India for providing the support to conduct the research work.
Availability of data and material
All data generated in the present study has been included in the submitted manuscript.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almeida DT, Viana TV, Costa MM, Silva CD, Feitosa S. Effects of different storage conditions on the oxidative stability of crude and refined palm oil, olein and stearin (Elaeisguineensis) Food Sci Technol. 2019;39:211–217. doi: 10.1590/fst.43317. [DOI] [Google Scholar]
- Bhardwaj K, Verma N, Trivedi RK, Bhardwaj S, Shukla N. A novel approach for improvement of oxidative stability of flaxseed oil by blending with palm oil. Int J Adv Res. 2015;3:1399–1407. [Google Scholar]
- Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- Choudhary M, Grover K, Kaur G. Development of rice bran oil blends for quality improvement. Food Chem. 2015;173:770–777. doi: 10.1016/j.foodchem.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Das AK, Babylatha R, Pavithra AS, Khatoon S. Thermal degradation of groundnut oil during continuous and intermittent frying. J Food Sci Technol. 2013;50:1186–1192. doi: 10.1007/s13197-011-0452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean LL, Davis JP, Sanders TH. Groundnut (peanut) oil. In: Gunstone F, editor. Vegetable oils in food technology: composition, properties and uses. Oxford: Blackwell Publishing Ltd; 2011. pp. 225–242. [Google Scholar]
- De Boer AA, Ismail A, Marshall K, Bannenberg G, Yan KL, Rowe WJ. Examination of marine and vegetable oil oxidation data from a multi-year, third-party database. Food Chem. 2018;15:249–255. doi: 10.1016/j.foodchem.2018.01.180. [DOI] [PubMed] [Google Scholar]
- Falade AO, Oboh G, Okoh AI. Potential health implications of the consumption of thermally-oxidized cooking oils–a review. Pol J Food Nutr Sci. 2017;67:95–106. doi: 10.1515/pjfns-2016-0028. [DOI] [Google Scholar]
- Fan HY, Sharifudin MS, Hasmadi M, Chew HM. Frying stability of rice bran oil and palm olein. Int Food Res J. 2013;20:403–407. [Google Scholar]
- Farhoosh R, EsmaeilzadehKenari R, Poorazrang H. Frying stability of canola oil blended with palm olein, olive, and corn oils. J Am Oil Chem Soc. 2009;86:71–76. doi: 10.1007/s11746-008-1315-x. [DOI] [Google Scholar]
- Folch J, Lees M, Stanley GS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- Jagtap AA, Badhe YS, Hegde MV, Zanwar AA. Development and characterization of stabilized omega-3 fatty acid and micronutrient emulsion formulation for food fortification. J Food Sci Technol (New Delhi, India) 2020;3:1–9. doi: 10.1007/s13197-020-04614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galella G, Marangoni F, Risé P, Colombo C, Galli G, Galli C. n–6 and n–3 fatty acid accumulation in thp–1 cell phospholipids. Biochim Biophy Acta (BBA) Lipid Lipid Metab. 1993;1169:280–90. doi: 10.1016/0005-2760(93)90252-5. [DOI] [PubMed] [Google Scholar]
- Ghafoorunissa G. Role of trans fatty acids in health and challenges to their reduction in Indian foods. Asia Pac J Clin Nutr. 2008;17:212–215. [PubMed] [Google Scholar]
- Giuffrè AM, Caracciolo M, Zappia C, Capocasale M, Poiana M. Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil. Ital J Food Sci. 2018 doi: 10.14674/IJFS-1269. [DOI] [Google Scholar]
- Gulla S, Waghray K. Effect of storage on physico-chemical characteristics and fatty acid composition of selected oil blends. J Life Sci. 2011;3:35–46. doi: 10.1080/09751270.2011.11885167. [DOI] [Google Scholar]
- Hintze KJ, Tawzer J, Ward RE. Concentration and ratio of essential fatty acids influences the inflammatory response in lipopolysaccharide challenged mice. Prostaglandins LeukotEssent Fat Acids. 2016;111:37–44. doi: 10.1016/j.plefa.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Ichihara KI, Fukubayashi Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res. 2010;51:635–640. doi: 10.1194/jlr.D001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail R. Palm oil and palm olein frying applications. Asia Pac J Clin Nutr. 2005;14:414. [PubMed] [Google Scholar]
