Abstract
Objective
Studies have suggested that inflammation contributes to the pathogenesis of postoperative delirium, but previous results on the proinflammatory cytokine IL-8 in plasma are contradictory. Additionally, a significant fraction of IL-8 is bound to erythrocytes, but the relevance of whole blood IL-8 in delirium has not been studied. In this work, we analyzed the association of postoperative delirium with levels of unbound IL-8 in plasma and levels of IL-8 in whole blood in patients from two studies which were conducted in our department and have not been presented previously. We assessed the prognostic value of whole blood IL-8.
Methods
Plasma/whole blood IL-8 was measured at least once in N = 504 patients preoperatively, on day one (d1) and/or three months after surgery in the BioCog observational study. Whole blood IL-8 was measured in N = 64 patients from the PHYDELIO trial preoperatively, on d1 and d7 after surgery. For the determination of whole blood IL-8, EDTA-preserved blood samples underwent lysis by adding Triton-X100 surfactant. Plasma and whole blood IL-8 levels were assessed with two different immunoassay kits. Delirium was appraised systematically for seven postoperative days according to DSM criteria using two comparable protocols consisting of validated screening tools.
Results
Delirium occurred in 25% of BioCog and 14% of PHYDELIO patients. In BioCog, IL-8 was elevated on d1 and in delirious patients. A steeper postoperative increase in delirium was confounded by surgery-related factors. A crescendo-decrescendo pattern of whole blood IL-8 levels was observed in non-delirious patients with a peak on d1. This pattern was more distinct in delirious BioCog patients, but inverted in delirious PHYDELIO patients. Preoperative whole blood IL-8>318.4 pg/mL (reference <150 pg/mL) had adequate sensitivity (0.79/0.78) and specificity (0.53/0.67) for delirium in both samples.
Conclusion
Our results contribute to an inflammatory hypothesis of postoperative delirium.
1. Introduction
Delirium is an acute onset fluctuating state of mental confusion associated with disturbances in attention, awareness and cognition which may also be accompanied with changes in psychomotor behavior and the individual's emotional state (American Psychiatric Association, 2013). Postoperative delirium (POD) is a common complication in older patients, with incidence of up to 21% in patients over 65 years of age, and even 33% in those older than 85 years (Kotfis et al., 2018).
The pathogenesis of postoperative delirium is of multiple etiologies. Along with neuronal mechanisms, inflammation has been discussed (Androsova et al., 2015). It has been shown peripheral inflammation may lead to neuroinflammation and subsequent brain tissue damage (Qin et al., 2007; Waage et al., 1989). Vice versa, animal studies have shown that neuroinflammation is capable of triggering delirium, especially when neuronal damage is preexisting (Field et al., 2012).
Interleukin 8 (IL-8, CXCL8) is an ubiquitously produced cytokine. As a chemoattractant, one of its main functions is to guide neutrophils to the site of inflammation (Leonard and Yoshimura, 1990). Subclinical inflammation, including increased peripheral IL-8 levels, has been observed in patients with Alzheimer's disease, schizophrenia and major depressive disorder (Çakici et al., 2020; Qin et al., 2016). Preoperative cognitive impairment, depressive and other psychopathological symptoms as well as low-grade peripheral inflammation have been found to be risk factors for postoperative delirium. Beyond neuronal damage, disoriented inflammatory responses could mediate susceptibility to delirium in patients with neuropsychiatric diseases. (Dasgupta and Dumbrell, 2006; Knaak et al., 2019). Increased IL-8 has been associated with poorer cognitive function in healthy elderly (Baune et al., 2008). The research team hypothesized that peripheral IL-8 passing the blood-brain barrier might trigger an neuroinflammatory response and interfere with neurotransmitters (Baune et al., 2008; Narita et al., 2005).
Previous studies analyzing IL-8 in delirium yielded contradictory results. De Rooij and colleagues analyzed cytokine pattern in 185 patients ≥65 years with all-cause admission to the emergency department (ED) and found increased IL-8 in delirious patients (de Rooij et al., 2007). The authors suggested that peripheral cytokines passing the blood-brain barrier may interact with neuronal signaling, leading to cognitive deterioration. Van Munster and colleagues reproduced the result in 98 patients admitted for hip fracture from the same study and reported peak IL-8 values before delirium onset (Munster et al., 2008). McNeil and colleagues analyzed plasma IL-8 levels in 156 non-demented surgical and non-surgical patients ≥65 years at emergency department admission, but found no association with delirium duration (McNeil et al., 2019). Khan and colleagues analyzed 321 delirious ICU patients and found IL-8 plasma levels at delirium onset to be associated with duration and severity, though they did not include patients without delirium (Khan et al., 2020). The above-mentioned studies investigated ED patients in whom delirium may have already occurred before surgery or had infectious etiology.
Two studies measured IL-8 in plasma of 101 patients ≥60 years undergoing elective hip arthroplasty and 71 patients undergoing esophagectomy, but found no association with delirium independent of diseases severity (Cerejeira et al., 2012; Khan et al., 2021). Another group of researchers analyzed plasma IL-8 and postoperative delirium in a sample merged from two cohort studies (Ballweg et al., 2021; Casey et al., 2020). Both studies reported that no preoperative, but a perioperative increase in IL-8, was associated with delirium. Likewise, they observed an association of IL-8 values and neuronal damage. Plasma IL-8 levels on the first postoperative day held prognostic value for delirium.
IL-8 binds to the Duffy antigen on erythrocytes and only a minor fraction of the total circulating IL-8 can be measured in the blood plasma (Darbonne et al., 1991). This mechanism might prevent high levels of free chemokines in plasma which would result in disorientation of activated leukocytes (Springer, 1995). On the other hand, erythrocytes might pose as a vehicle or reservoir for IL-8 through which it may be disseminated systemically and exert effects on the central nervous system (Minten et al., 2014; Narita et al., 2005; Pruenster et al., 2009). Free IL-8 can be measured in plasma, however methods to assess whole blood IL-8, including erythrocyte-bound IL-8 after cell lysis, have been established as well (Friebe and Volk, 2008). Previous studies have not investigated of whole blood IL8 association with delirium, even though erythrocyte binding might impact the systemic effects of IL-8.
Preoperative anemia and intraoperative blood loss are known to increase the risk for POD and IL-8 binding could be one factor by which anemia facilitates delirium (Joosten et al., 2006; Marcantonio et al., 1998). One previous study suggested hematocrit to affect plasma IL-8 measurements (Neunhoeffer et al., 2011). Perioperative anemia could be a relevant confounding factor in studies on IL-8 and delirium.
At this juncture, our study group intended to add to the research field by investigating the association between perioperative IL-8 and delirium in two independent cohorts of older surgery patients. These samples have been recruited in our department and have not been previously presented. The analysis of two datasets allows for replication of our findings on the prognostic value of IL-8 in an independent sample. This facilitates an evaluation on how the study design affects the findings of IL-8 in postoperative delirium.
We hypothesized that IL-8 levels would be increased in patients with POD during the perioperative course. We further presumed that results would differ depending on the assessment method and the relevance of erythrocyte-bound IL-8 in delirium. To assess the independence of this association from confounding factors, we adjusted the analysis for several surrogate parameters for the extent of surgical trauma and assessed the relevance of perioperative hemoglobin levels as a confounding factor. In addition, the prognostic values of perioperative IL-8 levels from whole blood in POD risk prediction was appraised.
