Abstract
The aim of the present study was to evaluate the ability of fish collagen peptides (FCP) derived from the skin of great hammerhead shark (Sphyrna mokarran) in attenuating the high fat diet-alcohol induced hyperlipidemia. The oral supplementation of FCP in high fat diet-alcohol fed experimental rats confirmed the regulation of body weight to normal level. The FCP treated group revealed the efficient lipid lowering ability by enhancing the cholesterol metabolism. Western blot analysis of the lipid metabolic enzymes revealed that the oral-intake of FCP has down-regulated the expression levels of fatty acid synthase and 3-Hydroxy-3-methylglutaryl-CoA reductase (HMGCR). Simultaneously, the expression levels of Lecithin–cholesterol acyltransferase (LCAT) in liver was up-regulated. Histopathology analysis of liver tissues demonstrated that the FCP treated group maintained normal liver parenchyma with moderate inflammatory infiltration, whereas the statin treated group developed centrilobular fibrosis, atrophy of hepatocytes and moderate inflammatory infiltration. Oral dietary supplementation of FCP enhanced the activity levels of both superoxide dismutase and catalase enzymes and, lowered the levels of lipid peroxidation in liver tissues.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-021-05118-0.
Keywords: Fish collagen peptides, Hypolipidemia, Antioxidant, Hepatoprotective activity
Introduction
Alcohol consumption is considered as the third largest risk factor for disease burden worldwide, affecting 2.5 million deaths every year (WHO, 2014). Alcoholic hyperlipidemia and associated liver diseases cause around 4.5% of total death and 2% of total DALY (Disability adjusted life years). Consumption of alcoholic beverages with deep fried food is one of the most commonly observed social habit which may lead to fatty acid accumulation in liver by facilitating the fatty acid esterification to form triglycerides, phospholipids and cholesterol esters (Duly et al. 2015). This diminishes the conversion rate of cholesterol into bile salts resulting hypercholesterolemia. Hypercholesterolemia enhances the production of reactive oxygen species (ROS) eventually destructing the hepatic antioxidant defense system, tissue damage and organ dysfunction (El-Tantawy, 2015). Lipid accumulation is the prominent cause of alcoholic hepatitis (through the onset of inflammation and necrosis) and liver cirrhosis (characterized by fibrosis and distortion of normal architecture of liver) (Bruha et al. 2012). Heat oxidation of unsaturated fatty acids leads to the generation of hydroperoxides (Plasma-8-isoprostane) (Nawar, 1998). Dietary intake of PUFA rich fish oil along with alcohol generates microsomal dienes by methelene flanking at the regions of unsaturation. This increases the susceptibility to lipid peroxidation and consequential activation of P450 2E1 that leads to severe liver injury (Bruha et al. 2012). The hypolipidemic drug (statin) reduces the cholesterol biosynthesis by inhibiting the HMGCR in liver (Lim, 2013).
Collagen is the most abundant animal protein, rich in imino acids and hydrophobic amino acids (Kumar et al. 2017; Dara et al. 2020a). Previous reports by Woo et al. (2018), suggested that collagen hydrolysates possess anti-obese and lipid lowering bioactive ability. Bioactive peptides maintained proper liver functioning, up-regulated the nitric oxide levels by reducing the levels of pro-inflammatory cytokines (Koyama and Kusubata, 2013). Marine collagen peptides were found to be proficient in preventing the alcoholic liver injury (Lin et al. 2012). Great hammerhead shark (S. mokarran) is one of the highly demanded species for their fins, meat and liver oil world wide. Several studies demonstrated the in vitro bioactive properties such as antioxidant and anti hypertensive properties of peptides derived from fish skin (Dara et al. 2020b,2020c). Our previous studies revealed that the collagen peptides from the skin of great hammerhead shark possessed excellent radical scavenging potential (Divya et al. 2018a) which might be suitable as a dietary component to neutralize the diet induced oxidative stress and related complications. No reports are available on the ability of fish collagen peptides to counteract the effect of heat oxidized fat and alcohol intake to the best of our knowledge. Thus, in the present study, an attempt was made to evaluate the ability of FCP to counteract the thermally oxidized high fat diet-alcohol induced alteration in lipid metabolism and oxidative stress induced liver damage.
Materials and methods
Skin of great hammer head shark was collected from local fish market (Thopumpady fish harbor, Kochi, Kerala, India). Atorvastatin was purchased from local medical store. Proteolytic enzymes (Pepsin from porcine gastric mucosa, Papain from papaya latex, Protease from Bacillus licheniformis antibodies used for western blotting and ELISA kits were purchased from Sigma Aldrich. Transfer stacks for western blot, BCIP/NBT solution, pre-cast gels and reagents used for SDS-PAGE analysis were procured from Thermofisher Scientific. The chemicals used were analytical grade.
HPLC system (HITACHI L-2130) equipped with fluorescence detector (HITACHI L-2485) and column oven (HITACHI L-2350) and two Shimadzu LC-10AT VP pumps, Light microscope (Leica DM 750) equipped with camera (Leica ICC50 HD), Lyophilizer (Lark), Mini electrophoretic gel tank (vertical) (Life Technologies), Power pack (Bio-Rad PowerPac HC), Spectrophotometer (Shimadzu UV-1601), Western blotting apparatus (iBlot 2 dry blotting system Life Technologies),
Preparation of fish collagen peptides (FCP)
Acid soluble collagen (ASC) was extracted from the skin of great hammerhead shark (S. mokkaran), according to the method described by Divya et al. (2018a); Divya et al. (2018b). The skin was treated with 0.1 N NaOH for 72 h at 4 °C to remove the non-collagenous impurities. The alkali-treated skins were washed with chilled distilled water to remove the traces of alkali until the solution attains neutral pH. The resultant swollen skins were treated with 0.5 M acetic acid for 72 h at 4 °C with gentle stirring to complete the extraction process. The solubilized` protein content was salted out by adding 2.8 M NaCl into the solution with continuous stirring. The precipitated collagen content was dialyzed against 0.5 M acetic acid for 3 days at 4 °C and then dialyzed against distilled water for the complete removal of salt, acid and other impurities. The samples were characterized for the yield of extraction and the purity of ASC extract was analyzed through the determination of total protein content using microkjeldahl method (AOAC 2000) as well as collagen content was quantified using Sirius Red dye binding method (Taskiran et al. 1999).
The amino acid composition of ASC was determined using an amino acid analyser (HPLC system) according to method described by Ishida et al. (1981). The flow rate was constant at 0.4 ml/min, the oven temperature was set at 60 °C also the fluorescence excitation and emission wavelengths were 340 nm and 450 nm, respectively. The samples for the analysis of amino acid composition were prepared by digesting the lyophilized collagen in 6 N HCl at 110 °C for 24 h under nitrogen.
