Abstract
Renealmia alpinia (Rottb.) Maas pulp was processed by spray drying using Maltodextrin (MDX), and Gum Arabic (GA), and the mixture of both encapsulating agents (MDX-GA). Yield, moisture, water activity (aw), apparent and bulk densities, size and morphology of capsules, color, and antioxidant potential (antioxidant activity, total carotenoids, and phenolic compounds) were analyzed. The encapsulates were incorporated as pigments in yogurt and the stability of antioxidant compounds (1, 7, 14, 21, and 28 days of storage) and the sensory properties were evaluated. The yields of all formulations (MDX, GA, MDX-GA) were around 17.86% with low moisture and aw range values (2.62–3.29% and 0.276–0.309, respectively). The microcapsules presented multiples particle sizes (0.67–27.13 µm) with irregular and smooth surfaces. Furthermore, these capsulates preserved yellow color and the retention of carotenoids was significantly higher with MDX (34.12 mg/100 g of powder), while the phenolic compounds and antioxidant activity increased with GA (474.17 mg GAE/100 g and 552.63 mg TE/100 g of powder, respectively). The main compounds β-carotene and gallic acid were identified and quantified in positive and negative mode respectively using LC–MS/MS. Finally, the addition of the encapsulated pigments to yogurt allowed to obtain a yellow coloration and the yogurt added with MDX–GA presented the best formulation with not significant changes in antioxidant activity and acceptable sensory attributes up 28 days of storage.
Keywords: Renealmia alpinia, Pigments, Carotenoids, Spray drying, Antioxidant retention
Introduction
A variety of plants and fruits are used for therapeutic purposes along with their edible properties. Their high concentrations of secondary metabolites (carotenoids, chlorophylls, among others) lead to beneficial effects such as antioxidant and hypoglycemic (Gul et al. 2015). Renealmia alpinia (Rottb.) Maas fruit belongs to the Zingiberaceae family, the pulp consists of intense yellow color arils that are associated with a high content of carotenoids (Lognay et al. 1991; Luna-Guevara et al. 2017). Currently, R. alpinia consumption and use are limited, commonly it is considered wasted or it is used as feed for livestock. However, the pulp, peel, and seeds have the potential to be used as a food ingredient. Previously, we evaluated the pericarp pigments of this fruit, a high concentration of anthocyanins is present with multiple foods uses as a natural colorant (Jimenez-Gonzalez et al. 2018). However, there is a lack of information about the use of R. alpinia pulp carotenoid-rich pigments that could be used in the food industry as appearance enhancers to increase the consumer's acceptability and product demand (Al-Shabib et al. 2017). Additionally, the use of carotenoids as dietary supplements and natural pigment have increased, due to their bioactive properties and antioxidant potential (Rodriguez-Amaya 2016). Some reports have concluded that regular consumption of these antioxidants reduces cancer risk (Gul et al. 2015; Gonçalves et al. 2016).
Nowadays, carotenoids can be an alternative for replacing synthetic colorants like tartrazine (FD & C Yellow No. 5, T102) which is used as a yellow colorant in beverages like yogurt, snacks, mustard, ice-cream or pharmaceutical products (Gul et al. 2015). Tartrazine has been associated with health problems such as hyperactivity, attention deficit disorder, hypersensitivity, asthma, and long-term use can promote the development of tumors (Al-Shabib et al. 2017).
Furthermore, the stability of natural compounds such as carotenoids depends on their concentration, chemical characteristics (number of double bonds, type of molecule, and structural isomers) and environment (processing and storage conditions) (Rodriguez-Amaya 2016; Rodriguez-Amaya 2019). According to Gul et al. (2015), the encapsulation process represents an interesting alternative to promotes the stability of antioxidants compounds, including carotenoids.
There are different encapsulation methods such as molecular inclusion, coacervation, complex coacervation, freeze-drying, extrusion, fluidized bed, etc (Jyothi et al. 2010). The spray drying method consists of covering the active compound with polymeric agents like maltodextrin or gum arabic, being these the most used materials. These polymeric compounds form a three-dimensional matrix that reduces the interaction of core compounds with the environment, decreasing their degradation (Gharsallaoui et al. 2007; Bakry et al. 2016). Additionally, the encapsulation process allows the obtaining a fine powder easy to incorporate into food formulations. Some studies about carotenoid encapsulation include zeaxanthin from Goji Berry fruit, cactus mucilage nanoemulsions and red pepper extract and cantaloupe carotenoids emulsions (de Campo et al. 2019; Šeregelj et al. 2019; Medeiros et al. 2020). However, there are no studies about R. alpinia pulp encapsulation and its potential application as food colorant for food. This study aimed to evaluate the encapsulated pigments and antioxidant compounds from R. alpinia pulp preserved through spray drying and to estimate their potential to be used as an additive and functional colorant in yogurt.
Materials and methods
Plant material
Renealmia alpinia fruit (30 kg) was collected in Cuetzalan del Progreso in the North of Puebla State, Mexico and only damage-free fruits, with similar maturity stages, were used. Pericarp and seeds were manually removed from the pulp. Finally, samples (pulp) were placed in polyethylene bags and hermetic plastic containers and stored at − 20 °C avoiding light exposure until further use.
Microencapsulation
Extracts and feeding mix preparation
Pigment extracts were obtained by pulp extrusion, which was first filtered through a cloth and Whatman #1 filter paper placed in a Büchner funnel attached to a vacuum flask; the filtrate was stored under freezing (7 °C) and dark conditions. The feed mix consisted of pulp extract and encapsulating agents (EA): Maltodextrin (MDX) with dextrose equivalents of 4.0–7.0 (Sigma-Aldrich, USA) and Gum Arabic (GA) (Meyer, Mexico). All mixtures were homogenized at room temperature using a magnetic stirrer for 30 min and adjusted to the total soluble solids: pulp extract (5°Bx) and some of the encapsulates agents: MDX (15°Bx), MDX (7.5°Bx)–GA (7.5°Bx) and GA (15°Bx).
