Abstract
Quinoa is gaining more attention throughout the world because of its high nutritional, antioxidant and antimicrobial impacts. This study aimed to develop a novel functional Kishk prepared from wheat burghul replacement with quinoa seeds at 0, 25, 50, 75, and 100% levels. Changes in chemical, microbial, and sensory properties were followed during storage at room temperature for 3 months. The obtained results revealed that Kishk samples fortified with quinoa seeds had higher protein (17.18–18.37%), fat (3.00–5.99%), ash (6.64–8.01%) and fiber (1.32–2.05%) compared to control sample (16.52, 2.82, 6.00 and 1.18%), respectively for fresh samples. Furthermore, incorporation of quinoa into Kishk formulations improved amino acid profile, mineral contents, antioxidant activity and total phenols. However, addition of quinoa affected the color attributes and significant decreases in L* and b* values were noticed compared to control sample. During the storage period, overall bacterial and lactic acid bacteria counts for all samples were reduced. Coliform, mould and yeast counts of all fresh samples were less than 10 CFU/g and not detected throughout the storage. Sensory evaluation results revealed that Kishk fortified with 50% quinoa seeds exhibited good sensory properties. Therefore, fortification with quinoa could improve nutritional and functional properties of fermented dairy products.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-021-05110-8.
Keywords: Kishk, Quinoa seeds, Wheat burghul, Laban Rayeb, Fermented dairy product, Functional properties
Introduction
Functional ingredients include dietary fiber, proteins, carotenoids, polyunsaturated fatty acids, phenolic compounds, minerals, vitamins, prebiotics, and probiotics are potentially beneficial compounds found naturally in or applied to foods (Betoret et al. 2011). Many traditional food products, containing fruits, vegetables, soya, whole grains and fermented dairy products have been found to contain components with potential health benefits.
One of the main popular foods in Upper Egypt is the Egyptian Kishk. In Egypt and the eastern Mediterranean, Kishk is widely consumed and is regarded as an important diet in many populations. It is natural, nutritious and has an appealing taste for the consumers. Laban Zeer (fermented milk) combined with boiled, dried and crushed whole wheat grains (Burghul) are the essential ingredients of Kishk. It is rich in nutrients and considered as a source of various vitamins, growth factors and other beneficial constituents (EL-Gendy 1983). Different types of cereals such as rice, wheat, corn, or sorghum were combined with natural local sour milk like Laban Khad, Laban Zeer, or Laban Rayeb to produce the Kishk. Laban Rayeb is one of the fermented dairy milk which widely consumed in Egypt, obtained from natural lactic acid fermentation of milk. A good quality Laban Rayeb is a firm and uniform consistency with a clean acid taste and a smooth, glossy surface and free from cracks or gas bubbles.
Quinoa is widely cultivated for its seed, a pseudo-cereal grain which is one of the worldwide common foods. It is a gluten free grain and considered one of the best sources of vegetal protein (with high bioavailability for all essential amino acids), as its protein levels are close to those found in milk and higher than those present in cereals such as wheat, rice and maize (Gordillo-Bastidas et al. 2016). It is a good source of minerals with significant quantities of potassium, calcium, magnesium, copper, iron, and manganese and, the ratio of calcium and phosphorus is better than those of conventional cereals. Furthermore, the seeds of quinoa are rich in lipids which contain high unsaturated fatty acids such as linoleic and linolenic acids and antioxidants, with five-fold higher levels than other cereals (Koziol 1992). Due to its high nutritional quality, quinoa has recently been used as a novel functional food ingredient. It is used in the preparation of salads, soups, porridges, stews, fried patties, drinks, breakfast cereals, granola bars, beer, snacks, bread, and biscuits.
Keeping in mind the necessity for increasing the nutritional quality, additional plant food sources are needed. Wheat based diets are poor regarding essential amino acid content especially lysine as the first limiting amino acid. Thus, whole grains have the potential to make a good contribution in this respect as recognized sources of essential amino acids, dietary fiber, minerals, vitamins, and antioxidants. Several studies have been carried out to enhance the nutritional quality of Kishk by replacing burghul using various kinds of cereal such as oats, freekeh and chickpeas (Nassar et al. 2016). Therefore, this study aimed to use quinoa seeds, as a novel functional food ingredient, in partially or complete replacement of wheat burghul for producing Kishk with high nutritional characteristics. The influence of quinoa seeds on Kishk properties was assessed by determining the physicochemical, microbial, minerals contents, color, and sensory properties.
Materials and methods
Materials
Cow milk used in Laban Rayeb preparation was obtained from dairy farm at El-Kharga City, New Valley Governorate, Egypt. Quinoa seeds genotype Regalona were obtained from the New Valley Research Station (Desert Research Center), Egypt. Wheat burghul and salt were purchased from local market in Asuuit City, Asuuit Governorate, Egypt. All chemicals were of analytical grade and purchased from Sigma-Aldrich Co. This study (main experiment) was carried out in laboratories of Dairy Science Department, Faculty of Agriculture, New Valley University and some chemical compositions were carried out in Food Technology Department, Faculty of Agriculture, Suez Canal University, Egypt.
Methods
Manufacture of Laban Rayeb
The raw cow milk was filtered and then left up to 48 h at 25 ± 2 °C for coagulation. After gelation, the resultant product is called “Rayeb” according to the method described by EL-Gendy (1983). For preparation of Laban Rayeb samples which will use in Kishk making, the milk was filtered and heated to 73 °C for 15 s in water bath and cold down to 37 °C. The starter culture (lactic acid bacteria was isolated from the prepared Rayeb) was added at 1% under sanitary conditions. The inoculated milk was left at room temperature for 24 h until curdling completed. The produced Laban Rayeb contains total solid 9.21%, protein 2.65%, fat 2.74%, ash 0.65%, acidity 1.25%, pH value 4.85 and carbohydrate 3.17%.
The resultant Laban Rayeb was filtered through cheesecloth (double layer) to be concentrated (23.45% total solids) and used in making of the Kishk.
Preparation of grains
Wheat burghul and quinoa seeds were cleaned from straw, dirt and weed seeds. After that, they washed using tap water several times (this step was carried out to remove the saponins from quinoa). The grains were soaked in water in a large pan for 24 h at room temperature. Then the cereals were heated until boiling for 30 min. The cooked cereals were washed with water to remove starch and peels.
Manufacture of Kishk
In five formulas Kishk samples were prepared as following: B, Control Kishk made from 100% wheat burghul; BQB, wheat burghul was replaced by 25% quinoa seeds; BQ, wheat burghul was replaced by 50% quinoa seeds; QBQ, wheat burghul was replaced by 75% quinoa seeds and Q, wheat burghul was replaced by 100% quinoa seeds. A mixture of wheat burghul and quinoa seeds were mixed with concentrated Laban Rayeb at level of 1:2 (w/w) respectively, then kept 1 day at room temperature for fermentation. The next day, the amount of Laban Rayeb was increased until the ratio reaches 1:4 (w/w) and the mixture was kneaded again. The salt was added to the mixture with a ratio of 1%. Afterwards, the mixture was formed into small balls, placed on plates, and then dried in a hot air oven at 50 °C ± 2 for 48 h. The resultant Kishk samples were packed in plastic bags and stored at room temperature for 90 days (Tamime et al. 1997). When it was fresh and after 1, 2, and 3 months, resultant Kishk samples were analyzed for physicochemical, microbial, and sensory properties. Furthermore, minerals, amino acids, antioxidant activity, and color attributes were analyzed when fresh.
Chemical analysis
The total solids, protein, ash, fiber, acidity, and pH values of wheat burghul, quinoa seeds and Kishk samples were analyzed according to Association of Official Analytical Chemists (2007). The carbohydrate content was calculated by difference according to the following equation: Carbohydrate % = 100 − (% moisture + % protein + % ash + % fat + % fiber).