For the preparation of fish collagen peptides, the ASC extract was primarily heat denatured by incubating in a boiling water bath for 30 min prior to the enzymatic fragmentation. Then it was hydrolyzed by three consecutive enzymes including, pepsin, papain and protease. The crude hydrolysate after enzymatic digestion was subjected to fractionation by passing through sephadex G-25 packed gel filtration column (flow rate 0.5 ml/min). The fractions were collected and characterized for in-vitro antioxidant activity (ABTS radical scavenging activity assay) according to the method described by Lee et al. (2014). The peptides fractions with maximum radical scavenging activity were pooled together and subjected to anion exchange fractionation by passing through DEAE-Sephadex A-25 resins (flow rate 0.5 ml/min). 0.2 M tris–HCl buffer (pH 8.3) with linear gradient concentration of NaCl (0.1–1.0 M) was used as the elution buffer. The fractions obtained were desalted by passing through sephadex G-25 column. The collected peptide fractions with maximum free radical scavenging activity were pooled together and subsequently lyophilized and, stored at − 20 °C, until further in vivo studies.
Animal experiment
Male Wistar strain albino rats weighing 250–300 g were selected for the animal experimental study. A total no. of twenty-four animals were randomly selected and divided into four groups. All animals were housed individually in hygiene and standard environmental conditions of temperature 28 ± 2 ˚C, humidity 60–70%, 12 h light and 12 h dark cycle in polypropylene cages. During the experimental period, the animals were fed with a standard diet (M/s Sai Foods, Bangalore, India; the diet contained carbohydrate 56.2%, crude protein 22%, ash 7.5%, total fat 4.2%, crude fibre 3%, glucose 2.5%, vitamin 1.8%, sand silica 1.4%, calcium 0.8%, phosphorus 0.6%, and provide metabolizable energy of 3600 kcal.) and water ad libitum. The rats were acclimatized for one week at room temperature (22–25 °C) with 12 h light/dark cycles, prior to begin with the experiment. The duration of the experiment was 60 days. The groups were treated as follows.
The optimum dosage regimen used for FCP administration was selected based on the previously conducted acute and sub-acute toxicity studies in our laboratory (Hema et al. 2016). In the present study, the experimental rats were orally administered with 600 mg FCP per kg body weight per day.
Normal control: Animals were fed with standard diet.
Positive control: Animals were fed high fat diet with daily alcohol consumption (Diet consumption: 15% pre-heated fish oil (w/w) and 15% butter fat (w/w) + 2% alcohol (v/v) along with standard diet) (Sreerekha et al. 2021).
FCP treated: FCP at a dosage of 600 mg per kg body weight per day along with high fat diet and alcohol (Diet consumption: 15% pre-heated fish oil (w/w) and 15% butter fat (w/w) + 2% alcohol (v/v) along with standard diet).
Statin treated: Atorvastatin at a concentration of 160 µg per kg body weight per day along with high fat diet and alcohol.
Body weight gain of experimental animals
The percentage of body weight gain of rats in each group was calculated from their weight gain during the period of experiment to the initial body weight.
| 1 |
where, W1-Initial body weight; W2-Final body weight after 60 days.
Sample collection
The experimental rats were maintained and performed as per the guidelines on the regulation of scientific experiments on animals of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India and approved by the Institutional Animal Ethics Committee of ICAR-Central Institute of Fisheries Technology. At the end of the experiment, rats were sacrificed by pentabarbitone anaesthesia (i/p administration (Dose: 0.02 mg/kg body weight); the excised liver tissue was washed with ice-cold saline solution. A portion of tissue was stored in 10% buffered formalin solution for histopathology analysis. The blood samples were collected in clean and dried tubes through cardiac puncture from normal and experimental group of rats without using any anticoagulant and the serum separated through centrifugation was used for the analyses of hematological parameters.
Lipid profile analysis of serum and liver tissue homogenate
The lipid profile includes total cholesterol (TC), triglycerides (TG), High density lipoprotein- cholesterol (HDL-C), Low density lipoprotein-cholesterol (LDL-C), and Very low-density lipoprotein- cholesterol (VLDL-C) in serum as well as cholesterol accumulation in liver tissue were analyzed. TC content was measured following the ferric chloride-sulphuric acid method (Zlatkis et al. 1953) with slight modifications. Liver homogenate for the determination of cholesterol accumulation was prepared in chloroform–methanol mixture (2:1) containing 0.01% butylated hydroxytoluene (BHT). Standard curve was plotted using cholesterol standard (R2 0.988 and y = 0.003x + 0.017). TG in serum samples were determined by the method described by Handel and Zilversmit, (1957) with suitable modifications. Triolein was used as standard. Serum levels of HDL-C were determined using phosphotungstate/Mg2+ method with slight modifications. Levels of LDL-C and VLDL-C in serum were estimated by the following method as described by Friedwald et al. (1972).
| 2 |
where K = 5, Triglyceride/Cholesterol ratio in VLDL-C is 5:1
| 3 |
where, Total Cholesterol = HDL-C + LDL-C + VLDL-C
Effect of FCP on HMG CoA reductase activity
Activity levels of HMGCR enzyme in hepatic tissues were determined by measuring the HMG Co-A /Mevalonate ratio (Rao and Ramakrishnan 1975). The indirect measurement of HMGCR activity includes, the ratio of absorbance of HMG Co-A to the absorbance of mevalonate in hepatic tissue homogenate. The tissue homogenate was mixed with equal volume of perchloric acid and centrifuged after 5 min incubation at room temperature. The supernatant was then treated with freshly prepared hydroxylamine reagent. Enzyme activity was inhibited by mixing with alkaline hydroxylamine reagent for control samples. The mixtures were then allowed to react with ferric chloride reagent, incubated for 10 min at room temperature and the absorbance was read at 540 nm against similarly treated saline-arsenate blank. The HMG Co-A/Mevalonate ratio is inversely proportional to the enzyme activity.
Western blotting analysis
The expression levels of FAS and HMGCR in hepatic tissues and LCAT in serum samples were analyzed by western blotting analysis. The tissue homogenate and serum samples were prepared using RIPA buffer before the gel run. The samples were transferred to nitrocellulose membrane using an iBlot 2 dry blotting system. Both the primary and secondary antibodies were diluted as per suggested by the kit manufacturers using 1X PBS solution. The membrane was treated with blocking buffer for 10 min and incubated for 12 h in primary antibodies (anti-FAS antibody, anti-LCAT antibody and anti-HMGCR antibody produced in rats) at 4 °C with continuous stirring. The membrane was washed 3 times using 1X PBS solution and treated with secondary antibody for 1 h at room temperature with continuous stirring. After treatment with secondary antibody (anti-rat IgG-Alkaline phosphatase antibody produced in goat) the membrane was washed 3 times using PBST solution and incubated in BCIP/NBT solution for 10 min at room temperature with continuous stirring. The bands obtained were analyzed and photographed.
