Abstract
In the present study drying of orange pomace was carried out at 50, 60 and 70 °Cand drying kinetics was evaluated. The characterization of the orange pomace powder dried at the three different temperatures was carried out. Modified page model was found to best fit the data on drying, whereas effective moisture diffusivity ranged from 3.34 × 10–10 to 1.06 × 10–9 m2/s and the activation energy obtained was 53.07 kJ/mol. The results from powder characterization showed that the chemical composition, water holding capacity and oil holding capacity were not influenced by temperature. The emulsifying activity, swelling capacity and crystallinity were improved by increasing the temperature of drying. The antioxidant capacity and vitamin C content were observed to decrease with increase in drying temperature. There were no noticeable changes in the functional groups or structure due to temperature.
Keywords: Orange pomace, Drying kinetics, Powder characterization
Introduction
Orange is the most abundant citrus fruit among the citrus family and mainly consists of mandarin orange (Citrus reticulata) and sweet orange (Citrus sinensis), are the most consumed healthy foodstuff and is rich in vitamin C which acts as a powerful natural antioxidant and helps in building the body’s immune system. It also contains some important and biologically active phytochemicals which helps in prevention of various diseases (Etebu and Nwauzoma 2014). Although it was originally cultivated South East Asia, now it is cultivated throughout the world in the tropical and sub-tropical regions. India ranks third in global orange production after Brazil and China. The world production of oranges is estimated to be 78.70 metric tonnes and that of India is 95.09 metric tonnes (FAOSTAT 2021). Approximately 26% of world’s citrus production is used industrially for extraction of juice (M’hiri et al. 2015). Citrus juice production creates large amount of solid wastes which includes peels, pomace and seeds among others and constitute 50–60 percent of the whole fruit (Zaker et al. 2017). Various useful phytochemicals like dietary fibres, essential oils, pigments, flavonoids etc. can be obtained from these by-products. The protein fibre, fat and ash contents of citrus by-products, peels and pulp obtained from different industries are 12.5, 75.7, 0.5 and 8.1; 10.2, 57.0, 2.2 and 3.3; 8.6, 7.3, 4.9 and 6.5 g/100 g respectively (Mahato et al. 2019). In spite of having such vast possibilities the wastes from orange juice processing mostly remains unutilized. At the present, these by-products are used mainly as animal feeds. Orange pomace (OP) contains a significant amount of antioxidant and is an excellent source of dietary fibre (García‐Pérez et al. 2005). Orange pomace contains approximately 31.83 and 14.11% insoluble and soluble fibres respectively (Nagarajaiah and Prakash 2016). As orange pomace is rich in fibre and other biologically active compounds, therefore, it can be used in the development of fibre-enriched food products for human consumption.
Orange pomace contains high amount of moisture which makes them perishable. Due to the high moisture content it is susceptible to microbial spoilage and it is also difficult to transport the wet pomace due to its large volumes which makes it difficult to utilize this waste for other industries. Therefore, the fresh orange pomace with high moisture content may be dried so that it can be stored for longer duration, can be easily transported and utilized for extraction of valuable compounds or for development of food products rich in fibre.
Drying is a unit operation involving simultaneous transfer of heat and mass, and is an integral part of food processing. Food products are dried to increase the storability of food products, facilitate transport and packaging by reducing the volume or to preserve the nutrients. Knowledge of the drying behaviour is necessary for the proper designing of the drying operation so that the process becomes efficient and is energy saving. Models describing the drying process are important for achieving this purpose (Al-Muhtaseb et al. 2010).
Consumption of dietary fibre on regular basis has been associated with prevention of gastrointestinal and cardiovascular diseases and is recommended for control of diabetes and obesity (Botelho et al. 2002). Constipation can be effectively treated by increasing the intake of fibre. The diet of average human is highly deficit in fibre and consumers now as days are health conscious, look for foods which are complete in all aspects. Therefore, a steady increase in fibre rich healthy food is expected in the future (García‐Pérez et al. 2005). Various food products can be developed utilizing the orange pomace and pomace from other fruits like yoghurt (García‐Pérez et al. 2005), bakery products (Sudha et al. 2007; Tańska et al. 2016), etc.
Although there are reported literature for drying of kinnow and lime peels (Mahawar et al. 2017), orange peel (Garcia-Amezquita et al. 2018; Manjarres-Pinzon et al. 2013) etc., but literature related to orange pomace drying is rare. Convective drying was selected as it is economical and easier as compared to novel drying methods as the major component of the by-products is the dietary fibre. Therefore, the present investigation was undertaken to analyse the drying behaviour of orange pomace, characterize the physico-chemical and functional properties of orange pomace dried at different temperatures.
Materials and methods
Raw material
Orange (Citrus reticulata) pomaces were collected from the juice processing vendor present inside Tezpur University Campus, Assam on a single day to avoid variation in quality. Seeds were separated from the orange pomace and were subjected to drying experiments.
Drying experiments
The experiments on drying were carried out at three different temperatures viz. 50, 60 and 70 °C in a laboratory tray dryer (Labotech, BDI-51, B. D. Instrumentation, Ambala, India) equipped with automatic temperature control device. The three temperatures were selected so that there is minimum loss of heat sensitive nutrients with faster drying times. Samples (500 g) were placed in the drying chamber. The thicknesses of the layers were kept constant 0.5 cm and air velocity was 1.3 m/s for all the temperatures. The average temperature and humidity of the ambient air during the experiment were 24 ± 3.2 °C and 65 ± 4.6% respectively. Drying was performed till the final moisture content (~10% wet basis) was attained which is considered to be microbiologically safe (Tze et al. 2012). The weight loss from the sample was noted at regular intervals of 10 min during the drying process. The curves for drying kinetics curves were obtained as a function of dimensionless moisture ratio (MR) (Al-Muhtaseb et al. 2010).
| 1 |
where Xe is equilibrium moisture content, X0 is initial moisture content, X(t) is the moisture content at any given time (kg water/kg dry matter) and MR is the moisture ratio. Xe was considered negligible as the value of Xe is comparatively smaller than X0 or Xt. Five thin layer drying models as shown in Table 1 were used to fit the experimental drying data.
Table 1.
