Abstract
Anthocyanins are considered as the largest group of water-soluble pigments found in the vacuole of plant cells, displaying range of colors from pink, orange, red, purple and blue. They belong to flavonoids, a polyphenolic subgroup. Application of anthocyanins in food systems as natural food colourants is limited due to the lack of stability under different environmental conditions such as light, pH, heat etc. Anthocyanins esterified with one or more acid groups are referred as acylated anthocyanins. Based on the presence or absence of acyl group, anthocyanins are categorized as acylated and nonacylated anthocyanins. Acylated anthocyanins are further classified as mono, di, tri, tetra acylated anthocyanins according to the number of acyl groups present in the anthocyanin. This review classifies common anthocyanin sources into non-acylated, mono-, di-, tri- and tetra-acylated anthocyanins based on the major anthocyanins present in these sources. The relative stabilities of these anthocyanins with respect to thermal, pH and photo stress in beverage systems are specifically discussed. Common anthocyanin sources such as elderberry, blackberry, and blackcurrant mainly contain nonacylated anthocyanins. Red radish, purple corn, black carrot also mainly contain mono acylated anthocyanins. Red cabbage and purple sweet potato have both mono and diacylated anthocyanins. Poly acylated anthocyanins show relatively higher stability compared with nonacylated and monoacylated anthocyanins. Several techniques such as addition of sweeteners, co-pigmentation and acylation techniques could enhance the stability of nonacylated anthocyanins. Flowers are main sources of polyacylated anthocyanins having higher stability, yet they have not been commercially exploited for their anthocyanins.
Keywords: Anthocyanin sources, Monoacylated anthocyanins, Diacylated anthocyanins, Triacylated anthocyanins, Tetraacylated anthocyanins
Introduction
Anthocyanins are water-soluble pigments belonging to the large group of flavonoids, a subclass of the polyphenol family (Choo 2019) and serve as natural food colourants. Anthocyanins can be found in fruits and vegetables sold in the market, such as red grape, blackcurrant, elderberry, blackberry, raspberry, black chokeberry, red cabbage, black carrot, purple corn, red radish, and purple-fleshed sweet potato.
Anthocyanins are derived from anthocyanidins; anthocyanins are glycosides of anthocyanidins. Most common anthocyanidins in plant kingdom are pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin (Choo 2019). Some anthocyanins are bonded with aromatic or aliphatic organic acids such as acrylic acid, coumaric acid, caffeic acid, malonic acid, lactic acid, acetic acid, succinic, tartaric acid, glutaric acid by ester bonds. This is referred as acylation of anthocyanins. The acylated anthocyanins are categorized mainly into four categories based on the number of acid groups present in them: monoacylated, diacylated, triacylated and tetraacylated. All di, tri, tetra acylated anthocyanins are referred as poly acylated anthocyanins. Anthocyanins with more than 4 acyl moieties are also present.
Many researches have revealed the unstable nature of nonacylated anthocyanins compared to acylated anthocyanins and rarely nonacylated anthocyanins have shown higher stability than acylated anthocyanins. Number of sugars groups also affect the stability of nonacylated anthocyanins. In strawberry, about 56% of the total anthocyanins are monoglucosides and nonacylated making those anthocyanins unstable (Gardeli et al. 2019). Cyanidin diglucosides in pomegranate showed comparatively higher storage stability compared to monoglucosides (Alighourchi et al. 2008).
Acylated anthocyanins show higher stability and antioxidant activity compared with their corresponding non acylated anthocyanin (Hu et al. 2014) and are reported to possess higher stability in aqueous solutions and are of interest to the food and beverage industry (Galaffu et al. 2015). The acylation makes the anthocyanins more stable in aqueous solutions through intramolecular co-pigmentation, protecting the flavylium chromophore from the nucleophilic attack of water and by generating steric hindrance (Trouillas et al. 2016). Acylation also helps to create an acidic environment that could help to maintain colour stability of anthocyanins (Zhao et al. 2017). When number of acid groups increases, the stability of the anthocyanins increases (Marpaung et al. 2019). However, the characteristics, the number, the binding site, and the spacial arrangement of acyl units affect the stability and antioxidant activity of acylated anthocyanins (Matsufuji et al. 2007). A number of studies investigated the antioxidant activity of acylated anthocyanins against oil oxidation. Tamura and Yamagami (1994) studied the antioxidant activity of monoacylated anthocyanins from Muscat Bailey which contains high amount of malvidin 3-O-(6-O-p-coumaroylglucoside)-5-glucoside. This study states that anthocyanins extracted from Muscat Bailey fruit showed higher antioxidant activity than (+) catechin and α-tocopherol. These monoacylated anthocyanins could significantly reduce the malonaldehyde formation from linoleic acid. Another study used anthocyanin extract from purple corn husk to reduce lipid peroxidation in mayonnaise. This anthocyanin extract demonstrated better antioxidant activity compared to chemical antioxidants such as butylated hydroxytoluene (BHT) and ethylenediaminetetraacetic acid (EDTA) (Li et al. 2014). Acylated anthocyanins could be an alternative source of natural antioxidants for use in the oil industry to retard oil oxidation besides other natural antioxidants such as rosemary extract.
There are a number of reviews on the sources of anthocyanin and stability of anthocyanins (Cortez et al. 2017) but there is limited information on a clear-cutting classification of anthocyanin sources based on their degree of acylation. Anthocyanins are used to colour beverages, confectionary, desserts, ice cream, fruit preparations, bakers jam and non-standard jellies and preserves, sherbets, ices, pops, yogurt, gelatine desserts, candy, and bakery fillings and toppings. This review classifies the anthocyanin sources based on their degree of acylation and compares the relative thermal, pH and photo stabilities of acylated and nonacylated anthocyanins in beverage systems.