Spray drying treatments
Mixtures were fed to a spray dryer (SEV Prendo, Puebla, Mexico) equipped with a 0.5 mm nozzle with a feed flow rate of 3 ± 1 g/min, which was provided by a peristaltic pump. The spray-dryer was operated at an inlet air temperature of 150 ± 2 °C and as outlet temperature 98 ± 2 °C, the atomization air pressure was fixed at 15 LPM and 50% aspiration. Mixtures were kept under constant agitation at 22 °C throughout the encapsulation process.
Physical analysis of encapsulates
Yield
Yield was calculated as grams of powder obtained after drying process per grams of solid contents (fruit extract solids + EA) and expressed as percentage of powder.
Water activity (aw) and moisture
Water activity was measured with the direct reading of dew point hygrometer (Aqualab Series 3TE, Washington, USA). Moisture content was obtained following the AOAC 925.10 method (AOAC 2000), by drying of the sample in a natural convection oven (BD 56, Binder, Tuttlingen, Germany) at 105 °C until reaching constant weight.
Apparent and bulk density
The tapped or apparent, and bulk densities were assessed according to the method proposed by Jangam and Thorat (2010), the volume (V) of powder was measured and the bulk density calculated (ρb = W/V). For the tap density (ρt = W/ Vc), the cylinder with powder was tapped (about 8 min) on the surface of a bench until reaching a compacted volume (Vc).
Morphology and size
A scanning electron microscope (SEM) (JEOL JSM-6610-LV, Akyshama, Japan) was used for morphology and size determinations, using the backscattered electron technique with a 10 kV acceleration. The morphology and size of particles were reported.
Color
Microencapsulated color parameters (CIE L*, a*, b*) were determined with a colorimeter (HunterLab, Color Flex 45/0 Spectrophotometer, Washington, USA), and were used to calculate the indices Chroma and °Hue: .
Stability of antioxidant properties
The samples were stored at two temperatures (4 and 25 °C) and the antioxidant potential was evaluated at 1, 7, 14, and 28 days. Determinations of antioxidant properties were made by reconstituting 20 mg of powder with 20 mL of distilled water and 5 min vortex agitation (CScientific, Mod. VTX-5, Mexico City, Mexico) to break the capsules. The antioxidant properties as total carotenoids (mg β-carotene/100 g of powder), phenolic compounds (mg GAE/100 mg powder) contents, identification and quantification of β-carotene and gallic acid (mg/100 g powder), and antioxidant activity (mg TE/100 g powder) were evaluated.
Total carotenoids
Total carotenoids determination was made according to Desobry et al. (1997) with some modifications. Five grams of powder were mixed with 1 mL distilled water and high-speed agitation for 3 min. Carotene extraction was made with 3 mL of hexane and 5 min agitation. The absorbance readings were made with a UV–Vis spectrophotometer (Jenway, 640 UV/Vis, New Jersey, USA) at 454 nm. The carotenoids were calculated according to Beer’s law ; where A is abs545, ε is the molar extinction coefficient of β-carotene in hexane (139 × 103 L mol−1 cm−1), C is the concentration of β-carotene, l is the path length (1 cm). Total carotenoids were expressed as mg of β-carotene/100 g of powder.
Phenolic compounds
The phenolic compounds were determined in 1 mL of sample mixed with 1 mL of Folin-Ciocalteu (0.1 M, Sigma Aldrich). After letting rest for 3 min in dark conditions 1 mL of Na2CO3 (0.05%, Golden Bell, Mexico) was added. The absorbances were obtained at 765 nm UV–Vis after 30 min. A blank for each sample was prepared in the same way except the Folin reagent. The calculations were made from a calibration curve (5, 10, 15, 20, 25 ppm gallic acid) (Golden Bell, Mexico) with equation, R2 ; where y is the Abs at 765 nm and x is the concentration as gallic acid equivalents (GAE) (Gao et al. 2000).
Identification and quantification of β-carotene and gallic acid
Microencapsulate samples (0.1 g) were dissolved in 2 mL dichloromethane and afterwards filtered through a 0.2 μm nylon syringe filter. A Waters (Milford, MA, USA) LC–MS/MS (Liquid Chromatography with Tandem Mass Spectrometry) was used for the identification and quantification of β-carotene and gallic acid. An Acquity UPLC BEH C18 (2.1 × 100 mm, 1.7 μm) column was used for compound separation. Mobile phases were 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in methanol (B). The column was maintained at 40 °C. A flow rate of 0.45 mL/min and an injection volume of 5 μL were used. Total run time of 14.5 min with 99% A at 0.5 min, 20% A at 13.5 min, and 99% A at 14 min.
Quattro Premier XE triple quadrupole mass spectrometer fitted with electrospray ionization (ESI) was used in positive mode for β-carotene and negative mode for gallic acid. Mass spectrometer optimization was conducted by direct infusion of stock standard solution (50 mg/L) to select precursor and product ions. The calibration curves (3–300 µg/mL) of standards were used for quantification.
Antioxidant activity
The antioxidant activity was determined with the inhibition of freed radical or DPPH scavenging, following the methodology reported by Nalawade and Gajjar (2016) with some modifications. Using 1 mL of radical DPPH (0.004%) (Sigma-Aldrich, Mexico) and 1 mL extract. The mixture was incubated in the dark at room temperature for 30 min. The changes in the absorbance at 517 nm were analyzed with a UV–Vis spectrophotometer (Jenway 640 UV/Vis, UK). The inhibition percent was estimated using the following expression: . The calibration curve was prepared with Trolox (Sigma-Aldrich) and obtained the following equation y = 6.27x − 1.30 with; where y is the % inhibition and x is the Trolox concentration expressed as Trolox equivalent (TE).
Incorporation of the pigments and yogurt assessments
Yogurt preparation
Before the incorporation of the pulp capsules into a food matrix (a commercial unflavored natural yogurt), the encapsulated pulp was microbiologically analyzed (molds and yeasts, aerobic mesophylls, total coliforms, data not shown) to ensure the product´s safety. Then, yogurt samples were added with 5 g/L of encapsulated pigments and high-speed vortexed for 1 min and stored at 4 °C (refrigeration) in dark conditions for 1, 7, 14, 21, and 28 days. Color and antioxidant activity were analyzed at each evaluation time according to the methods described above.