Thin layer drying models used for fitting the drying data
| Sl.No | Model | Equation | References |
|---|---|---|---|
| 1 | Newton | Lewis (1921) | |
| 2 | Henderson and pabis | Henderson and Pabis (1961) | |
| 3 | Page | Page (1949) | |
| 4 | Modified page | Overhults et al. (1973) | |
| 5 | Logarithmic | Yağcıoğlu et al. (1999) |
MR moisture ratio; a, c, n: drying coefficients; k: drying constant; t: drying time
Coefficient of determination (R2), sum of square of error (SSE) and root mean square error (RMSE) were determined for the fitted models. The best model was selected by comparing primarily the values of R2. Apart fromR2, RMSE values were compared to see the relative goodness of the fit. Curve fitting tool of MATLAB was used for determining the model parameters.
The effective moisture diffusivity (Deff) was calculated according to Fick’s law using the following equation and for long drying times:
| 2 |
where, Deff is the effective moisture diffusivity (m2/s), t is drying time (min) and L is the thickness of half the drying layer (m).
The activation energy (Ea) of the diffusion process was obtained using Arrhenius equation (Akdaş and Başlar 2015):
| 3 |
where, D0 is the pre-exponential factor of Arrhenius equation (m2/s), Ea is the activation energy (J/mol), R is the gas constant (8.3145 J/mol K) and T is the drying temperature (K).
The orange pomace after drying was ground using laboratory grinder and passed through 80 mesh sieve and stored at 7 °C till further analysis.
Chemical analysis of orange pomace powders
The moisture, ash, fat and crude fibre content of the orange pomace powders were measured using AOAC methods (AOAC 1990). Kjeldahl method (AACC 1990) was used to determine protein content (N × 6.25). The values for total carbohydrates were obtained by difference method.
Water holding capacity (WHC) and oil holding capacity (OHC) of pomace powders
Water holding capacity (WHC) and oil holding capacity (OHC) were measured according to the method described by Selani et al. (2014) with modifications. One gram of orange pomace powder was weighed into pre-weighed dry centrifuge and mixed with 10 mL of distilled water (for WHC) or soybean oil (Fortune, Adani Wilmar Limited, Ahmedabad, India) (for OHC), centrifuged at 3000 × g for 15 min. The supernatant was carefully decanted and the centrifuge tube with sediment was weighed. The WHC and OHC are calculated as follows:
| 4 |
| 5 |
Emulsifying activity (EA) of pomace powders
EA was determined following the method of Huber et al. (2016) with modifications. Orange pomace powder (0.5 g) was taken into a dry centrifuge tube and 25 mL of distilled water was added. To it 25 mL of sunflower oil was added and homogenized for 10 min. The dispersion was centrifuged at 1200 rpm for 5 min. EA was calculated as % v/v basis. EA was calculated as follows,
| 6 |
Swelling capacity of pomace powders
The swelling capacity of the orange pomace powders were determined as per the method described by Robertson et al. (2000). Orange pomace powder (0.2 mg) was accurately weighed and transferred into a calibrated cylinder. After that 10 mL of distilled water was then added to the powder. The contents of the cylinder were properly mixed and incubated at room temperature for 18 h. After incubation the packed volume was measured and swelling capacity was reported as millilitres per gram of OP powder.
Ascorbic acid of pomace powders
Determination of Ascorbic acid was done by 2,6-Dichlorophenol-indophenol dye method (AOAC 2005). A working standard solution was prepared by mixing ascorbic acid with 4% oxalic acid solution (100 μg/mL). 5 mL of working standard was mixed with 10 mL of 4% oxalic acid solution which was titrated against standardized 2,6-dichlorophenolindophenol sodium dye solution until persistent pink colour occurred. Similarly, a sample of 5 g was added in 10 mL of oxalic acid (4%) and titration was carried out with the dye solution. Ascorbic was content was calculated as follows,
| 7 |
where, V1 = Titrated value of working standard solution and V2 = Titrated value of sample solution.
Antioxidant activity of pomace powders
It was determined by scavenging activity against 1,1-Diphenyl-2-picrylhydrazyl (DPPH) as described by Sancho et al. (2015). 1 g of sample was added to 20 mL acetone (20% aqueous). The diluted sample extract (0.3 mL) was mixed with 1.5 mL of methanolic solution containing DPPH radicals (4.73 mg/mL of methanol). The DPPH radical activity was measured by monitoring continuously the decrease of absorption at 515 nm. The scavenging percentage of DPPH radical was calculated according to the following formula:
| 8 |
where, ADPPH = Absorbance of the DPPH solution and.As = Absorbance of the mixture of methanolic extract and DPPH.
Color analysis of pomace powders
Colorimetric CIE (L*, a* and b*) measurements of pomace powders were conducted using a Hunterlab colorimeter (UltraScan VIS, Hunter Associates Laboratory Inc., Reston, VA, USA) as with that for pomace powders. L* values shows the lightness/darkness. a* values denotes redness (+ a) to greenness (− a), while b* values denotes yellowness (+ b) to blueness (− b).
Fourier Transform Infrared (FTIR) spectroscopy of pomace powders
FT-IR instrument (Spectrum 100, Perkin Elmer, SA) was used to obtain the FT-IR spectra of the orange pomace powders. The spectra were obtained in absorbance mode from 4000 to 400 cm−1 the resolution was 0.44 cm−1. Before analysis the powder samples were mixed with dry and pure KBr (1:100, w/w), were thoroughly ground and pellets were formed by compression.
X-ray diffraction (XRD) analysis of pomace powders
XRD analysis of the OP powder were done in X-ray diffractometer (Miniflex, Japan)by exposing them to X-ray beam at 15 mA and 30 kV and were scanned from 5 ° to 70 ° (2θ) at a scan rate of 2 ° min−1. Calculation of crystallinity index (CrI) was done from the heights of the peak at 200 (I002, 2θ = 22.6 °) and the minimum of the intensity between the peaks at 200 and 110 (Iam, 2θ = 18 °) using the Segal method (Neto et al. 2013). I002 represents crystalline nature, while Iam represents the amorphous nature.
| 9 |
Powder morphology
The morphology of the pomace powders were examined under Scanning Electron Microscope (SEM) (Jeol, JSM 6390 LV, Singapore). SEM was done by taking specified amount of the powder randomly from the three samples. The powder particles were coated with thin platinum layer after mounting them on circular aluminium stubs using double sided adhesive tape and were evaluated by the microscope at an accelerating voltage of 15 kV and the magnification was 2000X.
Thermal analysis
Thermogravimetry (TG) of the OP powder was recorded by a TGA instrument. The temperature was increased from 25 to 800 °C with a linear heating rate 10 °C/min and the weight of sample was approximately 5 mg.