Histopathology analysis of liver tissue
Histological changes occurred on the hepatic tissue during the induction of hyperlipidemia, and the effect of FCP on hepatic tissue, liver tissues after hematoxylin and eosin (H&E) staining were observed using a light microscope.
In-vivo antioxidant assay
The samples were analyzed for the SOD and catalase enzymes according to the methods described by Mishra and Fridovich (1972) and Takahara et al. (1960) with slight modifications. The percentage of inhibition of SOD in terms of its capacity to prevent the free radical mediated autoxidation of epinephrine to form adrenochrome. Samples diluted in 0.1 M carbonate-bicarbonate buffer were mixed with epinephrine and spectrophotometric measurements of the change in absorbance were read at 480 nm for 180 s (Sun and Zigman 1978). The percentage of inhibition and the enzyme activity was calculated according to the equations given below.
| 4 |
where, ‘A’ is the absorbance of epinephrine at 480 nm, ‘∆A’ is the change in absorbance occurred per minute.
| 5 |
where, PI is percentage of inhibition, v is volume of serum/tissue homogenate (ml) and c is protein concentration in sample (mg/ml).
The catalase enzyme activity measurement was carried out from the rate of decomposition of hydrogen peroxide substrate. The decomposition was measured from the decrease in absorbance at 240 nm. The enzyme activity was measured using the equation given below.
| 6 |
where, ‘∆A’ is the change in absorbance at 240 nm per minute, ‘c’ is protein concentration in tissue or serum (mg/ml) and molar extinction coefficient of hydrogen peroxide is 43.6 M−1 cm−1.
The levels of lipid peroxidation of hepatic tissues of experimental rats were measured using thiobarbituric acid reactive substances (TBARS) assay method (Buege et al.1978) with slight modifications. The level of lipid peroxidation was expressed in terms of concentration of malonaldehyde (mM). The concentration of malonaldehyde formed was calculated using an extinction coefficient of 1.56 × 102 M−1 cm−1.
Statistical analyses
The experiments were carried out in triplicates independently. The mean and standard deviation values were calculated using the statistics programme SPSS (SPSS.16.0 for windows, SPSS Inc., Chicago, IL).
Results and discussion
Characterization of acid soluble collagen
The yield of ASC obtained from the skin of S. mokarran was found to be 41.0 ± 0.2% (dry weight basis). Previous investigations from our laboratory recorded a yield of 73.4% of ASC from airbladder of striped cat fish (Divya et al. 2018b). The total protein and collagen content of obtained ASC extract was found to be 96.6% and 937.9 ± 1.8 µg of collagen content/mg of ASC extract. The quantification of total collagen content using Sirius Red dye binding method can be correlated with the amount of hydroxyproline in the molecule (Taskiran et al. 1999). The time of extraction and temperature of extraction used for the extraction process determines the extent of solubilization of collagen. Moreover, the source of raw material, method of pre-treatment, and parameters of the extraction process influence the yield of collagen (Kumar et al. 2017).
The amino acid composition analysis and the chromatogram of obtained ASC are depicted in Supplementary material Table 1 and Fig. 1. The amino acid composition analysis in the present study is in-accordance with the acid soluble collagen obtained from the air bladder of P hypophthalmus (Divya et al. 2018b). The obtained collagen contains high glycine content which is similar with other pervious reports (Dara et al. 2020a; Kumar et al. 2017). Moreover, it was noticed that the obtained ASC contains 22.8% of imino acids content (Proline + Hydroxyproline), whereas the amino acids such as tryptophan, cysteine and methionine were found to be absent. It has been reported that hydroxy proline determines the functional properties and plays important role in the stabilization of triple helical strands due to its hydrogen bonding ability of its hydroxyl groups. The presence of carboxylic amino acids such as aspartic acid and glutamic acid determines the hydrophilic nature of collagen molecule (Dara et al. 2020c). Further, the obtained ASC has good levels of total essential amino acids such as histidine, threonine, valine, phenylalanine, isoleucine, leucine and lysine. The protein molecules with higher hydrophobic amino acid content were reported to possess antioxidant activity and anti-hypertensive activity (Dara et al. 2020b). In the present study, hydrophobic amino acids such as alanine, valine, phenylalanine, isoleucine and proline were present in the extracted ASC. In the present study, the total essential amino acid and total hydrophobic amino acid content of ASC obtained from the skin of great hammerhead shark (S. mokarran) was found to be 17.4% and 26.5%, respectively. The hydrophobic amino acid content in bioactive molecules depends on the molecular size of predominant peptides (Dara et al. 2020c). Further, the hydrophobic amino acid content is one of the major factors that influences radical scavenging activity (Dara et al. 2020b).
Fig. 1.
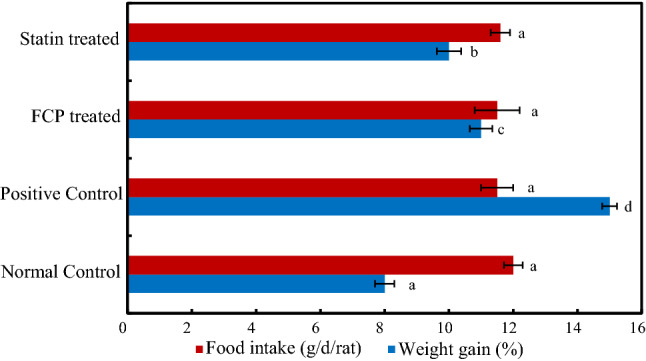
Graphical representations of the percentage of body weight gain during the experimental period in rats and the mean value of food intake in g/day/rat. *Mean values ± standard deviation, n = 6. Values that have different superscript letters differ significantly (P < 0.05) with each other
The crude fish collagen hydrolysate was found to have 88.5% radical scavenging activity which is equivalent to 19.7 µg/ml BHA. The collagen hydrolysates were subjected to gel filtration and column anion exchange chromatography to collect peptide fractions. The purified peptide fraction found to have maximum free radical scavenging activity of 94.6%. It has been reported that the several factors such as type of enzyme, substrate and peptide composition might influence the ABTS radical scavenging activity (Dara et al. 2020b). Nevertheless, in the present study the ABTS radical scavenging activities of the peptide fraction reflects the similar ability in quenching ABTS radical. Overall, the amino acid composition analysis demonstrated that the ASC extracted from the skin of great hammerhead shark (S. mokarran) was rich in hydrophobic amino acids and thus it can be considered as a remarkable source of bioactive peptides. The peptide fraction with 94.6% free radical scavenging activity was selected for further in vivo evaluation.