The calorific value of Kishk samples was calculated from the proximate analysis results using the following equation:
Minerals and amino acids analyses
The mineral contents of Kishk samples including calcium (Ca), phosphorus (P), potassium (K), Magnesium (Mg), iron (Fe), zinc (Zn), and copper (Cu) elements were determined using Perkin-Elmer Atomic Absorption Spectrophotometer as reported by AOAC (2007).
The amino acid contents were analyzed as reported by Association of Official Analytical Chemists (2007) using High Performance Amino Acid Analyzer (Biochrom 20, Auto sampler version, Amersham Pharmacia Biotech., Sweden). Sample (100 mg) was hydrolyzed with 5 mL of 6 N HCl at 110 °C for 24 h. The hydrolyzed sample was re-dissolved in Na citrate buffer (pH 2.2) and filtered using a 0.2 µm membrane filter then injected into the amino acid analyzer. The contents of the various recovered amino acids were presented as grams per 100 g of protein.
Preparation of methanolic extracts
The samples were homogenized to obtain fine powder. The resultant powder (2.5, 5.0, 7.5, or 10.0 g) and methanol (40 mL) were placed in flasks, kept in a magnetic agitator overnight, and then filtered through filter paper (Whatman No. 1). The methanolic extracts represented 50, 125, 187.5, and 250 mg of sample per mL, respectively.
Total antioxidant capacity was measured using DPPH radical scavenging activity method, according to Ravichandran et al. (2013). Sample extract (0.1 mL) was mixed with 3.9 mL of DPPH solution (6 × 10–5 M), and left to react for 30 min. The absorbance of the mixture was measured at 515 nm using a spectrophotometer (model 6505 UV/Vis, JENWAY, UK). The DPPH solution without extract was analyzed as a control. The antioxidant activity was calculated as follows:
where Ac is the absorbance of control and As is the absorbance of sample at 515 nm.
The IC50 value (mg/mL) was defined as the concentration of the extract which was required to quench 50% of the initial amount of DPPH· under the given experimental conditions.
The total phenolic compounds content was determined according to Barros et al. (2011). An aliquot of the extract solution was mixed with Folin-Ciocalteu reagent (5 mL, previously diluted with water 1:10 v/v) and sodium carbonate (75 g/L, 4 mL). The tubes were vortexed for 15 s and allowed to stand for 30 min. Absorbance was measured at 765 nm using a spectrophotometer (model 6505 UV/Vis, JENWAY, UK). Gallic acid was used as standard and the results were expressed as mg of gallic acid equivalents (GAE) per g.
Color attributes determination
Color attributes of the samples were estimated by measuring the L* (100 = white; 0 = black), a* (+ , red; −, green) and b* (+ , yellow; −, blue) values using a Minolta color reader (Minolta Camera, Co., Ltd., Osaka, Japan). Values are the mean of three determinations.
Enumeration of microorganisms
Counts of microorganisms were determined and examined by the method of Marshall (1992). For the enumeration of total aerobic bacteria (TAB), lactic acid bacteria (LAB), yeast and mould (YM) and coliform bacteria, samples of Kishk powder (10 g) were dispersed in 90 mL sterile physiological saline solution (0.85%). The count of TAB was enumerated by Plate Count Agar (Oxoid) after incubation at 37 °C for 48 h. LAB was counted on MRS agar containing 0.1 g/L of cycloheximide after incubation at 30 °C for 72 h in anaerobic conditions. Dichloran Rose Bengal Chloramphenicol Agar (Oxoid) was used for YM enumeration and plates were incubated at 25 °C for 5 days. Coliform bacteria were enumerated on Violet Red Bile Agar (Oxoid) after incubation at 37 °C for 24 h.
Sensory evaluation
Kishk samples were sensory evaluated by the staff members of the Dairy Science Department, Faculty of Agriculture, New Valley University. The samples were prepared as soups by mixing dried Kishk (20 g) with 170 mL of water, then heated with stirring to boiling for few mins, and cooled to 40 °C. The samples were introduced in identical glass containers for organoleptic panelists and served at 40 °C. The scoring system for sensory attributes was as follows: the flavor (1–45 points), body and texture (1–30 points), appearance and color (1–15 points) and acidity (1–10 points), according to the method of Abou-Donia et al. (1991). In another trial, 5% spice mixture containing tomato paste, paprika, onion powder, peppers red hot at ratio 1:2:2:1 respectively, were added to soups to improve the soups taste.
Statistical analysis
The obtained data were expressed as mean ± SD of triplicates and subjected to analysis of variance (ANOVA) and Duncan’s multiple range test was used to detect significant differences between samples. Significant differences were defined at p < 0.05. All analyses were performed using SPSS program (version 17.0 SPSS Inc).
Results and discussion
Chemical composition of raw materials
The chemical compositions of wheat burghul and quinoa seeds are presented in Table 1. The quinoa seeds have higher protein (14.10%), fat (6.17%), ash (3.80%) and fiber contents (3.67%) than those in wheat burghul; protein (10.95%), fat (2.10%), ash (1.95%) and fiber (1.35%). On the other hand, wheat burghul has higher carbohydrate content than quinoa seeds. In general, the chemical composition of quinoa seeds was in a good agreement with the data obtained by other authors (Miranda et al. 2013), and that of wheat burghul was in agreement with those obtained by of Yousif et al. (2018). Furthermore, Navruz-Varli and Sanlier (2016) found that protein content of quinoa seeds was higher than those found in rice, corn, rye, barley, sorghum, and wheat. Quinoa is better known than other grains for its protein quality; quinoa protein is made up of amino acids, of which eight are considered important for both children and adults alike (Repo-Carrasco-Valencia et al. 2003). Quinoa protein can provide more than 150% and 200% of schoolchildren and adults requirements, respectively (Yamani and Lannes 2012). The fiber content in quinoa seeds is higher than that reported previously in wheat or rice and are comparable with those determined for legumes (Sobota et al. 2020). Dietary fiber is considered necessary for optimal digestive health, and imparts various functional benefits. Moreover, quinoa seeds are rich in fat, which represents a source for calories, and helps in the absorption of fat-soluble vitamins such as vitamin E. Dini et al. (1992) reported that lipid content of quinoa seeds is 14.5% with an unsaturated level of about 70%, having linoleic and oleic acids in percentages of 38.9% and 27.7% respectively.
Table 1.