2. Methods
2.1. Study design and procedures
2.1.1. BioCog
The BioCog project (Biomarker Development for Postoperative Cognitive Impairment in the Elderly study, www.biocog.eu) is a prospective multicenter cohort study with the aim to develop a biomarker-based algorithm for risk prediction of post-operative delirium and cognitive dysfunction. Patients ≥65 years of European descent presenting for elective surgery with an expected duration >60min were included after giving informed consent. Exclusion criteria were MMSE score ≤23 points in the screening, neuropsychiatric morbidity and sensory impairment interfering with neurocognitive testing, centrally acting medication, homelessness or expected unavailability for a follow-up assessment, participation in another clinical intervention study during hospital stay, accommodation in an institution due to official or judicial order (Winterer et al., 2018).
The study was conducted in line with the declaration of Helsinki. All procedures were approved by the local medical ethics committees of the study centers in Berlin, Germany (EA2/092/14) and Utrecht, Netherlands (14–469). The study was registered at clinicaltrials.gov (NCT02832193). Recruitment took place between November 2014 and April 2017.
2.1.2. PHYDELIO
PHYDELIO (Perioperative PHYsostigmine prophylaxis for liver resection patients at risk for DELIrium and postOperative cognitive dysfunction) is a single center, double blinded, two-armed, randomized placebo-controlled phase IV trial: Patients were randomized either to receive physostigmine or placebo starting after induction of anesthesia for 24 h. Patients 18 years or older scheduled for liver resection were included after giving informed consent. Exclusion criteria were lactation or pregnancy, accommodation in an institution due to official or judicial order, staff member of the Charité-Universitätsmedizin Berlin, lack of German language proficiency or illiteracy, scheduled non-anatomic liver or wedge resection, ASA-PS ≥ IV, preoperative delirium, MMSE ≤23, relevant visual and hearing impairment, regular intake of psychotropic drugs (including sleeping medication and benzodiazepines) or established psychiatric disease and contraindications to physostigmine application (see supplement) (Spies et al., 2021).
After baseline assessment and consent, patients were pseudonymised and stratified by ASA score </≥ III and type of liver resection (right/left hepatectomy and extended hepatectomy/trisegmentectomy). Physostigmine (Anticholium®, Köhler comp, Germany) was administrated in a dose of 0.02 mg/kg body weight as bolus and 0.01 mg/kg body weight per hour intravenously or placebo with identical appearance for 24 h after induction of anesthesia.
The study was conducted in line with the declaration of Helsinki. It was approved by the “Landesamt für Gesundheit und Soziales Berlin” (Berlin, Germany) ethics committee on January 15, 2009, and a written informed consent was obtained from all participants. The study is registered, number ISRCTN18978802 and EudraCT 2008-007237-47. Recruitment took place between August 2009 and September 2016 at the Charité- Universitätsmedizin Berlin in Germany.
2.1.3. Delirium assessment
Independently from routine hospital procedures, POD assessments were conducted during the first seven days after surgery. For this purpose, POD was defined according to DSM-IV-TR (PHYDELIO) and DSM-5 (BioCog) criteria (American Psychiatric Association, 2002, 2013).
The screening for delirium was initiated post-surgery and repeated twice per day at 8:00am and 7:00pm (±1 h) up to seven days after surgery. All study visits were either made by study physicians, or by trained study nurses and study assistants under supervision of a study physician.
In the BioCog study, patients were considered delirious if they presented with:
-
•
≥2 cumulative points on the Nursing Delirium Screening Scale (Nu-DESC) (Gaudreau et al., 2005) and/or
-
•
a positive Confusion Assessment Method (CAM) score on a peripheral ward (Inouye et al., 1990) and/or
-
•
a positive CAM for the Intensive Care Unit (CAM-ICU) score on an intensive care unit (Ely et al., 2001) and/or
-
•
patient's chart review showing descriptions of delirium (e.g. confused, agitated, drowsy, disorientated, delirious, received antipsychotic therapy) (Saczynski et al., 2014).
In the PHYDELIO study, patients were considered delirious if they presented with:
-
•
≥2 cumulative points on the Nu-DESC in the recovery room and/or
-
•
a positive CAM score on the peripheral ward and/or
-
•
a positive CAM-ICU score on an intensive care unit and/or
-
•
a positive Delirium Detection Score in the ICU (Radtke et al., 2010) and/or
-
•
a positive Intensive Care and Delirium Screening Checklist in the ICU (Bergeron et al., 2001) and/or
-
•
Delirium Rating Scale ≥18, and/or NuDesc on the peripheral ward (Trzepacz, 1999) and/or
-
•
patient chart review showing signs of delirium.
2.2. IL-8 measurement
Total IL-8 content was determined by lysis of 100 μl EDTA whole blood with 100 μl 1% Triton-X100 in RPMI medium. IL-8 in the resulting blood lysate was measured with the IMMULITE® IL-8 immunoassay (DPC Biermann, Germany). The reference range for IL-8 in whole blood samples of healthy adults is < 150 pg/mL (Reinsberg et al., 2000).
IL-8 was measured using a commercially available assay (ProcartaPlex Multiplex Immunoassay kit, Thermo Fisher Scientific Inc, Carlsbad, CA, USA) in plasma samples from the BioCog study.
For the BioCog study, blood samples were collected immediately before surgery, on the first postoperative day, as well as three months after surgery. Blood was collected in supine position by trained clinic staff according to a standard operating procedure. Plasma samples were immediately sent to laboratories adjacent to the respective hospital site for analysis of hemoglobin. Additional plasma samples and whole blood samples were frozen at −80 °C for later analysis of IL-8. Apart from IL-8, several other inflammatory parameters were assessed on genome, transcriptome and proteome levels, but research into these subjects will published elsewhere.
In the PHYDELIO study, blood samples were obtained immediately before surgery, as well as on the first and seventh days post-surgery. Apart from IL-8, C-reactive protein and procalcitonin have been assessed, but preliminary results were negative and these biomarkers have not been considered for this work (Spies et al., 2021).
2.3. Statistical analysis
As a first step, IL-8 levels from all available sample were analyzed using simple non-parametric tests. Then, patients with serial IL-8 measurements were included in mixed-effects linear model analysis. Finally, IL-8 levels were analyzed as predictors of delirium in ROC analyses.
Each analysis was conducted including data from as many blood samplings as possible, though samplings from the BioCog dataset were incomplete. The number of included samples will be stated with the respective result.
Since they give additional information on the certainty of a point estimate (e.g., regression coefficient), confidence intervals (CI) were reported in addition to p-values. Especially for the small PHYDELIO sample, significance may result from a large effect size even in the presence of considerable uncertainty (wide confidence intervals). This may indicate that results from an identical replication study could yield different point estimates.
CIs were calculated from 50 000 bootstrapped samples. Statistical analysis was conducted using the boot, Hmisc, lme4, ROCit and DescTools packages for R 4.0.
2.3.1. Unadjusted analyses
Correlations of plasma and whole blood IL-8 levels were assessed using Spearman's ρ. In order to compare IL-8 levels at three different sampling time points between patients with postoperative delirium and without delirium, the Wilcoxon rank sum test for independent samples was applied. These statistical methods were chosen since IL-8 levels were not normally distributed. The level of significance p < 0.05 was adjusted for six independent test (two sample types at measured at three time points: 0.05/6 ≈ 0.008) in the BioCog study and three independent tests (one sample type measured at three time points: 0.05/3 ≈ 0.016) in the PHYDELIO study. Wilcoxon's signed rank test was also used to test for statistically differences in IL-8 levels between patients with different perioperative medication (Supplementary Tables S6 and S7).