Sources of acylated and nonacylated anthocyanins
Plant components such as fruits, vegetables, flowers, leaves, yams, cereals etc. may contain anthocyanins. Depending on the location, in which anthocyanins are in a plant, it can be predicted whether the anthocyanins are acylated or not. Anthocyanins that are mainly present in fruits and cereals of a plant are nonacylated. Vegetables, berries and yams mainly contain monoacylated anthocyanins whereas flowers and leaves mainly contain tri and tetra acylated anthocyanins. Sources of acylated and nonacylated anthocyanins and the predominant anthocyanins found in respective sources are summarised in Table 1.
Table 1.
Sources of acylated and nonacylated anthocyanins
| Anthocyanin | Sources | Major compounds | References |
|---|---|---|---|
| Nonacylated anthocyanins | Elderberry | Mainly cyanidin-3-O-glucoside and cyanidin-3-O-sumbuside | Koley et al. (2019) |
| Blackcurrant | Mainly delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside | Yang et al. (2019) | |
| Mulberry | Mainly cyanidin-3-glucoside | Kim and Lee (2020) | |
| Blackberry | Mainly cyanidin-3-O-glucoside | Benedek et al. (2020) | |
| Blue berry | Mainly cyanidin, delphinidin, malvidin and peonidin based anthocyanins | Lohachoompol et al. (2004) | |
| Grape | Mainly delphinidin, malvidin, cyanidin, petunidin, and peonidin based anthocyanins | Song et al. (2013) | |
| Strawberry | Mainly cyanidin-3-glucoside, pelargonidin-3-glucoside and pelargonidin-3-rutinoside | Octavia and Choo (2017) | |
| Monoacylated anthocyanins | Purple carrot | Mainly cyanidin based anthocyanins acylated with synapic acid, ferulic acid and coumaric acid | Tsutsumi et al. (2019) |
| Black carrot | Mainly cyanidin based anthocyanins acylated with coumaric acid, ferulic acid, and sinapic acid | Koley et al. (2019) | |
| Red radish | Mainly pelargonidin based anthocyanins acylated with coumaric, ferulic, or caffeic acid | Koley et al. (2020) | |
| Red cabbage (Specially Matured Primero and Azurro varieties) | Mainly cyanidin based anthocyanins acylated with sinapic, ferulic or coumaric acid | Strauch et al. (2019) | |
| Purple sweet potato | Mainly cyanidin and peonidin based anthocyanins acylated with caffeic, ferulic or hydroxyl benzoic acid | Kurata et al. (2019) | |
| Black goji berry | Mainly petunidin based anthocyanins acylated with coumaric acid | Tang and Giusti (2018) | |
| Nitraria tangutorun Bobr fruit | Mainly cyanidin, delphinidin and pelargonidin based anthocyanins acylated with coumaric acid or caffeic acid | Sang et al. (2019) | |
| Purple corn | Mainly cyanidin, pelargonidin and peonidin based anthocyanins acylated with malonic acid | Chatham et al. (2020) | |
| Diacylated anthocyanins | Purple sweet potato | Mainly peonidin based anthocyanins acylated caffeic, ferulic and hydroxybenzoic acid | Kurata et al. (2019) |
| Matured Buscaro and Bandolero red cabbage varieties | Mainly cyanidin derived anthocyanins acylated with coumaric, caffeic, ferulic and sinapic acid | Strauch et al. (2019) | |
| Red radish | Mainly pelargonidin based anthocyanins acylated with coumaric, ferulic and caffeic acid | Li et al. (2020) | |
| Red flowers of Clematis | Mainly cyanidin based anthocyanins acylated with caffeic acid, malonic acid, calleoic acid and succinic acid | Hashimoto et al. (2011) | |
| Triacylated anthocyanins | Purple-violet flowers of Matthiola longipetala | Mainly cyanidin based anthocyanins acylated with ferulic acid, malonic acid and sinapic acid | Tatsuzawa (2014) |
| Red–purple flowers of Iberis umbellata L: candycane rose and candycane red | Mainly pelargonidin based anthocyanins acylated with sinapic, ferulic and coumaric acid | Tatsuzawa (2019) | |
| Ionopsidium acaule flower petals | Mainly cyanidin based anthocyanins acylated with coumaric acid, malonic acid and ferulic acid | Tatsuzawa et al. (2014) | |
| Leaves of Rhoeo spathacea (Swartz) | Mainly cyanidin based anthocyanin acylated with ferulic acid | Tan et al. (2014) | |
| Clitoria ternatea petals | Mainly delphinidin based anthocyanins acylated with malonic and coumaric acid | Jeyaraj et al. (2020) | |
| Tetraacylated anthocyanins | Red–purple flowers of Iberis umbellata L candycane rose and candycane red | Mainly cyanidin and pelargonidin based anthocyanins acylated with malonic acid, coumaric acid, ferulic acid, sinapic acid and hydroxycinnamic acid | Tatsuzawa (2019) |
| Gynura bicolor leaves | Mainly cyanidin based anthocyanins acylated with caffeic acid and malonic acid | Shimizu et al. (2010) |
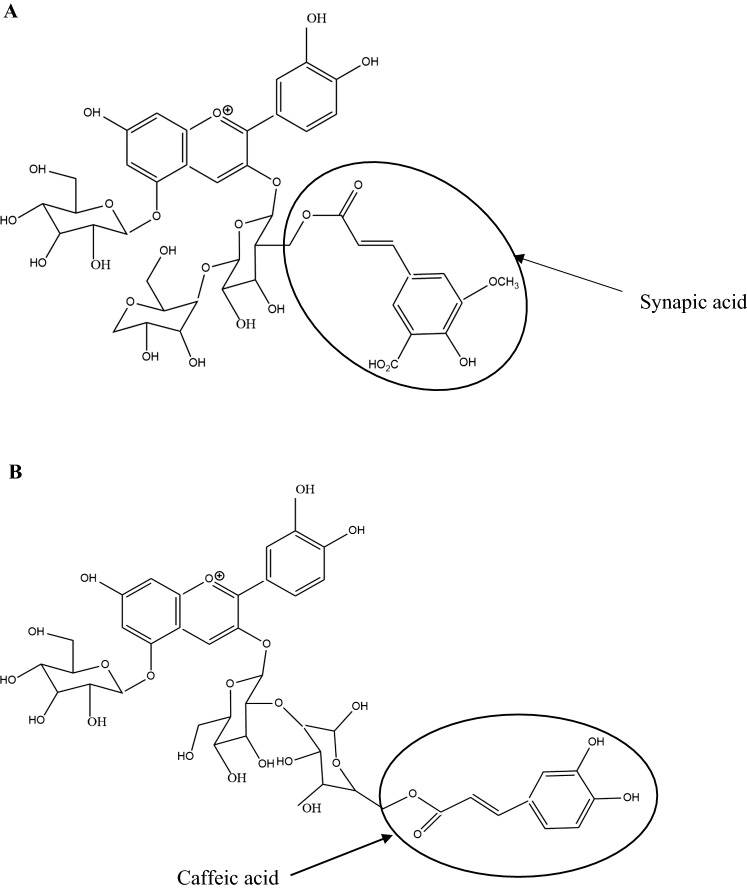
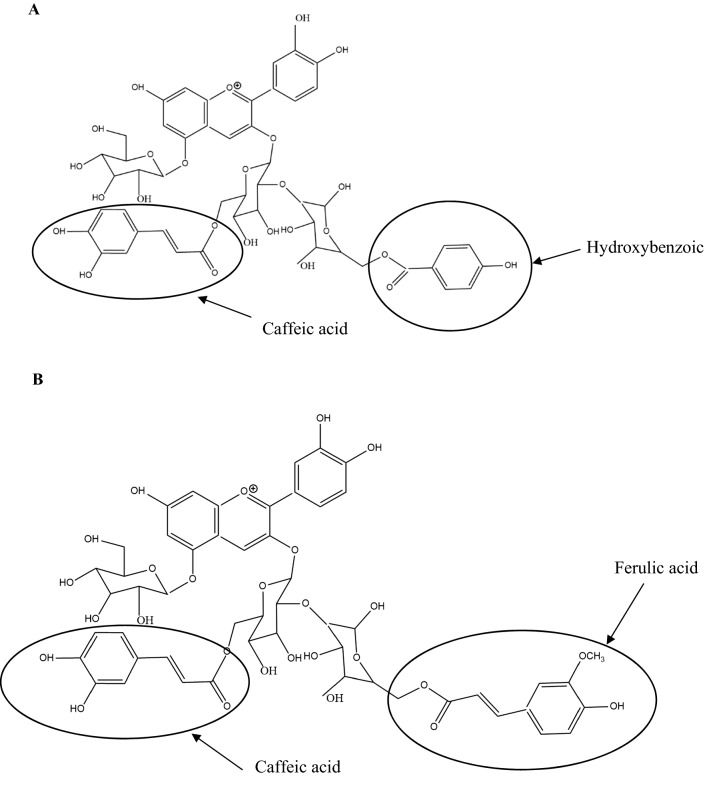
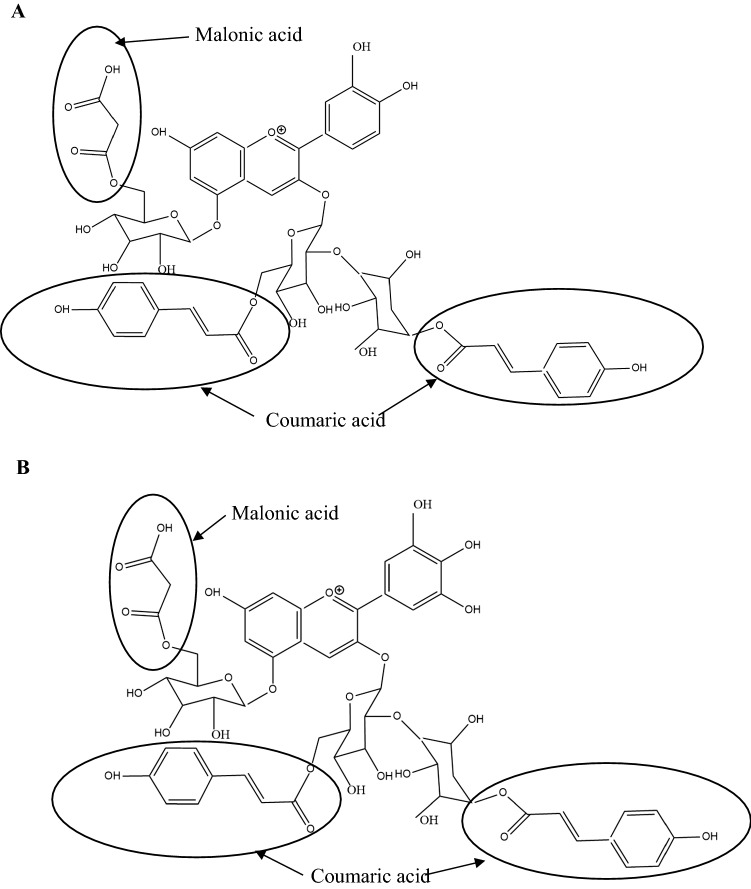
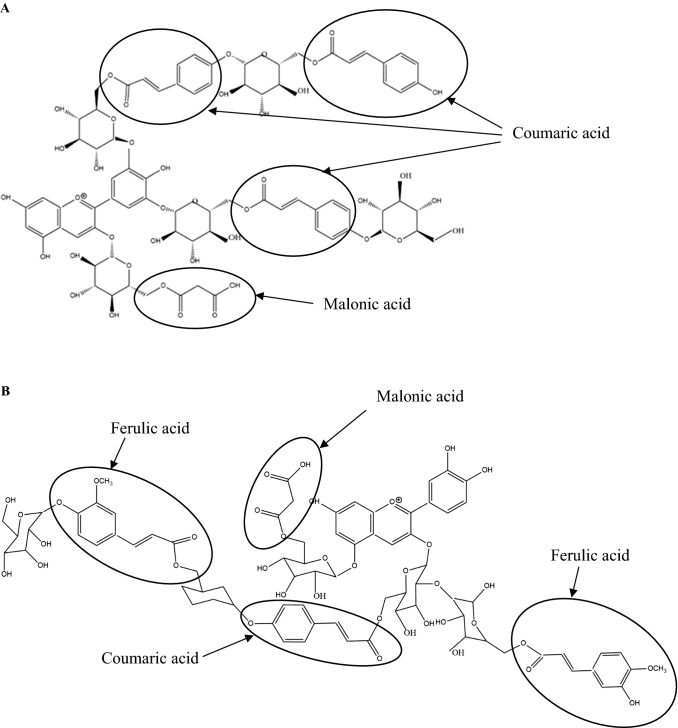
Nonacylated anthocyanins do not have aliphatic or aromatic acid groups connected to them. Examples of nonacylated anthocyanin found in pomegranate (Fig. 1a) and grapes (Fig. 1b) are shown in Fig. 1. Monoacylated anthocyanins contain only one aliphatic or aromatic acid group. Examples of monoacylated anthocyanin found in red cabbage (Fig. 2a) and purple sweet potato (Fig. 2b) are shown in Fig. 2. Diacylated anthocyanins contain two aliphatic or aromatic acid groups. Examples of diacylated anthocyanin found in purple sweet potato (Fig. 3a) and red radish (Fig. 3b) are shown in Fig. 3. Triacylated anthocyanins contain 3 acid groups connected to their anthocyanin glycoside. Examples of triacylated anthocyanin found in Ajuga reptans flower are shown in Fig. 4. Tetraacylated anthocyanins contain 4 acid groups in their anthocyanin glycoside. Examples of tetraacylated anthocyanin found in blue pea flower (Fig. 5a) and flowers of Iberis umbellate (Fig. 5b) are shown in Fig. 5. Many researchers suggest that polyacylated anthocyanins are more stable compared with other anthocyanins because of the ability of intramolecular copigmentation and imposing steric hindrance (Fenger et al. 2020).