Sensory analysis
The visual aspect, odor, flavor, and general acceptability of samples yogurts (pigment-free, added with artificial colorant and encapsulated extract) were evaluated by 50 untrained panelists (15 men and 35 women) within age range 19–35 years old. The yogurt samples with food-grade artificial colorant were equalized according to the yellowish color of the products with encapsulated extract (5 g/L). A hedonic scale of 7 points (1 = dislike extremely and 7 = like extremely) was used and the samples were randomly given to the panelists.
Statistical analysis
All analyses and formulations were made in triplicate and the results were statistically analyzed using analysis of variance (ANOVA, α = 0.05) and Tukey’s test for pairwise comparison of the mean values with the software Statistix 8.1 (Tallahassee, FL).
Results and discussion
Physical properties of encapsulated pigments
Yield
There were no significant differences (P > 0.05) between yields results (17–18%) with the use of three encapsulation agents (EA) (Table 1). The values can be attributed to the initial concentration of solids in the feed and other conditions as the low glass transition temperature (Tg) of organic acids and some sugars extracts contained in the sample. According to Luna-Guevara et al. (2017) the pulp of R. alpinia contains 10.3% of carbohydrates and 0.3% of titratable acidity (related to the organic acids content). The presence of these compounds and higher drying temperatures over Tg could produce sticky drops that attached to the drying chamber, impeding the retention in the collector of spray dry equipment (Can Karaca et al. 2016). Krishnaiah et al. (2012), reported yields between 14.5 and 39.1% in the encapsulation of noni (Morinda citroflora) using maltodextrin; the authors suggested that this parameter depends on the ratio between core and wall material in the feed. Another study on encapsulation of lutein with different EA (MDX, MDX–GA, GA) showed higher yields than those obtained in this study 21.3, 21, and 32.7%, respectively at 185 °C as drying temperature (Álvarez-Henao et al. 2018). Although the same report suggests that these values were relatively low, which are attributed to the reduction in the heat and mass transfers due to the high feed rates, during the drying spray process. Rutz et al. (2013) suggested that molecular interactions like dipole–dipole and hydrogen bonds between the extract and wall materials improving the stability of pigments, and enhancing the protection of the core material.
Table 1.
Physical and color properties in microencapsulated pulp of Renealmia alpinia with different coating agents
| Parameter | MDX | MDX–GA | GA |
|---|---|---|---|
| Yield (%) | 17.17 ± 0.2 a | 18.01 ± 4.2 a | 18.43 ± 2.2 a |
| Moisture (%) | 3.29 ± 0.03 a | 2.62 ± 0.77 a | 2.93 ± 1.64 a |
| aw | 0.26 ± 0.02 a | 0.19 ± 0.09 a | 0.16 ± 0.03 a |
| Bulk density(g/cm3) | 0.30 ± 0.02 a | 0.27 ± 0.05 a | 0.26 ± 0.02 a |
| Tapped density (g/cm3) | 0.45 ± 0.01 a | 0.46 ± 0.04 a | 0.46 ± 0.02 a |
| Color | |||
| L* | 70.02 ± 5.1 b | 75.85 ± 1.7 a | 75.96 ± 1.7 a |
| a* | − 1.90 ± 0.4 a | − 2.17 ± 0.5 a | − 2.01 ± 0.2 a |
| b* | 45.75 ± 1.4 b | 47.31 ± 4.1 b | 51.64 ± 2.5 a |
| Chroma | 45.79 ± 1.4 b | 47.31 ± 4.1 b | 51.64 ± 2.5 a |
| Hue | 92.37 ± 0.5 a | 92.65 ± 0.6 a | 92.23 ± 0.2 a |
± represents standard deviation of n = 3. The comparison of means was carried out between treatments in the same row. Different letters in the same row means significant differences (P < 0.05). Unencapsulated pulp extract L* = 48.04 ± 4.87; a* = 18.43 ± 3.9; b* = 71.93 ± 6.48
Water activity and moisture
Two of the main factors related to the microencapsulation of carotenoids and phenolic compounds are moisture and water activity (aw) (Santana et al. 2016). In this study, the aw and moisture values had no significant difference (P > 0.05) (Table 1). The results of aw were lower than 0.3 which suggest stable microencapsulates, attributable to the lower water molecules mobility (Oberoi and Sogi 2015). Moisture contents were similar across all formulations (2.6–3.2%), which are consistent with those reported using the agents MDX and GA–MDX combination (Carneiro et al. 2013). On the other hand, Quek et al. (2007) considered moisture as the most important parameter for the storage of encapsulated bioactive compounds, due to high sugar content induces the clumping of the particles.
Apparent and bulk density
According to Kingwatee et al. (2015) and Luna-Guevara et al. (2017), density is an important parameter, due to, its relation with the fluidity, transport, storage, and packaging of encapsulates. In this study, all formulations exhibited similar values with no significant difference (P < 0.05) between tapped or apparent and bulk density, which ranged around 0.45–0.46 and 0.26–0.30 g/cm3, respectively (Table 1). The temperature, flow rate (process conditions) and the encapsulates agents can influence on the density of powders (Khalilian Movahhed and Mohebbi 2016). While, Goula and Adamopoulos, (2010) reported that the apparent and bulk density values depend to particle size, particle agglomeration and the arrangement inside the cylinder used for the analysis of these parameters.