Chemical composition, antioxidant activity, total phenols, minerals and amino acid content of wheat burghul and quinoa seeds
| Component | Wheat burghul | Quinoa seeds |
|---|---|---|
| Chemical composition | ||
| Moisture (%) | 10.67 ± 0.23b | 9.65 ± 0.51a |
| Dry matter (%) | 89.33 ± 0.23a | 90.35 ± 0.51b |
| Protein (%) | 10.95 ± 0.35a | 14.10 ± 0.40b |
| Fat (%) | 2.10 ± 0.28a | 6.17 ± 0.32b |
| Ash (%) | 1.95 ± 0.35a | 3.80 ± 0.10b |
| Fiber (%) | 1.35 ± 0.64a | 3.67 ± 0.32b |
| Carbohydrate (%) | 72.97 ± 1.15b | 62.61 ± 0.79a |
| Acidity (%) | 0.22 ± 0.06a | 0.32 ± 0.14a |
| pH | 6.67 ± 0.02a | 6.51 ± 0.16a |
| Antioxidant activity (DPPH) | ||
| Scavenging activity—IC50 (mg/mL) | 271.67 ± 1.87a | 146.00 ± 1.87b |
| Total phenolic compounds (mg GAE/100 g) | ||
| Total phenols | 35.03 ± 1.47b | 119.55 ± 0.45a |
| Minerals (mg/100 g) | ||
| Calcium | 100.87 ± 3.67a | 156.30 ± 9.04b |
| Phosphorus | 430.57 ± 14.32b | 397.70 ± 19.38a |
| Potassium | 513.43 ± 38.57a | 876.48 ± 184.19b |
| Magnesium | 64.80 ± 16.00a | 141.15 ± 30.02b |
| Iron | 4.60 ± 0.26a | 10.83 ± 6.75b |
| Zinc | 1.53 ± 1.07a | 4.35 ± 0.54b |
| Copper | 0.42 ± 2.79a | 1.17 ± 0.24b |
| Amino acid (g/100 g Protein) | ||
| Threonine | 2.85 ± 0.07a | 3.33 ± 0.35b |
| Valine | 4.30 ± 0.14a | 4.27 ± 0.32a |
| Isoleucine | 3.20 ± 0.00a | 3.60 ± 0.26b |
| Leucine | 6.80 ± 0.00b | 6.43 ± 0.47a |
| Tyrosine | 1.37 ± 0.13a | 3.20 ± 0.71b |
| Phenylalanine | 3.80 ± 0.08a | 4.71 ± 0.25b |
| Histidine | 2.15 ± 0.21a | 3.03 ± 0.15b |
| Lysine | 2.65 ± 0.07a | 5.87 ± 0.42b |
| Methionine | 1.75 ± 0.07a | 3.27 ± 0.25b |
| Tryptophan | 1.10 ± 0.14b | 0.73 ± 0.15a |
Means (three different determinations) ± standard deviation (SD)
The pH and acidity values of quinoa seeds (6.51 and 0.32%) are similar to those of wheat burghul (6.67 and 0.22%), respectively. These values are important when food additive is added as a replacement for another grain crops which will not affect the characteristics of the final product. The pH value for quinoa fell within the range reported by Pellegrini et al. (2018) for six commercial genotypes of quinoa (6.42–6.63).
Table 1 illustrates the DPPH· radical scavenging activities (expressed as IC50) and total phenolic compounds content in quinoa and wheat burghul samples. The results indicated that quinoa has lower IC50 almost two times (146.00 mg/mL) than that of wheat burghul (271.67 mg/mL) this can be attributed to the higher phenolic compounds in quinoa (119.55 mg GAE/100 g) compared to wheat burghul (35.03 mg GAE/100 g). Therefore, quinoa seeds can be utilized as a natural potent antioxidant.
3.2 Minerals and amino acid content of wheat burghul and quinoa seeds
The minerals content of wheat burghul and quinoa seeds are shown in Table 1. Quinoa is a good source of minerals. It had the greatest macro elements; K, Mg and Ca contents and microelements; Fe, Zn, and Cu than wheat burghul. Furthermore, K was found to be the most abundant minerals and Cu was the lowest one. K is an important chemical element that helps to prevent muscle weakness, respiratory insufficiency, and hypotension in humans. Quinoa seeds are rich in minerals than other grains and contain high levels of Mg, Ca, Zn, Fe, and Cu when compared to the recommended daily allowances (Repo-Carrasco-Valencia et al. 2003).
Table 1 illustrates the essential amino acids content in wheat burghul, and quinoa seeds. The data revealed that quinoa seeds are the highest in the contents of amino acids such as lysine, threonine, isoleucine, tyrosine, phenylalanine, histidine and methionine. On the other hand, wheat burghul was higher in valine, leucine and tryptophan contents than quinoa seeds. Miranda et al. (2012) found that quinoa proteins are rich in the essential amino acids, especially lysine, methionine, and threonine which are the limiting amino acids in most traditional cereal grains. Moreover, the essential amino acid content of quinoa protein is equal to or greater than that of FAO/WHO/UNU (1985) reference patterns and similar to the amino acid composition of milk casein. Additionally, Vega-Gálvez et al. (2010) found that the essential amino acid content of quinoa protein was higher than other whole cereal grains, such as wheat, barley, rice, and/or corn.
Chemical composition of Kishk samples
The chemical composition of the resultant quinoa and wheat burghul Kishk samples are shown in Table 2. Significant differences were noticed in the chemical compositions among Kishk samples. Use of quinoa seeds (Q, 100%) as a substitute for wheat burghul gave a product with high moisture, fat, protein, ash, and fiber values whilst, low carbohydrate content compared to the control (B, 100% wheat burghul) and the rates increased with increasing the substitution level of quinoa seeds (BQB, BQ, QBQ and Q). The data declared that incorporation of 100% of quinoa seeds (Q) increased the protein, fat, ash, and fiber contents in fresh Kishk by around 10.07, 52.92, 25.09, 42.44%, respectively but decreased carbohydrate by around 17.50%. Results of Kishk blends with quinoa seeds were close to the data previously reported by Curti et al. (2017) who found that yogurt fortified with quinoa resulted in an increment in the nutrients and produced yogurt with higher protein, ash, fiber, and lipids contents than that of the control yogurt. The results also indicated that moisture content increased with increasing quinoa seeds levels. This might be due to the starch in quinoa added some functional properties like water holding capacity and high viscosity which may increase the moisture holding in Kishk samples. The higher protein, fat, ash, and fiber content of quinoa-Kishk samples can be attributed to the higher contents of these constituents in quinoa seeds than in wheat burghul. The total carbohydrate content of Kishk samples blended with quinoa seeds (25–100%) was lower than that in control due to the content of protein, lipids, ash, and fiber in Quinoa-Kishk being higher than wheat Kishk. On the other hand, moisture, protein, and fat were decreased whereas ash, fiber, and carbohydrate, were increased during storage.
Table 2.