Fig. 1.
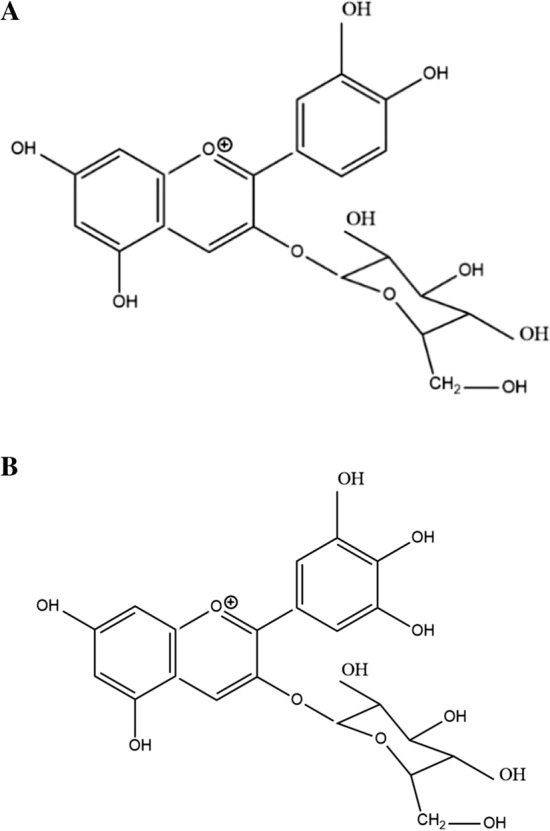
Examples of nonacylated anthocyanin a Cyanidin-3-glucoside, b Delphinidin-3-glucoside
Fig. 2.
Examples of monoacylated anthocyanins a Cyanidin-3-(synapoyl) diglucosideside-5-glucoside, b Cyanidin-3-(6″-caffeoyl sophoroside)-5-glucoside
Fig. 3.
Examples of diacylated anthocyanins a Cyanidin-3-O-(6-O-(E)-caffeyl-2-O-(6-O-(E)-p-hydroxybenzoyl-β-D-glucopyranoside)-β-D-glucopyranoside)-5-O-β-D-glucopyranoside, b Cyanidin-3-O-[6-O-(E)-caffeoyl-2-O-(6-(E)-feruloyl-β-D-glucopyranosyl)-β-D-glucopyranoside]-5-O-β-D-glucopyranoside
Fig. 4.
Examples for triacylated anthocyanins a Cyanidin-3-O-(2-O-(6-O-(E)-p-coumaryl-β-D glucopyranosyl)-(6-O-(E)-p-coumaryl-β-D-glucopyranosyl)-5-O-(6-O-malonyl)-β-D-glucopyranoside, b Delphinidin-3-O-(2-O-(6-O-(E)-p-coumaryl-β-D glucopyranosyl)-(6-O-(E)-p-coumaryl-β-D-glucopyranosyl)-5-O-(6-O-malonyl)-β-D-glucopyranoside
Fig. 5.
Examples of tetraacylated anthocyanins a Ternatin B2, b Cyanidin 3-2-(2-(feruloyl)-glucosyl)-6-(4-(6-(4-(glucosyl)-feruloyl)-glucosyl)-p-coumaroyl)-glucoside)-5-6-(malonyl)-glucoside
European Union has categorized anthocyanin under the classification number E163. Grape skin extract 163(ii), black current 163(iii), elder berry 163(ix) are the commonly used fruit sources for anthocyanin extraction. Black carrot 163(vi), red radish 163(viii), red cabbage 163(v), purple sweet potato 163(vii) and purple corn 163(iv) are the vegetable sources that are used as commercial anthocyanin sources. Hibiscus 163(x) is the most used flower source to extract anthocyanin (IACM 2020). From the above list of commercially used anthocyanins, grape skin, black current, elderberry mainly contain nonacylated anthocyanins whereas black carrot, red radish, red cabbage, purple sweet potato and purple corn mainly contain monoacylated and diacylated anthocyanins. These anthocyanins are mainly used to in beverage, confectionary and dairy industries (IACM 2020). This review mainly focuses on the stability of acylated and nonacylated anthocyanins in beverage systems.
Anthocyanin as food colours, are permitted in all around the world, but there are differences with respect to what sources and degree of purity are permitted in different counties. For example, grape skin extract is extracted from the remaining residue, left after grape is pressed for grape juice or wine production. Grape color extract is obtained as a by-product from the juice processing of Concord grapes. The anthocyanins are extracted from precipitated lees produced during the storage of Concord grape juice. Using grape color extract anthocyanins is limited only to non-beverage food in America whereas anthocyanins extracted from grape skin can also be used in alcoholic and non-alcoholic beverages (Wrolstad and Culver 2012).
Stability of anthocyanins
The main drawback of using anthocyanins, as food colorants is the lack of stability. The stability of anthocyanins is affected by several factors such as temperature, pH, light and presence of complexing agents in the system. Most anthocyanins are stable in the pH range 4.5–5 (Wrolstad and Culver 2012). Therefore, their application is limited for food products having this pH range. According to Wrolstad and Culver (2012) most anthocyanins cannot be used in milk-based beverages since the pH range of such beverages is around 6–7. Anthocyanins extracted from vegetable sources such as black carrot, red radish, red cabbage, purple sweet potato and purple corn demonstrate significant stability attributed to their acylated anthocyanins (Giusti and Wrolstad 2003). Therefore, when selecting an anthocyanin colorant for a beverage system, its stability in the beverage system must be clearly understood.
Thermal stability
Temperature is a critical parameter in food industry because many important thermal processes such as pasteurization, sterilization, incubation, blanching, freezing etc. are involved in food manufacturing. Some foods undergo high temperature treatments (e. g: pasteurization, sterilization) and some foods require cold storage (e. g: frozen desserts) during manufacturing and storing. Generally, thermal processes above 50 °C cause partial or complete degradation of anthocyanins (Escher et al. 2020). Therefore, when anthocyanin containing food materials are taken for food manufacturing, the thermal stability of those anthocyanins should be taken into consideration.
When considering nonacylated anthocyanins, they are highly unstable to heat. They degrade rapidly even at cold storage. The nonacylated anthocyanins fraction of unprocessed pomegranate juices from 5 pomegranate varieties showed degradation during 10 days of storage at 4 °C. The average percentages of degradation of delphinidin-3,5-diglucoside, delphinidin-3-glucoside, pelargonidin-3-glucoside and pelargonidin-3,5-diglucoside in pomegranate juices were as high as 83%, 68.7%, 56.75% and 34.4%, respectively. However only cyanidin-3,5-diglucoside showed a lower degradation percentage of 23.0% (Alighourchi et al. 2008) whereas Clitoria ternatea polyacylated anthocyanins reduced only down to about 80% in 30 days at 5 °C and the color intensity remained almost unchanged for about 1 year (Lee et al. 2011). More than 80% of total anthocyanins of purple sweet potato was preserved when stored at 4 and 25 °C for 15 days (Chen et al. 2019). This could be attributed to the high amount of acylated anthocyanins in purple sweet potato. In general, degradation reactions happen at a slower rate in refrigerated conditions, but the nonacylated anthocyanins in pomegranate degraded in significant amounts compared with acylated anthocyanins of blue pea flower and purple sweet potato. Food manufacturing usually involves blanching, pasteurization or sterilization step that uses a thermal treatment over 60 °C for a certain time period. The stability of anthocyanins in this temperature range must be studied when they are used in food manufacturing. Blackberry anthocyanins degraded at a high rate when they are heated at 60 °C and the rate constant increased with increasing temperature and heating time (Fernandes et al. 2018). The degradation half-life of blue berry anthocyanins drastically reduced from 180.5 to 5.1 h when the temperature increased from 40 to 80 °C (Kechinski et al. 2010) but blue pea flower anthocyanins at pH 3.6 and 5.4 showed high stability at 60 °C for 6 h (Escher et al. 2020). This temperature range lye around pasteurization temperature used in low temperature long time pasteurization (LTLT: 63 °C for 30 min). Pasteurization is a critical step in food manufacturing. Nonacylated anthocyanin isolated from black goji (Lycium ruthenicum) reduced down its content to 62% when stored at 35 °C for 10 days, whereas monoacylated anthocyanin isolated from Nitraria Tangutorum Bobr and black goji berry reduced their contents only down to 80–85% under same conditions. The total anthocyanin content (94.9 mg mL−1) of red raspberry reduced by 90% when they were stored at 37 °C for 30 days (Chen et al. 2020b). The nonacylated anthocyanin fraction of red cabbage showed a degradation constant of 6.9 h−1 which was higher than those of monoacylated (4.1 h−1) and diacylated anthocyanin fractions (0.8 h−1) when heated at 50 °C in a neutral solution (Fenger et al. 2019). The thermal stability of acylated anthocyanins could be explained by intramolecular copigmentation phenomena. The hydrophobic interaction created between anthocyanin skeleton and acyl groups and overlapping of delocalized π-electron cloud could protect the anthocyanin molecules from nucleophilic water molecules, preventing the formation of colourless carbinol (Sari et al. 2015). These results are evidence of the unstable nature of nonacylated anthocyanins in the temperature ranges used in food manufacturing and storage. Therefore, anthocyanin sources containing higher amount of acylated anthocyanins are more suitable to be used in the food industry.