Statistical analysis
All experiments were carried out in three replications. Single factor analysis of variance (ANOVA) was carried out for the experimental data. Significant differences between the data were obtained by Duncan’s multiple range tests using SPSS 20 (IBM Analytics, USA).
Results and discussion
Modelling of drying kinetics of orange pomace and estimation of effective moisture diffusivity and activation energy
The initial moisture content of the fresh pomace was found to be 91.23 ± 0.24% (wet basis). The pomace samples were dried in tray dryer until the moisture contents were reduced to approximately 10% (wet basis). The drying kinetics was significantly affected by the drying temperature. The time required for drying at 50, 60 and 70 °C were 316, 288 and 119 min respectively. It can be seen that the time required for drying at 70 °C was much lower as compared to time required at 50 or 60 °C. The experimental data for moisture contents were converted to moisture ratios (MR) using Eq. 1 and plotted against time (Fig. 1). The dimensionless MR data were fitted to five different thin-layer drying models. The model coefficients along with root mean square error (RMSE), Sum of square of errors (SSE) and coefficient of determination (R2) values for the different models are given in Table 2. The model giving the highest R2 and the lowest RMSE and SSE values can be described as the best thin layer drying model for the experimental data. Accordingly, the Modified Page model can be called the best model to describe the drying of orange pomace with R2, SSE and RMSE values of 0.999, 0.005, 0.002 respectively. The drying constant (k) describes the drying behaviour of orange pomace and an increase in the value of k with temperature indicates an increase in drying potential i.e. faster drying at elevated temperatures (Jha and Sit 2020; Barbosa et al. 2020). Mathematical models are important in designing new systems or for improving the existing ones (Babalis and Belessiotis 2004).
Fig. 1.
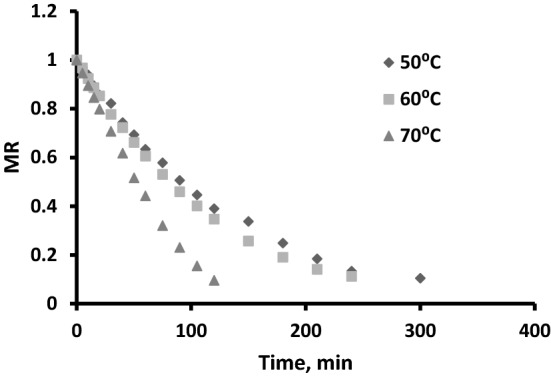
Drying curve (MR vs Time) for drying of orange pomace at different temperatures
Table 2.
Estimated model and statistical parameters obtained for the various models by fitting the drying data for orange pomace
| T (°C) | Model and statistical parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Newton | Henderson-Pabis | Page | ||||||||||||
| k | R2 | SSE | RMSE | k | a | R2 | SSE | RMSE | k | n | R2 | SSE | RMSE | |
| 50 | 0.009 | 0.985 | 0.031 | 0.042 | 0.010 | 1.043 | 0.992 | 0.025 | 0.035 | 0.003 | 1.261 | 0.997 | 0.005 | 0.016 |
| 60 | 0.010 | 0.993 | 0.016 | 0.030 | 0.011 | 1.032 | 0.995 | 0.026 | 0.028 | 0.005 | 1.184 | 0.998 | 0.015 | 0.014 |
| 70 | 0.013 | 0.984 | 0.030 | 0.184 | 0.014 | 1.048 | 0.983 | 0.033 | 0.038 | 0.005 | 1.345 | 0.998 | 0.019 | 0.019 |
| T (°C) | Model and statistical parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modified-Page | Logarithmic | ||||||||||
| k | n | R2 | SSE | RMSE | k | a | c | R2 | SSE | RMSE | |
| 50 | 0.009 | 1.161 | 0.997 | 0.004 | 0.016 | 0.010 | 1.032 | 2.515 × 10-13 | 0.985 | 0.025 | 0.031 |
| 60 | 0.010 | 1.179 | 0.998 | 0.004 | 0.015 | 0.102 | 1.068 | 2.144 × 10-9 | 0.994 | 0.013 | 0.022 |
| 70 | 0.016 | 1.385 | 0.999 | 0.005 | 0.020 | 0.128 | 1.175 | -0.151 | 0.995 | 0.014 | 0.033 |
Knowledge of effective moisture diffusivity (Deff) is required to model the mass transfer process during drying operations. The Deff during drying of orange pomace at 50, 60 and 70 °C are 3.34 × 10–10, 3.93 × 1–10 and 1.06 × 10–9 m2/s respectively (Table 3). The Deff increased with increase in temperature shows the increase in vapour pressure inside the pomace samples. The values of Deff obtained in the present investigation lies within the range of 10–12–10–8 m2/s reported for food materials during drying (Abano et al. 2011). The amount of Energy required to activate moisture diffusion in a drying process is known as the activation energy (Ea) and determination of Ea is important in drying applications. The Ea for moisture diffusion obtained in the present investigation for orange pomace drying is 53.07 kJ/mol. The activation energy obtained in the present study was comparable to that obtained by Al-Muhtaseb et al. (2010) for tomato pomace drying (52.1 kJ/mol).
Table 3.
Effective moisture diffusivities and activation energy of drying
| Temperature ( °C) | Deff (m2/s) | Ea (kJ/mol) |
|---|---|---|
| 50 | 3.34 × 10–10 | 53.07 |
| 60 | 3.93 × 1–10 | |
| 70 | 1.06 × 10–9 |
Chemical composition of orange pomace powder dried at different temperatures
The composition of the orange pomace powders dried at different temperatures are presented in Table 4. As the drying was carried out at all temperatures till the moisture contents reached approximately 10%, therefore the moisture contents of the pomace powders were found to vary from 9.42 ± 0.10 to 9.74 ± 0.22%. The differences in the moistures contents were not statistically significant. Similarly, significant differences were not observed in the other parameters as well, like ash content, fat content, protein content, total carbohydrate and crude fibre content for the pomaces dried at different temperatures. As was expected drying at temperatures from 50 to 70 °C does not affect the composition, since drying only removes water from the wet samples and does not induce any chemical changes. The ash, fat, protein, total carbohydrate and crude fibre contents of the pomaces dried at different temperatures ranged from 4.67 ± 0.28 to 5.52 ± 0.32%, 3.12 ± 0.20 to 3.58 ± 0.17%, 4.39 ± 0.55 to 5.33 ± 0.16%, 86.23 ± 0.36 to 87.05 ± 0.43% and 58.71 ± 0.19 to 59.07 ± 0.11% respectively. The ash, fat and protein contents observed in the present study were found to be close to the values reported by de Moura et al. (2017) for orange pomace (ash-3.26%, fat-4.29%, protein-4.84%), but were different from the values reported by Nagarajaiah and Prakash (2016) for orange pomace (ash-2.65%,protein-8.45%). The crude fibre content found in the present study were higher than those reported by both de Moura et al. (2017) and Nagarajaiah and Prakash (2016). Differences in composition may be attributed to variety or cultivar of the fruit and may also be influenced by growing conditions and type of soil in which the plant grows (Figuerola et al. 2005; Selani et al. 2014). Thus from the composition of the orange pomace in the present study it can be inferred that orange pomace can be utilized as a promising ingredient for development of fibre rich products.
