Abstract
One hundred clinical isolates of Cryptococcus neoformans from human immunodeficiency virus (HIV)-infected and non-HIV-infected patients from Brazil, Chile, and Venezuela were separated according to varieties and tested for antifungal susceptibility. A high susceptibility to antifungal agents was observed among all the isolates. The electrophoretic karyotyping of 51 strains revealed good discrimination among Cryptococcus neoformans var. neoformans strains.
An increase in the incidence of cryptococcosis has been reported in recent years and is associated with the growing population of immunocompromised patients and the human immunodeficiency virus (HIV) epidemic. In Brazil, 4.5% of all opportunistic infections in AIDS patients have been reported as being caused by Cryptococcus neoformans (6). Distribution of serotypes and varieties is considered to be regionally specific, but Cryptococcus neoformans var. neoformans serotype A has been recovered from approximately 99% of all AIDS patients in most countries (7). The evaluation of antifungal susceptibility of C. neoformans is of great interest, considering the high frequency and the severe clinical manifestations of this infection (1). DNA typing methods have shown a high genetic heterogeneity among clinical and environmental isolates of C. neoformans (3, 9). Most studies in Latin America have been limited to clinical and epidemiological aspects (10, 11), and only few investigations have studied the molecular epidemiology of clinical isolates from this area (3). Therefore, we studied clinical isolates of C. neoformans isolates from three countries in South America according to their varieties, serotypes, antifungal susceptibilities, and genomic diversity, as determined by electrophoretic karyotype (EK).
One hundred clinical isolates C. neoformans from Brazil (69 isolates), Venezuela (20 isolates), and Chile (11 isolates) were investigated. Sixty strains were obtained from HIV-positive patients, 16 were obtained from HIV-negative patients, and for 24 patients, the data on HIV status were not available. The isolates were identified to species level based on micromorphological and biochemical characteristics (5). Canavanine-glycine-bromthymol blue agar medium was used for the differentiation of the varieties (4), and serotyping was performed by slide agglutination test (Crypto Check; Iatron Co., Japan). All the isolates were tested by broth microdilution method, performed according to the NCCLS guidelines (8) for amphotericin B (AMB), fluconazole (FLC), itraconazole (ITC), and flucytosine (5FC). Broth microdilution testing was performed with RPMI 1640 with l-glutamine, without bicarbonate, and buffered with MOPS (morpholinepropanesulfonic acid) at pH 7.0. Candida parapsilosis ATCC 22019 was included on each test as quality control strain. Breakpoints for azole and 5FC MICs were defined as the lowest drug concentration resulting in a prominent decrease in turbidity, compared with that in the growth control (drug-free) well. The AMB MICs were defined as the lowest concentration able to inhibit any visual growth. Karyotype analysis was done by counter-clamped homogeneous electrophoresis (CHEF DRII). The chromosomal DNA extractions were prepared according to previous protocols (3). Pulsed-field gel electrophoresis was carried out at 6 V/cm at 13.5°C with pulses of 60 to 120 s for 27 h. A Saccharomyces cerevisiae chromosomal DNA size standard was inserted in each gel as a molecular weight standard. Isolates were considered different if any readily detectable band did not match. A computer analysis program (Vilber Loumart, Marnes la Valle, France) was used to determine a dendrogram based on the Dice coefficient of similarity (5%).
Eighty-nine isolates were identified as C. neoformans var. neoformans, and 11 were identified as Cryptococcus neoformans var. gattii. All C. neoformans var. gattii strains were from HIV-negative patients. Serotyping of 62 isolates of C. neoformans var. neoformans identified 60 (96.8%) strains as serotype A. Among the 11 C. neoformans var. gattii strains, nine isolates were serotype B. No particular serotype distribution was related to any geographic areas, although three isolates, serotype AD, were found only among the Chilean isolates. Our data were consistent with several reports in the literature that referred to C. neoformans var. neoformans serotype A as predominant worldwide, especially in AIDS patients (7, 12). However, detailed surveys are needed to ascertain the prevalence of serotypes in Latin America countries.
All C. neoformans isolates were susceptible to AMB; 99% of C. neoformans var. neoformans and 73% of C. neoformans var. gattii isolates were susceptible to FLC. For 5FC, 90% of the isolates were susceptible, 9% were intermediate, and one strain, from Venezuela, was resistant. MIC ranges for C. neoformans var. neoformans were as follows, respectively: AMB, 0.125 and 0.5 μg/ml; FLC; 4 and 8 μg/ml; ITC, 0.06 and 0.125 μg/ml; 5FC, 4 and 4 μg/ml. MIC ranges for C. neoformans var. gattii were as follows: AMB, 0.25 and 0.5 μg/ml; FLC, 8 and 16 μg/ml; ITC, 0.125 and 0.25 μg/ml; 5FC, 2 and 8 μg/ml. The susceptibility profiles of C. neoformans isolates obtained from HIV-infected and non-HIV-infected patients, as well as those of the isolates from different countries, were very similar and consistent with studies in the literature (2; S. Cordoba, M. Melhem, S. Pukinskas, M. A. Martins, S. Nery, B. Calvo, W. Vivot, M. Soria, G. Davel, and L. L. Rodero, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1508, 1999). Of note, the MIC90s of azoles obtained with C. neoformans var. gattii were higher than the values obtained with C. neoformans var. neoformans. However, these values represented only one tube dilution; additional studies are needed for further conclusions.
