Summary
Background
The aberrant brain network that gives rise to the phantom sound of tinnitus is believed to determine the effectiveness of tinnitus therapies involving neuromodulation with repetitive transcranial magnetic stimulation (rTMS) and sound therapy utilizing tailor-made notch music training (TMNMT). To test this hypothesis, we determined how effective rTMS or TMNMT were in ameliorating tinnitus in patients with different functional brain networks.
Methods
Resting-state functional MRI was used to construct brain functional networks in patients with tinnitus (41 males/45 females, mean age 49.53±11.19 years) and gender-matched healthy controls (22 males/35 females, mean age 46.23±10.23 years) with independent component analysis (ICA). A 2 × 2 analysis of variance with treatment outcomes (Effective group, EG/Ineffective group, IG) and treatment types (rTMS/TMNMT) was used to test the interaction between outcomes and treatment types associated with functional network connections (FNCs).
Findings
The optimal neuroimaging indicator for responding to rTMS (AUC 0.804, sensitivity 0.700, specificity 0.913) was FNCs in the salience network-right frontoparietal network (SN-RFPN) while for responding to TMNMT (AUC 0.764, sensitivity 0.864, specificity 0.667) was the combination of FNCs in the auditory network- salience network (AUN-SN) and auditory network-cerebellar network (AUN-CN).
Interpretation
Tinnitus patients with higher FNCs in the SN-RFPN is associated with a recommendation for rTMS whereas patients with lower FNCs in the AUN-SN and AUN-CN would suggest TMNMT as the better choice. These results indicate that brain network-based measures aid in the selection of the optimal form of treatment for a patient contributing to advances in precision medicine.
Funding
Yuexin Cai is supported by Key R&D Program of Guangdong Province, China (Grant No. 2018B030339001), National Natural Science Foundation of China (82071062), Natural Science Foundation of Guangdong province (2021A1515012038), the Fundamental Research Funds for the Central Universities (20ykpy91), and Sun Yat-Sen Clinical Research Cultivating Program (SYS-Q-201903). Yu-Chen Chen is supported by Medical Science and Technology Development Foundation of Nanjing Department of Health (No. ZKX20037), and Natural Science Foundation of Jiangsu Province (No. BK20211008).
Keywords: Tinnitus, Functional magnetic resonance imaging, Functional network connection, Transcranial magnetic stimulation, Sound therapy
Research in context.
Evidence before this study
Tinnitus is a complex brain disorder frequently accompanied by emotional and cognitive symptoms and alterations in brain networks involved in perception, salience, memory, distress, and attention. There has been a growing interest in developing various therapies to treat patients with debilitating tinnitus; however, the success of different interventions has been variable possibly because the therapeutic intervention selected is ineffective in treating the aberrant neural activity responsible for the patient's tinnitus. The inconsistent results obtained from various therapeutic interventions suggest that the brain networks responsible for tinnitus may differ across tinnitus patients, an interpretation consistent with the diversity of brain imaging results seeking to identify the neural signature of tinnitus.
Added value of this study
We used neuroimaging measurements as pretreatment biomarkers to select the optimal neuromodulation intervention for precision treatments of tinnitus. Repetitive transcranial magnetic stimulation (rTMS) and tailor-made notch music training (TMNMT) are currently being investigated as interventions for chronic tinnitus, but a priori, it is unclear which is likely to be most effective in ameliorating tinnitus in a particular patient. To test the hypothesis that individual brain network-based measures could be used to determine whether TMNMT or rTMS would be more effective for a patient, our study on treatment responsiveness have employed different types of treatments and explored baseline neuroimaging differences between Responders and Non-Responder, providing evidence to identify the most effective treatment for an individual subject.
Implications of all the available evidence
Our study indicates that significant differences in the pretreatment brain networks predicted the clinical outcomes of rTMS or TMNMT for tinnitus patients. Pre-treatment network-level resting-state signatures might represent a powerful tool for deciding whether a tinnitus patient is likely to benefit from rTMS versus TMNMT. These results suggest that neuroimaging may serve as promising for selecting the optimal neuromodulation intervention for precision treatments of tinnitus and other neurological treatments by identifying brain networks connections.