Table 4.
Proximate composition, ascorbic acid content, antioxidant activity, water holding capacity, oil holding capacity, swelling capacity, emulsifying activity, colour and crystallinity of orange pomace powders
| Sample | Moisture (%wb) | Ash (% db) | Fat (% db) | Protein (% db) | Total carbohydrates (% db) | Crude fibre (% db) | Ascorbic acid (mg/100 g | Antioxidant activity (% DPPH radical scavenging activity) |
|---|---|---|---|---|---|---|---|---|
| OP dried at 50 °C | 9.94 ± 0.22a | 4.98 ± 0.59a | 3.12 ± 0.31a | 4.85 ± 0.31a | 87.05 ± 0.43a | 59.06 ± 0.11a | 119.36 ± 0.91a | 64.22 ± 0.81a |
| OP dried at 60 °C | 9.82 ± 0.41a | 5.54 ± 0.32a | 3.28 ± 0.44a | 4.95 ± 0.25a | 86.23 ± 0.36a | 58.92 ± 0.87a | 114.42 ± 1.07b | 60.97 ± 0.29b |
| OP dried at 70 °C | 9.88 ± 0.26a | 4.68 ± 0.28a | 3.58 ± 0.37a | 4.69 ± 0.64a | 87.05 ± 0.40a | 58.90 ± 0.49a | 110.23 ± 1.09c | 59.95 ± 0.35b |
| WHC (g/g dry sample) | OHC (g/g dry sample) | Swelling capacity (mL/ g dry sample) | Emulsifying activity (%) | L* | a* | b* | Crystallinity (%) |
|---|---|---|---|---|---|---|---|
| 3.03 ± 0.11a | 1.02 ± 0.09a | 13.37 ± 0.41b | 49.22 ± 0.36b | 77.12 ± 0.61b | 7.69 ± 0.11a | 36.51 ± 0.22a | 15.99 ± 0.21b |
| 3.18 ± 0.23a | 1.18 ± 0.06a | 16.46 ± 0.47a | 53.84 ± 0.71a | 81.16 ± 0.98a | 3.78 ± 0.40b | 25.06 ± 0.43c | 14.99 ± 0.14b |
| 3.46 ± 0.41a | 1.25 ± 0.08a | 17.30 ± 52a | 55.25 ± 0.44a | 77.34 ± 0.58b | 5.29 ± 1.29b | 29.86 ± 0.34b | 19.23 ± 0.51a |
OP orange pomace
Values reported as Mean ± Std. Dev. of three replications
Means followed by same small letter superscripts within a column are not significantly different (p > 0.05)
Ascorbic acid content and antioxidant activity of orange pomace dried at different temperatures
There are many fruits having good amount of vitamin C, but citrus fruits have higher vitamin C content compared to other fruits. The vitamin C content of the dried pomace powders varied from 110.23 to 119.36 mg/100 g dry matter as compared to that of fresh pomace which contained much higher amount of vitamin C (132.56 ± 1.63 mg/100 g dry matter). It can be seen from Table 4 that the loss of ascorbic acid increased significantly with temperature of drying. Degradation of vitamin C might be attributed to thermal destruction and oxidation during drying, as vitamin C is susceptible to both heat and oxidation (Akdaş and Başlar 2015). Ong and Law (2011) also observed drastic reduction in vitamin C content of salak fruit at higher drying temperatures. Similarly, Stéger-Máté et al. (2011) reported highest vitamin C content in black currant dried at lower temperature. The reduction in vitamin C content was more when the temperature was increased from 50 to 60 °C, as compared to when the temperature was increased from 60 to 70 °C inferring that above 60 °C the effect of temperature becomes lesser. Although Nagarajaiah and Prakash (2016) higher vitamin C content in orange pomace compared to pomaces from other fruits, but the vitamin C contents observed in the present study were found to be much higher. The higher vitamin C contents in the present study might be the result of milder drying conditions as well as difference in cultivar used in the study. The higher vitamin C content of the dried orange pomaces will make it useful for enrichment of various food products and will also enhance the bioavailability of iron present in the food (Tańska et al. 2016; Lyu et al. 2020).
Dietary fibres rich in antioxidants may help to combat various disorders. Hence, it becomes necessary to have knowledge of the antioxidant activity of citrus fruit pomace powder. The antioxidant activities of the dried orange pomace powders are presented in Table 4 and are much lower compared to that of fresh pomace (82.31 ± 1.47% DPPH radical scavenging activity). Temperature of drying had a profound effect on the antioxidant activities dried pomaces. The antioxidant activities were found to decrease as drying temperature increased which might be due to degradation of heat sensitive bioactive compounds including vitamin C. The highest antioxidant activity was observed for the samples dried at 50 °C. Similar trend was observed by Vardin and Yilmaz (2018) for dried pomegranate arils. As with vitamin C, decrease in antioxidant activity was mainly observed when the temperature was increased from 50 to 60 °C and when the temperature was further increased to 70 °C, significant differences were not observed.