Effect of enrichment with quinoa seeds on the chemical composition of Kishk samples during storage
| Kishk samples | Storage period (month) | Moisture | Protein | Fat | Ash | Fiber | Carbohydrate | Gross energy (kcal) |
|---|---|---|---|---|---|---|---|---|
| B | Zero | 9.69 ± 0.23cA | 16.52 ± 0.42dA | 2.82 ± 0.19cA | 6.00 ± 0.25dD | 1.18 ± 0.03cC | 63.79 ± 0.08aA | 346.62 ± 0.76bA |
| One | 8.75 ± 0.45cB | 16.11 ± 0.24dAB | 2.66 ± 0.11cAB | 6.75 ± 0.07eC | 1.21 ± 0.02cC | 64.51 ± 1.22aA | 346.45 ± 4.20aB | |
| Two | 6.81 ± 0.32dC | 15.69 ± 0.32dBC | 2.54 ± 0.11 dB | 7.52 ± 0.11 dB | 1.29 ± 0.01cB | 66.15 ± 0.15aA | 350.25 ± 1.38bB | |
| Three | 6.13 ± 0.13dD | 15.13 ± 0.15cC | 2.43 ± 0.12cB | 8.00 ± 0.24dA | 1.33 ± 0.02bA | 66.97 ± 1.31aA | 350.31 ± 4.25aB | |
| BQB | Zero | 9.50 ± 0.21cA | 17.18 ± 0.21cA | 3.00 ± 0.14cA | 6.64 ± 0.13cD | 1.32 ± 0.26bC | 62.36 ± 0.26bA | 345.14 ± 0.70bA |
| One | 9.19 ± 0.18cA | 16.86 ± 0.13cB | 2.91 ± 0.19cA | 7.20 ± 0.13dC | 1.36 ± 0.12bB | 62.48 ± 0.70aB | 343.58 ± 4.01aB | |
| Two | 7.61 ± 0.16cB | 16.52 ± 0.15cBC | 2.88 ± 0.13cA | 7.97 ± 0.13cB | 2.15 ± 0.25bAB | 62.86 ± 0.47aAB | 343.45 ± 0.43aB | |
| Three | 7.30 ± 0.15cB | 16.29 ± o.16bC | 2.77 ± 0.14cA | 8.31 ± 0.13cdA | 2.45 ± 0.15aA | 62.87 ± 0.42bAB | 341.60 ± 1.05aB | |
| BQ | Zero | 10.62 ± 0.32bA | 17.60 ± 0.31bcA | 4.33 ± 0.39bA | 7.15 ± 0.10bC | 1.88 ± 0.13aC | 58.41 ± 0.21cA | 343.05 ± 2.40aA |
| One | 9.98 ± 0.14bB | 17.32 ± 0.26bA | 4.16 ± 0.19bA | 7.50 ± 0.18cB | 2.19 ± 0.05aB | 58.85 ± 2.56bAB | 342.14 ± 9.33aB | |
| Two | 8.02 ± 0.19cC | 16.74 ± 0.36cB | 3.96 ± 0.24bA | 8.03 ± 0.16cB | 2.42 ± 0.10aA | 60.82 ± 0.64cB | 345.91 ± 2.66bB | |
| Three | 7.52 ± 0.11cD | 16.38 ± 0.23bB | 3.96 ± 0.24bA | 8.50 ± 0.20cA | 2.58 ± 0.16aA | 61.06 ± 0.79cAB | 345.44 ± 4.98aB | |
| QBQ | Zero | 11.05 ± 0.18abA | 17.99 ± 0.15abA | 4.34 ± 0.28bA | 7.47 ± 0.25bC | 1.98 ± 0.14aB | 57.18 ± 0.15eC | 339.71 ± 2.57cAB |
| One | 10.37 ± 0.22abB | 17.52 ± 0.26ab | 4.29 ± 0.23bA | 8.00 ± 0.14bB | 2.13 ± 0.05abB | 57.69 ± 0.24bB | 339.43 ± 1.88aB | |
| Two | 8.71 ± 0.14bC | 17.35 ± 0.22aB | 4.07 ± 0.18bA | 8.30 ± 9.17bB | 2.24 ± 0.06abAB | 59.33 ± 0.20bA | 343.35 ± 1.56aA | |
| Three | 8.33 ± 0.21bD | 17.04 ± 0.19aB | 3.84 ± 0.24bA | 8.96 ± 0.16bA | 2.47 ± 0.29aA | 59.36 ± 0.19cA | 340.18 ± 1.30aAB | |
| Q | Zero | 11.29 ± o.25aA | 18.37 ± 0.44 aA | 5.99 ± 0.13aA | 8.01 ± 0.12aD | 2.05 ± 0.12aB | 54.29 ± 0.66dA | 344.55 ± 1.31aA |
| One | 10.66 ± 0.41aB | 17.89 ± 0.17aA | 5.71 ± 0.15aA | 8.46 ± 0.07aC | 2.20 ± 0.18aB | 55.08 ± 1.07bA | 343.25 ± 6.12aB | |
| Two | 9.30 ± 0.38aC | 17.24 ± 0.45abB | 5.33 ± 0.21aB | 8.76 ± 0.13aB | 2.45 ± 0.08aA | 56.93 ± 0.62dA | 344.59 ± 0.92aBC | |
| Three | 9.04 ± 0.10aC | 17.03 ± 0.24aB | 5.04 ± 0.13aB | 9.27 ± 0.15aA | 2.54 ± 0.12aA | 57.08 ± 0.71dA | 341.83 ± 2.58aC |
Results are the mean of three different determinations ± standard deviation. Means that are followed by the same letter in the row and the same capital letter in the column did not differ significantly (p < 0.05). B, Control Kishk made from 100% wheat burghul; BQB, replaced at 25% Quinoa seeds; BQ, replaced at 50% Quinoa seeds; QBQ, replaced at 75% Quinoa seeds; Q, replaced at 100% Quinoa seeds
The gross energy values of Kishk samples are shown in Table 2. Small differences were noticed for the gross energy values between the Kishk samples when processed fresh and during storage. As the chemical compositions vary within and between ingredients, the measured component may often differ. Fat, protein and carbohydrates in foods provide energy for body functions and physical activities. It could be seen that the differences between Kishk samples with respect to energy values, may be due to the greater level of fat and protein in quinoa seeds and opposite the greater level of carbohydrates in wheat burghul. It is recognized that carbohydrates, protein, and fat are considered as fuel for all organisms which contribute to about 55–75, 10–15, and 15–30% respectively, of energy required by the organisms (WHO 1990).
Figure 1 shows the acidity and pH values for the different Kishk samples. The rates of acidity were increased and pH values were decreased throughout the storage. Moreover, acidity values were higher in Kishk samples fortified with quinoa seeds (BQB, BQ, QBQ and Q), than control sample (B) when processed fresh and up to the end of storage. It can be concluded that the hydrolysis of quinoa proteins by LAB during fermentation could enhance their growth and metabolic activity toward the release of organic acids during storage periods (Moore et al. 2008). Significant decrease in pH and an increase in acidity was found for Kishk samples prepared from quinoa seeds up to level 100%. Curti et al. (2017) found that yogurt samples fortified with quinoa flour revealed a much greater decrease in the pH value and increases in the total acidity values comparatively with the non-supplemented control sample during the fermentation process, possibly because of the high amino acid and mineral contents of quinoa flour that are required for yogurt starter culture to develop.
Fig. 1.
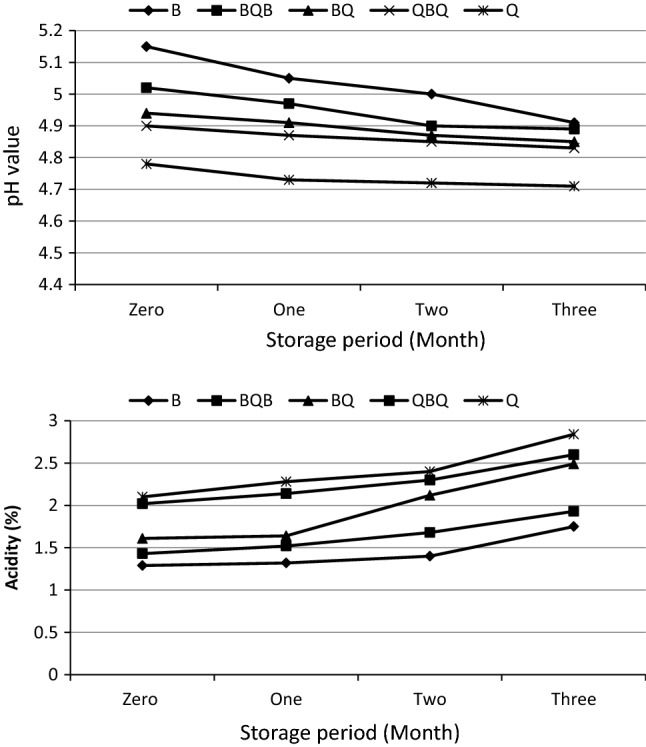
Effect of enrichment with quinoa seeds on pH values and acidity (%) of kishk during storage. B, Control Kishk made from 100% wheat burghul; BQB, replaced at 25% Quinoa seeds; BQ, replaced at 50% Quinoa seeds; QBQ, replaced at 75% Quinoa seeds; Q, replaced at 100% Quinoa seeds
Minerals content of fresh Kishk samples
Mineral contents of fresh Kishk made with various ratios of quinoa seeds were presented in Table 3. Significant differences in minerals content between control (B) and all blends with quinoa seeds (BQB, BQ, QBQ and Q) were noticed. The results indicated that K, P, Mg and Ca were the most predominant elements and Fe, Zn and Cu were the lowest among all Kishk samples. Mineral contents in Kishk samples were increased as levels of quinoa seeds increased in Kishk formulation. Kishk sample contains 100% quinoa seeds (Q) were higher in K, Mg and Ca than control (B, 100% wheat burghul). The increase in these minerals in quinoa Kishk samples might be due to the high mineral content of quinoa seeds than those in wheat burghul. It was previously reported increases in the mineral contents after the addition of quinoa flour into some foods such as tarhana and bread (Demir 2014; Bilgiçli and İbanoğlu 2015). Ballester-Sánchez et al. (2019) reported similar behavior with regard to the minerals content of bakery products fortified with quinoa. Besides, the bioavailable forms of Ca, Mg, and K are present in quinoa, so their content is considered sufficient for a healthy diet.