Effect of FCP on body weight and lipid profile
The results of body weight gain analysis are represented in Fig. 1. The positive control group animals exhibited significantly increased levels of body weight gain as compared to normal control group (P ≤ 0.05). The rate of body weight gain of positive control group rats was 48% higher than normal control group rats. The oral supplementation of FCP considerably reduced 30% body weight compared to normal control group. Similarly, statin treatment reduced the weight gain rate to 17%. In addition, FCP supplementation did not make any significant difference in the levels of food intake among the groups (12.06 g/day/rat) (P ≤ 0.05). The results indicated that the FCP can be considered as potent dietary component which have ability to regulate the levels of diet induced body weight gain into a normal level without reducing the levels of food intake (Woo et al. 2018).
The serum samples collected from the experimental rats were analyzed for their lipid profile including TC, TG, HDL-C, LDL-C and VLDL-C. The lipid profiling of normal control rats was found to be, TC (110.7 mg/dl), TG (64.8 mg/dl), HDL-C (56.5 mg/dl), LDL-C (41.2 mg/dl) and VLDL-C (13.0 mg/dl), respectively (Fig. 2A). The administration of high fat diet along with alcohol caused a significant increase in the concentrations of TC (77.9%), TG (221%), LDL-C (169%) and VLDL-C (221%) compared to normal control group rats (P ≤ 0.05). High fat diet leads to change in the dietary cholesterol metabolism by forming in-vivo reactive oxygen species (ROS) and lipid peroxides that elevates oxidative stress. This oxidative stress is a key factor that connects hyperlipidaemia with atherosclerotic cardiovascular disease (Sreerekha et al., 2021). The results of the lipid profile analysis in samples of experimental rats clearly indicated the effective induction of hyperlipidemia in high fat diet-alcohol fed animals. The level of HDL-C was significantly diminished by 21.6% in positive control group rats as compared to normal control group rats. FCP treatment on high fat rats normalized the levels of lipid profile TC (135.4 mg/dl), TG (88.8 mg/dl), LDL-C (26.5 mg/dl), VLDL-C (17.8 mg/dl) and HDL-C (65.0 mg/dl), respectively (P ≤ 0.05). Statin treatment also found to normalize the serum lipid profiles. The increased levels of HDL-C in FCP treated group rats, indicate the improved cholesterol transportation and enhanced utilization in tissues. Certain peptide fragments rich in glycine and proline viz. anticoagulant tetrapeptide (Pro-Gly-Pro-Leu) and bioactive tripeptides (Ile-Pro-Pro and Val-Pro-Pro) along with plant sterol was reported to possess cholesterol lowering ability as well as down regulate the levels of LDL-C (Miasoedov et al. 2013). In the present study, the results of the lipid profile analysis clearly establish the lipid lowering ability of FCP.
Fig. 2.
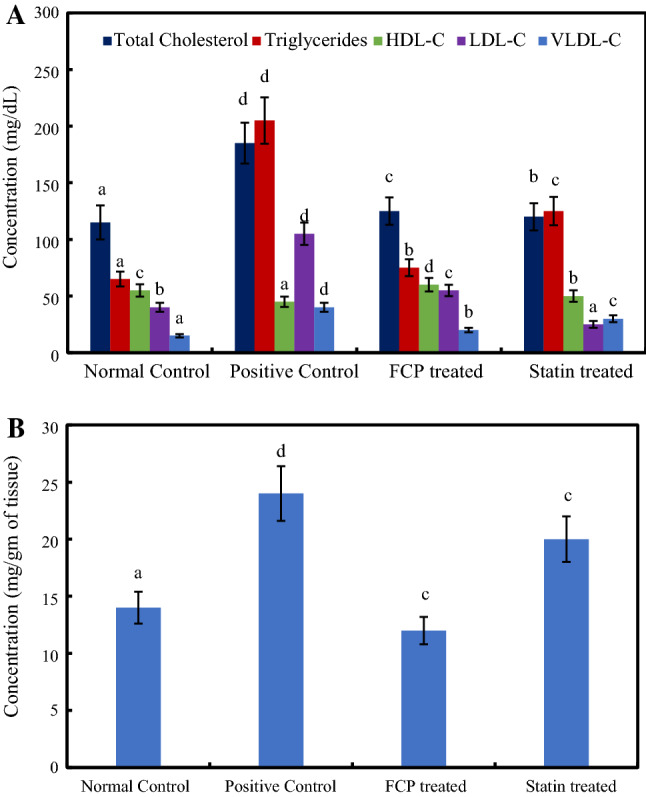
A Lipid profile of serum samples B Levels of cholesterol accumulation in the hepatic tissues of experimental rats. *Mean values ± standard deviation, n = 6. Values that have different superscript letters differ significantly (P < 0.05) with each other
An augmented level of accumulated cholesterol was observed in the hepatic tissues of positive control group animals, while FCP treatment significantly reduced the levels of cholesterol deposition even after high fat diet intake (P ≤ 0.05) (Fig. 2B). Hyperlipidemia and related hepatic lipid accumulation are the major cause of hepatic steatosis, prolonged condition can lead to dyslipidemia. Liver plays a major role in maintaining the systemic lipid homeostasis and thus, hepatic tissue damage impairs the lipid metabolism (El-Tantawy 2015). Hence this has become the main strategy of bioactive molecules to improve the lipid profile by preventing the occurrence of hepatic dysfunction and associated diseases. Several plant proteins which are deficient in methionine, rich in arginine and lysine, were reported to exhibit hypolipidemic properties (Carroll and Kurowska, 1995). In fact, the amino acid composition analysis of FCP demonstrated the absence of sulfur containing amino acids methionine and cysteine. The amino acid composition of the ASC sample revealed that the arginine:lysine ratio is 1.13 (Supplementary material Table 1 and Fig. 1) which is comparably higher than standard protein casein (0.46) (Ngatchic et al. 2016). The lipid lowering ability of FCP can also be related to the presence of bioactive peptides. Even though FCP intake regulated the cholesterol deposition in hepatic tissue, statin causes the drastic reduction of circulating LDL-C concentration by up-regulating the LDL-C receptor levels in hepatic tissues. Clinical reports suggest that the possibility of liver damage by the prolonged statin treatment (Argo et al. 2008). Overall, compared to positive control group, the FCP treated group showed the significant hypolipidemic effect on lowering serum lipid levels, improving lipid metabolism and protecting plasma membrane of hepatocytes. This shows the efficiency and efficacy of fish collagen bioactive peptides.
