It has been hypothesized that immune-related adverse events (irAEs) are a biomarker for treatment effect and a positive prognostic indicator in patients receiving immune checkpoint inhibitors (ICIs). ICI-induced thyroiditis is a common irAE that has been associated with improved survival.1–3 We investigated whether the prolonged survival associated with ICI-induced thyroiditis was merely a product of immortal time bias using time-varying Cox proportional hazard modeling and conditional landmark analysis in a retrospective cohort study of consecutive adults who received ICIs between 2010 and 2019. Immortal time bias can occur when a cohort study is designed so that follow-up includes a period of time where participants in the exposed group cannot experience the outcome.
The follow-up period began with the first ICI exposure; patients were followed until death or to June 2020. Patients were excluded if they had a history of thyroid disease determined by ICD9/10 codes or were prescribed thyroid replacement therapy at baseline. We defined ICI-induced hypothyroidism as the initiation of thyroid replacement therapy after day 14 of ICI therapy.4 Thyrotoxicosis was defined by concurrent thyroid stimulating hormone <0.5 ug/dl and free T4 >1.9 ng/dl. We carried out time-independent and time-varying Cox proportional hazards models and survival analysis with and without a conditional landmark to demonstrate the effect of immortal time bias on the estimate of the effect5,6 (Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2021.05.357).
We identified 9419 patients receiving ICIs; after exclusions, 6596 were included. Mean age was 64 years (standard deviation 13), 57% male, and 89% white, non-Hispanic. Patients were followed for a median of 9.6 months (interquartile range 3.6–19.9 months) (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.05.357). Thyroiditis developed in 1155 (17%). Median time to thyroiditis was 3.1 months (interquartile range 1.5–6.2 months).
Multivariable models were adjusted for age, sex, race, cancer type, ICI class, pre-existing rheumatologic disease, and Charlson comorbidity score. ICI-induced thyroiditis was independently associated with a large improvement in overall survival in the time-independent model [adjusted hazard ratio (aHR) 0.47, 95% confidence interval (CI) 0.44–0.53, P < 0.001]. The association with improved survival remained consistent, albeit with a lower effect estimate with time-varying Cox models (aHR 0.80, 95% CI 0.71–0.89, P < 0.001) (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.05.357).
In the survival analysis, without applying a conditional landmark, thyroiditis was associated with a large reduction in death: HR 0.44 (0.39–0.49, P < 0.001). When a 6-month landmark was applied, a weaker, but statistically significant improvement in overall survival was found: HR for death 0.76 (95% CI 0.65–0.88, P < 0.001) (Figure 1). Applying a 3-month landmark yielded a very similar result: HR 0.78 (95% CI 0.67–0.92, P = 0.002). A 6-month landmark analysis was used to determine the association between thyroid dysfunction and overall survival in each malignancy subgroup (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2021.05.357). Patients with lung cancer demonstrated the strongest relationship between thyroiditis and overall survival (HR for death 0.56 [95% CI 0.40–0.79], P < 0.001). The relationship was least in breast, melanoma, and genitourinary tumors.
Figure 1.