Morphology and particle size
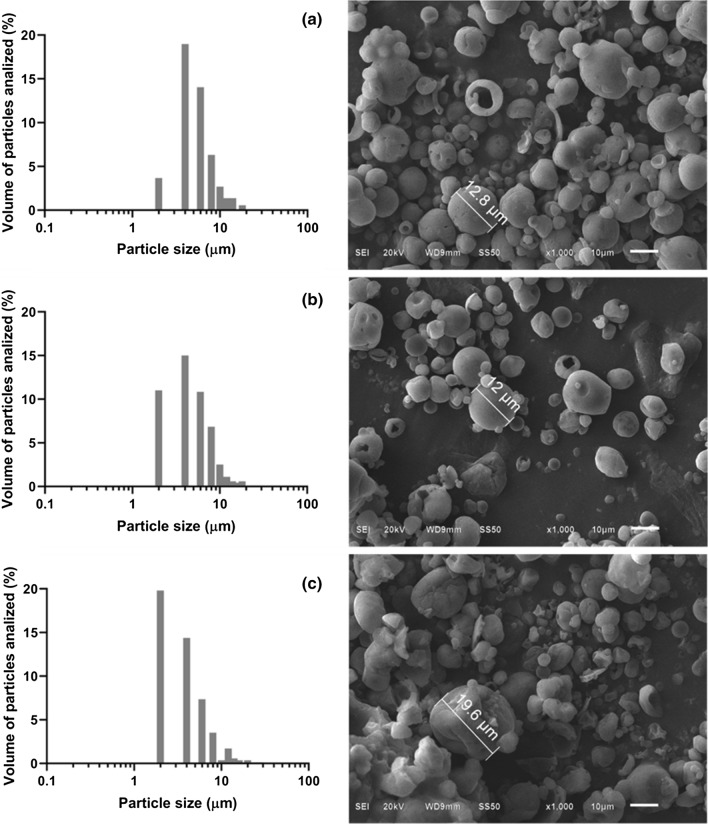
These parameters affect several technological properties including bulk density, flowability, compaction, rehydration, and solubility, which have an impact on the possible applications of the powders. In this study, all formulations evidenced agglomerations and adherence of small particles over the surface of the bigger ones. The bonding between the particles might be related to the low glass transition temperature (Tg) of the wall materials (GA and MDX) (Etzbach et al. 2020). Furthermore, the microcapsules showed sphered shape and some particles presented both types of surfaces smooth without deformation or irregular with some shrinkages (Fig. 1a–c). The microphotographs with GA revealed imperfect and collapse particles (Fig. 1c), this tendency has been observed by other authors and it has been associated with high drying temperatures and mass transfer of the inner water to the outside (Yu and Lv 2019; Zhang et al. 2020). While MDX encapsulations improved the surface of the particles (Fig. 1a, b).
Fig. 1.
Size and morphology of the particles obtained with the different coating agents (a) MDX, (b) MDX–GA and (c) GA
Regarding the particle size of the microcapsules was between 1.1 and 20.6 µm (MDX), 0.1–17.5 µm (MDX–GA), 0.6–27.1 µm (GA) (Fig. 1). These differences could be attributed to the viscosity of the EA mixture, in accordance with Di Battista et al. (2015) an increase of this parameter helps to reduce the size of the capsules. The values were similar to those reported by Pereira et al. (2019) with powders obtained by spray-dried of juçara pulp (mean diameters 11.5–21.5 μm). However, 90% of the particles presented maximum values between 8 and 10 µm, and 50% of the capsules measured less than 4 µm. Although the capsules with higher particle sizes were with GA as EA, all formulations showed different particle sizes which contributed to stabilizing of the wall, due to the small spaces were filled out by particles with lesser size.
Color
The L*, a* and b* parameters were used to determine the color loss in the encapsulates by the drying process and the EA used. The color measures of capsules showed significant changes (P < 0.05), specifically, the L* values increased with the addition of GA (Table 1), these results are similar to that reported by Daza et al. (2016). Besides, brighter L*(75.96) and yellowish b*(51.64) capsules were obtained with this EA, which is associated with its natural yellow color. The Hue results were around 90° confirming the yellow color of all formulations. Regarding the Chroma values, they showed significant differences among EA with the storage times, the higher values belonged to GA encapsulates (22.9–24.1) (Table 1). The yellow intensity in the encapsulates depended on different factors such as the type and concentration of the encapsulating agent, drying conditions, and chemical changes that occurred through the drying (reduction in double bonds, isomerization, or oxidation) (Daza et al. 2016).
Antioxidant activity of microencapsulates
Total carotenoids
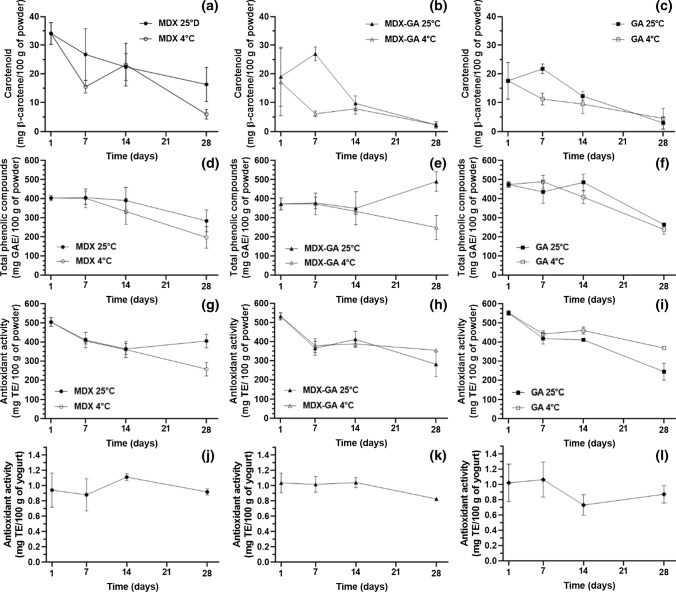
The initial values of the encapsulated carotenoids were 34.1 ± 3.8 (MDX), 19.0 ± 10.3 (MDX–GA), 17.5 ± 6.4 (GA) mg of β-carotene/100 g powder (Fig. 2a–c). Different authors suggest that EA and drying conditions (temperature and oxygen exposure) affect the final contents of carotenoids (Coronel-Aguilera and San Martín-Gonzalez 2015; Oberoi and Sogi 2015). Previous studies reported values around 12.6–28.5 mg β-carotene/100 g of powder with MDX and sodium caseinate and fluidized bed coating with hydroxypropyl cellulose (Coronel-Aguilera and San Martín-Gonzalez 2015). The stability of the encapsulated carotenoids was evaluated under two storage temperatures (4 and 25 °C), a reduction of carotenoids was observed in all formulations and both storage conditions (Fig. 2a–c), Rutz et al. (2013) suggested that the stability of antioxidant compounds depends on the effectiveness of the coating, since surface compounds tend to degrade easily. The formulation with MDX showed to be the most stable, the encapsulates maintained 47% retention (final concentration 16.32 ± 6.02 mg β-carotene/100 g) at 25 °C for 28 days (Fig. 2a). While the rest of the formulations reduced carotenoids with retention percentages close to 20%. The mixture MDX–GA showed the lowest carotenoids concentrations 2.18 ± 0.69 and 2.25 ± 1.11 mg β-carotene/100 g of powder with both temperatures 4 and 25 °C with 28 days of storage, respectively (Fig. 2b). The stability of MDX encapsulates is related to the concentration of this agent and its high-water solubility, which can contribute to reducing the viscosity of feed dispersion favoring the atomization and drying of the liquid feed (Sansone et al. 2011). The encapsulating agents such as some gums, maltodextrins, and gelatins can be used alone or combined in different proportions (Drusch and Mannino 2009). The powder´s stability in this study was related to the combination of the carriers used (MDX, GA and MDX-GA). Which generates the formation of structures denser and more continuous in the capsules preventing oxygen transfer through the system and thus retard the oxidation of bioactive compounds (Loksuwan 2007).