Table 3.
Effect of enrichment with quinoa seeds on minerals content, amino acid, antioxidant activity, total phenols and color attributes of fresh Kishk samples
| Component | Kishk samples | ||||
|---|---|---|---|---|---|
| B | BQB | BQ | QBQ | Q | |
| Minerals (mg/100 g) | |||||
| Calcium | 173.33 ± 0.79e | 188.13 ± 0.92c | 210.32 ± 1.80b | 222.11 ± 2.30a | 182.32 ± 0.91d |
| Phosphorus | 527.26 ± 0.85a | 495.46 ± 0.55b | 476.93 ± 1.79c | 462.35 ± 1.96d | 452.60 ± 0.80e |
| Potassium | 681.46 ± 0.77e | 715.21 ± 4.03d | 812.99 ± 2.43c | 875.26 ± 1.79b | 964.53 ± 1.76a |
| Magnesium | 86.30 ± 0.93e | 121.28 ± 1.83d | 171 ± 0.71c | 232.47 ± 1.69b | 261.17 ± 1.60a |
| Iron | 4.44 ± 0.23e | 6.61 ± 0.10d | 9.08 ± 0.09c | 13.50 ± 0.50b | 15.33 ± 1.17a |
| Zinc | 1.37 ± 0.16e | 1.91 ± 0.04d | 2.62 ± 0.09c | 3.36 ± 0.07b | 4.12 ± 0.15a |
| Copper | 0.41 ± 0.02c | 0.62 ± 0.04b | 0.64 ± 0.12b | 0.87 ± 0.09a | 0.99 ± 0.015a |
| Amino acid (g/100 g protein) | |||||
| Threonine | 3.15 ± 0.09b | 3.17 ± 0.04b | 3.28 ± 0.15ab | 3.23 ± 0.12ab | 3.410.03a |
| Valine | 4.71 ± 0.03a | 4.66 ± 0.21a | 4.62 ± 0.11a | 4.56 ± 0.10a | 4.53 ± 0.20a |
| Isoleucine | 3.42 ± 0.02a | 3.48 ± 0.23a | 3.57 ± 0.16a | 3.63 ± 0.24a | 3.72 ± 0.06a |
| Leucine | 7.40 ± 0.05a | 7.15 ± 0.12b | 7.12 ± 0.10b | 7.00 ± 0.12b | 7.04 ± 0.07b |
| Tyrosine | 1.55 ± 0.04e | 2.14 ± 0.07d | 2.46 ± 0.12c | 2.94 ± 0.06b | 3.37 ± 0.07a |
| Phenylalanine | 4.11 ± 0.13c | 4.25 ± 0.10c | 4.56 ± 0.11b | 4.71 ± 0.07b | 4.91 ± 0.10a |
| Histidine | 2.31 ± 0.09d | 2.44 ± 0.13 cd | 2.62 ± 0.19bc | 2.74 ± 0.09b | 3.1 ± 20.09a |
| Lysine | 3.05 ± 0.06d | 3.32 ± 0.11d | 4.53 ± 0.31c | 5.46 ± 0.11b | 6.17 ± 0.06a |
| Methionine | 1.52 ± 0.10e | 2.26 ± 0.09d | 2.66 ± 0.08c | 3.44 ± 0.09b | 4.64 ± 0.13a |
| Tryptophan | ND | ND | ND | ND | ND |
| Antioxidant activity (DPPH) | |||||
| Scavenging activity-IC50 (mg/mL) | 290.27 ± 2.01a | 245.64 ± 2.51b | 193.23 ± 2.04c | 170.68 ± 1.45d | 149.87 ± 0.59e |
| Total phenolic compounds (mg GAE/100 g) | |||||
| Total phenols | 33.45 ± 1.13d | 49.04 ± 2.03e | 64.56 ± 1.51c | 81.13 ± 1.02b | 111.64 ± 1.58a |
| Color attributes | |||||
| L* | 68.08 ± 1.61a | 61.20 ± 2.77b | 56.53 ± 0.84c | 52.40 ± 1.86d | 46.96 ± 1.72d |
| a* | 3.67 ± 0.20d | 3.79 ± 0.11 cd | 4.04 ± 0.16c | 4.34 ± 0.11b | 4.76 ± 0.12a |
| b* | 23.60 ± 0.93a | 20.48 ± 1.12b | 17.45 ± 1.29c | 14.50 ± 0.64d | 13.79 ± 0.98d |
Results are the mean of three different determinations ± standard deviation. Means that are followed by the same letter in the row did not differ significantly (p < 0.05). ND, not detected. B, Control Kishk made from 100% wheat burghul; BQB, replaced at 25% Quinoa seeds; BQ, replaced at 50% Quinoa seeds; QBQ, replaced at 75% Quinoa seeds; Q, replaced at 100% Quinoa seeds. All measurements were taken for fresh samples (at zero time)
Amino acid content of fresh Kishk samples
The main characteristic of quinoa seeds is the unique quality of its amino acid composition. Table 3 shows the amino acid composition of Kishk samples made from wheat burghul replacement with different ratios of quinoa seeds (25–100%). It is clears that incorporation of quinoa in Kishk formulations had improved the amino acid profile of the resultant Kishk samples. An increase in the essential amino acids; threonine, tyrosine, isoleucine, phenylalanine, histidine, lysine, and methionine was noticed by the addition of quinoa seeds to Kishk formulations but the contents of valine and leucine were lower when compared with the control (B). The values of leucine followed by valine, phenylalanine and lysine were higher than other amino acids. Tryptophan was not detected in all samples, because of acid hydrolysis during the digestion of samples for analysis or by destroying during the processing of Kishk samples as a result of fermentation and drying. Quinoa protein is characterized as a better amino acid balance due to the presence of methionine, lysine, and cysteine in relatively higher amounts than that are deficient in cereal proteins such as wheat and corn. Hence, quinoa is one of plant foods that able to provide human body with all essential amino acids with values close to those adopted by FAO and its amino acid composition is similar to that of milk protein (Koziol 1992).
Antioxidant activity and total phenolic compounds
The DPPH· radical scavenging activities (expressed as IC50) and total phenolic compounds contents of samples made from wheat burghul and quinoa seeds are shown in Table 3. It can be seen that the substitution of wheat burghul with quinoa seeds resulted in increased antioxidant activity of Kishk, i.e. lower IC50 values and higher total phenolic compounds. The obtained IC50 values depended on the level of substitution (BQB, BQ, QBQ and Q), and decreased dose-dependently and significant differences (p < 0.05) between IC50 values at all levels of substitution with quinoa were noticed. Increased antioxidant activity of quinoa-enriched Kishk could be attributed to the significantly higher total phenolic content and in all samples as a result of quinoa inclusion in Kishk samples (Table 3). These results are in parallel with those obtained by Jan et al. (2018) who found an increase in total antioxidants in cookies supplemented with quinoa flour. These results suggested that quinoa seeds possess advanced amounts of antioxidant activity and phenolic compounds.