The nonacylated anthocyanins from jambolan (Syzygium cumini) fruit had poor stability to thermal stress in a model beverage system (pH 3), but acylated anthocyanins that were synthesized enzymatically, from nonacylated anthocyanins of jambolan fruit, had higher stability even at 80–90 °C in the same beverage system (Sari et al. 2015). In industrial beverage production this temperature range is used for pasteurization. Nonacylated cyanidin-3-glucoside, cyanidin-3-rutinoside, cyanidin-3,5-diglucoside extracted from purple corn husk, trueno fruit and bottlebrush flowers, respectively, were esterified enzymatically to synthesize five new acylated anthocyanins that showed better thermal stability at 85 °C compared to their non acylated form (Fernandez-Aulis et al. 2020). All these are underutilized anthocyanin sources that could be used in the food and beverage industry. This technique could be considered as a remedy to improve the stability of nonacylated anthocyanins and utilize them in a much useful manner.
The thermal stability of nonacylated anthocyanins is also affected by the other ingredients in a beverage system. Nonacylated anthocyanins degrade at a high rate in beverage systems containing ascorbic acids and oxidizing agents. Ascorbic acid accelerates the degradation of nonacylated anthocyanins. At 40 °C, degradation rate of rubired grape anthocyanins increased nearly by 10 folds with the addition of 100 ppm ascorbic but further addition of ascorbic acid (up to 400 ppm) did not alter the degradation rate significantly. However, at higher temperatures the effect of ascorbic acid content on anthocyanin degradation became negligible (Song et al. 2013) due to the thermal degradation of ascorbic acid. Unlike other anthocyanidin-3-O-glucosides in rubired grape, the degradation rate of cyanidin-3-O-glucoside extract was higher in both temperatures 40 °C and 60 °C with 500 ppm ascorbic acid. Strawberry anthocyanins are very hear sensitive and presence of ascorbic acid in strawberry makes the nonacylated anthocyanins unstable restricting the colour stability of strawberry products in the market (Sadilova et al. 2009). Not only nonacylated anthocyanins, ascorbic acid reduced the thermal stability of acylated anthocyanins as well. The degradation half-life of purple sweet potato anthocyanins heated at 90 °C reduced from 502 to 135 min upon addition of 0.6% ascorbic acid. (Xu et al. 2019). The reason for this is the condensation reaction and formation of hydrogen peroxide during ascorbic acid degradation. Beverage systems containing oxidizing metal ions increase the degradation rate of nonacylated anthocyanins. Nonacylated anthocyanins of grape in a vitamin enriched water system containing zinc (II) and chromium (III) showed high degradation rates at higher temperatures (80 °C) but not in relatively lower temperatures (40 °C and 60 °C), whereas acylated anthocyanins from purple sweet potato showed relative higher stability in same beverage system (Song et al. 2013) demonstrating the stability of acylated anthocyanins to heat stress. Therefore, when using anthocyanin sources containing nonacylated anthocyanins in the food industry, one should avoid using ascorbic acid or ingredients containing oxidizing metal ions.
Like nonacylated anthocyanins, the thermal stability of acylated anthocyanins is affected by the ingredients in the medium as well. Red cabbage extract containing a high amount of diacylated anthocyanins acylated with p-coumaric, ferulic and sinapic acids showed higher thermal stability at 80 °C in pH 3 buffer solution when compared with 3 nonacylated anthocyanin sources like blackcurrant, grape skin and elderberry. The stability of red cabbage anthocyanins were two times lower in non-carbonated soft drink medium (pH 3.0) than in buffer solution. The reason could be the effect of sugar and ascorbic acid in the beverage system (Dyrby et al. 2001). This suggests that red cabbage cultivars with higher proportions of diacylated anthocyanins can be used as a colourant in non-carbonated beverage systems. Some ingredients further increase the thermal stability of acylated anthocyanins. Anthocyanin extract of Clitoria ternatea (Blue pea) flower showed high thermal stability to its polyacylated anthocyanins. Blue pea anthocyanins were stable at 5 and 45 °C for 15 days and at 100 °C for 80 min and presence of benzoic acid has increased the stability (Lee et al. 2011). Therefore, when using blue pea anthocyanins as a food colourant, benzoic acid could be used as a preservative.
However, despite of acylation, the thermal stability of anthocyanins depends on the pH of the medium. This dependency is subjective on the anthocyanin source from which the anthocyanins were extracted. Some anthocyanins show higher thermal stability in lower pH whereas some anthocyanins reduce their thermal stability in lower pH. Thermal degradation of red radish diacylated anthocyanins were dependant on pH of the medium. At pH 3, the diacylated anthocyanins were significantly more stable to heat than monoacylated anthocyanins (Matsufuji et al. 2007). In contrast, purple sweet potato anthocyanins were unstable at very low pH. The half-life of purple sweet potato anthocyanins was high in pH 3 than pH 2 but again reduced when pH raised from 5 to 6. Anthocyanins extracted from purple sweet potato anthocyanins added in to apple juice (pH 3.5) and pear juice (pH 4.2) were more stable to thermals stress during heating at 80–100 °C, but was unstable in lemon juice (pH 2.2) (Li et al. 2013). Regardless whether the anthocyanins were acylated or nonacylated, the most suitable pH range where these anthocyanins show highest stability ranges between pH 3 and pH 5.
In summary, acylated anthocyanins show higher stability in a broad temperature range: in low temperatures such as 4 °C, moderate temperatures such as 35 °C and in high temperatures such as 60, 80 and 100 °C where most of the thermal processes (e. g: pasteurization, blanching etc.) are practised in the food industry whereas nonacylated anthocyanins are stable only up to 35 to 40 °C limiting their applications. But there are several ways to increase the stability of nonacylated anthocyanins, and those techniques will be discussed later in this review.
pH stability
Every food has a pH value and pH is an important quality controlling parameter in food industry. If a food product does not achieve the intended pH value while manufacturing, it would affect badly on the shelf life and the sensory quality of that food product. On the other hand, pH of a system significantly affects the stability of anthocyanins. With the change in pH of the medium, the anthocyanin structure also changes. In acidic pH the flavylium ion exists, when pH increases flavylium ion turns to colourless carbinol pseudo base and in alkaline pH the anionic quinoidal base and the calcone exist (Liu et al. 2014). This transformation makes it easy for water to attack the anthocyanin core, thus making the anthocyanins unstable.