Water holding capacity and oil holding capacity of orange pomace dried at different temperatures
The WHC and OHC of the orange pomaces dried at different temperatures are shown in Table 4. The WHC increased from 3.03 ± 0.11 to 3.46 ± 0.08 g/g where as OHC increased from 1.01 ± 0.05 to 1.24 ± 0.04 g/g dried pomace when the temperature was increased from 50 to 70 °C. Although, both WHC and OHC increased with temperature, but significant differences could not be observed, indicating that temperature of drying has little effect on these properties. Water holding capacity of an food ingredient is one of the important functional properties, as it able to increase the volume and alter the texture and viscosity of food products and also helps in reducing calories by reducing uptake of fat and changing fat digestion (Gunness and Gidley 2010; Rubio-Senent et al. 2015). Oil absorption capacity on the other hand is related to the degree of acetylation and esterification of the molecules, due to the increase in their hydrophobicity (Rubio-Senent et al. 2015; de Moura et al. 2017). The WHC observed in the present study were found to be higher than that of blue grapes pomace (2.95 g/g) reported by Nagarajaiah and Prakash (2016) and lemon peel (1.74–1.85 g water/g sample), orange peel (1.65 g water/g sample) and apple pomace (1.62–1.87 g water/g sample) reported by Figuerola et al. (2005), but lower than pineapple pomace (4.83 g/g) and orange pomace (5.18 g/g) reported by Nagarajaiah and Prakash (2016) and pineapple pomace (5.32 g/g) reported by Selani et al. (2014). The OHC observed in this study was found to be close to peach pomace (1.02–1.11 g oil/g sample) as reported by Grigelmo-Miguel et al. (1999), but was lower when compared to pineapple pomace (2.01 g/g) reported by Selani et al. (2014). The difference in the values reported might be because these properties depend on various factors such as the difference in the structure of the polysaccharide present in the sample, porosity, particle size etc. (Elleuch et al. 2011) and also on the method of analysis or preparation of the samples. As ingredients with high WHC can be used as thickening agent or for reducing syneresis in food products contain high amount of water and ingredients with a high OHC can be used as emulsifying agent, therefore the results of the present study suggests that orange pomace powder can find application in products in which syneresis need to be reduced or improvement of texture but cannot be used where stabilization of emulsion is required.
Emulsifying activity and swelling capacity of orange pomace dried at different temperatures
The swelling capacities of the orange pomace powders dried at different temperatures are shown in Table 4. The swelling capacity was lowest for the orange pomace dried at 50 °C and increased significantly for the pomace dried at 60 °C. Significant differences were not observed for pomace dried at 60 and 70 °C. This might be due to the fact that increasing the drying temperature from 50 to 60 °C might affect the porosity and particle size of the final powder formed, which is an important parameter affecting the hydration properties of the pomace (Carvalho et al. 2009; Elleuch et al. 2011). The results of emulsifying activity of the orange pomace powders are shown in Table 4. As with the swelling capacity it can be observed that emulsifying activity also increased significantly when drying temperature was increased from 50 to 60 °C and significant difference was not observed when the temperature was further increased. Shape of the particles is an important parameter affecting the emulsifying property of fibres (Huber et al. 2016) and in the present study increasing the drying temperature from 50 to 60 °C might affect the shape of the particles, thereby affecting the emulsifying activity. As per Wong and Cheung (2005) fibres with emulsifying activity above 50%, may be considered as good emulsifying agents, therefore the results of the present study indicates that the orange pomace dried at 60 and 70 °C can serve as emulsifying agents.
Colour of orange pomace dried at different temperatures
The colour parameters L*, a* and b* for the orange pomaces dried at different temperatures are shown in Table 4. It can be seen that the L* value increased when the temperature of drying was increased from 50 to 60 °C and then decreased when the temperature was increased from 60 to70 °C, while a* and b* values initially decreased with drying temperature and then again increased with further increase in temperature. Highest L* value was observed for pomace dried at 60 °C, while highest a* and b* values were observed for pomace dried at 50 °C. The higher b* values of all the samples shows that samples were more yellow in colour. The initial increase in lightness (L*) when temperature was increased from 50 to 60 °C might be attributed to reduction in enzymatic browning caused by polyphenol oxidase at higher temperatures, and then decrease in L* value with further increase in temperature might be due to non-enzymatic browning (Maillard reaction) occurring at higher temperatures (Abano et al. 2011). The lower values of a* and b* for the pomaces dried at 60 and 70 °C compared to pomace dried at 50 °C indicates that enzymatic browning is more responsible for development of redness and yellowness in the samples than non-enzymatic browning. The results of the present study are in agreement with the results obtained by Al-Muhtaseb et al. (2010) where they observed an increase in L* value of tomato pomace when drying temperature was increased from 40 to 70 °C and then decreased when the temperature was increased to 80 °C. Similar trend was also observed for L*, a* and b* values during drying of tomato slices by Abano et al. (2011).
FT-IR analysis of orange pomace dried at different temperatures
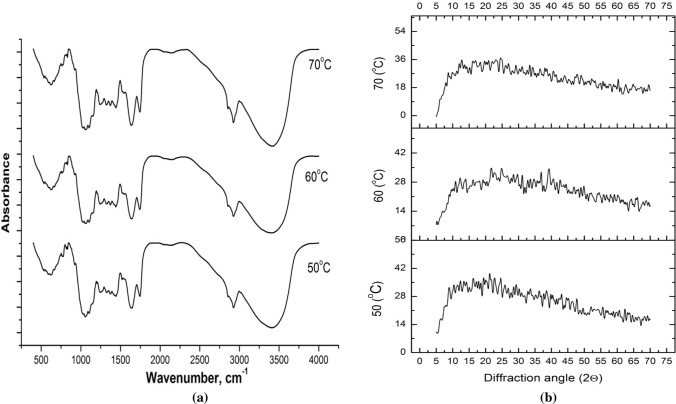
The FT-IR spectra helps in identifying the functional groups present in a sample and to determine the whether modification has taken place due to processing. The FT-IR spectra for the orange pomace powders were obtained to recognise the functional groups present in pomace powder and whether temperature of drying affects the functional group or not (Fig. 2a). It has been observed that there is no major deviation in the position as well as intensity of the peaks in the FT-IR spectra of the pomace powders dried at 50, 60 and 70 °C. For the orange pomace powders intense peaks can be seen near 3300, 3000, 2900, 1740, 1690, 1600, 1530, 1400, 1230, 1050 and 636 cm−1. The absorption band near 3300 cm–1 characterises the OH or NH group in pomace. The band detected near 3000 and 2900 cm–1 are due to symmetrical and asymmetrical stretching vibration of C–H bond and are assigned to –CH3 or –CH2 groups in carboxylic acid. At around1400 cm–1, their bending vibration can be observed. The peak at 1530 cm−1 represents secondary amine group. The C=C stretch of alkene, aromatic or amino acids are represented by the peak at 1600 cm–1. The C=O stretching band of aldehyde is observed near 1740 cm–1 and C–O stretch of alcohols, ethers and esters can be observed near the peak of 1400 cm−1. The presence of phenol or tertiary alcohol, C–O stretch, and primary amine and CN stretch can be seen near 1230 cm–1, 1050 cm–1 and 636 cm–1 respectively (Pathak et al. 2017; Mohapatra et al. 2017). The carboxylic acid helps in curing of various diseases such as ulcers, headache, wound etc. and is mainly contributed by pectin, cellulose or lignin present in the pomace. Similarly, hydroxyl group in pomace is responsible for adsorption of anionic impurities (Pathak et al. 2017).