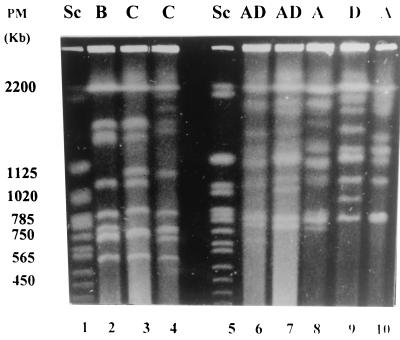
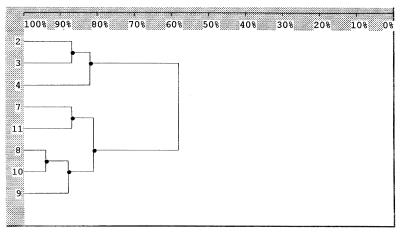
EK was performed with 40 C. neoformans var. neoformans strains (25 from Brazil, 10 from Venezuela, and 5 from Chile) and 11 C. neoformans var. gattii strains (8 from Brazil and 3 from Venezuela). C. neoformans var. neoformans presented 18 profiles, while only 3 were observed with C. neoformans var. gattii. Among the 25 Brazilian isolates of C. neoformans var. neoformans, 9 profiles were identified. The Venezuelan C. neoformans var. neoformans strains had six EK profiles represented by four unique profiles. Two of the predominant profiles of the Brazilian collection were also identified among Venezuelan strains. Interestingly, five distinctive profiles were observed only in the five Chilean C. neoformans var. neoformans isolates. In a previous study, Franzot et al. (3) also observed a broad diversity among Brazilian isolates. In our study, among the 11 C. neoformans var. gattii strains, EK identified three different profiles and all 7 isolates from the Brazilian collection exhibited the same profile. No association between EK profiles and HIV disease or serotypes was observed (Fig. 1 and 2). The distances between the two varieties and serotypes generated by Dice coefficients are shown in Fig. 2. Among the 18 profiles revealed by EK for C. neoformans var. neoformans, proper profiles were observed in each country that were not identified in others. Thus, further analysis is necessary to evaluate the significance of geographic distribution in the molecular epidemiology of C. neoformans in Latin America.
FIG. 1.
EK profiles of C. neoformans var. gattii and C. neoformans var. neoformans. The serotypes are indicated above the lanes. PM, molecular size; Sc, S. cerevisiae DNA marker.
FIG. 2.
Dendrogram corresponding to Fig. 1. The values were generated from the Dice coefficients and illustrate the relatedness of varieties and serotypes of C. neoformans var. neoformans and C. neoformans var. gattii isolates. Numbers 2, 3, and 4 represent C. neoformans var. gattii serotypes B, C, and C, respectively. Numbers 7, 8, 9, 10, and 11 represent C. neoformans var. neoformans serotypes AD, AD, A, D, and A, respectively. Values at the top are percent similarity.
In conclusion, C. neoformans serotype A appeared to be the most prevalent agent of cryptococcosis in Latin America. Despite minor differences of antifungal susceptibility exhibited by the two varieties of C. neoformans, most isolates were susceptible to all drugs tested. Our results provide evidence of a higher diversity of genotypes among C. neoformans var. neoformans isolates than among C. neoformans var. gattii isolates.
Acknowledgments
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP- 14.518-7), Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq- 522144/96-9), Brazil, Japan International Cooperative Agency (JICA), and Chiba University, Chiba, Japan.
REFERENCES
- 1.Barchiesi F, Hollis R, Messer S A, Scalise G, Rinaldi M G, Pfaller M A. Electrophoretic karyotype and in vitro antifungal susceptibility of Cryptococcus neoformans isolates from AIDS patients. Diagn Microbiol Infect Dis. 1995;23:99–103. doi: 10.1016/0732-8893(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 2.Franzot S P, Hamdan J S. In vitro susceptibilities of clinical and environmental isolates of Cryptococcus neoformans to five antifungal drugs. Antimicrob Agents Chemother. 1996;40:822–824. doi: 10.1128/aac.40.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franzot S P, Hamdan J S, Currie B P, Casedevall A. Molecular epidemiology of Cryptococcus neoformans in Brazil and the United States: evidence for both local genetic differences and a global clonal population structure. J Clin Microbiol. 1997;35:2243–2251. doi: 10.1128/jcm.35.9.2243-2251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon-Chung K J, Polacheck I, Bennett J E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C) J Clin Microbiol. 1982;15:535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacaz C S, Porto E, Martins J E C. Micologia médica. Fungos, actinomicetos e algas de interesse médico. Sao Paulo, Brazil: Sarvier; 1991. Técnicas micológicas e imunológicas; pp. 545–608. [Google Scholar]
- 6.Ministério da Saúde. Programa nacional de doenças sexualmente transmissíveis, Brasília. Brasil Bol Epidemiol AIDS. 1999;anoI(01):44. [Google Scholar]
- 7.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 9.Perfect J R, Magee B B, Magee P T. Separation of chromosomes of Cryptococcus neoformans by pulsed-field gel electrophoresis. Infect Immun. 1989;57:2624–2627. doi: 10.1128/iai.57.9.2624-2627.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozembaum R, Gonçalves A J R, Wanke B, Caiuby M J, Clemente H, Lazera M S, Monteiro P C F, Londero A T. Cryptococcus neoformans varieties as agents of cryptococcosis in Brazil. Mycopathologia. 1992;199:133–136. doi: 10.1007/BF00448809. [DOI] [PubMed] [Google Scholar]
- 11.Rozembaum R, Gonçalves A J R. Clinical epidemiological study of 171 cases of cryptococcosis. Clin Infect Dis. 1994;18:369–380. doi: 10.1093/clinids/18.3.369. [DOI] [PubMed] [Google Scholar]
- 12.Steenbergen J N, Casadevall A. Prevalence of Cryptococcus neoformans var. neoformans (serotype D) and Cryptococcus neoformans var. grubii (serotype A) isolates in New York City. J Clin Microbiol. 2000;32:1974–1976. doi: 10.1128/jcm.38.5.1974-1976.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]