2.3.2. Mixed-effects models of perioperative IL-8 trajectories
To adjust for confounding factors in serial measurements, we applied linear mixed-effects models with subject as a random effect and IL-8 levels as the dependent variable. Plasma IL-8 data were square-root transformed and, similarly, whole blood IL-8 data were log-transformed before analysis to approximate normal distribution based on histogram inspection. (For illustrative purposes, regression coefficients were re-transformed to a natural scale for a female 72-year-old patient in BioCog and a female 59-year-old patient in PHYDELIO.)
Basic mixed-effects models included delirium incidence, a linear and quadratic term for time as well as the interaction thereof as the independent variables of interest. Age and sex were included as covariates.
The conjunction of perioperative factors with IL-8 levels before and one day after surgery was analyzed in the BioCog sample. As additional confounding factors the following were considered: type of surgery (non-peripheral with opening of body cavities vs. peripheral), duration of anesthesia (>4 h), preoperative ASA physical status (ASA-PS ≥ III) and hemoglobin levels. Interactions were dropped from the model when they were not significant. Explicitly, the following models were analyzed:
Model 1 and 2: Whole blood IL-8 levels from all three assessment points in the BioCog and PHYDELIO studies, adjusted for sex and age.
Model 3 and 4: Whole blood and plasma IL-8 levels from the first two assessment points of the BioCog study, adjusted for sex and age.
Model 5 and 6: Whole blood and plasma IL-8 levels from the first two assessment points of the BioCog study, adjusted for sex and age, surgery type, ASA-PS, anesthesia duration and hemoglobin levels.
There were not sufficient patients from the BioCog study with complete plasma IL-8 samplings from all three assessment time points (N = 29) for modeling trajectories until the third assessment at follow-up after three months. PHYDELIO data have not been adjusted for treatment group (physostigmine or placebo), since neither did treatment influence delirium incidence, nor did IL-8 levels differ significantly between treatment groups (Spies et al., 2021).
For mixed-effects regression models, the regression coefficients (β) have been reported with 95% CI. In order to adjust for measurement of both plasma and whole blood IL-8 in the same sample from the BioCog study, 95% CIs were adjusted to 97.5% CIs (two measurements, accepted α-error = 0.05, 1–0.05/2 = 0.975).
2.3.3. Receiver operating curves for whole blood IL-8
To determine potential cut-off values for preoperative IL-8 levels in whole blood as well as the postoperative increase in the BioCog study, empirical receiver operating curves (ROC) were employed. Postoperative IL-8 increase was defined as the difference in IL-8 concentrations (Δc) between the first postoperative day (cd1) and the baseline measurement (cd0): Δc(IL-8) = cd1(IL-8)-cd0(IL-8). Since two prognostic markers were assessed, 95% CIs were adjusted to 97.5% CIs. Cut-off values were chosen according to Youden's index. Test performance metrics (sensitivity, specificity, accuracy, negative and positive predictive value) were calculated for the proposed cut-off values in the BioCog study. In an independent replication procedure, those same test metrics were determined for whole blood IL-8 in the PHYDELIO study. Since plasma IL-8 was not measured in PHYDELIO and thus validation was not possible, the prognostic value for plasma IL-8 has not been analyzed in this instance.
3. Results
3.1. Description of study samples
In the BioCog study, N = 504 patients were at least once assessed for IL-8 level, regardless of time point or measurement method. Of those, 25% (n = 125) developed delirium. A full description of the PHYDELIO sample has been published previously (Spies et al., 2021). Of N = 64 patients included in the PHYDELIO study, N = 32 (50%) participated in the intervention arm of the trial and N = 9 (14%) developed delirium. Table 1, Table 2 and Supplementary Table S4 describe the BioCog and PHYDELIO study samples. Hemoglobin levels were significantly lower in delirious patients from the BioCog study at any time point (see Supplementary Table S5).
Table 1.
BioCog sample characteristics in N = 504 patients with at least one IL-8 measurement. See also Supplementary Table S5.
| Median | Interquartile range | Minimum-maximum range | |
|---|---|---|---|
| Age (y) | 72 | 69–73 | 65–91 |
| ASA-PS score | II | II-III | I-III |
| Duration of anesthesia (min) | 223 | 147–345 | 10–1669 |
| Length of ICU stay (d) | 0 | 0–1 | 0–54 |
| Length of hospital stay (d) | 8 | 5–13 | 1–131 |
| Preoperative Hb (g/dL) | 12.9 | 11.5–14.0 | 6.7–17.00 |
| Hb on postoperative day 1 (g/dL) | 11.1 | 9.5–12.4 | 5.8–15.6 |
| Hb at follow-up (g/dL) | 13.5 | 12.5–14.4 | 9.4–16.6 |
| N | Frequency | ||
| Sex (female) | 223 | 44% | |
| Non-peripheral surgery | 258 | 51% | |
| Regional anesthesia only | 18 | 4% | |
| Combined general and regional anesthesia | 132 | 26% | |
| Preoperative intake of benzodiazepines | 105 | 21% | |
| Benzodiazepine for premedication | 56 | 12% | |
| Anticholinergic medication before surgery | 134 | 27% | |
| Anticholinergic medication on day of surgery | 222 | 67% | |
| Anticholinergic medication on the first postoperative day | 299 | 62% |
ASA-PS - American Society of Anesthesiologists physical status, d - days, g/dL - gram per deciliter, Hb - hemoglobin min - minutes, y - years.
Table 2.
PHYDELIO sample characteristics in all N = 64 patients.
| Median | Interquartile range | Minimum-maximum range | |
|---|---|---|---|
| Age (y) | 59 | 52–66 | 27–80 |
| ASA-PS score | II | II-II | I-III |
| Duration of surgery (min) | 281 | 219–358 | 60–559 |
| Length of ICU stay (d) | 1 | 1–3 | 1–42 |
| Length of hospital stay (d) | 14 | 9–24 | 6–98 |
| Preoperative Hb (g/dL) | 12.1 | 11.2–13.0 | 7.7–16.0 |
| Hb at the end of surgery (g/dL) | 10.5 | 9.3–11.9 | 5.8–14.2 |
| N | Frequency | ||
| Sex (female) | 25 | 39% | |
| Intraoperative Pringle maneuver | 25 | 40% | |
| Intraoperative methylprednisolone | 51 | 80% | |
| Received packed blood cells | 15 | 23% | |
| Received fresh frozen plasma | 39 | 61% | |
| Received packed thrombocytes | 1 | 2% |
Abbreviations: ASA-PS - American Society of Anesthesiologists physical status, d - days, g/dL - gram per deciliter, Hb-hemoglobin min - minutes, y - years.
3.2. Unadjusted analyses
Table 3 depicts IL-8 levels for all three sampling time points in both studies, including data distribution and correlation between plasma and whole blood IL-8 levels in the BioCog study. Plasma and whole blood IL-8 levels showed moderate to high correlations before surgery and on postoperative day 1. No statistically significant differences in IL-8 levels could be detected between PHYDELIO treatment arms (Supplementary Table S7).