Generally, anthocyanins are unstable in neutral and alkaline pH, they are stable in low pH range (Fenger et al. 2020). Interestingly, monoacylated anthocyanins of black goji are stable in both acidic and alkaline environment. Monoacylated anthocyanins from black goji showed high stability from pH 7 to 9, whereas the saponified anthocyanins (acyl grouped removed) degraded faster in the neutral and alkaline pH (Tang and Giusti 2018). Hu et al (2014) also observed the stability of monoacylated anthocyanins from black goji at alkaline pH. In solutions with pH 5 and 7, petunidin-3, 5-O-diglucosides, a nonacylated anthocyanin from black goji reduced by 85% in 12 days, whereas the acylated anthocyanin, petuinidin-3-O-rutinoside (trans-p-coumaroyl)-5-glucosides) reduced only by 50% after 12 days. Bucaro and Bandolero red cabbage cultivars have shown much lower anthocyanin degradation rates in neutral and alkaline pH which could be explained by the high content of diacylated anthocyanins in them. These diacylated anthocyanins rich extracts better matched the colours of indigo carmine at neutral pH (Ahmadiani et al. 2014). Purple sweet potato anthocyanins, containing a large fraction of diacylated anthocyanins have been stable for 15 days in pH 2, 4 and 6 (Li et al. 2015). A functional beverage was produced using blue pea anthocyanin extract. The optimum pH range for this functional beverage was pH 3.5–4.0 and the beverage could be preserved at room temperature for 28 days without using any preservatives (Lakshan et al. 2019). In another study, a model beverage (pH 2.8) prepared with 13% sucrose and red sweet potato anthocyanins containing diacylated anthocyanins demonstrated better colour stability compared to model beverage prepared with blackberry anthocyanins containing nonacylated anthocyanins (Francis 1987). The pH stability of acylated anthocyanins could be explained by the protection provided by the acyl groups to anthocyanin chromophore, by π-stacking interaction and self-associations so that it limits the hydration of the electrophilic sites in anthocyanin chromophore (Fenger et al. 2019). Specifically, for diacylated anthocyanins, they demonstrate intramolecular sandwich type stacking protecting the anthocyanin choromophore from nucleophilic water molecules (Giusti and Wrolstad 2003). Bridle and Timberlake (1997) explained the pH stability of polyacylated anthocyanins from blue pea flower whereby when the pH of the medium increases, the presence of acyl groups prevents the hydrolysis of flavylium ion to less stable carbinol pseudo base form, instead form the blue colour quinoidal form having less sensitivity to pH changes in mildly acidic or neutral condition. The pH value of all beverages varies around this pH range, making this diacylated anthocyanin source a suitable source of anthocyanin for beverages. The stability of diacylated anthocyanins at higher pH also depends on the acyl group. At higher pH anthocyanins diacylated with p-coumaric or ferulic acids are more stable than ones diacylated with caffeic acids (Matsufuji et al. 2007).
Some studies show that the pH stability of acylated anthocyanins is not always higher than the nonacylated anthocyanins. In a study, cyanidin dominant anthocyanin extract and pelargonidin dominant anthocyanin extracts from purple corn-with high percentages of acylated anthocyanins (57% and 64%, respectively)—majority were monoacylated, when added in a beverage system (pH 3), monoacylated anthocyanins degraded at a higher rate compared with nonacylated anthocyanins, both at 25 °C and 40 °C over the storage period. The reason is, in anthocyanins from corn, the majority of acylation is aliphatic not aromatic. This research states that even the copigmentation of purple corn monoacylated anthocyanins with purple corn C-glycosyl flavone had no effect on anthocyanin half-life but nonacylated anthocyanins had a prolonged half-life in copigmented system (Chatham et al. 2020). Considering the above studies, we can conclude that petunidin derived monoacylated anthocyanins and polyacylated anthocyanins are more suitable to be used as colourants in the beverage industry than nonacylated anthocyanins and pelargonidin derived monoacylated anthocyanins.
Photo stability
Light is an unavoidable environmental factor that affects the stability of anthocyanins. Along the food processing chain and during storage the anthocyanins get exposed to photo stress. Anthocyanins are generally stable to photo stress in acidic medium, but nonacylated anthocyanins isolated from black goji berry showed poor photo stability even in low pH whereas acylated anthocyanins showed higher photo stability. Among anthocyanins stored in a pH 3 solution at 35 °C, after 10 days, nearly 90% of acylated anthocyanins from Nitraria Tangutorum Bobr and Lycium ruthenicum Murray were retained when stored in light whereas nonacylated anthocyanin content reduced to 79% when stored in dark and to 64% when stored exposed to light (Hu et al. 2014). These results explain the unstable nature of nonacylated anthocyanins to photo stress. Nonacylated anthocyanins from jambolan fruit had poor stability to photo stress in a model beverage system (pH 3). Acylated anthocyanins synthesized enzymatically, from nonacylated anthocyanins of jambolan fruit, had high stability under fluorescent light for 6 days (Sari et al. 2015). The photo stability of some monoacylated anthocyanins from red radish was independent of the pH between pH 3 and 5 (Matsufuji et al. 2007). The photodegradation occurs when photons are absorbed by molecules with the resulting cleavage of bonds. The aromatic acyl groups in acylated anthocyanins work as a filter that absorb photons and reduce the sensitivity of acylated anthocyanins to photo stress (Dyrby et al. 2001).
Photo stability of most anthocyanins depend on the pH of the medium. At pH 3, the diacylated anthocyanins from red radish were significantly more stable to photo stress than monoacylated anthocyanins. Red radish anthocyanins kept at pH 3 and 5 for 20 days subjected to light exposure had more than 60% of anthocyanins retained but in pH 7, anthocyanin content reduced to 40% within 1 day (Matsufuji et al. 2007). Another research was conducted using red radish anthocyanins in a model beverage (pH-3.5) and it demonstrated a degradation half-life of 22 weeks at 25 °C (Giusti et al. 1999). Generally, pH of beverages is acidic. This suggest the applicability of acylated anthocyanins in acidic beverage systems.
Surprisingly, unlike thermal stress, ascorbic acid reduces the photo stress induced degradation rates in both acylated and nonacylated anthocyanins. Addition of 100 ppm ascorbic acid reduced the degradation rates of purple sweet potato anthocyanin and grape anthocyanin from 2.73 × 10−3 to 1.58 × 10−3 min−1 at photo stress of 500 W/m2 min−1 (Song et al. 2013). This happens because of the scavenging effect of ascorbic acid on the peroxyl radicals formed from anthocyanins due to photo stress (Ay et al. 2019). This effect of ascorbic acid also depends on the atmospheric composition in contact with anthocyanin solution. Nitrogen gas lowers the stability of both acylated and nonacylated anthocyanins when ascorbic acid is present in solution. However, if ascorbic acid is not present in the solution, nitrogen filled atmosphere increases the stability of anthocyanins (Ay et al. 2019). Reducing the headspace and filling the headspace with an inert gas such as nitrogen may help to increase the stability of anthocyanins in beverage systems.
Some evidence shows that the acylated anthocyanins are not always more stable to photo-stress than nonacylated anthocyanins. Diacylated anthocyanins extracted from purple sweet potato showed higher degradation rates for photo stress in a vitamin enriched water system compared with nonacylated anthocyanins extracted from grape. Under a photo stress of 500 W m2 min−1 the degradation rate of purple sweet potato anthocyanins was 2.02 × 10–3 min−1 whereas for grape anthocyanins the rate was 1.00 × 10–3 min−1. Under a photo stress of 750 W m2 min−1 the degradation rate of purple sweet potato anthocyanins was 2.40 × 10–3 min−1 whereas for grape anthocyanins the rate was 1.85 × 10–3 min−1.This study found that due to photo stress, the diacylated anthocyanin (peonidin-3-dicaffeoylsophoroside-5-glucoside) converted in to monoacylated anthocyanins by removing a caffeic acid group resulting an unstable nature in purple sweet potato anthocyanins (Song et al. 2013). Surprisingly, blue pea anthocyanins having a significant amount of polyacylated anthocyanins show highly unstable nature to photo stress. Blue pea anthocyanins in a solution of pH 3.6 and 5.4 exposed to light showed a retention percentage as low as 34.4% and 48.3% whereas solutions kept in dark showed high retention percentages over 90% (Escher et al. 2020). Both monoacylated and diacylated anthocyanins from purple sweet potato degraded nearly by 87% when stored at 25 °C exposed to light within a period of 6 months (Quan et al. 2019). Therefore, when using blue pea and purple sweet potato anthocyanins in beverages it is recommended to use amber colour or covered containers that prevent light penetration.
As a whole it is clear that acylated anthocyanins demonstrate higher stability than nonacylated anthocyanins with a few exceptions. When using acylated anthocyanins in the food industry, by applying low temperature long time pasteurization (63.5 °C for 30 min), using translucent or opaque packaging, maintaining the pH of the product in the range 3–5 and storing the finished products around 25 °C could further improve the stability of acylated anthocyanins. When using acylated anthocyanins as natural food colourants, one must be careful about the presence of other ingredients used for manufacturing the food product since ingredients such as reducing agents and oxidizing agents can affect the stability of anthocyanins in different manners. Still, flowers containing high amount of polyacylated anthocyanins have not been used as commercial anthocyanin sources. It is recommended to search for more flower sources containing high content of polyacylated anthocyanins and feasible techniques to improve the stability of currently used anthocyanins.
Techniques to increase the stability of nonacylated anthocyanins
Even though nonacylated anthocyanins are relatively unstable, food industry cannot avoid using those anthocyanin sources in food manufacturing because they demonstrate high nutritional profiles and attractive colours. On the other hand, the availability of nonacylated anthocyanin sources is higher compared to polyacylated anthocyanin sources. Therefore, it is important to find out remedial techniques to overcome the unstable nature of these nonacylated anthocyanins and to use them effectively in food manufacturing.
One of the techniques is adding sweeteners to beverage systems. The instability of nonacylated anthocyanins could be controlled by adding sweeteners such as honey and maltose syrup into beverages. Sweeteners reduce the water availability by reducing available nucleophilic water molecules that can attack the anthocyanin core, and thus reduce the anthocyanin degradation. Honey reduces the anthocyanin degradation in another way. Polyphenols, amino acids and organic acids present in honey act as copigments and protect the anthocyanin chromophore (Ertan et al. 2018). Adding honey or maltose syrup is preferred than using sucrose, because during storage, sucrose could be degraded to form products such as fructose, which reduces the anthocyanin stability (Kopjar and Piližota 2011). However, the effect of adding sweeteners like honey, on other physical properties of the beverage such as turbidity and colour should be taken in to account.