Western blotting analysis
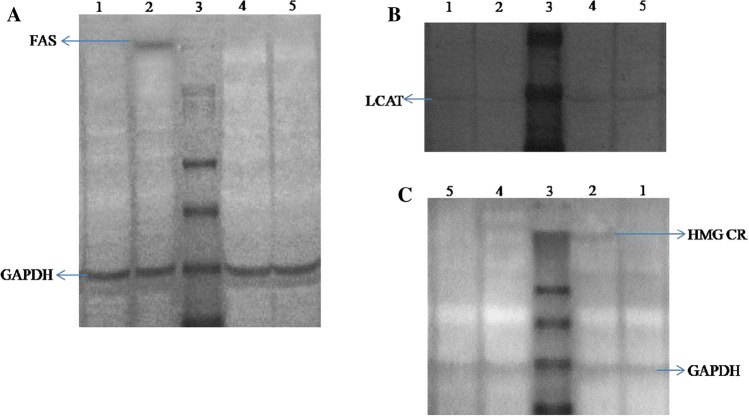
HMG-CoA reductase, Fatty acid synthase and LCAT are the major enzymes involved in lipid metabolism. FAS signaling pathway regulates fatty acid synthesis and further leads to triglycerides synthesis. FAS, the cytosolic multienzyme complex involved in the de novo biosynthesis of fatty acids from acetyl coenzyme A and malonyl coenzyme A using NADPH as a coenzyme (Chakravarty et al. 2004). FAS plays critical role in the secretion of TG to blood stream (Bao et al. 2016). The expression levels of metabolic enzymes are the key regulatory markers to investigate the lipid lowering ability of a biomolecule. Cellular level expressions of these three important lipid metabolic enzymes were carried out using western blotting analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as positive control. Figure 3A illustrates the enhanced expression levels of FAS in positive control group rats, nevertheless, soon after the FCP ingestion the expression levels of FAS in liver tissue were significantly decreased. Induction of hyperlipidemia was previously demonstrated to increase the TG levels in serum. It can be associated with the enhanced expression levels of FAS in hepatic tissues of positive control group rats. Previous reports proposed that the various bioactive peptides or protein hydrolysates have potential hypotriglyceridemic ability to down-regulate the expression levels of FAS (Ruiz et al. 2014). The disappearance of FAS band in FCP treated groups revealed the down regulation or inhibition of lipogenic transcription factors of FAS lipogenic gene pathway.
Fig. 3.
Expression levels of (A) Fatty acid synthase (B) Lecithin-Cholesterol Acyltransferase and (C) HMG Co-A Reductase in serum and liver tissue homogenates of experimental rats Lane 1-Normal Control, Lane 2-Positive Control, Lane 3-Protein marker, Lane 4-FCP treated and Lane 5-Statin treated
LCAT is a lipoprotein associated enzyme, essential for the normal maturation of HDL molecules, interconversion and rearrangements of all lipoprotein classes and, is also involved in reverse cholesterol transport. It is abundantly present in circulation as bound with lipoproteins that catalyses the formation of cholesteryl esters with lipoprotein thereby promotes its transportation (Glomset et al. 1966). Figure 3B demonstrates the up-regulated expression of LCAT levels in the serum samples of FCP treated rats. Further, the expression levels of LCAT were diminished in positive control group animals. Expression levels of the metabolic enzyme in statin treated group animals were comparable to that of FCP treated group. The reduction of total cholesterol in FCP treated groups indicates the down regulating of LCAT by modulating HDL-C maturation and maintaining reverse cholesterol transport. The enhanced LCAT expression can be linked with the increased levels of serum HDL-C as explained previously. FCP is capable to stimulate the transportation and catabolism of dietary cholesterol in serum.
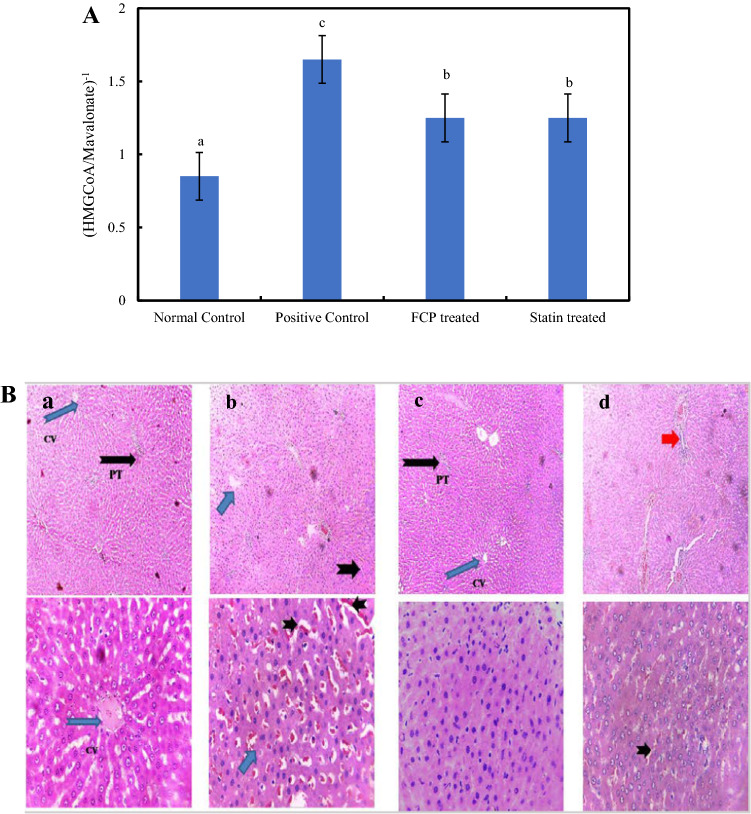
HMGCR is the abundantly expressed cholesterol biosynthetic enzyme that catalyses the rate limiting step in the pathway (Liang and Vaziri, 2003). It mediates the reduction of HMG CoA to mevalonate, the precursor molecule of cholesterol biosynthesis (Siperstein and Guest 1960). Expression levels of the HMGCR enzyme was displayed in Fig. 3C showing a clear band in lane 2 at the region of 97 kDa. Expression levels of HMGCR were up-regulated in high fat diet induced hyperlipidemic rats and it was normalized by FCP treatment. The enzymatic activity and expression levels of enzyme correlate with each other indicating the enzyme inhibitory activity of FCP and, thereby regulating the cellular cholesterol biosynthesis. The increased levels of serum cholesterol regulate the expression levels of HMGCR through feedback inhibition. Several specific binding proteins are involved in the activation of transcription factors mediating the biosynthesis of HMGCR (Ji et al. 2011). In the present study, positive control group animals exhibited significantly higher expression and activity levels of HMGCR indicating the augmented cholesterol biosynthesis, which can be related to the increased serum lipid profiles. The reduced serum concentration of TC, TG, LDL-C and VLDL-C in the FCP treated animals might have lowered the expression levels of HMGCR. Indirect assessment of the HMGCR activity was carried out in liver homogenate by measuring the ratio of HMG CoA/mevalonate, which is inversely related to the enzyme activity. Figure 4A demonstrates the activity levels of HMGCR in liver tissues under experimental conditions. The induction of hyperlipidemia caused significant increase in the activity levels of the enzyme HMGCR, pointing out the up-regulated cholesterol biosynthetic pathway. Meanwhile, FCP treatment significantly regulated the enzyme activity in liver tissue (P ≤ 0.05).