Table 3.
Whole blood and plasma IL-8 levels from the BioCog and PHYDELIO studies.
| Preoperative | Postoperative day 1 | Follow-up | ||
|---|---|---|---|---|
| BioCog | ||||
| Whole blood (pg/mL) | Median (IQR) | 346 (227–567) | 748 (410–1806) | 295 (184–418) |
| Min.-Max. (N) | 65-4368 (N = 178) | 125-17524 (N = 159) | 92-736 (N = 54) | |
| Plasma (pg/mL) | Median (IQR) | 376 (267–606) | 652 (334–1253) | 305 (247–678) |
| Min.-Max. (N) | 3-3939 (N = 178) | 4-18049 (N = 367) | 3-2654 (N = 72) | |
| Spearman's ρ | 0.47 | 0.56 | <0.01 | |
| p-value (N) | 0.0002∗ (N = 59) | <0.0001∗ (N = 90) | >0.99 (N = 9) | |
| PHYDELIO | ||||
| Preoperative | Postoperative day 1 | Postoperative day 7 | ||
| Whole blood (pg/mL) | Median (IQR) | 221 (122–472) | 763 (411–1095) | 363 (198–811) |
| Min.-Max. (N) | 33-12640 (N = 64) | 140.4–6320 (N = 64) | 33-12640 (N = 64) | |
Abbreviations: IQR - interquartile range, pg/mL – picogram per milliliter.
∗ p-values are significant after adjusting for three independent tests (pad.<0.017).
Wilcoxon's signed-rank test indicated significant higher IL-8 levels at baseline in whole blood samples from delirious patients in the BioCog (W = 2219, p < 0.001) and PHYDELIO (W = 121, p = 0.015) studies. On the first postoperative day, IL-8 was significantly higher in both plasma (W = 9911, p = 0.008) and whole blood (W = 1867, p < 0.001) samples from delirious patients in the BioCog study. Beyond this day, no significant differences between patients with and without delirium could be identified (see Supplementary Figs. S1–S3).
3.3. Mixed-effects models of whole blood IL-8 trajectories until follow-up
Using mixed effects models including a quadratic term for time (model 1 and 2), we analyzed the perioperative course of IL-8 at all three time points in N = 45 patients (n = 12 with delirium) with whole blood IL-8 samples at each assessment time point from the BioCog study and the complete sample from the PHYDELIO study. There was a significant negative quadratic effect of time in both BioCog (β = −0.96 [−1.10; −0.82], p < 0.001) and PHYDELIO (β = −0.55 [−0.87; −0.24], p = 0.001) data, implying a crescendo-decrescendo development of IL-8 in the perioperative period. Additionally, a significant time-dependent linear decrease of IL-8 (β = −0.11 [−0.21; −0.003], p = 0.044) was found in the BioCog dataset. The expected IL-8 trajectory for a non-delirious patient in the BioCog study was 414 pg/mL at baseline, 854 pg/mL on the first postoperative day and 406 pg/mL at follow up after three months. In PHYDELIO, the expected trajectory was 205 pg/mL at baseline, 784 pg/mL on the first, and 346 pg/mL on the seventh postoperative day. We found a significant main effect of delirium (β = 0.62 [0.37; 0.87], p < 0.001) on IL-8 concentrations and a steeper crescendo-decrescendo pattern in delirious patients in the BioCog study (βtime2·delirium = −0.45 [−0.72; −0.17], p = 0.002). Expected IL-8 levels of a delirious patient were higher by +307 pg/mL and +1311 pg/mL at baseline and on the first postoperative day compared to non-delirious patients, but slightly lower (−18 pg/mL) at follow-up. There was a significant interaction of time and delirium in PHYDELIO, which resulted a complete inversion of the crescendo-decrescendo pattern observed in non-delirious patients (βtime2·delirium = 1.06 [0.44; 1.69], p = 0.001). Expected IL-8 concentrations in a delirious patient from the PHYDELIO study were 1755 pg/mL at baseline, 1212 pg/mL on the first and 2321 pg/mL on the seventh postoperative day. Fig. 1 presents the trajectories of IL-8 in delirious and non-delirious patients from both studies. A description of the full models is given in Supplementary Table S1.
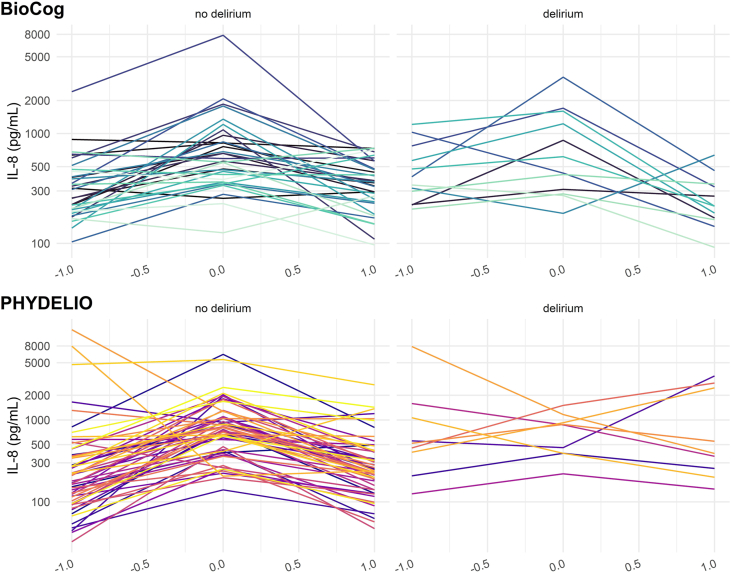
Fig. 1.
Whole blood IL8 in 45 patients with complete sampling at three assessment time points in the BioCog study and all 64 patients from the PHYDELIO study. Individual IL-8 trajectories in the perioperative course are shown on an log-transformed scale. Both studies show a temporary postoperative IL-8 increase in patients without delirium, with visible regredience at the seventh postoperative day. Patients with delirium have more individual pattern in the short term postoperative period (PHYDELIO) and a delayed decline of IL-8 (BioCog).