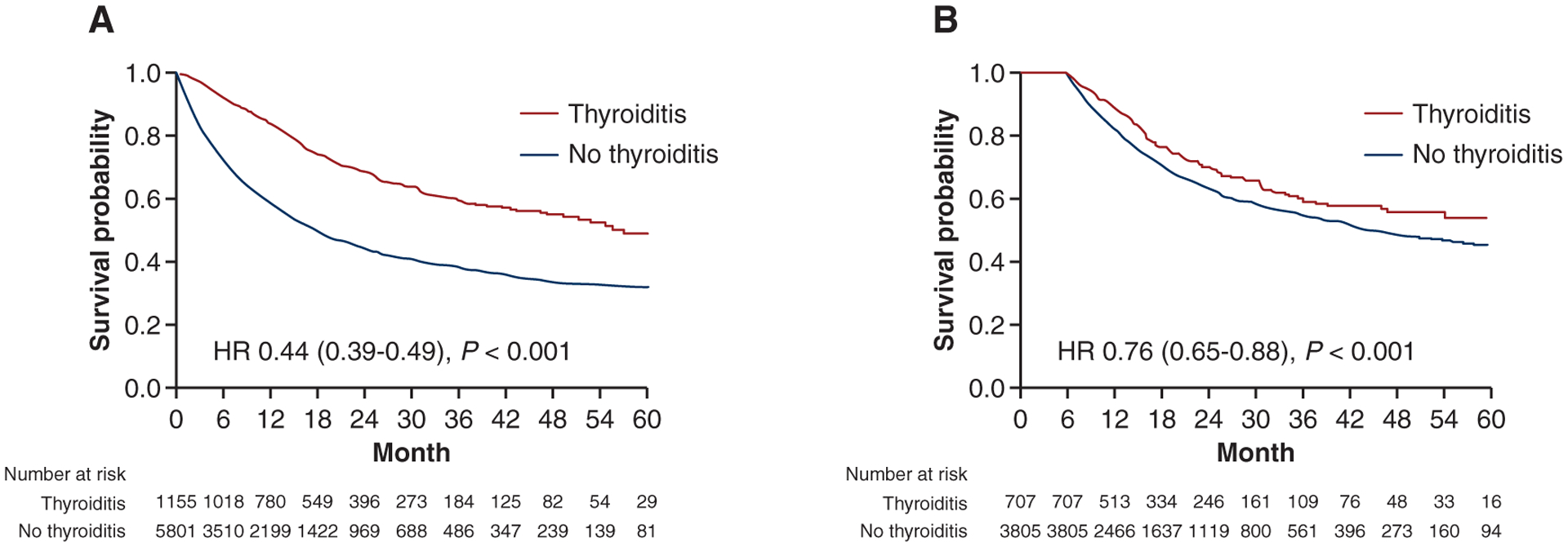
Kaplan–Meier survival curves without a landmark (1A) and using a 6-month landmark analysis (1B). For the conditional landmark analysis, 2444 patients did not survive until the 6-month landmark and were excluded. Of the 4512 remaining patients, 707 developed thyroiditis within 6 months, whereas 3805 patients either never developed thyroiditis or developed it after the 6-month landmark. Most patients received programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors (87%), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors (5%), and 8% received combined PD-1/CTLA-4.
HR, hazard ratio.
In conclusion, after accounting for immortal time bias, we showed a 20% reduction in the aHR for death in patients who develop ICI-induced thyroiditis. The association between thyroiditis and overall survival varied by tumor type, but was strongest in patients with lung cancer, possibly related to the shared developmental origin of thyroid and lung epithelia. Our study demonstrates the large effect of immortal time bias. Future studies with large cohorts are needed to examine the association of other irAEs with survival and must utilize methods that account for mmortal time bias.
Supplementary Material
FUNDING
This work was supported by National Institutes of Health (NIH) [grant number T32 DK007028] (to MR); NIH [grant number K23 DK 117014] (to MES) and the Claflin Distinguished Scholars Award. TGN is supported by NIH [grant numbers R01HL137562-, R01HL130539, K24HL150238] and in part, through a kind gift from A. Curtis Greer and Pamela Kohlberg. The NIH had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. LZ is supported by Spanish Society of Medical Oncology (no grant number) and a gift from Christine Olsen.
DISCLOSURE
LZ serves as a consultant for Merck and reports a grant from SEOM to study immune-related toxicities. TGN has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, H3-Biomedicine, and AbbVie, outside of the current work. TGN also reports consultant fees from Bristol-Myers Squibb (BMS) for a Scientific Advisory Board focused on myocarditis related to immune checkpoint inhibitors and an unrestricted grant from AstraZeneca to study atherosclerosis related to immune checkpoint inhibitors. OR reports research support from Merck. Speaker for activities supported by educational grants from BMS and Merck. Consultant for Merck, Celgene, Five Prime, GlaxoSmithKline, Bayer, Roche/Genentech, Puretech, Imvax, Sobi. In addition, he has a patent ‘Methods of using pembrolizumab and trebananib’ pending. MJM has served as a consultant and/or received honorarium from AstraZeneca, Nektar Therapeutics, Catalysis Pharmaceuticals, Immunai. RJS has been a consultant/served on advisory boards for AstraZeneca, Eisai, Merck, Novartis, Oncosec, Pfizer, Replimune and received research funding from Merck. All other authors have declared no conflicts of interest.
REFERENCES
- 1.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 2.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? a systematic review and meta-analysis. BMC Med. 2020;18:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14:e0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suissa S Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