Copigmentation is an association of anthocyanins with other compounds, metal ions or with the anthocyanin itself (Zhao et al. 2020). Copigmentation contributes to the color expression and stabilization of anthocyanins in beverages (Chen et al. 2020a). A study shows that by adding copigments like gallic acid and plant extracts such as extracts of rose leaf, cherry stem, pomegranate rind, sour cherry stem, containing copigments, could increase the stability of nonacylated anthocyanins of strawberry nectar sweetened with sucrose and maltose syrup. Here the anthocyanin degradation is hindered via 2 mechanisms: sweeteners reduces the water availability and the co pigmentation effect of compounds in plant extracts. But for strawberry nectar sweetened with honey, these copigments reduced the anthocyanin stability (Ertan et al. 2020). This could be explained by the competition among copigment molecules in extracts and honey to bind with anthocyanins. Therefore, for beverage systems consisting of nonacylated anthocyanins like cyanidin-3-glucoside, pelargonidin-3-glucoside and pelargonidin-3-rutinoside their storage stability could be increased by adding natural extracts of sour cherry stem and pomegranate rind together with sweeteners such as maltose syrup. However, sucrose syrup makes pelargonidin based anthocyanins unstable (Ertan et al. 2020), therefore some other sweetener should be used when manufacturing beverages with fruits containing pelargonidin as their major anthocyanin.
Pasteurization can reduce the degradation of nonacylated anthocyanins. Degradation rates of nonacylated anthocyanins in pasteurized pomegranate juice were lower during cold storage for 10 days compared with raw juice attributed to heat induced copigmentation with native copigments and self-association of anthocyanins (Alighourchi et al. 2008). Therefore, we suggest that pasteurization of fruit juices with nonacylated anthocyanins to control their degradation.
Adding hydrocolloids into beverage systems containing anthocyanins, can stabilize the anthocyanins. Purple rice bran anthocyanins showed very high degradation rate at 90 °C in pH 6 but carboxymethyl cellulose, xanthan gum, modified starch, and gum arabic reduced the degradation of purple rice bran anthocyanins. Modified starch showed the greatest effect (Das et al. 2020). Steric hindrance imposed by the hydrocolloids and the electrostatic interactions between hydrocolloids and the flavylium ion protect the flavylium ion from nucleophilic water molecules (Buchweitz et al. 2013). As mentioned previously, ascorbic acid increases the thermal degradation of both acylated and nonacylated anthocyanins. Hydrocolloids also can retard the anthocyanin degradation caused by ascorbic acid. Gum Arabic was able to reduce the anthocyanin degradation caused by ascorbic acid, in a model beverage system (pH 3) containing purple carrot anthocyanins. During a period of 5 days at 40 °C under light, the degradation rate constant reduced from 0.309/day down to 0.116/day when 1.5% gum Arabic was added to model beverage system. Anthocyanin could have interacted with the glycoprotein fractions of gum Arabic through hydrogen bonding, resulting in improved stability (Chung et al. 2016). Addition of high acyl gellum gum increased the degradation half-life of purple sweet potato anthocyanins from 135 to 274 min at 90 °C (Xu et al. 2019). Biopolymers such as bovine serum albumin, whey protein and soy protein increase the thermal stability of anthocyanins. Addition of 200 mg mL−1 whey protein and 25 mg mL−1 soy protein significantly reduced the degradation rate constant of purple sweet potato anthocyanins in a model beverage system but addition of β-cyclodextrin slightly increased the degradation rate constant. When the model beverage was heated at 100 °C for 30 min its degradation rate constant was 1.103 min−1, addition of whey protein and soy protein reduced the rate constant to 0.751 min−1 and 0.569 min−1 respectively, but addition of 500 mg mL−1 β-cyclodextrin increased the degradation rate constant up to 1.431 min−1. However, addition of 50 mg mL−1 whey protein, 50 mg mL−1 soy protein and 2500 mg mL−1 β-cyclodextrin increased the storage stability of the model beverage system with purple sweet potato anthocyanin (Quan et al. 2020). Chung et al. (2015) suggested that biopolymers such as whey proteins complexed with anthocyanins through hydrogen bonding and enhanced the stability of anthocyanins. Adding grape skin pectin in to solution containing grape anthocyanins reduced the degradation rate constant from 0.6 to 0.032 h−1 when heated at 60 °C and from 1.15 to 1.05 h−1 when heated at 80 °C (Fernandes et al. 2020). Hydrocolloids may be protecting the flavylium cation from water molecules, thus preventing anthocyanin degradation (Das et al. 2020). Buchweitz et al. (2013) reported that the strong interactions between the carboxylate groups and free electron pair of amide groups of pectin molecules with the flavylium ion may protect the anthocyanin molecule from nucleophilic water attack and subsequent degradation. This could be used in food industry when developing food products with purple rice bran or its anthocyanins.
Acylation is another approach to increase the stability of nonacylated anthocyanins. Acylated anthocyanins could be synthesized from nonacylated anthocyanins by chemical or enzyme assisted esterification. Out of the two techniques, enzyme esterification is considered better since the chemical method degrade anthocyanins and has poor selectivity (Yang et al. 2019). Enzymatically synthesized new acylated anthocyanins, from purple corn husk, trueno fruit and bottlebrush flowers (Fernandez-Aulis et al. 2020), from black currant anthocyanins (Yang et al. 2019), and from jambolan fruit showed better thermal and photo stability compared to their non acylated form. However, acylation reduced the antioxidant activity of jambolan anthocyanins (Sari et al. 2015). However, enzymatic acylation of blueberry anthocyanins with coumaric acid and caffeic acid increased the antioxidant activity and thermal stability of native blueberry anthocyanins. Both anthocyanin fractions acylated with p-coumaric acid and caffeic acid showed significantly low fading degrees at 25, 40 and 60 °C during a period of 96 h, compared with native blueberry anthocyanins. Adding p-coumaric acid and caffeic acid increased the antioxidant activity of blueberry anthocyanins measured by DPPH radical scavenging activity by 6.56% and 15.21%, respectively (Liu et al. 2020). Therefore, acylation always increases the stability of anthocyanins but if affects the antioxidant activity of the anthocyanins in different ways.
Conclusion and future prospective of research
Most of the commercial anthocyanin sources contain nonacylated anthocyanins or monoacylated anthocyanins as their prominent anthocyanins. The stability of these nonacylated anthocyanins is relatively low compared with acylated anthocyanins. Monoacylated anthocyanins are more stable than nonacylated anthocyanins but the stability is affected by several factors. Despite the acylation, the stability of anthocyanins to thermal stress depends on the pH of the medium whereas some acylated anthocyanins showed pH independent stability for photo-stress. Several plant extracts containing copigments, sweeteners and colloidal substances have been able to increase the stability of nonacylated anthocyanins, but we have to consider the cost factor and the effect of these copigments on the sensory attributes of the food product. Acylation of nonacylated anthocyanins may be an approach to increase their stability in food systems but the process seems quite complicated and expensive restricting its application in industrial scale. All anthocyanins are stable in acidic pH and unstable in alkaline pH, yet some polyacylated anthocyanins showed pH independent stability. Polyacylated anthocyanins have relatively higher stabilities compared to nonacylated anthocyanins and monoacylated anthocyanins but still these sources have not been exploited on a commercial scale. Finally, we propose that, future studies should concentrate more on extracting polyacylated anthocyanins from natural sources, especially flowers.
Funding
This work was funded by School of Science, Monash University Malaysia.