3.4. Mixed-effects models of whole blood and plasma IL-8 trajectories until the first postoperative day
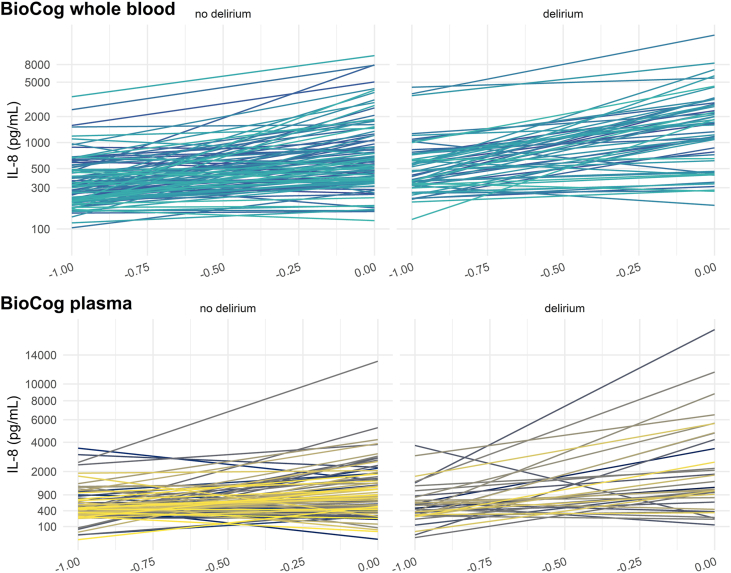
Since the majority of IL-8 values was lacking for the follow-up assessment after three months in the BioCog study, the IL-8 increase from baseline to the first postoperative day was analyzed separately. Whole blood IL-8 increase was analyzed in N = 157 patients (n = 57 with delirium, model 3) and perioperative plasma IL-8 increase in N = 127 patients (n = 35 with delirium, model 4). IL-8 was significantly higher in delirious patients (whole blood: β = 0.48 [0.23; 0.74], p < 0.001, +363 pg/mL, plasma: β = 5.11 [1.33; 8.88], p = 0.002, +289 pg/mL) and increased from baseline to the first postoperative day (whole blood: β = 0.85 [0.70; 1.00], p < 0.001 + 657 pg/mL, plasma: β = 9.86 [6.64; 13.18], p < 0.001, +553 pg/mL). A statistically significant difference in postoperative IL-8 increase between delirious and non-delirious patients was only found for plasma samples (β = 8.59 [1.96; 15.25], p = 0.004). IL-8 concentration were expected to increase by 284 pg/mL in a non-delirious patient between baseline and the first postoperative day, and by 865 pg/mL in a delirious patient. Fig. 2 shows the postoperative IL-8 surge for both plasma and whole blood values. Full models descriptions can be found in Supplementary Table S2. Please note that whole blood and plasma concentrations underwent different transformations before analysis, thus limiting the direct comparability of point estimates.
Fig. 2.
IL-8 trajectories in serial whole blood measurements of N = 157 patients and serial plasma measurements in N = 127 patients from the BioCog study. Individual perioperative IL-8 trajectories are shown on a log-transformed scale for whole blood measurements and a square root-transformed scale for plasma values.
3.5. Mixed-effects models of whole blood and plasma IL-8 trajectories adjusted for perioperative parameters
We analyzed IL-8 levels with adjustment for perioperative factors (type of surgery, duration of anesthesia, ASA-PS score and hemoglobin level) in whole blood samples of N = 146 patients (n = 56 with delirium, model 5) and plasma samples of N = 103 patients (n = 32 with delirium, model 6) from the BioCog study. IL-8 levels in both whole blood (β = 0.67 [0.51; 0.84], p < 0.001, +464 pg/mL) and plasma (β = 7.48 [3.67; 11.36], p < 0.001, +387 pg/mL) were significantly increased after surgery. Patients with delirium presented significantly higher levels of IL-8 in whole blood (β = 0.30 [0.04; 0.54], +204 pg/mL), but not in plasma levels. The interaction between delirium and sampling time point did not reach statistical significance.
At any measurement time, IL-8 concentrations in whole blood were found to be higher in patients with non-peripheral surgery (β = 0.34 [0.11; 0.56], p = 0.004, +232 pg/mL) and anesthesia duration >4 h (β = 0.32 [0.10; 0.54], p = 0.006 + 218 pg/mL). The postoperative increase in IL-8 concentration was steeper in patients with non-peripheral surgery (β = 0.38 [0.12; 0.64], p = 0.005, postoperative increase +278 pg/mL in peripheral and +713 pg/mL in non-peripheral surgery) and anesthesia duration >4 h (β = 0.53 [0.27; 0.79], p < 0.001, postoperative increase +236 pg/mL after ≤4 h and +772 pg/mL after >4 h anesthesia).
IL-8 levels in plasma samples were higher in patients with non-peripheral surgery at any time (β = 4.54 [1.29; 7.81], p = 0.007, +235 pg/mL). Higher hemoglobin levels were associated with lower plasma IL-8 levels (β = −1.02 [−1.86; −0.22], p = 0.014, −51 pg/mL per 1 g/dL hemoglobin), and this association was found to be stronger on the first postoperative day (β = −2.39 [−3.83; −0.97], p = 0.001, change per 1 g/dL hemoglobin +7 pg/mL at baseline and −126 pg/mL on the first postoperative day). See Supplementary Table S3 for details.
In an exploratory analysis, spurious associations of IL8 levels with perioperative intake of anticholinergic medication were detected (Supplementary Tables S6 and S7). Adding anticholinergic medication to the model did not yield any significant main effects or interactions with time. The relation of delirium and whole blood IL-8 was only slightly diminished after adding anticholinergic medication to the model (β = 0.22, p = 0.052).
3.6. ROC analysis and prognostic performance
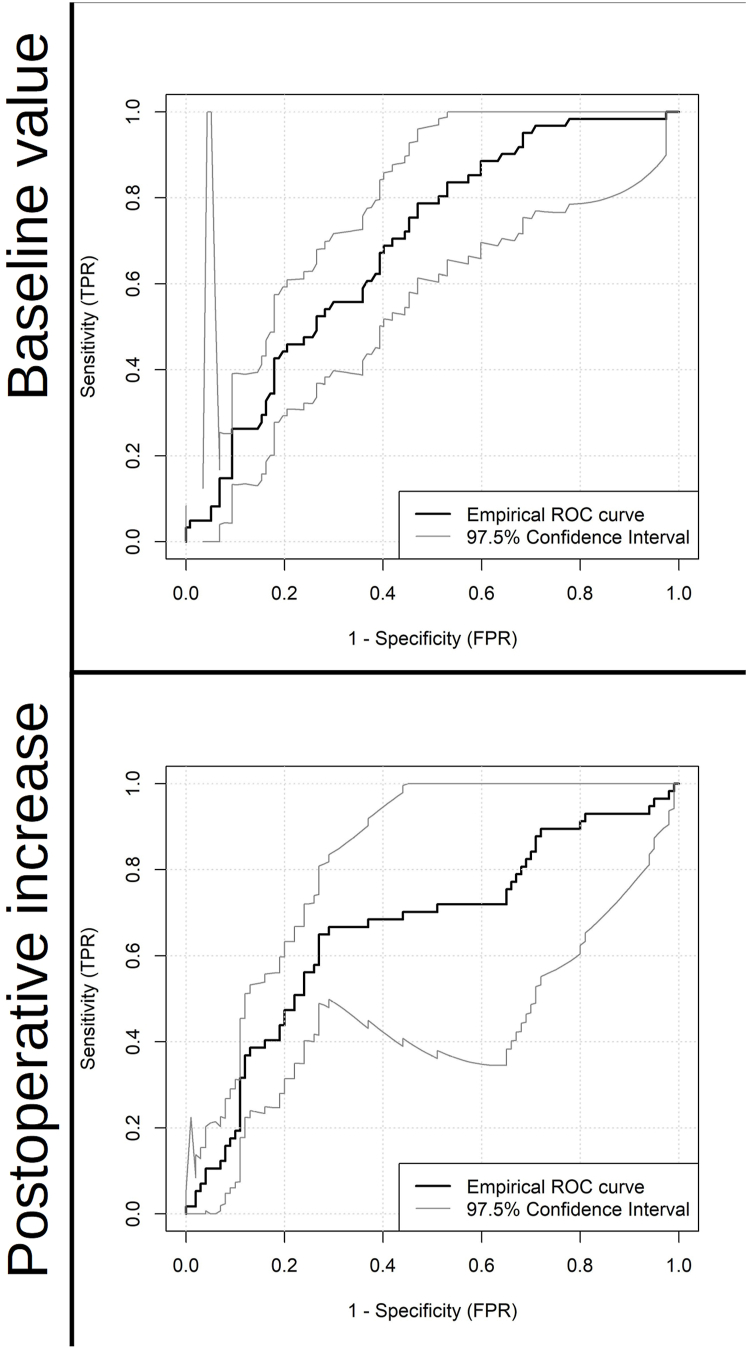
Preoperative IL-8 levels and the IL-8 increase from baseline to the first postoperative day in whole blood samples predicted postoperative delirium significantly better than chance. The AUCs of the empirical ROC were 0.69 (0.59; 0.79) and 0.66 (0.56; 0.77), respectively (Fig. 3). Youden's index suggested a cut-off value of >318.4 pg/mL (IY = 0.32) for baseline IL-8 and >600 pg/mL (IY = 0.38) for the perioperative IL-8 rise.