Fig. 4.
A Effect of FCP on the activity levels of HMGCR in liver tissues of experimental rats. *Mean values ± standard deviation. Values that have different superscript letters differ significantly (P < 0.05) with each other. B. Microscopic evaluation of the histopathology sections of hepatic tissues of experimental animals at low (10X) and high (40X) magnification. a Normal Control b Positive Control c FCP treated and (d) Statin treated. Short blue arrow indicates micro vesicular steatosis, Short black arrow shows inflammatory infiltration and Red arrow point out bridging fibrosis
Histopathology analysis
Histological changes in hepatic tissues of experimental rats were demonstrated in Fig. 4B. The liver tissues of normal control group animals exhibited normal liver parenchyma along with sparse inflammatory infiltration. The administration of high fat diet with alcohol caused the formation of periportal microvesicular steatosis, which is an earlier reversible stage of liver cirrhosis. It is histologically characterized by the accumulation of lipids in hepatic tissues with moderate inflammatory infiltration. Alcohol consumption along with high fat diet was previously reported to induce liver damage by the outbreak of gram-negative bacteria in intestine. It causes an increased permeability to lipopolysaccharides which trigger inflammatory responses (Kirpich et al. 2017). It also reduces the mitochondrial levels of oxidized nicotinamide adenine dinucleotide (NAD+), thus disturbing the rate of β-oxidation in liver, leads to the formation of hepatic steatosis (Gu et al. 2015).
FCP ingestion was found to prevent the formation of steatosis providing a normal liver parenchyma with moderate inflammatory infiltration. Similarly, statin treatment also declined lipid accumulation and maintained the normal liver parenchyma. Meantime, it resulted in the occurrence of architectural distortion of hepatic tissue forming centrilobular bridging fibrosis. Atrophy was noticed in hepatocytes with moderate inflammatory infiltration. Hepatic atrophy can be characterized by decreased cytoplasm to nuclei ratio. Previous studies demonstrated the capability of marine collagen peptides to reverse the alcohol induced liver damage in rats (Lin et al. 2012).
In vivo antioxidant activity
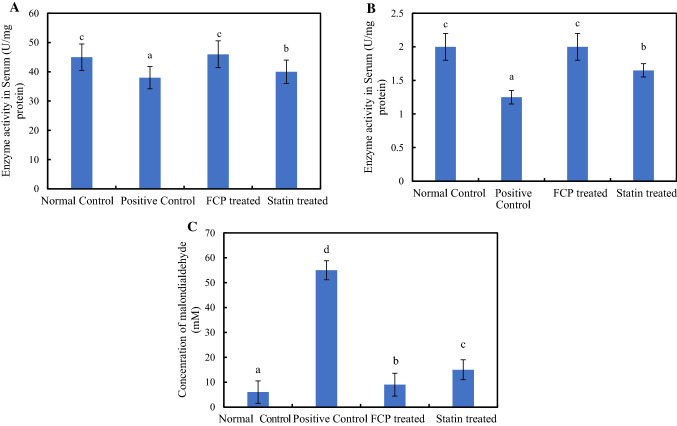
Figure 5A, B illustrate the activity levels of the antioxidant defense enzymes SOD and catalase in serum. Administration of heat oxidized fat with alcohol significantly reduced the enzyme activities of both SOD and catalase (P ≤ 0.05). Oxidative stress occurs as a result of generation of reactive oxidative species, which exceeds the free radical scavenging capability of tissue antioxidant defense system. Prolonged exposure to increased levels of reactive oxidative species formation is well known to inhibit the functionality of enzymatic and non-enzymatic antioxidant mechanisms in tissues, which in turn results in metabolic dysfunction and deterioration of organs. Since the hepatic system is the prime metabolic site involved in the detoxification process of toxic metabolites, its prolonged exposure to abundant levels of highly reactive oxygen species during hyperlipidemic condition may lead to hepatic tissue injury (Varma et al. 2004). The cell membranes are mainly composed of lipid conjugates especially polyunsaturated fatty acids, which are highly susceptible to oxidation. Thus, the ingestion of oxidized oil and alcohol increases the rate of membrane lipid peroxidation in liver. SOD enzyme neutralizes the superoxide free radicals into hydrogen peroxide, consecutively catalase enzyme mediates the conversion of hydrogen peroxide to water and oxygen (Guo et al. 2009). Marine collagen peptides were previously reported to exhibit antioxidant activity to counteract alcohol induced oxidative stress in Wistar rats (Lin et al. 2012). These class of bioactive peptide include metal ion binding proteins which chelates metal ions and, simultaneously prevent the formation of free radicals. In the present study, the oral supplementation of FCP improved the enzyme activity to normal levels comparable to statin treated group. The graphical representation of the results obtained from the TBARS assay was shown in Fig. 5C. The induction of oxidative stress increased the rate of membrane lipid peroxidation in hepatic tissues of experimental rats approximately 10 times than that of normal control rats. Since a decline in the rate of lipid peroxidation was observed in FCP fed groups, it is evident that FCP intake reduced the level of diet induced oxidative stress in hepatic tissues.
Fig. 5.
A Superoxide dismutase enzyme B Catalase enzyme C Membrane lipid peroxidation of hepatic tissues in experimental rats. *Mean values ± standard deviation. Values that have different superscript letters differ significantly (P < 0.05) with each other
The diagrammatic representation of the possible mechanism and action of FCP in attenuating the diet induced hyperlipidemia and hepatic tissue damage is demonstrated in Supplementary material Fig. 2. The dietary intake of thermally oxidized oil along with alcohol induced the oxidative stress, elevated the expression levels of biosynthetic enzymes FAS and HMGCR also declined the expression levels of cholesterol transportation enzyme LCAT. The increased levels of oxidative stress caused inflammatory infiltration in hepatic tissues, stimulated the accumulation of fat globules and formation of microvesicular steatosis and eventually lead to the distortion of hepatic tissue architecture by the formation of bridging fibrosis. Oral administration of FCP reversed the action of diet induced oxidative stress by regulating the expression levels of metabolizing enzymes FAS, HMGCR and LCAT into normal level. It also found to be capable to prevent the oxidative stress induced hepatic tissue damage and maintained the normal liver parenchyma, diminished the fat accumulation and inflammatory infiltration.