Fig. 2.
A. Stability of antioxidant compounds of microencapsulated pulp extract stored at 4 °C and 25 °C: Total carotenoids (a) MDX, (b) MDX–GA, (c) GA; Total phenolic compounds d) MDX, (e) MDX–GA, (f) GA; and antioxidant activity (g) MDX, (h) MDX–GA, (i) GA and B. Antioxidant activity of yogurt added with microencapsulated pulp extract stored at 4 °C: (j) MDX, (k) MDX–GA and (l) GA
Total phenolic compounds
Total phenolic compounds presented significant differences (P < 0.05) after encapsulation (Fig. 2d–f). The highest values corresponded to powders with GA (474.17 ± 13.83 mg GAE/100 g) on the first day of storage and the treatment MDX–GA maintained up to 14 days with values of 390 ± 68.61 mg GAE/100 g and increased their concentrations until 485.71 ± 42.83 mg GAE/100 g stored at 25 °C (Fig. 2e). The degradation of phenolic compounds was more evident under conditions of 4 °C at 28 days of storage and all encapsulating agents with reductions of 48%, 66%, 50% for MDX, MDX–GA, and GA, respectively (Fig. 2d–f). According to Rutz et al. (2013) the degradation reactions can be attributed to the relative humidity and temperature during the storage of the encapsulates, which could promote water mobility. Also, despite the encapsulation process decrease the oxygen interactions this could be due to the poor stability of antioxidants compounds such as polyphenols.
Identification and confirmation of antioxidant compounds
The compounds identified by LC–MS/MS, which presented higher content into the encapsulates, were β-carotene (91–101 µg/g dry weight) and gallic acid (25–40 µg/g dry weight) (Table 2). Whereby it is confirmed that encapsulation provided important protection for antioxidant pigments. The obtained results agree with our previous studies (Luna-Guevara et al. 2017), where R. alpinia fruit had high concentrations of carotenoids in the pulp, contributing to provide a yellowish pigmentation.
Table 2.
Evaluation of β-carotene and gallic by LC–MS/MS from Renealmia alpinia microencapsulates
| Encapsulation material | β-carotene (µg/g dry weight) | Gallic acid (µg/g dry weight) |
|---|---|---|
| MDX | 100.8 ± 2.56a | 39.5 ± 0.0843a |
| GA | 100.0 ± 0.735a | 38.7 ± 1.92a |
| MDX*GA | 91.2 ± 4.01a | 24.9 ± 0.317b |
± represents standard deviation of n = 3. Different capital letters in the same column for each encapsulation agent indicate significant differences (P < 0.05)
Antioxidant activity
The antioxidant activity of microencapsulated R. alpinia extract depended on the phenolic compounds mainly (Fig. 2d–f). All formulations did not show significant differences (P > 0.05) on the first day, however, the changes were observed among three treatments with both temperatures 4 °C and 25 °C after 28 days (Fig. 2g–i). The reductions are attributed to factors such as time and storage conditions, coating agents, and bioactive compounds present in the extract (Krishnaiah et al. (2012). The best encapsulation and storage conditions were MDX, 25 °C and MDX–GA and GA at 4 °C. The most significant reductions were 48, 47, and 55% achieved with MDX at 4 °C and MDX–GA, GA at 25 °C, respectively after 28 days of storage.
Incorporation of encapsulates to yogurt
Color
The visual color of all samples was similar to commercial pineapple yogurts, L* values were approximated to 80 in all treatments which show high lightness. The yogurts presented a yellow tone Hue close to 100° with the incorporation of encapsulated extract, these results were higher than natural pigment-free yogurt (Hue = 96.5°), while saturation of color decreased (Chroma = 28.8). Besides, these indexes showed that encapsulated pigment color remain stable during all the storage time (28 d), only with the mixture MDX–GA had a significant change (19.12–19.91 values) (Table 3). Wallace and Giusti (2008) related the color changes. with carotenoid rearrangements to other isomers without color and environmental conditions that lead to oxidation of these pigments. However, color stability has been investigated by Coronel-Aguilera and San Martín-González (2015) who added encapsulated β-carotene to yogurt and observed that color remained during the storage period of 4 weeks.
Table 3.