Fig. 3.
Empirical receiver operating curves for predictive performance of preoperative IL-8 levels (top, N = 178) and postoperative IL-8 increase (bottom, N = 157 for plasma) with 98.75% CIs. Postoperative increase was defined as the difference between IL-8 concentrations (Δc) between the first postoperative day (cd1) and the baseline measurement (cd0): Δc(IL-8) = cd1(IL-8)-cd0(IL-8).
Table 4, Table 5 provide sensitivity, specificity, positive and negative predictive values as well as accuracy for preoperative IL-8 levels and for the postoperative increase of IL-8 concentration in whole blood which were derived from the ROC analysis of the BioCog data. Preoperative IL-8 showed similar predictive performance in both samples. Only the specificity (0.67 vs. 0.53) tended to be slightly higher in the PHYDELIO data compared to the test metrics estimated in the BioCog data. Perioperative IL-8 rise showed considerably lower sensitivity, specificity and accuracy in PHYDELIO compared to BioCog.
Table 4.
Test performance metrics for preoperative whole blood IL-8 concentration >318.4 pg/mL with 95% confidence intervals.
| BioCog (N = 178) | Phydelio (N = 64) | |
|---|---|---|
| Sensitivity | 0.79 (0.69; 0.87) | 0.78 (0.45; 0.94) |
| Specificity | 0.53 (0.44; 0.62) | 0.67 (0.54; 0.78) |
| PPV | 0.47 (0.37; 0.56) | 0.28 (0.14; 0.48) |
| NPV | 0.83 (0.73; 0.90) | 0.95 (0.83; 0.99) |
| Accuracy | 0.62 (0.54; 0.69) | 0.68 (0.57; 0.79) |
Abbreviations: PPV - positive predictive value, NPV - negative predictive value.
Table 5.
Test performance metrics for perioperative whole blood IL-8 increase >600 pg/mL with 95% confidence intervals.
| BioCog (N = 157) | Phydelio (N = 64) | |
|---|---|---|
| Sensitivity | 0.65 (0.52; 0.76) | 0.11 (0.02; 0.43) |
| Specificity | 0.73 (0.64; 0.81) | 0.60 (0.47; 0.72) |
| PPV | 0.57 (0.46; 0.69) | 0.04 (0.01; 0.21) |
| NPV | 0.78 (0.69; 0.86) | 0.80 (0.66; 0.90) |
| Accuracy | 0.70 (0.62; 0.77) | 0.53 (0.41; 0.65) |
Abbreviations: PPV - positive predictive value, NPV - negative predictive value.
4. Discussion
4.1. IL-8 in postoperative delirium
We found that before and immediately after surgery, patients who developed postoperative delirium had higher IL-8 concentrations in plasma samples and whole blood samples. Our results suggest that particularly IL-8 is involved in the peripheral immune reaction to surgical trauma in patients with postoperative delirium.
Whole blood IL-8 measurements from two different studies were included in our analysis. In both samples, IL-8 showed an increase from baseline to the first postoperative days with a subsequent decline in the immediate (PHYDELIO) and intermediate (BioCog) postoperative period. The crescendo-decrescendo pattern was significantly different between patients with and without delirium. In the BioCog sample, this pattern was found to be more pronounced in patients with delirium, suggesting a more severe inflammatory reaction. On the contrary, individual IL-8 level trajectories in delirious patients from the PHYDELIO study showed more heterogeneity, which appeared as an IL-8 “dip” on the first postoperative day in the statistical models. In part, the observed differences in results from both studies may be accounted for differing follow-up periods in BioCog (three months) and PHYDELIO (seven days). Consequently, we conclude that the IL-8 reaction in patients with delirium likely lasts for several days to weeks and that IL-8 levels normalize in the course of weeks to months. Furthermore, differences in sample characteristics may have played are role, since only patients with hepatectomy were included in PHYDELIO. Elevated IL-8 levels have been reported in patients with hepatocellular carcinoma and chronic liver disease (Pan et al., 2020; Zimmermann et al., 2011). In these patients, intrahepatic IL-8 synthesis has been reported and postoperative decrease of IL-8 levels in delirious patients may reflect the extent of resection rather than an inflammatory reaction to surgical trauma. Thus, delirium in patients with postoperative IL-8 dipping in PHYDELIO may be related to postoperative liver insufficiency.
After adjustment for surgery-related variables and perioperative changes in hemoglobin levels, the interaction between delirium and sampling time was no longer significant in the BioCog data. Hence, the steeper IL-8 increase in delirious patients after surgery is mainly related to the extent of the operative intervention rather than individual patient characteristics. Both plasma and whole blood IL-8 levels were generally higher in patients with postoperative delirium from the BioCog study, but the relation was no longer observed in plasma samples after adjustment for perioperative factors. This may be due to a higher pathophysiological relevance of whole blood IL-8 including its erythrocyte bound fraction compared to free IL-8 in plasma, although our observational data do not proof causality. Notably, different immunoassays have been used for plasma samples and whole blood samples, introducing considerable bias into the analyses. We observed lower hemoglobin levels to be associated with higher IL-8 levels in plasma samples, especially after surgery. Previous studies suggested that measurements of plasma IL-8 could be affected by hematocrit (Neunhoeffer et al., 2011). The stronger relation observed on the postoperative day may be a statistical phenomenon, explained by the higher variability of IL-8 and hemoglobin levels after surgery. Higher values of unbound IL-8 might be measured in patients with low erythrocyte counts, especially after surgery.
Previously, the literature has reported conflicting results with regard to IL-8 involvement in delirium. Results from the BioCog study agree with reports of two large observational studies which reported higher perioperative IL-8 increase in delirious patients, although our results suggest that the perioperative IL-8 increase is confounded by surgery characteristics (Ballweg et al., 2021; Casey et al., 2020). Additionally, our results suggest that delirious patients have increased whole blood IL8 levels throughout the perioperative period.
Some of the previous studies reporting the absence of an independent association of IL-8 and delirium had significantly shorter follow-up periods after surgery (Cerejeira et al., 2012; Khan et al., 2021). The half-life of IL-8 has been reported to be considerably longer compared to other cytokines (Colditz et al., 1990; DeForge et al., 1992). Thus, associations of IL-8 levels and delirium may become apparent only after a sufficiently long follow-up period.
Van Munster already reported increased IL-8 levels in delirious ED patients with hip fracture, but did not differentiate between delirium at admission and postoperative delirium, which limits the comparability to our study. (Munster et al., 2008). Various diseases and disorders may account for delirium in ED patients, e.g. infection and intoxication, and hip fracture may likely rather be a consequence of delirium than its trigger.