Conclusion
The results of the present study demonstrated that the dietary supplementation of FCP attenuate the high fat diet induced hyperlipidemic aberrations in liver. The FCP consumption regulated the levels of body weight gain and lipid profile in experimental rats. The cellular expression levels of lipid metabolic enzymes were regularized towards near normal. The oral supplementation of FCP also counteracted hyperlipidemia mediated peroxidative deterioration of lipid milieu through upregulation of hepatic antioxidant defense status. The utilization of fish skin originated collagen peptides will be a promising component as a nutraceutical to ameliorate oxidative stress and hepatic dysfunctions in the present-day lifestyle change associated healthcare problems.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the Director, ICAR-Central Institute of Fisheries Technology (ICAR-CIFT), Cochin, Kerala, India for providing the facilities to carry out this work and also for granting permission to publish the data acquired from the study. The authors would like to express their sincere gratitude to ICAR for providing funds to carry out the research work under ICAR-National Fellow Scheme. The authors are grateful to Dr. Rohit and Dr. Reghu Nandan, Clinical Pathologists, MedHelix Laboratory for doing histopathology analysis and interpretation of the data. The authors are grateful to the Dr. B. Ganesan (Chief Technical Officer, Animal lab facility), Mrs. P. A. Jaya (Technical Officer), Mr. P. Suresh (Senior Technician), Mrs. N. Lekha (Technical Officer) ICAR-Central Institute of Fisheries Technology (CIFT), Cochin, Kerala for providing technical support to carry out the analyses.
Abbreviations
- ABTS
2,2′Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- ASC
Acid soluble collagen
- BHT
Butylated hydroxytoluene
- CPCSEA
Committee for the Purpose of Control and Supervision of Experiments on Animals
- ELISA
Enzyme linked immunosorbent assay
- FAS
Fatty acid synthase
- FCP
Fish collagen peptides
- FCH
Fish collagen hydrolysate
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- H&E
Haematoxylin and eosin
- HDL-C
High density lipoprotein- cholesterol
- HMG CoA
3-Hydroxy-3-methylglutaryl-CoA
- HMGCR
3-Hydroxy-3-methylglutaryl-CoA reductase
- LCAT
Lecithin–cholesterol acyltransferase
- LDL-C
Low density lipoprotein-cholesterol
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NC
Normal control
- PBS
Phosphate buffered saline
- PC
Positive control
- PUFA
Poly unsaturated fatty acid
- ROS
Reactive oxygen species
- SDS-PAGE
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SOD
Superoxide dismutase
- Stat
Statin
- TBARS
Thiobarbituric acid reactive substances
- TC
Total cholesterol
- TG
Triglyceride
- VLDL-C
Very low-density lipoprotein- cholesterol
Author contributions
Research concept- Dr. DKV, Dr. RA, Research design- Dr. DKV, Dr. RA, Dr SM, Supervision- Dr RA and Dr SM and Dr CNR, Experiments- Dr. DKV, Dr. PRS, Data analysis and Interpretation- Dr. DKV, Dr. PKD, Ms. MRJ, Dr. RA, Dr SM, Writing article- Dr. DKV, Dr. PKD, Ms. MRJ, Critical review- Dr SM, Dr CNR, Article editing- Dr. PKD, Ms. MRJ, Final approval- Dr SM, Dr CNR.
Funding
ICAR-National Fellow Scheme.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The experimental rats were maintained and performed as per the guidelines on the regulation of scientific experiments on animals of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India and approved by the Institutional Animal Ethics Committee of ICAR-Central Institute of Fisheries Technology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anandan R, Ganesan B, Obulesu T, Mathew S, Asha KK, Lakshmanan PT, Zynudheen AA. Antiaging effect of dietary chitosan supplementation on glutathione-dependent antioxidant system in young and aged rats. Cell Stress Chaperon. 2013;18:121–125. doi: 10.1007/s12192-012-0354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (2000) Official methods of analysis of AOAC International, 17th edn. AOAC, Gaithersburg, MD, USA
- Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology. 2008;48:662–669. doi: 10.1002/hep.22402. [DOI] [PubMed] [Google Scholar]
- Bao L, Hu L, Zhang Y, Wang Y. Hypolipidemic effects of flavonoids extracted from Lomatogonium rotatum. Exp The Med. 2016;11:1417–1424. doi: 10.3892/etm.2016.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruha R, Dvorak K, Petrtyl J. Alcoholic liver disease. World J Hepatol. 2012;4(3):81–90. doi: 10.4254/wjh.v4.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege JA, Aust SD, Fleischer S, Packer L. Microsomal lipid peroxidation. Biomembranes-Part C: Biological oxidations, Academic press; 1978. pp. 302–310. [Google Scholar]
- Carroll KK, Kurowska EM. Soy consumption and cholesterol reduction: review of animal and human studies. J Nutr. 1995;12:594–597. doi: 10.1093/jn/125.3_Suppl.594S. [DOI] [PubMed] [Google Scholar]
- Chakravarty B, Gu Z, Chirala SS, Wakil SJ, Quiocho FA. Human fatty acid synthase: Structure and substrate selectivity of the thioesterase domain. PNAS. 2004;101(44):15567–15572. doi: 10.1073/pnas.0406901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dara PK, Raghavankutty M, Sebastian N, Chatterjee NS, Mathew S, Ravishankar CN, Anandan R. Rheological, physico-chemical, and surface-active properties of gelatin extracted from bigeye tuna (Thunnus obesus) skin waste. J Aq Food Product Tech. 2020;29(5):428–444. [Google Scholar]
- Dara PK, Elavarasan K, Shamasunadar BA. Characterization of antioxidant and surface-active properties of gelatin protein hydrolysates obtained from croaker fish skin. Int Aq Res. 2020;12:116–126. [Google Scholar]
- Dara PK, Elavarasan K, Shamasundar BA. Improved utilization of croaker skin waste and freshwater carps visceral waste: conversion of waste to health benefitting peptides. Int J Peptide Res Therap. 2020;26:2641–2651. [Google Scholar]
- Divya KV, Sreerekha PR, Elavarasan K, Mathew S, Ravi Shankar CN, Anandan R. Determination of electrophoretic subunit pattern and peptide mapping of collagen and collagen peptides extracted from skin of hammerhead shark (Sphyrnae mokkaran) SF J Anal Biochem. 2018;1:1–8. [Google Scholar]
- Divya KV, Sreerekha PR, Tejpal CS, Asha KK, Mathew S, Ravishankar CN, Anandan R. Extraction and characterization of acid soluble collagen (ASC) from airbladder of striped cat fish (Pangasius hypophthalmus) Int J Fish Aquat Stud. 2018;6(4):310–318. [Google Scholar]