Color parameters of yogurt enriched with carotenoid pigments from Renealmia alpinia
| Encapsulation material | Time (days) | L* | a* | b* | Chroma | °Hue |
|---|---|---|---|---|---|---|
| MDX | 1 | 79.9 ± 3.7 Ab | − 4.3 ± 0.5 Aa | 20.7 ± 1.3 Ab | 21.2 ± 1.4 Ab | 101.7 ± 0.6 Aab |
| 7 | 79.5 ± 4.4 Aa | − 4.2 ± 0.6 Aa | 19.6 ± 2.4 Ab | 20.0 ± 2.5 Ab | 102.1 ± 0.6 Aa | |
| 14 | 76.1 ± 8.3 Aa | − 4.2 ± 0.6 Aa | 19.0 ± 3.4 Ab | 19.4 ± 3.5 Ab | 102.6 ± 0.7 Aab | |
| 28 | 79.4 ± 3.3 Aa | − 4.6 ± 0.5 Aa | 20.0 ± 1.6 Aab | 20.5 ± 1.6 Aab | 103.1 ± 1.1 Aa | |
| MDX–GA | 1 | 81.6 ± 0.5 Aa | − 4.1 ± 0.46 Aa | 18.6 ± 0.9 Ab | 19.1 ± 1.0 Ab | 102.5 ± 0.7 Aa |
| 7 | 80.5 ± 2.3 Aa | − 4.3 ± 0.31 Aa | 19.3 ± 0.8 Ab | 19.8 ± 0.8 Ab | 102.5 ± 1.0 Aa | |
| 14 | 80.3 ± 1.8 Aa | − 4.2 ± 0.36 Aa | 18.6 ± 0.7 Ab | 19.1 ± 0.7 Ab | 102.9 ± 0.9 Aa | |
| 28 | 80.8 ± 1.8 Aa | − 4.6 ± 0.18 Aa | 19.3 ± 1.2 Ab | 19.9 ± 1.2 Ab | 103.4 ± 1.1 Aa | |
| GA | 1 | 79.5 ± 0.6 Aa | − 4.5 ± 0.1 Aa | 23.4 ± 1.4 Aa | 23.8 ± 1.4 Aa | 101.0 ± 0.5 Bb |
| 7 | 79.9 ± 1.0 Aa | − 5.0 ± 0.5 Aa | 23.6 ± 2.1 Aa | 24.1 ± 2.1 Aa | 102.0 ± 0.3 Aa | |
| 14 | 79.6 ± 0.5 Aa | − 4.7 ± 0.3 Aa | 23.4 ± 1.3 Aa | 23.8 ± 1.3 Aa | 101.5 ± 0.2 ABb | |
| 28 | 80.2 ± 1.1 Aa | − 4.8 ± 0.4 Aa | 22.5 ± 1.3 Aa | 22.9 ± 1.4 Aa | 102.1 ± 0.5 Aa |
± represents standard deviation of n = 3. Different capital letters in the same column for each encapsulation agent indicate significant differences for the storage times (P < 0.05). Different lowercase letters in the same run for the same time indicate significant differences between the encapsulation agents at the same time (P < 0.05)
Antioxidant activity of yogurt added with microcapsules
Figure 2j–l reveals the stability of the antioxidant activity of yogurts during 28 d of storage, presenting a slight reduction at 4 °C, probably to some conditions such as pH and acidity could produce instability in these products. Another report by Rascón et al. (2011) confirms the advantages of carotenoids encapsulation from paprika oleoresin, which were subjected to high relative humidity environments and water absorption during storage. The antioxidant stability is attributed to the matrix coating by the polymeric structure which limits the oxygen interaction and reduces environment interactions to protect antioxidant molecules. While Chen et al. (2017) report that the encapsulation of bioactive compounds allows its gradual liberation when these have been incorporated into food matrices. However, for dairy products, the antioxidant activity may be attributed to the bioactive peptides released during the fermentation in products (Taha et al. 2017).
Sensory evaluation
In general, the yogurts showed better values in the attribute of visual acceptability, the highest results were achieved with the treatment MDX–GA (5.24 ± 1.06) and this mixture did not present statistical difference (p < 0.05) in odor, flavor, and general acceptability compared to yogurt added with the artificial colorant (Table 4). However, the judges detected a strange aftertaste with yogurt with MDX, which is distinctive from the pulp. Finally, the use of pigments as a natural colorant is common in dairy products to improve sensory characteristics could be a good source to increase the bioactive compounds (Coronel-Aguilera and San Martín-Gonzalez 2015).
Table 4.
Sensory analysis of yogurts added with natural encapsulated pigments and colorant
| Sample | Visual acceptability | Odor | Flavor | General acceptability |
|---|---|---|---|---|
| Yogurt pigment free | 5.34 ± 1.37a | 5.31 ± 1.54a | 5.45 ± 1.64a | 5.45 ± 1.53a |
| MDX | 4.97 ± 1.21a | 3.41 ± 1.18b | 3.03 ± 1.55b | 3.07 ± 1.39b |
| MDX–GA | 5.24 ± 1.06a | 4.28 ± 1.41ab | 3.93 ± 1.46b | 4.03 ± 1.56b |
| GA | 4.8 ± 1.27a | 4.13 ± 1.43b | 3.73 ± 1.7b | 3.83 ± 1.66b |
| Yellow colorant | 5.3 ± 1.32a | 5.17 ± 1.29a | 5.27 ± 1.74a | 5.33 ± 1.58a |
± standard deviation of n = 50
Different letters in the same column means significant different (P < 0.05)
Conclusion
This study evaluated the microencapsulation of Renealmia alpinia (Rottb.) Maas pulp pigment and antioxidant compounds by spray-drying and their incorporation in yogurt. The results showed that the physical properties such as yield, moisture, and density of encapsulates did not present significant difference, while the treatments with GA had the high values of L* and b* with yellow and brilliant powders. The stability of the pigments and antioxidant compounds in the encapsulates depended on the storage conditions and the encapsulating agent used. The best formulations were MDX at 25 °C and MDX–GA at 4 °C and 25 °C. However, the MDX-GA formulation remained stable in both storage temperatures, hence it can be considered the most suitable to obtain encapsulated pigments. Additionally, this mixture presented the highest levels of organoleptic preference, especially in visual and general acceptability of yogurt during sensory tests. Furthermore, the yogurt color was stable and the antioxidant activity variations were low during the evaluation time, in comparison to MDX and GA treatments. Finally, the encapsulation and protection of pigments and antioxidant compounds of an unappreciated fruit like R. alpinia represent an innovative alternative for future studies and their possible additions to food matrices.
Acknowledgements
The authors thank LBT Miguel Angel Benítez Luna for his participation in providing language help and writing assistance of the paper, and the Food Analysis Laboratory Intema SA de CV for its contribution in R. alpinia compounds identification.
Author contributions
OJG: Development of experimental research and article writing; JJLG: Assistance for the development of encapsulation experiments; MMRR: Identification and confirmation of antioxidant compounds by LC–MS / MS (Liquid Chromatography with tandem Mass Spectrometry); DLV: Assistance for the development of the antioxidant compounds experiments; MLLG: Planning the research project and writing the paper.
Funding
The authors thank Vicerrectoría de Investigación y Estudios de Posgrado of Benemerita Universidad Autonoma de Puebla, whom supported the research (Project, 00108).
Data availability
There is availability of data and images in the manuscript.