- Jaswir I, Kitts DD, Man YB, Hassan TH. Physico-chemical stability of flaxseed oil with natural antioxidant mixtures during heating. J Oleo Sci. 2005;54:71–79. doi: 10.5650/jos.54.71. [DOI] [Google Scholar]
- Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–1041. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods-a review. J Food Sci Technol. 2014;51:2289–2303. doi: 10.1007/s13197-012-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohikamali S, Alam MS. Improvement in nutritional quality and thermal stability of palm olein blended with macadamia oil for deep-fat frying application. J Food Sci Technol. 2019;56:5063–5073. doi: 10.1007/s13197-019-03979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvis A, Šimon P, Dubaj T, Sládková A, Ház A, Jablonský M, Sekretár S, Schmidt Š, Kreps F, Burčová Z, Hodaifa G. Determination of the thermal oxidation stability and the kinetic parameters of commercial extra virgin olive oils from different varieties. J Chem. 2019 doi: 10.1155/2019/4567973. [DOI] [Google Scholar]
- Mostafa RA, Moharram YG, Attia RS, El-Sharnouby SA. Utilization of some vegetable oil blends rich in omega-3 fatty acids: Biological evaluation. Emir J Food Agric. 2013;2013:320–330. doi: 10.9755/ejfa.v25i5.12092. [DOI] [Google Scholar]
- Salehipour M, Javadi E, Reza JZ, Doosti M, Rezaei S, Paknejad M, Nejadi N, Heidari M. Polyunsaturated fatty acids and modulation of cholesterol homeostasis in THP-1 macrophage-derived foam cells. Int J Mol Sci. 2010;11:4660–4672. doi: 10.3390/ijms11114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semwal AD, Arya SS. Studies on the stability of some edible oils and their blends during storage. Int J Food Sci Technol. 2001;38:515–518. [Google Scholar]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhang LJ, Li H, Gu Y, Li FF, Jiang LN, Liu F, Ye J, Li Q. Polyunsaturated fatty acid relatively decreases cholesterol content in THP-1 macrophage-derived foam cell: partly correlates with expression profile of CIDE and PAT members. Lipid Health Dis. 2013;12:111. doi: 10.1186/1476-511X-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symoniuk E, Ratusz K, Krygier K. Comparison of the oxidative stability of linseed (Linumusitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J Food Sci Technol. 2016;53:3986–3995. doi: 10.1007/s13197-016-2398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmizi AH, Ahmad K. Feasibility of continuous frying system to improve the quality indices of palm olein for the production of extruded product. J Oleo Sci. 2015;64:1259–1266. doi: 10.5650/jos.ess15131. [DOI] [PubMed] [Google Scholar]
- Tarmizi AH, Ismail R. Comparison of the frying stability of standard palm olein and special quality palm olein. J Am Oil Chem Soc. 2008;85:245–251. doi: 10.1007/s11746-007-1184-8. [DOI] [Google Scholar]
- Wang S, Wu D, Lamon-Fava S, Matthan NR, Honda KL, Lichtenstein AH. In vitro fatty acid enrichment of macrophages alters inflammatory response and net cholesterol accumulation. Br J Nutr. 2009;102:497–501. doi: 10.1017/S0007114509231758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Noncommunicable diseases progress monitor 2017. Geneva: WHO; 2017. [Google Scholar]
- Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–391. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]
- Zhao G, Etherton TD, Martin KR, Heuvel JP, Gillies PJ, West SG, Kris-Etherton PM. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun. 2005;336:909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated in the present study has been included in the submitted manuscript.