Color analysis of fresh Kishk samples
Color of foods is an important feature and determines the acceptance or rejection of final product by the consumer. The color of processed Kishk samples is measured using the L* a* b* values and presented in Table 3. It could be noticed that using of quinoa seeds in Kishk formulations affected the color values and significant differences were noticed between the color attributes of the different Kishk samples. Control Kishk (B) had the highest L* value (68.08) whilst Kishk (Q, 100% quinoa seeds) were the lowest one (46.96) and lightness L* values decreased by increasing the ratio of quinoa seeds in Kishk formulas. These results may be attributed to the light color of wheat burghul and dark color of quinoa seeds; in addition, some minerals like Fe and Cu catalyzed some non-enzymatic browning reactions which lower the color values of Kishk incorporated with quinoa seeds (Bilgiçli and İbanoğlu 2015). On the other hand, darker color of quinoa is probably due to its higher ash content and phenolic pigments (Tang et al. 2015). The a* values (redness) indicated that Kishk prepared using quinoa seeds at different levels was different in its color from those of control. The a* values of Kishk samples were significantly increased (p < 0.05) when higher levels of quinoa seeds were added. Similarly, Wang et al. (2015) found that the darkness and redness of bread increased when the levels of quinoa flour were raised. The b* values (yellowness) in control Kishk was a significant increase when compared to other treatments. Color of bulghul is mainly due to natural pigments (carotenoids) that are present at different levels in wheat which, give it yellowish color lead to increase customer acceptability. The b* value showed a significant reduction when the proportion of quinoa seeds was increased. The obtained results are similar to those reported by Abd-Rabou et al. (2020) who found that addition of quinoa seeds to wheat grains caused increase in the values of redness a* and decrease in the values of lightness L* and yellowness b* in all fortified camel milk kishk samples. Similarly, Abou-Zaid et al. (2012) reported that increasing the percentage of added quinoa meal to wheat flours, caused decrease in the values of lightness L* and yellowness b* in all fortified samples.
Microbiological analysis
Despite the extended period of fermentation and drying of the Kishk samples, no spoilage or contamination by pathogenic microbes were observed, because the nature of Kishk product in terms of acidity and low water activity did not support the growth of these microorganisms (Tamime et al. 1997). The results of the microbial analyses of Kishk samples are given in Table 4. It could be noticed that total bacterial and lactic acid bacteria counts of experimental Kishk samples were higher than that of control Kishk (B) in fresh and through the storage period. Dallagnol et al. (2013) reported that hydrolysis of quinoa proteins was faster, reaching 40–100%, while hydrolysis of wheat protein has only 0–20% after 8 h of incubation, resulted in greater quantities of peptides and free amino acids were found in quinoa compared to wheat. Moreover, the nature of starch of the quinoa seeds may also influenced the microbial growth. Although, quinoa seed has a considered amount from Saponin most of it concentrated in the husk that is nutritional component approximately 4.7–30.8 g/kg had antifungal and antibacterial activities (Gómez-Caravaca et al. 2014). However, washing, soaking, and other processing steps for quinoa seeds preparation represent a way of decreasing the Saponin component (Quispe-Fuentes et al. 2013). As expected quinoa seeds used in Kishk did not affect the total bacterial and lactic acid bacteria counts but the enumeration of microorganisms of Kishk samples increased with quinoa seeds incorporation. However, total bacterial and lactic acid bacteria counts of all samples were decreased during the storage period. This decrease could be evidently attributed to the increase in acidity which controlled the rate of bacterial growth or acted as a bactericidal agent. In addition, this may be attributed to the decrease of moisture content in samples that would inhibited the growth of microorganisms. Regarding the coliform counts, mould and yeast counts of all fresh samples were less than 10 CFU/g and not detected throughout the storage period. The Kishk samples have some special characteristics including low pH (3.3–5.0) and moisture content (6–10%) resulted in a harsh environment for pathogenic microbes. In such conditions, food spoilage may not occur and the shelf life will extend.
Table 4.
Effect of enrichment with quinoa seeds on microbiological quality of kishk during storage
| Microbiological analysis | Storage period (month) | Kishk samples | ||||
|---|---|---|---|---|---|---|
| B | BQB | BQ | QBQ | Q | ||
| Total bacterial count (Log CFU/g) | Zero | 8.04 ± 0.10dA | 8.34 ± 0.11bA | 8.58 ± 0.04cA | 8.76 ± 0.01bA | 8.95 ± 0.09aA |
| One | 8.00 ± 0.06dA | 8.12 ± 0.11cdB | 8.25 ± 0.07cB | 8.62 ± 0.04bB | 8.77 ± 0.05aA | |
| Two | 7.23 ± 0.11cB | 7.43 ± 0.03bC | 7.66 ± 0.05aC | 7.72 ± 0.03aC | 7.81 ± 0.16aB | |
| Three | 6.15 ± 0.05cC | 6.17 ± 0.06cD | 6.23 ± 0.05cD | 6.38 ± 0.03bD | 7.57 ± 0.08aC | |
| Lactic acid bacteria (Log CFU/g) | Zero | 7.13 ± 0.01eA | 7.22 ± 0.02dA | 7.31 ± 0.02cA | 7.43 ± 0.03bA | 7.59 ± 0.04aA |
| One | 6.88 ± 0.02cB | 6.85 ± 0.05cB | 7.14 ± 0.03bB | 7.17 ± 0.01bB | 7.38 ± 0.03aB | |
| Two | 6.05 ± 0.04eC | 6.34 ± 0.03dC | 6.47 ± 0.06cC | 6.64 ± 0.09bC | 6.76 ± 0.02aC | |
| Three | 5.22 ± 0.09dD | 5.44 ± 0.03cD | 5.68 ± 0.03bD | 5.61 ± 0.04bD | 5.83 ± 0.04aD | |
| Coliform bacteria, mold and yeast counts (CFU/g) | Zero | < 10 | < 10 | < 10 | < 10 | < 10 |
| One | ND | ND | ND | ND | ND | |
| Two | ND | ND | ND | ND | ND | |
| Three | ND | ND | ND | ND | ND | |
Results are the mean of three different determinations ± standard deviation. Means that are followed by the same letter in the row and the same capital letter in the column did not differ significantly (P < 0.05). ND, not detected. B, Control Kishk made from 100% wheat burghul; BQB, replaced at 25% Quinoa seeds; BQ, replaced at 50% Quinoa seeds; QBQ, replaced at 75% Quinoa seeds; Q, replaced at 100% Quinoa seeds
Sensory evaluation
Consumer rejection of any of the sensory characteristics could negatively influence the overall perception of food. Organoleptic characteristics of Kishk samples are shown in Table 5. Panelists had evaluated the flavor, body and texture, appearance, and color as well as acidity during the storage period. We noticed that wheat bulghul substitution by quinoa seeds affected the sensory properties of Kishk samples. There were slight differences (p < 0.05) between the soup samples of Kishk in flavor and texture scores. However, significant differences existed with regard to appearance and color as well as acidity, this is may be due to the unconventional color and sour taste that are different from those of wheat Kishk. Statistically, 25% supplemented with quinoa seeds containing sample (BQB) had the same sensory scores compared to control Kishk. Also, addition of quinoa seeds improved all sensorial properties. Tang et al. (2015) reported that quinoa protein has WHC higher than that of oat, soybean, and wheat proteins, thus in various food applications it is expected that quinoa will improve the texture characteristics. Moreover, Demir and Kılınç 2017) found that addition of quinoa flour improved all sensorial properties of cookies samples. Also, the authors stated that more favorable cookies can be made using quinoa flour up to levels 20%. These results showed that more satisfying Kishk can be manufactured using quinoa seeds up to levels 50% (BQ), and being preferred over the control sample. It is clear that, the addition of quinoa seeds more than 50% resulted in lower total acceptability. Curti et al. (2017) found that addition of higher concentrations of quinoa flour into yogurts formulations resulted in reduction in acceptability of aroma and flavor. Although the color of the final product was darkness and thus not so appealing but, sensory color values of Kishk soup samples containing quinoa seeds were not significantly different and most of the soups samples were accepted in position of sensory color values and have the potential to be well received by consumers.