Declarations
Conflicts of interest
The authors have not conflict of interest.
Consent to participate
The authors considered the consent statements.
Consent for publication
The authors considered the consent for publication.
Ethical approval
The authors approve the ethical requirement.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
O. Jimenez-Gonzalez, Email: oscar_j123@hotmail.com
J. J. Luna-Guevara, Email: juanj.luna@correo.buap.mx
M. M. Ramírez-Rodrigues, Email: milena.ramirez@udlap.mx
D. Luna-Vital, Email: armando-luna@hotmail.com
M. L. Luna-Guevara, Email: maria.luna@correo.buap.mx
References
- Al-Shabib NA, Khan JM, Khan MS, Ali MS, Al-Senaidy AM, Alsenaidy MA. Synthetic food additive dye “Tartrazine” triggers amorphous aggregation in cationic myoglobin. Int J Biol Macromol. 2017;98:277–286. doi: 10.1016/j.ijbiomac.2017.01.097. [DOI] [PubMed] [Google Scholar]
- Álvarez-Henao MV, Saavedra N, Medina S, Jiménez Cartagena C, Alzate LM, Londoño-Londoño J. Microencapsulation of lutein by spray-drying: characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018;256:181–187. doi: 10.1016/j.foodchem.2018.02.059. [DOI] [PubMed] [Google Scholar]
- AOAC (Official Methods of Analysis) (2000) Association of Official Analytical Chemists, Washington, DC
- Bakry AM, Abbas S, Ali B, Majeed H, Abouelwafa MY, Mousa A, Liang L. Microencapsulation of oils: a comprehensive review of benefits, techniques, and applications: encapsulation of marine, vegetable, essential oils. Compr Rev Food Sci F. 2016;15:143–182. doi: 10.1111/1541-4337.12179. [DOI] [PubMed] [Google Scholar]
- Can Karaca A, Guzel O, Ak MM. Effects of processing conditions and formulation on spray drying of sour cherry juice concentrate: spray drying of sour cherry juice concentrate. J Sci Food Agr. 2016;96:449–455. doi: 10.1002/jsfa.7110. [DOI] [PubMed] [Google Scholar]
- Carneiro HCF, Tonon RV, Grosso CRF, Hubinger MD. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J Food Eng. 2013;115:443–451. doi: 10.1016/j.jfoodeng.2012.03.033. [DOI] [Google Scholar]
- Chen J, Li F, Li Z, McClements DJ, Xiao H. Encapsulation of carotenoids in emulsion-based delivery systems: enhancement of β-carotene water-dispersibility and chemical stability. Food Hydrocoll. 2017;69:49–55. doi: 10.1016/j.foodhyd.2017.01.024. [DOI] [Google Scholar]
- Coronel-Aguilera CP, San Martín-González MF. Encapsulation of spray dried β-carotene emulsion by fluidized bed coating technology. LWT-Food Sci Technol. 2015;62:187–193. doi: 10.1016/j.lwt.2014.12.036. [DOI] [Google Scholar]
- Daza LD, Fujita A, Fávaro TCS, Rodrigues Ract JN, Granato D, Genovese MI. Effect of spray drying conditions on the physical properties of Cagaita (Eugenia dysenterica DC.) fruit extracts. Food Bioprod Process. 2016;97:20–29. doi: 10.1016/j.fbp.2015.10.001. [DOI] [Google Scholar]
- de Campo C, Queiroz Assis R, Marques da Silva M, Haas Costa TM, Paese K, Guterres SS, de Oliveira Rios A, Flôres SH. Incorporation of zeaxanthin nanoparticles in yogurt: influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chem. 2019;301:125230. doi: 10.1016/j.foodchem.2019.125230. [DOI] [PubMed] [Google Scholar]
- Desobry SA, Netto FM, Labuza TP. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J Food Sci. 1997;62:1158–1162. doi: 10.1111/j.1365-2621.1997.tb12235.x. [DOI] [Google Scholar]
- Di Battista CA, Constenla D, Ramírez Rigo MV, Piña J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015;286:193–201. doi: 10.1016/j.powtec.2015.08.016. [DOI] [Google Scholar]
- Drusch S, Mannino S. Patent based review on industrial approaches for the microencapsulation of oils rich in polyunsaturated fatty acids. Trends Food Sci Technol. 2009;20:237–244. doi: 10.1016/j.tifs.2009.03.007. [DOI] [Google Scholar]
- Etzbach L, Meinert M, Faber T, Klein C, Schieber A, Weber F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr Res Nutr Food Sci. 2020;3:1–38. doi: 10.1016/j.crfs.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Ohlander M, Jeppsson N, Björk L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agr Food Chem. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40:1107–1121. doi: 10.1016/j.foodres.2007.07.004. [DOI] [Google Scholar]
- Gonçalves A, Estevinho BN, Rocha F. Microencapsulation of vitamin A: a review. Trends Food Sci Tech. 2016;51:76–87. doi: 10.1016/j.tifs.2016.03.001. [DOI] [Google Scholar]
- Goula AM, Adamopoulos KG. A new technique for spray drying orange juice concentrate. Innov Food Sci Emerg. 2010;11:342–351. doi: 10.1016/j.ifset.2009.12.001. [DOI] [Google Scholar]
- Gul K, Tak A, Singh AK, Singh P, Yousuf B, Wani AA, Yildiz F. Chemistry, encapsulation, and health benefits of β-carotene a review. Cogent Food Agric. 2015;1:1–12. doi: 10.1080/23311932.2015.1018696. [DOI] [Google Scholar]
- Jangam SV, Thorat BN. Optimization of spray drying of ginger extract. Dry Technol. 2010;28:1426–1434. doi: 10.1080/07373937.2010.482699. [DOI] [Google Scholar]
- Jimenez Gonzalez O, Ruiz Espinosa H, Luna Guevara JJ, Ochoa Velasco CE, Luna Vital D, Luna Guevara ML. A potential natural coloring agent with antioxidant properties: microencapsulates of Renealmia alpinia (Rottb.) maas fruit pericarp. NFS. 2018;13:1–9. doi: 10.1016/j.nfs.2018.08.001. [DOI] [Google Scholar]