Fig. 2.
FT-IR spectra a and XRD pattern b of the orange pomace powders dried at different temperatures
XRD analysis of orange pomace dried at different temperatures
The XRD pattern of the orange pomace dried at different temperatures is presented in Fig. 2b. XRD analysis was carried out to identify changes in crystallinity due to drying temperature. From the XRD patterns it can be seen that there are no significant crystalline peaks present for all the samples. According to Roncero et al. (2005), for the crystalline materials the intensity peak is maximum at 2θ angle between 22° and 23°, while for amorphous materials is between 18o and 19°. Therefore, the crystallinity index was obtained using the Segal’s empirical equation and is presented in Table 4. The crystallinity of the dried pomace powders were in the range of 14.99 ± 0.23–19.23 ± 0.14%. Zain et al. (2014) reported that the crystallinity of untreated pomelo albedo was 25.1% due to presence of high amount of lignin and hemicelluloses which increased to 57.1% on removal of these components. Therefore, it can be inferred that the low crystallinity of orange pomace powders might be due to higher content of hemicellulose and lignin in them. Significant differences in crystallinity were not observed for the pomaces dried at 50 and 60 °C, but increased for the pomace dried at 70 °C. This might be attributed to the faster removal of water from the pomace at higher temperature which preserves the crystalline structure resulting in higher crystallinity.
Thermo gravimetric analysis of orange pomace dried at different temperatures
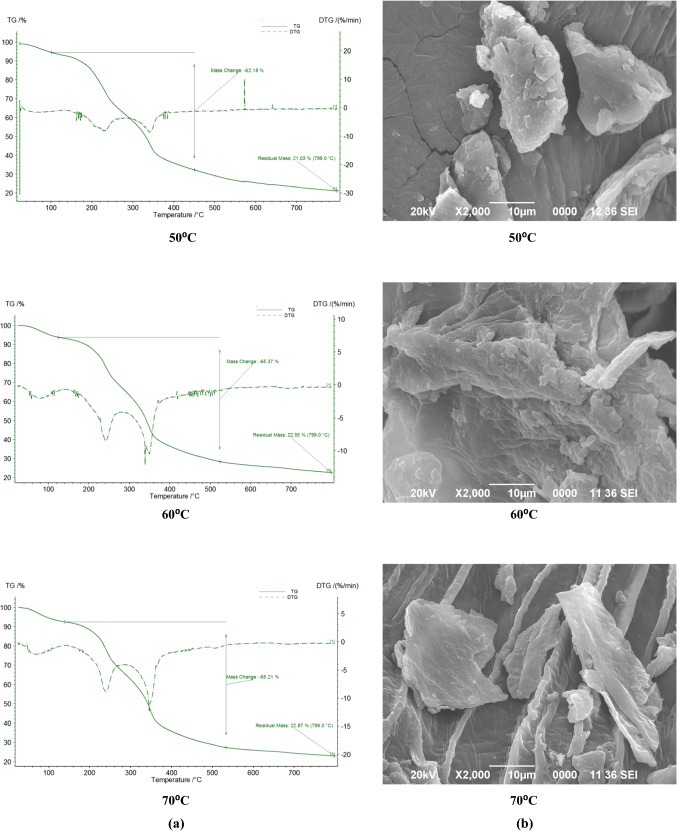
The thermal stability of the pomaces dried at different temperatures were determined using thermo gravimetric analysis. The curves for thermo gravimetric (TG) and derivative of thermogravimetric (DTG) are shown in Fig. 3a. Weight loss was not significant below 150 °C and first weight loss was seen between 150 and 200 °C. This change in weight was due to loss of moisture and other volatile components such as oils, pigments etc. The second weight loss can be seen between 250 and 400 °C and corresponds to degradation of cellulose and hemicelluloses. Weight loss was not significant after 700 °C as heat stable fixed carbons might be present. From the DTG curves it can be seen that there are two decomposition peaks, one between 200 and 250 °C and the other between 300 and 400 °C. Pomace dried at 50 °C showed weak peaks, whereas sharp peaks were observed for the pomaces dried at 60 and 70 °C. This difference might be attributed to the structural difference in the particles due to the different drying temperatures. The peak between 200 and 250 °C represents the decomposition of pectin and hemicelluloses, and the peak between 300 and 400 °C matches to the decomposition of cellulose. A flat tailing section was observed for all the DTG curves which shows the presence of lignin and is known to decompose steadily over a range of temperature. As the pomace powders are stable till 150 °C, these powders can be used for fibre enrichment of various food products including those requiring high processing temperatures such as bakery products.
Fig. 3.
Thermogravimetric curves a and b SEM images of orange pomace powders dried at different temperatures
SEM analysis of orange pomace dried at different temperatures
The structural variation of the particles in the orange pomace powders dried at different temperatures were observed using SEM images. The SEM images for the orange pomace powders are shown in Fig. 3b. It can be observed that the particles are irregular in shape with rough surfaces. The size and shape of the particles varied within a sample and among the different samples. The size of the particles in pomace dried at 60 °C was found to be larger than the particle of the pomaces dried at 50 and 70 °C. Apart from being larger in size the shape of the particles of the pomace powder dried at 60 °C was also found to be more irregular and rough with number of pores. From the SEM image of the pomace dried at 70 °C it can be observed that the particles have certain crystal and flaky structure and fibre strands are also visible. It is clear from the SEM images that drying temperature has definite effect on the structure of the particles. The differences in the size and shape of the particles might be attributed to the rate of drying as well as rate of various other reactions which are influenced by the amount of water and temperature of drying. Similar observations were noted by Hernández-Ortega et al. (2013) for microwave and conventional dried carrot pomace.
Conclusion
The present study investigated the drying behavior of orange pomace which a waste generated from the orange juice processing industry and to characterize the dried product with an aim to utilize it for enrichment of food products which generally lacks fibres like yoghurt. Increasing the drying temperature is necessary for any industrial process as it improves the mass transfer rate thereby reducing the drying time which is very important for any industrial process. From the present investigation it can be noticed that drying temperature has both positive and negative effects on the properties of the dried pomace. On one hand it improves the functional properties like emulsifying activity and swelling capacity, but on the other hand it decreases the antioxidant capacity and vitamin C content, which are important nutrients. From the present study 60 °C temperature is recommended for drying as this gives better functional properties of the pomace powders like swelling, emulsifying activity, colour etc., although there is some loss of nutrients like vitamin C and antioxidant activity. As pomace is rich in fibre, it can be utilized for development of fibre enriched food products.