Table 5.
Effect of enrichment with quinoa seeds on sensory evaluation of Kishk during storage
| Kishk samples | Storage period (month) | Flavor (45) | Body and texture (30) | Appearance and color (15) | Acidity (10) | Total acceptability (100) |
|---|---|---|---|---|---|---|
| B | Zero | 38.17 ± 1.04bA | 21.83 ± 1.76cB | 12.17 ± 0.76aA | 9.00 ± 0.50aA | 81.17 ± 3.21dAB |
| One | 41.33 ± 1.53aA | 25.33 ± 2.08abA | 14.00 ± 1.00aA | 9.50 ± 0.50aA | 90.17 ± 5.01bA | |
| Two | 42.00 ± 1.00aAB | 26.17 ± 0.76aA | 14.00 ± 1.00aA | 9.50 ± 0.50aA | 91.67 ± 0.76aAB | |
| Three | 38.00 ± 1.00bAB | 23.17 ± 0.76bcB | 13.00 ± 1.00aA | 8.67 ± 0.29aA | 82.83 ± 0.58cAB | |
| BQB | Zero | 37.00 ± 1.00bA | 25.17 ± 0.76bA | 12.17 ± 0.76bAB | 8.00 ± 0.50aAB | 82.33 ± 1.76bAB |
| One | 41.33 ± 0.76aAB | 26.00 ± 1.00abA | 14.00 ± 1.00abAB | 9.00 ± 1.00aB | 90.33 ± 3.40aAB | |
| Two | 42.00 ± 1.00aBC | 27.00 ± 0.50aA | 14.00 ± 1.00aAB | 8.83 ± 0.29aAB | 91.83 ± 0.76aBC | |
| Three | 38.33 ± 0.29bAB | 25.00 ± 1.00bA | 12.00 ± 1.00bB | 8.00 ± 1.00aAB | 83.33 ± 2.25bB | |
| BQ | Zero | 37.00 ± 0.50cA | 26.00 ± 0.50bA | 13.00 ± 0.87abA | 7.00 ± 0.50cBC | 83.00 ± 1.00cA |
| One | 42.00 ± 0.50aA | 27.00 ± 1.00abA | 14.00 ± 0.50aA | 8.00 ± 0.50bB | 91.00 ± 1.73bA | |
| Two | 43.00 ± 1.00aA | 28.00 ± 1.00aA | 14.00 ± 0.50aA | 9.00 ± 0.50aAB | 94.00 ± 1.32aA | |
| Three | 39.00 ± 1.00bA | 26.33 ± 0.76bA | 12.00 ± 0.50bB | 8.00 ± 0.50bAB | 85.33 ± 0.29cA | |
| QBQ | Zero | 35.00 ± 1.00 dB | 26.00 ± 1.00aA | 11.00 ± 1.00aBC | 6.00 ± 1.00bC | 78.00 ± 3.61cB |
| One | 39.00 ± 1.00bBC | 27.00 ± 1.00aA | 12.00 ± 1.00aBC | 7.00 ± 0.58abB | 85.00 ± 3.00abAB | |
| Two | 41.00 ± 0.58aBC | 27.00 ± 1.00aA | 12.00 ± 1.00aB | 8.00 ± 1.00aB | 88.00 ± 2.50aCD | |
| Three | 37.00 ± 1.0cB | 25.17 ± 1.00aA | 12.00 ± 0.50aB | 7.00 ± 0.50abBC | 81.17 ± 0.29bcBC | |
| Q | Zero | 33.00 ± 1.00cC | 24.50 ± 0.50cA | 11.00 ± 0.50bC | 6.00 ± 0.50bC | 74.50 ± 2.00dC |
| One | 38.17 ± 1.26abC | 26.33 ± 0.76abA | 11.33 ± 0.76abC | 7.00 ± 0.50abB | 82.83 ± 0.58bB | |
| Two | 39.67 ± 0.76aC | 27.50 ± 1.00aA | 12.00 ± 1.00aB | 8.00 ± 1.00aB | 87.67 ± 1.76aD | |
| Three | 37.00 ± 1.00bB | 25.50 ± 1.04bcA | 11.00 ± 0.50abB | 6.00 ± 1.00bC | 79.50 ± 1.04cC |
Results are the mean of three different determinations ± standard deviation. Means that are followed by the same letter in the row and the same capital letter in the column did not differ significantly (p < 0.05). B, Control Kishk made from 100% wheat burghul; BQB, replaced at 25% Quinoa seeds; BQ, replaced at 50% Quinoa seeds; QBQ, replaced at 75% Quinoa seeds; Q, replaced at 100% Quinoa seeds
In the second trial (Fig. S1), during sensory evaluation of Kishk samples which prepared as soups by using spices mixture (tomato paste, paprika, onion powder and peppers red hot) at level 5% to improving the taste. This treatment improved the score acceptably of soup properties in terms and enhanced the sensory properties. Some arbitrators reported that addition of the spices has enhanced the Kishk taste and the replacement of wheat bulghul with quinoa seeds could be increased to 100%.
Conclusion
The present study showed that inclusion of quinoa seeds into Kishk formulations increased the protein, fat, ash, fiber, mineral contents and antioxidants. In addition, the sensory evaluation results revealed that acceptability of the Kishk samples were enhanced by the addition of quinoa seeds. Generally, substitution levels up to 50% quinoa seeds received the most acceptable sensory scores for Kishk samples. Modification of the technological procedures afforded the inclusion of high levels of quinoa in Kishk processing and gave the possibility to develop a novel highly nutritive product. Thus, supplementation with quinoa is not only important in developing countries but also in developed countries where there is a need to introduce new and more nutritious food products and further studies could be performed to formulate other products with quinoa as raw material.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AOAC
Association of Official Analytical Chemists
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- TAB
Total aerobic bacteria
- LAB
Lactic acid bacteria
- FAO
Food and Agriculture Organization
- WHO
World Health Organization
- UNU
United Nations University
- CFU
Colony forming unit
Author contributions
All authors are equal contribution for this paper.