- Duly AMP, Alani B, Huang EW, Yee C, Haber PS, McLennan SV, Seth D. Effect of multiple binge alcohol on diet-induced liver injury ina mouse model of obesity. Nutr Diabetes. 2015;5:154. doi: 10.1038/nutd.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tantawy WH. Biochemical effects, hypolipidemic and antiinflammatory activities of Artemisia vulgaris extract in hypercholesterolemic rats. J Clin Biochem Nutr. 2015;57(1):33–38. doi: 10.3164/jcbn.14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedwald WT, Levy RJ, Fredricken DS. Estimation of VLDLcholesterol in the plasma without the use of preparative ultracentrifuge. Clin Chem. 1972;18:449. [PubMed] [Google Scholar]
- Glomset JA, Janssen ET, Kennedy R, Dobbins J. Role of plasma lecithin:cholesterolacyltransferase in the metabolism of high density lipoproteins. J Lipid Res. 1966;7:638–648. [PubMed] [Google Scholar]
- Gu J, Zhang Y, Xu D, Zhao Z, Zhang Y, Pan Y, Cao P, Wang Z, Chen Y. Ethanol-induced hepatic steatosis is modulated by glycogen level in the liver. J Lipid Res. 2015;56(7):1329–1339. doi: 10.1194/jlr.M056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Sun J, He H, Yu GC, Du J. Antihepatotoxic effect of corn peptides against Bacillus Calmette-Guerin/lipopolysaccharide- induced liver injury in mice. Food Chem Toxicol. 2009;47:2431–2435. doi: 10.1016/j.fct.2009.06.041. [DOI] [PubMed] [Google Scholar]
- Handel VE, Zilversmit DB. Micro method for the direct determination of serum triglycerides. J Lab Clin Med. 1957;50(1):152–157. [PubMed] [Google Scholar]
- Hema GS, Shyni K, Ganesan B, Joshy CG, Manu Prasad M, Suseela M. In vivo and in vitro studies on joint regenerative potential of fish skin derived collagen peptides. World J Pharm Res. 2016;5(7):916–930. [Google Scholar]
- Ishida Y, Fugita T, Asai K. New detection and separation method for amino acids by high performance liquid chromatography. J Chroma. 1981;204:143–148. doi: 10.1016/s0021-9673(00)81650-7. [DOI] [PubMed] [Google Scholar]
- Ji G, Zhao X, Leng L, Liu P, Jiang Z. Comparison of dietary control and atorvastatin on high fat diet induced hepatic steatosis and hyperlipidemia in rats. Lipids Health Dis. 2011;10:23. doi: 10.1186/1476-511X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, McClain CJ, Vatsalya V, Schwandt M, Phillips M, Falkner KC, Zhang L, Harwell C, George DT, Umhau JC. Liver injury and endotoxemia in male and female alcohol-dependent individuals admitted to an alcohol treatment program. Alcohol Clin Exp Res. 2017;41(4):747–757. doi: 10.1111/acer.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Kusubata M. Effects of collagen peptide ingestion on blood lipids in rats fed a high-lipid and high-sucrose diet. Food SciTech Res. 2013;19(6):1149–1153. [Google Scholar]
- Kumar DP, Chandra MV, Elavarasan K, Shamasundar BA. Structural properties of gelatin extracted from croaker fish (Johniussp) skin waste. Int J of Food Prop. 2017;20:S2612–S2625. [Google Scholar]
- Lee KJ, Song NY, Oh YC, Cho WK, Ma JY. Isolation and bioactivity analysis of ethyl acetate extract from Acer tegmentosum using in vitro assay and on-line screening HPLC-ABTS+ system. J Anal Met Chem. 2014;150509:1–15. doi: 10.1155/2014/150509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Vaziri ND. HMG-CoA reductase, cholesterol 7 alpha-hydroxylase, LCAT, ACAT, LDL receptor, and SRB-1 in hereditary analbuminemia. Kidney Int. 2003;64:192–198. doi: 10.1046/j.1523-1755.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- Lim SY. Role of statins in coronary artery disease. Chonnam Med J. 2013;49:1–6. doi: 10.4068/cmj.2013.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Zhang F, Yu Y, Jiang Q, Zhang Z, Wang J, Li Y. Marine collagen peptides protect against early alcoholic liver injury in rats. Br J Nutr. 2012;107:1160–1166. doi: 10.1017/S0007114511004211. [DOI] [PubMed] [Google Scholar]
- Miasoedov NF, Shubina TA, OberganTIu GME, Andreeva LA, Liapina LA. Cholesterol-lowering effect of the regulatory peptide Pro-Gly-Pro-Leu. VoprPitan. 2013;82(5):41–45. [PubMed] [Google Scholar]
- Misra HP, Fridovich T. The role of superoxide ion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- Mizushige T, Komiya M, Onda M, Uchida K, Hayamizu K, Kabuyama Y. Fish protein hydrolysate exhibits anti-obesity activity and reduces hypothalamic neuropeptide Y and agouti-related protein mRNA expressions in rats. Biomed Res. 2017;38(6):351–357. doi: 10.2220/biomedres.38.351. [DOI] [PubMed] [Google Scholar]
- Nawar WW. Volatile components of the frying process. Grasasy Aceites. 1998;49(3–4):271–274. [Google Scholar]
- Ngatchic JTM, Njintang NY, Bernard C, Oben J, Mbofung CM. Lipid-lowering properties of protein-rich mucuna product. Nutrire. 2016;41:2. [Google Scholar]
- Rao AV, Ramakrishnan S. Indirect assessment of hydroxymethylglutaryl Co-A reductase (NADPH) activity in liver tissue. Clin Chem. 1975;21(10):1523–1525. [PubMed] [Google Scholar]
- Ruiz JR, Ancona DB, Campos MS. Bioactive vegetable proteins and peptides in lipid-lowering; nutraceutical potential. Nutr Hosp. 2014;29(4):776–784. doi: 10.3305/nh.2014.29.4.7208. [DOI] [PubMed] [Google Scholar]
- Siperstein MD, Guest MJ. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreerekha PR, Dara PK, Vijayan DK, Chatterjee NS, Raghavankutty M, Mathew S, Ravishankar CN, Anandan R. Dietary supplementation of encapsulated anthocyanin loaded-chitosan nanoparticles attenuates hyperlipidemic aberrations in male wistar rats. Carb Poly Tech App. 2021;2:100051. [Google Scholar]
- Takahara S, Hamilton BH, Nell JV, Kobra TY, Ogawa Y, Nishimura ET. Hypocatalasemia: a new genetic carried state. J Clin Invest. 1960;39(4):610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskiran D, TaskiranE YH, Kutay F. Quantification of total collagen in rabbit tendonby the sirius red method. Tr J Med Sci. 1999;29:7–9. [Google Scholar]
- Varma PS, Aruna K, Rukkumani R, Menon VP. Alcohol and thermally oxidized pufa induced oxidative stress: role of N-acetyl cysteine. Ital J Biochem. 2004;53(1):10–15. [PubMed] [Google Scholar]
- WHO (2014) Global status report on alcohol and health. World Health Organization
- Woo M, Song YO, Kang K, Noh JS. Anti-Obesity effects of collagen peptide derived from skate (Raja kenojei) Skin through regulation of lipid metabolism. Mar Drugs. 2018;16:306. doi: 10.3390/md16090306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953;41(3):486–492. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.