Finally, previous studies did not investigate whole blood IL-8 levels which may have a particular pathophysiological relevance for delirium.
4.2. Prognostic value of IL-8
Our study evidently favors the use of preoperative whole blood IL-8 concentration over the perioperative IL-8 increase to estimate delirium risk in patients. Generally, we do not recommend using IL-8 as a single biomarker for delirium performance, due to its moderate efficacy. Nevertheless, we were able to reproduce sensitivity and specificity of baseline levels in two independent samples. Our results suggest that in patients with baseline IL-8 >320 ng/mL, which has approximately 80% sensitivity for delirium, further evaluation of delirium risk factors and preventive measures should be considered. Contrasting, we were not able to reproduce a high sensitivity for a postoperative IL-8 increase by > 600 ng/mL, which is in accordance with the heterogeneous IL-8 trajectories observed in delirious patients from PHYDELIO. The negative predictive value (NPV) of a postoperative IL-8 increase was acceptable (80%) and may provide a benefit for a limited group of well-defined patients with an a-priori low risk for delirium and without significant liver disease. Previous studies suggested IL-8 on the first postoperative day to be relevant for prognosis, but our findings from the PHDYDELIO study suggest that perioperative IL-8 trajectories depend on the investigated population and may not be useful in some highly selected patient groups (Ballweg et al., 2021).
4.3. Limitations
In our study, IL-8 concentrations have been quantified with two different methods and we have discussed how the measurement method may affect the interpretation of results, assuming that whole blood IL-8 includes erythrocyte-bound IL-8, whereas plasma IL-8 does not. Nevertheless, several other parameters could influence the difference between plasma and whole blood IL-8 values. For instance, different test kits have been used for plasma and whole blood IL-8 measurements, which confounds our results significantly.
It is prudent to acknowledge the differences between the two studies we analyzed here. In contrast to BioCog, PHYDELIO was not purely observational, but a randomized controlled trial in a highly selected population, which limits the generalizability of our results. The BioCog samples suffers from a large number of missing values, which likely introduces bias. Follow-up times differed significantly between both samples and limit the comparability of data collected at the third assessment.
The PHYDELIO sample is small and the delirium incidence was low. Thus, evidence from this study is limited. To reflect uncertainty of results from this sample, we reported CIs. It is important to note that after log- or square root-transformation of IL-8 values for regression analyses, the CIs were distorted and may appear narrower than for data on a natural scale.
Moreover, IL-8 levels reflect only one aspect of a complex inflammatory reaction to surgical trauma in delirious patients. Apart from IL-8, several other inflammatory biomarkers have been assessed in PHYDELIO and BioCog, but were not analyzed in this study. We considered IL-8 of particular interest due to preliminary findings from the PHYDELIO study (Spies et al., 2021). Nevertheless, results of the comprehensive inflammatory -omics panel collected in BioCog are pending, but may have considerable impact on the interpretation of the findings presented here.
5. Conclusions
The current study suggests a significant role for IL-8 in postoperative delirium. Whole blood IL-8 seems to be more relevant than plasma IL-8, suggesting that the erythrocyte-bound fraction of IL-8 might have a pathophysiological role in delirium. IL-8 could be considered as an additional biomarker in delirium risk evaluation and management programs.
Disclosure of interests and funding
The BioCog study has received funding from the European UnionSeventh Framework Program [FP7/2007–2013] under grant agreement n° 602461. Georg Winterer and Claudia Spies are currently licensing a Class IIa medical device (web-based software tool for multivariate risk prediction of postoperative delirium and cognitive dysfunction in clinical practice). This diagnostic software includes a pending patent application. Georg Winterer is also founder and CEO of Pharmaimage Biomarker Solutions GmbH Berlin (Germany). None of the other authors have a conflict of interest to declare. PHYDELIO is an investigator-initiated trial funded by Köhler GmbH. The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
Special thanks to Dr. Maria Lietz and Jaqueline Lehmann for revising the manuscript with regard to language and style.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100419.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- American Psychiatric Association . fifth ed. APA Press; Washington DC: 2013. Diagnostic and Statistical Manual. [Google Scholar]
- American Psychiatric Association . 2002. Diagnostic and Statistical Manual IV - Text Revision, 6. Printing. Washington, DC. [Google Scholar]
- Androsova G., Krause R., Winterer G., Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front. Aging Neurosci. 2015;7:112. doi: 10.3389/fnagi.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweg T., White M., Parker M., Casey C., Bo A., Farahbakhsh Z., Kayser A., Blair A., Lindroth H., Pearce R.A., Blennow K., Zetterberg H., Lennertz R., Sanders R.D. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br. J. Anaesth. 2021;126:458–466. doi: 10.1016/j.bja.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune B.T., Ponath G., Golledge J., Varga G., Arolt V., Rothermundt M., Berger K. Association between IL-8 cytokine and cognitive performance in an elderly general population--the MEMO-Study. Neurobiol. Aging. 2008;29:937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Bergeron N., Dubois M.J., Dumont M., Dial S., Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- Çakici N., Sutterland A.L., Penninx B.W.J.H., Dalm V.A., de Haan L., van Beveren N.J.M. Altered peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Brain Behav. Immun. 2020;88:547–558. doi: 10.1016/j.bbi.2020.04.039. [DOI] [PubMed] [Google Scholar]
- Casey C.P., Lindroth H., Mohanty R., Farahbakhsh Z., Ballweg T., Twadell S., Miller S., Krause B., Prabhakaran V., Blennow K., Zetterberg H., Sanders R.D. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerejeira J., Nogueira V., Luís P., Vaz-Serra A., Mukaetova-Ladinska E.B. The cholinergic system and inflammation: common pathways in delirium pathophysiology. J. Am. Geriatr. Soc. 2012;60:669–675. doi: 10.1111/j.1532-5415.2011.03883.x. [DOI] [PubMed] [Google Scholar]
- Colditz I.G., Zwahlen R.D., Baggiolini M. Neutrophil accumulation and plasma leakage induced in vivo by neutrophil-activating peptide-1. J. Leukoc. Biol. 1990;48:129–137. doi: 10.1002/jlb.48.2.129. [DOI] [PubMed] [Google Scholar]