Declarations
Conflict of interests
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadiani N, Robbins RJ, Collins TM, Giusti MM. Anthocyanin contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity Stages. J Agric Food Chem. 2014;62:7524–7531. doi: 10.1016/j.jfca.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Alighourchi H, Barzegar M, Abbasi S. Anthocyanins characterization of 15 Iranian pomegranate (Punica granatum L.) varieties and their variation after cold storage and pasteurization. Eur Food Res Technol. 2008;227:881–887. doi: 10.1007/s00217-007-0799-1. [DOI] [Google Scholar]
- Ay E, Morlet-Savary F, Graff B, Galopin C, Gérard V, Ogren T, Lalevée J. Thermal and photochemical stability of anthocyanins from black carrot, grape juice, and purple sweet potato in model beverages in the presence of ascorbic acid. J Agric Food Chem. 2019;67:5647–5660. doi: 10.1021/acs.jafc.9b01672. [DOI] [PubMed] [Google Scholar]
- Benedek C, Bodor Z, Merrill VT, Kókai Z, Gere A, Kovacs Z, Dalmadi I, Abrankó L. Effect of sweeteners and storage on compositional and sensory properties of blackberry jams. Eur Food Res Technol. 2020 doi: 10.1007/s00217-020-03564-2. [DOI] [Google Scholar]
- Bridle P, Timberlake C. Anthocyanins as natural food colours-selected aspects. Food Chem. 1997;58:103–109. doi: 10.1016/S0308-8146(96)00222-1. [DOI] [Google Scholar]
- Buchweitz M, Speth M, Kammerer DR, Carle R. Impact of pectin type on the storage stability of black currant (Ribes nigrum L.) anthocyanins in pectic model solutions. Food Chem. 2013;139:1168–1178. doi: 10.1016/j.foodchem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Chatham LA, Howard JE, Juvik JA. A natural colorant system from corn: flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2020;310:125734. doi: 10.1016/j.foodchem.2019.125734. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lin C, Chen MH, Chiang PY. Stability and quality of anthocyanin in purple sweet potato extracts. Foods. 2019;8:393. doi: 10.3390/foods8090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lou L, Chen Y, Ye X, Zhu Y, Chen J. Copigmentation effect of three phenolic acids on color and thermal stability of chinese bayberry anthocyanins. Food Sci Nutr. 2020;8:3234–3242. doi: 10.1002/fsn3.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Du J, Li ML, Li CM. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT Food Sci Technol. 2020;128:109448. doi: 10.1016/j.lwt.2020.109448. [DOI] [Google Scholar]
- Choo WS. Fruit pigment changes during ripening. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. The Netherlands: Elsevier; 2019. pp. 117–123. [Google Scholar]
- Chung C, Rojanasasithara T, Mutilangi W, McClements DJ. Enhanced stability of anthocyanin-based color in model beverage systems through whey protein isolate complexation. Food Res Int. 2015;76:761–768. doi: 10.1016/j.foodres.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Chung C, Rojanasasithara T, Mutilangi W, Mcclements DJ. Enhancement of colour stability of anthocyanins in model beverages by gum Arabic addition. Food Chem. 2016;201:14–22. doi: 10.1016/j.foodchem.2016.01.051. [DOI] [PubMed] [Google Scholar]
- Cortez R, Luna-Vital DA, Margulis D, de Mejia EG. Natural pigments: stabilization methods of anthocyanins for food applications. Compr Rev Food Sci Food Saf. 2017;16:180–198. doi: 10.1111/1541-4337.12244. [DOI] [PubMed] [Google Scholar]
- Das AB, Goud VV, Das C. Degradation kinetics of anthocyanins from purple rice bran and effect of hydrocolloids on its stability. J Food Process Eng. 2020;43:e13360. doi: 10.1111/jfpe.13360. [DOI] [Google Scholar]
- Dyrby M, Westergaard N, Stapelfeldt H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001;72:431–437. doi: 10.1016/S0308-8146(00)00251-X. [DOI] [Google Scholar]
- Ertan K, Türkyılmaz M, Özkan M. Effect of sweeteners on anthocyanin stability and colour properties of sour cherry and strawberry nectars during storage. J Food Sci Technol. 2018;55:4346–4355. doi: 10.1007/s13197-018-3387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertan K, Türkyılmaz M, Özkan M. Color and stability of anthocyanins in strawberry nectars containing various co-pigment sources and sweeteners. Food Chem. 2020;310:e125856. doi: 10.1016/j.foodchem.2019.125856. [DOI] [PubMed] [Google Scholar]
- Escher GB, Wen M, Zhang L, Rosso ND, Granato D. Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L. (butterfly pea) blue petals. Food Chem. 2020;331:127341. doi: 10.1016/j.foodchem.2020.127341. [DOI] [PubMed] [Google Scholar]
- Fenger JA, Moloney M, Robbins RJ, Collins TM, Dangles O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019;10:6740–6751. doi: 10.1039/c9fo01884k. [DOI] [PubMed] [Google Scholar]
- Fenger JA, Robbins RJ, Collins TM, Dangles O. The fate of acylated anthocyanins in mildly heated neutral solution. Dyes Pigm. 2020;178:108326. doi: 10.1016/j.dyepig.2020.108326. [DOI] [Google Scholar]
- Fernandes A, Rocha MAA, Santos LMNBF, Brás J, Oliveira J, Mateus N, De Freitas V. Blackberry anthocyanins: β-cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018;245:426–431. doi: 10.1016/j.foodchem.2017.10.109. [DOI] [PubMed] [Google Scholar]
- Fernandes A, Brandão E, Raposo F, Maricato É, Oliveira J, Mateus N, Coimbra MA, De Freitas V. Impact of grape pectic polysaccharides on anthocyanins thermostability. Carbohydr Polym. 2020;239:116240. doi: 10.1016/j.carbpol.2020.116240. [DOI] [PubMed] [Google Scholar]
- Fernandez-Aulis F, Torres A, Sanchez-Mendoza E, Cruz L, Navarro-Ocana A. New acylated cyanidin glycosides extracted from underutilized potential sources: enzymatic synthesis, antioxidant activity and thermostability. Food Chem. 2020;309:125796. doi: 10.1016/j.foodchem.2019.125796. [DOI] [PubMed] [Google Scholar]
- Francis FJ. Stability of anthocyanins from sweet-potatoes in a model beverage. J Food Sci. 1987;52:1753–1754. doi: 10.1111/j.1365-2621.1987.tb05927.x. [DOI] [Google Scholar]
- Galaffu N, Bortlik K, Michel M. An industry perspective on natural food colour stability. In: Scotter MJ, editor. Colour additives for foods and beverages. Oxford: Woodhead Publishing; 2015. pp. 91–130. [Google Scholar]
- Gardeli C, Varela K, Krokida E, Mallouchos A. Investigation of anthocyanins stability from pomegranate juice (Punica granatum L. Cv Ermioni) under a simulated digestion process. Medicines. 2019;6:31434230. doi: 10.3390/medicines6030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochem Eng J. 2003;14:217–225. doi: 10.1016/s1369-703x(02)00221-8. [DOI] [Google Scholar]
- Giusti MM, Wrolstad RE, Rodriguez-Saona LE, Wrolstad RE. Color and pigment stability of red radish and red-fleshed potato anthocyanins in juice model systems. J Food Sci. 1999;64:451–456. doi: 10.1111/j.1365-2621.1999.tb15061.x. [DOI] [Google Scholar]
- Hashimoto M, Suzuki T, Iwashina T. New acylated anthocyanins and other flavonoids from the red flowers of Clematis cultivars. Nat Prod Commun. 2011;6:1631–1636. doi: 10.1177/1934578X1100601118. [DOI] [PubMed] [Google Scholar]
- Hu N, Zheng J, Li WC, Suo YR. Isolation, stability, and antioxidant activity of anthocyanins from Lycium ruthenicum murray and Nitraria tangutorum bobr of Qinghai-Tibetan plateau. Sep Sci Technol. 2014;49:2897–2906. doi: 10.1080/01496395.2014.943770. [DOI] [Google Scholar]
- International Association of Colour Manufacturer (2020). https://iacmcolor.org/color-profile/anthocyanins/. Accessed 6 July 2020
- Jeyaraj EJ, Lim YY, Choo WS. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J Food Sci Technol. 2020 doi: 10.1007/s13197-020-04745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechinski CP, Guimarães PVR, Noreña CPZ, Tessaro IC, Marczak LDF. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J Food Sci. 2010;75:173–176. doi: 10.1111/j.1750-3841.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- Kim I, Lee J. Variations in anthocyanin profiles and antioxidant activity of 12 genotypes of mulberry (Morus spp.) fruits and their changes during processing. Antioxidants. 2020;9:242. doi: 10.3390/antiox9030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koley TK, Srivastava S, Tripathi YB, Banerjee K, Oulkar D, Goon A, Singh B. High-resolution LCMS profiling of phenolic compounds of indian black carrot and evaluation of its effect on antioxidant defense and glucose metabolism in animal model. Agric Res. 2019;8:481–489. doi: 10.1007/s40003-018-0389-4. [DOI] [Google Scholar]
- Koley TK, Khan Z, Oulkar D, Singh BK, Maurya A, Singh B, Banerjee K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab J Chem. 2020;13:1355–1366. doi: 10.1016/j.arabjc.2017.11.007. [DOI] [Google Scholar]
- Kopjar M, Piližota V. Prevention of thermal degradation of anthocyanins in blackberry juice with addition of different sugars. CyTA-J Food. 2011;9:237–242. doi: 10.1080/19476337.2010.522735. [DOI] [Google Scholar]