Acknowledgements
The authors acknowledge the financial help received from UGC-SAP (DRS-I) and DST-FIST for carrying out the work.
Abbreviations
- ANOVA
Analysis of variance
- CrI
Crystallinity index
- D0
Pre-exponential factor of Arrhenius equation (m2/s)
- Deff
Effective moisture diffusivity (m2/s)
- DPPH
1,1-Diphenyl-2-picrylhydrazyl
- DTG
Derivative of thermogravimetric
- Ea
activation energy (J/mol)
- EA
Emulsifying ability
- FTIR
Fourier transform infra-red
- MR
Moisture ratio
- OHC
Oil holding capasity
- OP
Orange pomace
- R2
Coefficient of determination
- RMSE
Root mean square error
- SEM
Scanning electron microscopy
- SSE
Sum of square of error
- TG
Thermogravimetric
- WHC
Water holding capacity
- X(t)
Moisture content at any given time
- X0
Initial moisture content
- Xe
Equilibrium moisture content
- XRD
X-ray diffraction
Authors' contribution
SMA: Formal analysis, investigation, methodology, writing- original draft, validation; AA: Formal analysis, investigation, methodology, writing- original draft, validation; NS: Conceptualization, Data curation, project administration, resopurces, supervision, visualization, writing- review & editing.
Funding
The authors acknowledge the financial help received from UGC-SAP (DRS-I) and DST-FIST for carrying out the work.
Data availability
Data will be available on request.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Syeda Muntazima Afrin and Arijit Acharjee have contributed equally towards the manuscript.
References
- AACC American Association of Cereal Chemists (1990) Approved methods of the AACC (7th ed.). St. Paul, MN. Method 46–12 (Protein)
- Abano EE, Ma H, Qu W. Influence of air temperature on the drying kinetics and quality of tomato slices. J Food Process Technol. 2011;2(5):2–9. [Google Scholar]
- Akdaş S, Başlar M. Dehydration and degradation kinetics of bioactive compounds for mandarin slices under vacuum and oven drying conditions. J Food Process Preserv. 2015;39(6):1098–1107. [Google Scholar]
- Al-Muhtaseb AAH, Al-Harahsheh M, Hararah M, Magee TRA. Drying characteristics and quality change of unutilized-protein rich-tomato pomace with and without osmotic pre-treatment. Ind Crops Prod. 2010;31(1):171–177. [Google Scholar]
- Association of Official Analytical Chemists (2005) Vitamin C in foods. Official Method 967.26. In: AOAC official methods of analysis, 18th ed. Association of Official Analytical Chemists, Gaithersburg, MD, USA, p. 45.1.16
- AOAC Official methods of analysis (15th ed.). Washington, DC: Association of Official Analytical Chemists. (1990). Method 935.29 (Moisture), Method 963.15 (fat), Method 923.03 (Ash), Method 962.09 (Crude fibre)
- Babalis SJ, Belessiotis VG. Influence of the drying conditions on the drying constants and moisture diffusivity during the thin-layer drying of figs. J Food Eng. 2004;65(3):449–458. [Google Scholar]
- Barbosa AM, Rocha TA, Saldarriaga JF, Estiati I, Freire FB, Freire JT. Alternative drying of orange bagasse in vibrofluidized bed for use in combustion. Chem Eng Process-Process Intensif. 2020;152:107941. [Google Scholar]
- Botelho L, Conceição A, Carvalho VD, Botelho L, Carvalho VD. Caracterização de fibras alimentares da casca e cilindro central do abacaxi" smooth cayenne". Ciênc Agrotec. 2002;26:362–367. [Google Scholar]
- Carvalho AFU, Portela MCC, Sousa MB, Martins FS, Rocha FC, Farias DF, Feitosa JPA. Physiological and physico-chemical characterization of dietary fibre from the green seaweed Ulva fasciata Delile. Braz J Biol. 2009;69(3):969–977. doi: 10.1590/s1519-69842009000400028. [DOI] [PubMed] [Google Scholar]
- de Moura FA, Macagnan FT, Dos Santos LR, Bizzani M, de Oliveira Petkowicz CL, Da Silva LP. Characterization and physicochemical properties of pectins extracted from agroindustrial by-products. J Food Sci Technol. 2017;54(10):3111–3117. doi: 10.1007/s13197-017-2747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;124(2):411–421. [Google Scholar]
- Etebu E, Nwauzoma AB. A review on sweet orange (Citrus sinensis L Osbeck): health, diseases and management. Am J Res Com. 2014;2(2):33–70. [Google Scholar]
- FAOSTAT (2021) The Food and agriculture organization corporate statistical database, Food and Agriculture Organization of the United Nations
- Figuerola F, Hurtado ML, Estévez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91(3):395–401. [Google Scholar]
- Garcia-Amezquita LE, Tejada-Ortigoza V, Campanella OH, Welti-Chanes J. Influence of drying method on the composition, physicochemical properties, and prebiotic potential of dietary fibre concentrates from fruit peels. J Food Qual. 2018;2018:1–11. [Google Scholar]
- García‐Pérez FJ, Lario Y, Fernández‐López J, Sayas E, Pérez‐Alvarez JA, Sendra E. (2005). Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Research & Application: Endorsed by Inter‐Society Color Council, The Colour Group (Great Britain), Canadian Society for Color, Color Science Association of Japan, Dutch Society for the Study of Color, The Swedish Colour Centre Foundation, Colour Society of Australia, Centre Français de la Couleur, 30(6), 457–463
- Grigelmo-Miguel N, Gorinstein S, Martin-Belloso, O. Characterisation of peach dietary fibre concentrate as a food ingredient. Food Chem. 1999;65(2):175–181. [Google Scholar]
- Gunness P, Gidley MJ. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010;1(2):149–155. doi: 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- Hendorson SM. Grain drying theory (I) temperature effect on drying coefficient. J Agric Eng Res. 1961;6(3):169–174. [Google Scholar]
- Hernández-Ortega M, Kissangou G, Necoechea-Mondragón H, Sánchez-Pardo ME, Ortiz-Moreno A. Microwave dried carrot pomace as a source of fiber and carotenoids. Food Nutr Sci. 2013;4(10):1037–1046. [Google Scholar]