- Darbonne W.C., Rice G.C., Mohler M.A., Apple T., Hébert C.A., Valente A.J., Baker J.B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J. Clin. Invest. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta M., Dumbrell A.C. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J. Am. Geriatr. Soc. 2006;54:1578–1589. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- de Rooij S.E., van Munster B.C., Korevaar J.C., Levi M. Cytokines and acute phase response in delirium. J. Psychosom. Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- DeForge L.E., Fantone J.C., Kenney J.S., Remick D.G. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J. Clin. Invest. 1992;90:2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely E.W., Margolin R., Francis J., May L., Truman B., Dittus R., Speroff T., Gautam S., Bernard G.R., Inouye S.K. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit. Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- Field R.H., Gossen A., Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J. Neurosci. 2012;32:6288–6294. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A., Volk H.-D. Stability of tumor necrosis factor α, interleukin 6, and interleukin 8 in blood samples of patients with systemic immune activation. Arch. Pathol. Lab Med. 2008;132:1802–1806. doi: 10.1043/1543-2165-132.11.1802. [DOI] [PubMed] [Google Scholar]
- Gaudreau J.-D., Gagnon P., Harel F., Tremblay A., Roy M.-A. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J. Pain Symptom Manag. 2005;29:368–375. doi: 10.1016/j.jpainsymman.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- Joosten E., Lemiengre J., Nelis T., Verbeke G., Milisen K. Is anaemia a risk factor for delirium in an acute geriatric population? Gerontology. 2006;52:382–385. doi: 10.1159/000095126. [DOI] [PubMed] [Google Scholar]
- Khan B.A., Perkins A.J., Prasad N.K., Shekhar A., Campbell N.L., Gao S., Wang S., Khan S.H., Marcantonio E.R., Twigg H.L., Boustani M.A. Biomarkers of delirium duration and delirium severity in the ICU. Crit. Care Med. 2020;48:353–361. doi: 10.1097/CCM.0000000000004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.H., Lindroth H., Jawed Y., Wang S., Nasser J., Seyffert S., Naqvi K., Perkins A.J., Gao S., Kesler K., Khan B. Serum biomarkers in postoperative delirium after esophagectomy. Ann. Thorac. Surg. 2021;S0003–4975(21) doi: 10.1016/j.athoracsur.2021.03.035. 00558–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaak C., Vorderwülbecke G., Spies C., Piper S.K., Hadzidiakos D., Borchers F., Brockhaus W.-R., Radtke F.M., Lachmann G. C-reactive protein for risk prediction of post-operative delirium and post-operative neurocognitive disorder. Acta Anaesthesiol. Scand. 2019 doi: 10.1111/aas.13441. [DOI] [PubMed] [Google Scholar]
- Kotfis K., Szylińska A., Listewnik M., Strzelbicka M., Brykczyński M., Rotter I., Żukowski M. Early delirium after cardiac surgery: an analysis of incidence and risk factors in elderly (≥65 years) and very elderly (≥80 years) patients. Clin. Interv. Aging. 2018;13:1061–1070. doi: 10.2147/CIA.S166909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E.J., Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [Interleukin-8]) Am. J. Respir. Cell Mol. Biol. 1990;2:479–486. doi: 10.1165/ajrcmb/2.6.479. [DOI] [PubMed] [Google Scholar]
- Marcantonio E.R., Goldman L., Orav E.J., Cook E.F., Lee T.H. The association of intraoperative factors with the development of postoperative delirium. Am. J. Med. 1998;105:380–384. doi: 10.1016/S0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- McNeil J.B., Hughes C.G., Girard T., Ware L.B., Ely E.W., Chandrasekhar R., Han J.H. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minten C., Alt C., Gentner M., Frei E., Deutsch U., Lyck R., Schaeren-Wiemers N., Rot A., Engelhardt B. DARC shuttles inflammatory chemokines across the blood–brain barrier during autoimmune central nervous system inflammation. Brain. 2014;137:1454–1469. doi: 10.1093/brain/awu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster B.C.V., Korevaar J.C., Zwinderman A.H., Levi M., Wiersinga W.J., Rooij S.E.D. Time-course of cytokines during delirium in elderly patients with hip fractures. J. Am. Geriatr. Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- Narita M., Tanaka H., Togashi T., Abe S. Cytokines involved in CNS manifestations caused by mycoplasma pneumoniae. Pediatr. Neurol. 2005;33:105–109. doi: 10.1016/j.pediatrneurol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Neunhoeffer F., Lipponer D., Eichner M., Poets C.F., Wacker A., Orlikowsky T.W. Influence of gestational age, cesarean section and hematocrit on lnterleukin-8 concentrations in plasma and detergent-lysed whole blood of noninfected newborns. Transfus. Med. Hemotherapy. 2011;38:183–189. doi: 10.1159/000328860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Kaminga A.C., Wen S.W., Liu A. Chemokines in hepatocellular carcinoma: a meta-analysis. Carcinogenesis. 2020;41:1682–1694. doi: 10.1093/carcin/bgaa106. [DOI] [PubMed] [Google Scholar]
- Pruenster M., Mudde L., Bombosi P., Dimitrova S., Zsak M., Middleton J., Richmond A., Graham G.J., Segerer S., Nibbs R.J.B., Rot A. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B., Li L., Wang S., Wu J., Huang Y., Zhou P., Bai J., Zheng Y. Interleukin-8 gene polymorphism -251T>A contributes to Alzheimer's disease susceptibility. Medicine (Baltim.) 2016;95 doi: 10.1097/MD.0000000000005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.-S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F.M., Franck M., Schust S., Boehme L., Pascher A., Bail H.J., Seeling M., Luetz A., Wernecke K.-D., Heinz A., Spies C.D. A comparison of three scores to screen for delirium on the surgical ward. World J. Surg. 2010;34:487–494. doi: 10.1007/s00268-009-0376-9. [DOI] [PubMed] [Google Scholar]
- Reinsberg J., Dembinski J., Dorn C., Behrendt D., Bartmann P., van Der Ven H. Determination of total interleukin-8 in whole blood after cell lysis. Clin. Chem. 2000;46:1387–1394. [PubMed] [Google Scholar]
- Saczynski J.S., Kosar C.M., Xu G., Puelle M.R., Schmitt E., Jones R.N., Marcantonio E.R., Wong B., Isaza I., Inouye S.K. A tale of two methods: chart and interview methods for identifying delirium. J. Am. Geriatr. Soc. 2014;62:518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies C.D., Knaak C., Mertens M., Brockhaus W.-R., Shadenok A., Wiebach J., Kunzmann K., Feldheiser A., Pratschke J., Müller O., Kipping V., Fabian M., Abels W., Borchers F., Akyüz L., Ely E.W., Wernecke K.-D., Menon D.K., Piper S.K. Physostigmine for prevention of postoperative delirium and long-term cognitive dysfunction in liver surgery: a double-blinded randomised controlled trial. Eur. J. Anaesthesiol. 2021 doi: 10.1097/EJA.0000000000001456. [DOI] [PubMed] [Google Scholar]
- Springer T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- Trzepacz P.T. The Delirium Rating Scale. Its use in consultation-liaison research. Psychosomatics. 1999;40:193–204. doi: 10.1016/S0033-3182(99)71235-1. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J. Exp. Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G., Androsova G., Bender O., Boraschi D., Borchers F., Dschietzig T.B., Feinkohl I., Fletcher P., Gallinat J., Hadzidiakos D., Haynes J.D., Heppner F., Hetzer S., Hendrikse J., Ittermann B., Kant I.M.J., Kraft A., Krannich A., Krause R., Kühn S., Lachmann G., van Montfort S.J.T., Müller A., Nürnberg P., Ofosu K., Pietsch M., Pischon T., Preller J., Renzulli E., Scheurer K., Schneider R., Slooter A.J.C., Spies C., Stamatakis E., Volk H.D., Weber S., Wolf A., Yürek F., Zacharias N. In: Chief), Del Guerra A., Galderisi S., Guest, editors. vol. 50. 2018. pp. 34–39. (Personalized Risk Prediction of Postoperative Cognitive Impairment – Rationale for the EU-Funded BioCog Project. European Psychiatry, Workshop on Schizophrenia and Other Mental Disorders - S. Frangou). [DOI] [PubMed] [Google Scholar]
- Zimmermann H.W., Seidler S., Gassler N., Nattermann J., Luedde T., Trautwein C., Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.