- Kurata R, Sun HN, Oki T, Okuno S, Ishiguro K, Sugawara T. Sweet potato polyphenols. In: Mu TH, Singh J, editors. Sweet potato. New York: Academic Press; 2019. pp. 177–222. [Google Scholar]
- Lakshan SAT, Jayanath NY, Abeysekera WPKM, Abeysekera WKSM. A commercial potential blue pea (Clitoria ternatea L.) flower extract incorporated beverage having functional properties. Evid Based Complement Altern Med. 2019 doi: 10.1155/2019/2916914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PM, Abdullah R, Hung LK (2011) Thermal degradation of blue anthocyanin extract of Clitoria ternatea flower. Poster session presentation at 2nd international conference on biotechnolgy and food science, Bali Island, Indonesia
- Li J, Li XD, Zhang Y, Zheng ZD, Qu ZY, Liu M, Qu L. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem. 2013;136:1429–1434. doi: 10.1016/j.foodchem.2012.09.054. [DOI] [PubMed] [Google Scholar]
- Li CY, Kim HW, Li H, Lee DC, Rhee HI. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem. 2014;152:592–596. doi: 10.1016/j.foodchem.2013.11.152. [DOI] [PubMed] [Google Scholar]
- Li XL, Lv YP, He Q, He XL. Composition and color stability of anthocyanin-based extract from purple sweet potato. Food Sci Technol. 2015;35:468–473. doi: 10.1590/1678-457X.6687. [DOI] [Google Scholar]
- Li W, Pang X, Xiao J, Wang X, He R, Zhao X. Degradation kinetics of pelargonidin-3-(p-coumaroyl)diglucoside-5-(malonyl)glucoside and pelargonidin-3-(feruloyl)diglucoside-5-(malonyl)glucoside in red radish during air-impingement jet drying. LWT Food Sci Technol. 2020;127:109390. doi: 10.1016/j.lwt.2020.109390. [DOI] [Google Scholar]
- Liu S, Fu Y, Nian S. Buffering colour fluctuation of purple sweet potato anthocyanins to acidity variation by surfactants. Food Chem. 2014;162:16–21. doi: 10.1016/j.foodchem.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Liu JN, Zhuang YH, Hu YH, Xue S, Li H, Chen L, Fei P. Improving the color stability and antioxidation activity of blueberry anthocyanins by enzymatic acylation with p-coumaric acid and caffeic acid. LWT Food Sci Technol. 2020;130:8. doi: 10.1016/j.lwt.2020.109673. [DOI] [Google Scholar]
- Lohachoompol V, Srzednicki G, Craske J. The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J Biomed Biotechnol. 2004;2004:248–252. doi: 10.1155/S1110724304406123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpaung AM, Andarwulan N, Hariyadi P, Faridah DN. The difference in colour shifting of Clitoria ternatea L. flower extract at pH 1, 4, and 7 during storage. Curr Nutr Food Sci. 2019;15:694–699. doi: 10.2174/1573401314666180503152636. [DOI] [Google Scholar]
- Matsufuji H, Kido H, Misawa H, Yaguchi J, Otsuki T, Chino M, Yamagata K. Stability to light, heat, and hydrogen peroxide at different pH values and DPPH radical scavenging activity of acylated anthocyanins from red radish extract. J Agric Food Chem. 2007;55:3692–3701. doi: 10.1021/jf063598o. [DOI] [PubMed] [Google Scholar]
- Octavia L, Choo WS. Folate, ascorbic acid, anthocyanin and colour changes in strawberry (Fragaria× annanasa) during refrigerated storage. LWT Food Sci Technol. 2017;86:652–659. doi: 10.1016/j.lwt.2017.08.049. [DOI] [Google Scholar]
- Quan W, He W, Lu M, Yuan B, Zeng M, Gao D, He Z. Anthocyanin composition and storage degradation kinetics of anthocyanins-based natural food colourant from purple-fleshed sweet potato. Int J Food Sci Technol. 2019;54:2529–2539. doi: 10.1111/ijfs.14163. [DOI] [Google Scholar]
- Quan W, He W, Qie X, Chen Y, Zeng M, Qin F, He Z. Effects of β-cyclodextrin, whey protein, and soy protein on the thermal and storage stability of anthocyanins obtained from purple-fleshed sweet potatoes. Food Chem. 2020;320:126655. doi: 10.1016/j.foodchem.2020.126655. [DOI] [PubMed] [Google Scholar]
- Sadilova E, Stintzing FC, Kammerer DR, Carle R. Matrix dependent impact of sugar and ascorbic acid addition on color and anthocyanin stability of black carrot, elderberry and strawberry single strength and from concentrate juices upon thermal treatment. Food Res Int. 2009;42:1023–1033. doi: 10.1016/j.foodres.2009.04.008. [DOI] [Google Scholar]
- Sang J, Zhang Y, Sang J, Li CQ. Anthocyanins from Nitraria tangutorun: qualitative and quantitative analyses, antioxidant and anti-inflammatory activities and their stabilities as affected by some phenolic acids. J Food Meas Charact. 2019;13:421–430. doi: 10.1007/s11694-018-9956-4. [DOI] [Google Scholar]
- Sari P, Setiawan A, Siswoyo TA. Stability and antioxidant activity of acylated jambolan (Syzygium cumini) anthocyanins synthesized by lipase-catalyzed transesterification. Int Food Res J. 2015;22:671–676. [Google Scholar]
- Shimizu Y, Imada T, Zhang HL, Tanaka R, Ohno T, Shimomura K. Identification of novel poly-acylated anthocyanins from Gynura bicolor leaves and their antioxidative activity. Food Sci Technol Res. 2010;16:479–486. doi: 10.3136/fstr.16.479. [DOI] [Google Scholar]
- Song BJ, Sapper TN, Burtch CE, Brimmer K, Goldschmidt M, Ferruzzi MG. Photo-and thermodegradation of anthocyanins from grape and purple sweet potato in model beverage systems. J Agric Food Chem. 2013;61:1364–1372. doi: 10.1021/jf3044007. [DOI] [PubMed] [Google Scholar]
- Strauch RC, Mengist MF, Pan K, Yousef GG, Iorizzo M, Brown AF, Lila MA. Variation in anthocyanin profiles of 27 genotypes of red cabbage over two growing seasons. Food Chem. 2019;301:e125289. doi: 10.1016/j.foodchem.2019.125289. [DOI] [PubMed] [Google Scholar]
- Tamura H, Yamagami A. Antioxidative activity of monoacylated anthocyanins isolated from Muscat Bailey A grape. J Agric Food Chem. 1994;42:1612–1615. doi: 10.1021/jf00044a005. [DOI] [Google Scholar]
- Tan JBL, Lim YY, Lee SM. Rhoeo spathacea (Swartz) Stearn leaves, a potential natural food colorant. J Funct Foods. 2014;7:443–451. doi: 10.1016/j.jff.2014.01.012. [DOI] [Google Scholar]
- Tang P, Giusti MM. Black goji as a potential source of natural color in a wide pH range. Food Chem. 2018;269:419–426. doi: 10.1016/j.foodchem.2018.07.034. [DOI] [PubMed] [Google Scholar]
- Tatsuzawa F. Acylated cyanidin 3-sambubioside-5-glucosides from the purple-violet flowers of Matthiola longipetala subsp. bicornis (Sm) P. W. Ball. (Brassicaceae) Phytochem Lett. 2014;9:17–21. doi: 10.1016/j.phytol.2014.03.011. [DOI] [Google Scholar]
- Tatsuzawa F. Acylated pelargonidin glycosides from the red-purple flowers of Iberis umbellata L. and the red flowers of Erysimum × cheiri (L.) Crantz (Brassicaceae) Phytochemistry. 2019;159:108–118. doi: 10.1016/j.phytochem.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Tatsuzawa F, Takahashi N, Kato K, Shinoda K, Saito N, Honda T. Acylated cyanidin glycosides from the pale-violet flowers of Ionopsidium acaule (Desf.) Rchb. (Brassicaceae) Phytochem Lett. 2014;7:69–76. doi: 10.1016/j.phytol.2013.10.004. [DOI] [Google Scholar]
- Trouillas P, Sancho-Garcia JC, De Freitas V, Gierschner J, Otyepka M, Dangles O. Stabilizing and modulating color by copigmentation: insights from review theory and experiment. Chem Rev. 2016;116:4937–4982. doi: 10.1021/acs.chemrev.5b00507. [DOI] [PubMed] [Google Scholar]
- Tsutsumi A, Horikoshi Y, Fushimi T, Saito A, Koizumi R, Fujii Y, Osakabe N. Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food Funct. 2019;10:1726–1735. doi: 10.1039/c8fo02125b. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Culver CA. Alternatives to those artificial FD&C food colorants. Annu Rev Food Sci Technol. 2012;3:59–77. doi: 10.1146/annurev-food-022811-101118. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Fang S, Li YH, Zhang F, Shao ZP, Zeng YT, Meng YC. Effects of low acyl and high acyl gellan gum on the thermal stability of purple sweet potato anthocyanins in the presence of ascorbic acid. Food Hydrocoll. 2019;86:116–123. doi: 10.1016/j.foodhyd.2018.03.007. [DOI] [Google Scholar]
- Yang W, Kortesniemi M, Ma X, Zheng J, Yang B. Enzymatic acylation of blackcurrant (Ribes nigrum) anthocyanins and evaluation of lipophilic properties and antioxidant capacity of derivatives. Food Chem. 2019;281:189–196. doi: 10.1016/j.foodchem.2018.12.111. [DOI] [PubMed] [Google Scholar]
- Zhao CL, Yu YQ, Chen ZJ, Wen GS, Wei FG, Zheng Q, Xiao XL. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017;214:119–128. doi: 10.1016/j.foodchem.2016.07.073. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ding BW, Qin JW, He F, Duan CQ. Intermolecular copigmentation between five common 3-O-monoglucosidic anthocyanins and three phenolics in red wine model solutions: the influence of substituent pattern of anthocyanin B ring. Food Chem. 2020;326:126960. doi: 10.1016/j.foodchem.2020.126960. [DOI] [PubMed] [Google Scholar]