- Jyothi NVN, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan GY. Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul. 2010;27:187–197. doi: 10.3109/02652040903131301. [DOI] [PubMed] [Google Scholar]
- Khalilian Movahhed M, Mohebbi M. Spray drying and process optimization of carrot-celery juice. J Food Process Preserv. 2016;40:212–225. doi: 10.1111/jfpp.12598. [DOI] [Google Scholar]
- Kingwatee N, Apichartsrangkoon A, Chaikham P, Worametrachanon S, Techarung J, Pankasemsuk T. Spray drying Lactobacillus casei 01 in lychee juice varied carrier materials. LWT-Food Sci Technol. 2015;62:847–853. doi: 10.1016/j.lwt.2014.12.007. [DOI] [Google Scholar]
- Krishnaiah D, Sarbatly R, Nithyanandam R. Microencapsulation of Morinda citrifolia L. extract by spray-drying. Chem Eng Res Des. 2012;90:622–632. doi: 10.1016/j.cherd.2011.09.003. [DOI] [Google Scholar]
- Lognay G, Marlier M, Severin M, Haubruge E, Gibon V, Trevejo E. On the characterization of some terpenes from Renealmia alpinia Rottb. (Maas) oleoresin. Flavour Frag J. 1991;6:87–91. doi: 10.1002/ffj.2730060113. [DOI] [Google Scholar]
- Loksuwan J. Characteristics of microencapsulated β-carotene formed by spray drying with modified tapioca starch, native tapioca starch and maltodextrin. Food Hydrocoll. 2007;21:928–935. doi: 10.1016/j.foodhyd.2006.10.011. [DOI] [Google Scholar]
- Luna-Guevara ML, Ochoa Velasco CE, Hernández Carranza P, Contreras Cortes LEU, Luna-Guevara JJ. Composition, physico-chemical properties and antioxidant capacity of Renealmia alpinia (Rottb.) maas fruit. Rev Fac Cienc Agrar. 2017;50:377–385. [Google Scholar]
- Medeiros I, de Oliveira GLR, de Queiroz JLC, Gomes CC, de Carvalho FMC, de Souza Lima MCJ, Serquiz AC, Santos PPA, Camillo CdS, MAciel BLL, Morais AHA, Pasos TS. Safety and bioactive potential of nanoparticles containing Cantaloupe melon (Cucumis melo L.) carotenoids in an experimental model of chronic inflammation. Biotechnol Rep. 2020;28:e00567. doi: 10.1016/j.btre.2020.e00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalawade PB, Gajjar AK. Microencapsulation of lutein extracted from marigold flowers (Tagetes erecta L.) using full factorial design. J Drug Deliv Sci Tec. 2016;33:75–87. doi: 10.1016/j.jddst.2016.03.012. [DOI] [Google Scholar]
- Oberoi DPS, Sogi DS. Effect of drying methods and maltodextrin concentration on pigment content of watermelon juice powder. J Food Eng. 2015;164:172–178. doi: 10.1016/j.jfoodeng.2015.06.024. [DOI] [Google Scholar]
- Pereira DCS, Beres C, Gomes FS, Tonon RV, Cabral LMC. Spray drying of juçara pulp aiming to obtain a “pure” powdered pulp without using carrier agents. J Dry Technol. 2019;1:1–11. doi: 10.1080/07373937.2019.1625363. [DOI] [Google Scholar]
- Quek SY, Chok NK, Swedlund P. The physicochemical properties of spray-dried watermelon powders. Chem Eng Process. 2007;46:386–392. doi: 10.1016/j.cep.2006.06.020. [DOI] [Google Scholar]
- Rascón MP, Beristain CI, García HS, Salgado MA. Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and Soy protein isolate as wall materials. LWT-Food Sci Technol. 2011;44:549–557. doi: 10.1016/j.lwt.2010.08.021. [DOI] [Google Scholar]
- Rodriguez-Amaya DB. Natural food pigments and colorants. Curr Opin Food Sci. 2016;7:20–28. doi: 10.1016/j.cofs.2015.08.004. [DOI] [Google Scholar]
- Rodriguez-Amaya DB. Update on natural food pigments: a mini-review on carotenoids, anthocyanins, and betalains. Int Food Res J. 2019;124:202–205. doi: 10.1016/j.foodres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Rutz JK, Zambiazi RC, Borges CD, Krumreich FD, da Luz SR, Hartwig N, da Rosa CG. Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr Polym. 2013;98:1256–1265. doi: 10.1016/j.carbpol.2013.07.058. [DOI] [PubMed] [Google Scholar]
- Santana AA, Cano Higuita DM, de Oliveira RA, Telis VRN. Influence of different combinations of wall materials on the microencapsulation of jussara pulp (Euterpe edulis) by spray drying. Food Chem. 2016;212:1–9. doi: 10.1016/j.foodchem.2016.05.148. [DOI] [PubMed] [Google Scholar]
- Sansone F, Mencherini T, Picerno P, d’Amore M, Aquino RP, Lauro MR. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J Food Eng. 2011;105:468–476. doi: 10.1007/s11947-012-0944-0. [DOI] [Google Scholar]
- Šeregelj V, Tumbas Šaponjac V, Lević S, Kalušević A, Ćetković G, Čanadanović-Brunet J, Nedović V, Stajčić S, Vulić J, Vidaković A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J Microencapsul. 2019;36:704–714. doi: 10.1080/02652048.2019.1668488. [DOI] [PubMed] [Google Scholar]
- Taha S, El Abd M, De Gobba C, Abdel-Hamid M, Khalil E, Hassan D. Antioxidant and antibacterial activities of bioactive peptides in buffalo’s yoghurt fermented with different starter cultures. Food Sci Biotechnol. 2017;26:1325–1332. doi: 10.1007/s10068-017-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TC, Giusti MM. Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. J Food Sci. 2008;73:241–248. doi: 10.1111/j.1750-3841.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lv Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int J Food Prop. 2019;22:2009–2021. doi: 10.1080/10942912.2019.1701011. [DOI] [Google Scholar]
- Zhang J, Zhang C, Chen X, Quek SY. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J Food Eng. 2020;1:1–42. doi: 10.1016/j.jfoodeng.2019.109744. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is availability of data and images in the manuscript.