Funding
The authors have no support or funding to report.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Conflict of interest
Authors have no conflicts of interest to declare for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Rabou HS, Shehata MG, El Sohaimy SA, Awad SA. Functional probiotic quinoa camel milk kishk. J Food Process Preser. 2020;44:e14681. doi: 10.1111/jfpp.14681. [DOI] [Google Scholar]
- Abou-Donia SA, Attia IA, Khattab AA, EL-Shenawi Z, Formulation of dried cereal fermented milks with prolonged storage life. Egyptian J Dairy Sci. 1991;19:283–299. [Google Scholar]
- Abou-Zaid AA, El-Faham SY, Emam WH. Use of quinoa meal to produce bakery products to celiac and autism stuffs. Int J Sci Res. 2012;3:1344–1354. [Google Scholar]
- Ballester-Sánchez J, Millán-Linares MC, Fernández-Espinar MT, Haros CM. Development of healthy, nutritious bakery products by incorporation of quinoa. Foods. 2019;8:379. doi: 10.3390/foods8090379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros L, Cabrita L, Boas MV, Carvalho AM, Ferreira ICFR. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011;127:1600–1608. doi: 10.1016/j.foodchem.2011.02.024. [DOI] [Google Scholar]
- Betoret E, Betoret N, Vidal D, Fito P. Functional foods development: trends and technologies. Trend Food Sci Technol. 2011;22:498–508. doi: 10.1016/j.tifs.2011.05.004. [DOI] [Google Scholar]
- Bilgiçli N, İbanoğlu Ş. Effect of pseudo cereal flours on some physical, chemical and sensory properties of bread. J Food Sci Technol. 2015;52:7525–7529. doi: 10.1007/s13197-015-1770-y. [DOI] [Google Scholar]
- Curti CA, Vidal PM, Curti RN, Ramón AN. Chemical characterization, texture and consumer acceptability of yogurts supplemented with quinoa flour. Food Sci Technol. 2017;37:627–631. doi: 10.1590/1678-457x.27716. [DOI] [Google Scholar]
- Dallagnol AM, Pescuma M, De Valdez GF, Rollán G. Fermentation of quinoa and wheat slurries by Lactobacillus plan- tarum CRL 778: proteolytic activity. Appl Microbiol Biotechnol. 2013;97:3129–3140. doi: 10.1007/s00253-012-4520-3. [DOI] [PubMed] [Google Scholar]
- Demir MK. Use of quinoa flour in the production of gluten-free tarhana. Food Sci Technol Res. 2014;20:1087–1092. doi: 10.3136/fstr.20.1087. [DOI] [Google Scholar]
- Demir MK, Kılınç M. Utilization of quinoa flour in cookie production. Int Food Res J. 2017;24:2394–2401. [Google Scholar]
- Dini A, Rastrelli L, Saturnino P, Schettino OA. Compositional study of Chenopodium quinoa seeds. Nahrung. 1992;36:400–404. doi: 10.1002/food.19920360412. [DOI] [Google Scholar]
- EL-Gendy SM, Fermented foods of Egypt and the Middle East. J Food Prot. 1983;46:358–367. doi: 10.4315/0362-028X-46.4.358. [DOI] [PubMed] [Google Scholar]
- FAO/WHO/UNU (1985) Food and agriculture organization of the United States/World Health Organization/United Nations University. In: Proceedings of the energy and protein requirements. Report of a Joint FAO/WHO/UNU Meeting, World Health Organization, Geneva, Switzerland
- Gómez-Caravaca AM, Iafelice G, Verardo V, Marconi E, Caboni MF. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd) Food Chem. 2014;157:174–178. doi: 10.1016/j.foodchem.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Gordillo-Bastidas E, Díaz-Rizzolo DA, Roura E, Massanés T, Gomis R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: an integrative review. J Nutr Food Sci. 2016;6:497. doi: 10.4172/2155-9600.1000497. [DOI] [Google Scholar]
- Jan KN, Panesar PS, Singh S. Optimization of antioxidant activity, textural and sensory characteristics of gluten-free cookies made from whole indian quinoa flour. LWT. 2018;93:573–582. doi: 10.1016/j.lwt.2018.04.013. [DOI] [Google Scholar]
- Koziol MJ. Chemical composition and nutritional value of quinoa (Chenopodium quinoa Willd.) J Food Compos Anal. 1992;5:35–68. doi: 10.1016/0889-1575(92)90006-6. [DOI] [Google Scholar]
- Marshall RT. Standard methods for the examination of dairy products. 16. Washington DC: American Public Health Association; 1992. [Google Scholar]
- Miranda M, Vega-Gálvez A, Quispe-Fuentes I, Rodriguez MJ, Maureira H, Martinez EA. Nutritional aspects of six quinoa (Chenopodium quinoa willd.) ecotypes from three geographical areas of Chile. Chilean J Agric Res. 2012;72:175–181. doi: 10.4067/S0718-58392012000200002. [DOI] [Google Scholar]
- Miranda M, Vega-Gálvez A, Martínez EA, López J, Marín R, Aranda M, Fuentes F. Influence of contrasting environments on seed composition of two quinoa genotypes: nutritional and functional properties. Chilean J Agric Res. 2013;73:108–116. doi: 10.4067/S0718-58392013000200004. [DOI] [Google Scholar]
- Moore MM, Dal Bello F, Arendt EK. Sourdough fermented by Lactobacillus plantarum FST 1.7 improves the quality and shelf life of gluten-free bread. Eur Food Res Technol. 2008;226:1309–1316. doi: 10.1007/s00217-007-0659-z. [DOI] [Google Scholar]
- Nassar KS, Shamsia SM, Attia IA. Improvement of the nutritional value of cereal fermented milk: 1. Soft kishk like. J Food Process Technol. 2016;7:619–625. [Google Scholar]
- Navruz-Varli SN, Sanlier, Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.) J Ceram Sci. 2016;69:371–376. doi: 10.1016/j.jcs.2016.05.004. [DOI] [Google Scholar]
- Pellegrini M, Lucas-gonzales R, Ricci A, Fontecha J. Fatty acid, polyphenolic profile, techno-functional and anti- oxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind Crop Prod. 2018;111:38–46. doi: 10.1016/j.indcrop.2017.10.006. [DOI] [Google Scholar]
- Quispe-Fuentes I, Vega-Gálvez A, Miranda M, Lemus-Mondaca R, Lozano M, Ah-Hen KA. Kinetic approach to saponin extraction during washing of quinoa (Chenopodium quinoa Willd.) seeds. J Food Proc Eng. 2013;36:202–210. doi: 10.1111/j.1745-4530.2012.00673.x. [DOI] [Google Scholar]
- Ravichandran K, Saw NMMT, Mohdaly AAA, Gabr MMA, Kastell A, Riedel H, Cai Z, Knorr D, Smetanska I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res Int. 2013;50:670–675. doi: 10.1016/j.foodres.2011.07.002. [DOI] [Google Scholar]
- Repo-Carrasco-Valencia R, Espinoza C, Jacobsen SE. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule) Food Rev Int. 2003;19:179–189. doi: 10.1081/FRI-120018884. [DOI] [Google Scholar]
- Sobota A, Świeca M, Gęsiński K, Wirkijowska A, Bochnak J. Yellow- coated quinoa (Chenopodium quinoa Willd)—physicochemical, nutritional, and antioxidant properties. J Sci Food Agric. 2020;100:2035–2042. doi: 10.1002/jsfa.10222. [DOI] [PubMed] [Google Scholar]
- Tamime AY, Muir DD, Barclay MNI, Khas-Kheli M, Mcnulty D. Laboratory-made Kishk from wheat, oat and barley: 2. Compositional quality and sensory properties. Food Res Int. 1997;30:319–326. doi: 10.1016/S0963-9969(97)00055-0. [DOI] [Google Scholar]
- Tang Y, Li X, Zhang X, Chen B, Liu R, Tsao R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd genotypes. Food Chem. 2015;166:380–388. doi: 10.1016/j.foodchem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Vega-Gálvez AV, Miranda M, Vergara J, Uribe E, Puente L. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.) an ancient Andean grain: A review. J Sci Food Agric. 2010;90:2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- Wang S, Opassathavorn A, Zhu F. Influence of Quinoa flour on quality characteristics of cookie, bread and Chinese steamed bread. J Text Stud. 2015;46:281–292. doi: 10.1111/jtxs.12128. [DOI] [Google Scholar]
- AOAC (2007) Official methods of analysis, 18 ed. Revision 2, Helrich K (ed) Association of Official Analytical Chemists. Washington D.C. USA
- WHO (1990) Diet nutrition and prevention of chronic diseases report of a WHO study group. Technical Report series, vol 797. WHO, Geneva, pp 54–61 [PubMed]
- Yamani BV, Lannes SCD. Applications of quinoa (Chenopodium Quinoa Willd.) and Amaranth (Amaranthus Spp.) and their influence in the nutritional value of cereal based foods. Food Public Health. 2012;2:265–275. [Google Scholar]
- Yousif SI, Bayram M, Kesen S. Characterization of Volatile Compounds of Bulgur (Antep Type) Produced from Durum Wheat. J Food Qual. 2018;8564086:9. doi: 10.1155/2018/8564086. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