- Huber E, Francio DL, Biasi V, Mezzomo N, Ferreira SRS. Characterization of vegetable fiber and its use in chicken burger formulation. J Food Sci Technol. 2016;53(7):3043–3052. doi: 10.1007/s13197-016-2276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Sit N. Drying characteristics and kinetics of colour change and degradation of phytocomponents and antioxidant activity during convective drying of deseeded Terminalia chebula fruit. J Food Meas Charact. 2020;14(4):2067–2077. [Google Scholar]
- Lewis WK. The rate of drying of solid materials. Ind Eng Chem. 1921;13(5):427–432. [Google Scholar]
- Lyu F, Luiz SF, Azeredo DRP, Cruz AG, Ajlouni S, Ranadheera CS. Apple pomace as a functional and healthy ingredient in food products: a review. Processes. 2020;8(3):319. [Google Scholar]
- M’hiri N, Ioannou I, Ghoul M, Boudhrioua NM. Proximate chemical composition of orange peel and variation of phenols and antioxidant activity during convective air drying. J New Sci. 2015;9:881–890. [Google Scholar]
- Mahato N, Sinha M, Sharma K, Koteswararao R, Cho MH. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods. 2019;8(11):523. doi: 10.3390/foods8110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawar MK, Jalgaonkar K, Bibwe B, Ghodki B, Bhushan B. Mathematical modelling and drying kinetics of kinnow and sweet lime peels. Int J Chem Stud. 2017;5(6):885–888. [Google Scholar]
- Manjarres-Pinzon K, Cortes-Rodriguez M, Rodríguez-Sandoval E. Effect of drying conditions on the physical properties of impregnated orange peel. Braz J Chem Eng. 2013;30(3):667–676. [Google Scholar]
- Mohapatra HS, Dubey P, Chatterjee A, Kumar P, Ghosh S. Development of a fibrous assembly from orange peel extract: characterization and antibacterial activity. Cellul Chem Technol. 2017;51(7–8):601–608. [Google Scholar]
- Nagarajaiah SB, Prakash J. Chemical composition and bioactivity of pomace from selected fruits. Int J Fruit Sci. 2016;16(4):423–443. [Google Scholar]
- Neto WPF, Silvério HA, Dantas NO, Pasquini D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–soy hulls. Ind Crops Prod. 2013;42:480–488. [Google Scholar]
- Ong SP, Law CL. Drying kinetics and antioxidant phytochemicals retention of salak fruit under different drying and pretreatment conditions. Dry Technol. 2011;29(4):429–441. [Google Scholar]
- Overhults DG, White GM, Hamilton HE, Ross IJ. Drying soybeans with heated air. Trans ASAE. 1973;16(1):112. [Google Scholar]
- Page GE (1949) MSc. Thesis, Factors influencing the maximum rates of air drying shelled corn in thin layers, Purdue University
- Pathak PD, Mandavgane SA, Kulkarni BD. Fruit peel waste: characterization and its potential uses. Curr Sci. 2017;113(3):444–454. [Google Scholar]
- Robertson JA, de Monredon FD, Dysseler P, Guillon F, Amado R, Thibault JF. Hydration properties of dietary fibre and resistant starch: a European collaborative study. LWT-Food Sci Technol. 2000;33(2):72–79. [Google Scholar]
- Roncero MB, Torres AL, Colom JF, Vidal T. The effect of xylanase on lignocellulosic components during the bleaching of wood pulps. Biores Technol. 2005;96(1):21–30. doi: 10.1016/j.biortech.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Rubio-Senent F, Rodríguez-Gutiérrez G, Lama-Muñoz A, Fernández-Bolaños J. Pectin extracted from thermally treated olive oil by-products: characterization, physico-chemical properties, in vitro bile acid and glucose binding. Food Hydrocoll. 2015;43:311–321. [Google Scholar]
- Sancho SDO, da Silva ARA, Dantas ANDS, Magalhães TA, Lopes GS, Rodrigues S, da Costa JMC, Fernandes FAN, Silva MGDV. Characterization of the industrial residues of seven fruits and prospection of their potential application as food supplements. J Chem. 2015;264284:2015. [Google Scholar]
- Selani MM, Brazaca SGC, dos Santos Dias CT, Ratnayake WS, Flores RA, Bianchini A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014;163:23–30. doi: 10.1016/j.foodchem.2014.04.076. [DOI] [PubMed] [Google Scholar]
- Stéger-Máté M, Nótin B, Juhász R, Verasztó B, Jakab D, Monspart-Sényi J & Barta J (2011) Effect of vacuum drying on blackcurrant’s antioxidant components. In International congress on engineering and food. (pp. 22–26)
- Sudha ML, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007;104(2):686–692. [Google Scholar]
- Tańska M, Roszkowska B, Czaplicki S, Borowska EJ, Bojarska J, Dąbrowska A. Effect of fruit pomace addition on shortbread cookies to improve their physical and nutritional values. Plant Foods Hum Nutr. 2016;71(3):307–313. doi: 10.1007/s11130-016-0561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tze NL, Han CP, Yusof YA, Ling CN, Talib RA, Taip FS, Aziz MG. Physicochemical and nutritional properties of spray-dried pitaya fruit powder as natural colorant. Food Sci Biotechnol. 2012;21(3):675–682. [Google Scholar]
- Vardin H, Yilmaz FM. The effect of blanching pretreatment on the drying kinetics, thermal degradation of phenolic compounds and hydroxymethyl furfural formation in pomegranate arils. Ital J Food Sci. 2018;30:156–169. doi: 10.14674/IJFS-947. [DOI] [Google Scholar]
- Wong KH, Cheung PC. Dietary fibers from mushroom sclerotia: 1. Preparation and physicochemical and functional properties. J Agric Food Chem. 2005;53(24):9395–9400. doi: 10.1021/jf0510788. [DOI] [PubMed] [Google Scholar]
- Yağcıoğlu A, Değirmencioğlu A & Çağatay F (1999) Drying characteristics of laurel leaves under different drying conditions. In 7th Int Congress on Agricultural Mechanization and Enerdy (pp. 565–569)
- Zain NM, Yusop SM, Ahmad I. Preparation and characterization of cellulose and nanocellulose from pomelo (Citrus grandis) albedo. J Nutr Food Sci. 2014;5(1):334. [Google Scholar]
- Zaker MA, Sawate AR, Patil BM, Sadawarte SK, Kshirsagar RB. Utilization of orange (Citrus sinesis) peel powder as a source of dietary fibre and its effect on the cake quality attributes. Int J Agric Sci. 2017;13(1):56–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request.