Alt-text: Unlabelled box
Introduction
Subjective tinnitus is a phantom auditory sensation that occurs in the absence of external sound affecting 12–30% of adults.1 Approximately 1% suffer from severe or debilitating tinnitus for which they seek medical treatment resulting in a huge social and economic burden due to the paucity of effective treatments.2,3 Consistent with the idea that chronic tinnitus is a highly heterogeneous condition with various treatments, previous research suggested that different subtypes of tinnitus might arise from various mechanisms rather than being unitary phenomenon.4, 5, 6, 7, 8, 9, 10 Tinnitus was originally believed to originate from aberrant neural activity confined to the peripheral auditory system; however, recent studies indicate that tinnitus is associated with abnormal neural activity in widely distributed brain networks involving both auditory network (AUN) and extra-auditory structures such as frontal cortex, parahippocampus, cingulate cortex, and insula as well as the cerebellum.4,5,11, 12, 13, 14, 15
Most patients with tinnitus also have hearing loss. For this subtype of tinnitus, auditory deafferentation limits the brain of the acquired information from the outside environment. For compensation, the brain will attempt to fill in the missing information, which might involve changes in auditory cortex and parahippocampal cortex. Therefore, in a bottom-up compensatory model, cochlear damage that reduces the neural input to the central nervous leads to compensatory changes that give rise to maladaptive hyperactivity and hypersynchrony in the auditory pathway and other regions of the central nervous system, contributing to the generation and maintenance of tinnitus.16, 17, 18 Differently, some patients with tinnitus are not accompanied by hearing loss. Investigations on such groups of patients has provided evidence on a tinnitus subtype associated with a deficient top-down noise cancelling mechanism.19 Specifically, the ventromedial prefrontal cortex and the subcallosal anterior cingulate cortex are responsible for modulation of sensory signals, together with the descending projection to the thalamic reticular nucleus, involving the fronto–limbic–striatal top-down sound-inhibitory system that can filter out abnormally increased signals in the ascending auditory pathway.19 Tinnitus might occur when such suppression of irrelevant sensory signals failed.
Tinnitus is a complex brain disorder frequently accompanied by emotional and cognitive symptoms and alterations in brain networks involved in perception, salience, memory, distress, and attention.20,21 In large-scale human brain networks, the synchrony of distinct, but functionally coherent networks contribute to complex cognitive and emotional processes.22,23 Tinnitus has been linked to abnormal activity and interactions in multiple intrinsic brain networks, and variations in the involvement of specific components of these networks is believed to account for its heterogeneity.24 For example, increased activation of the salience network (SN) may prevent habituation of phantom sound resulting in continuous perception of tinnitus.25
The inherent neuroplasticity of the nervous system has been seized upon as a potential means to reverse the maladaptive organization of the tinnitus brain particularly by non-invasive neuromodulation,26 such as repetitive transcranial magnetic stimulation (rTMS) or sound-based interventions like tailor made notch music training (TMNMT).27 The efficacy of non-invasive neuromodulation interventions with rTMS or TMNMT has been inconsistent with some subjects showing improvement while others exhibiting minimal or transient benefit.26, 27, 28, 29 The inconsistent results suggest that the brain networks responsible for tinnitus may differ across tinnitus patients, an interpretation for the diversity of brain imaging results seeking to identify the neural signature of tinnitus.29, 30, 31, 32, 33 For example, Han et al.30 compared the baseline and post-treatment brain network architectures in patients with tinnitus and reported that baseline brain network measures of the thalamus, underlying interaction between auditory network and limbic network in tinnitus, could predict outcomes to sound based interventions. The use of pooled neuroimaging results to select a particular form of treatment for a patient may be of limited value because of inherent heterogeneity in the pooled data and variability in causal mechanism, which may explain why tinnitus treatments are often inconsistent.29, 30, 31, 32, 33 In addition, most studies on treatment responsiveness have only employed a single type of treatment and explored baseline neuroimaging differences between Responders and Non-Responder, making it difficult to identify the most effective treatment for an individual subject.
Independent component analysis (ICA) is a data-driven method based on a blind source separation algorithm.34 ICA is a promising technique in exploring the functional networks connectivity (FNC) in the brain, which describes the temporal and spatial characteristics of the underlying hidden components or networks for inter-network connectivity analysis. Compared to other functional connectivity analysis techniques such as seed-based analysis (the single interaction between the seed region and the entire voxel), or graph theory analysis (the topological properties of the region of interest within the whole brain related to a particular function), ICA investigates multiple simultaneous voxels to voxel interactions of distinct networks in the brain, which explores functional connectivity between large-scale independent networks rather than between single brain areas.35
TMNMT and rTMS are currently being investigated as interventions for chronic tinnitus, but a priori, it is unclear which is likely to be most effective in ameliorating tinnitus in a particular patient. To test the hypothesis that individual brain network-based measures could be used to determine whether TMNMT or rTMS would be more effective for a patient, we used resting-state functional MRI to identify brain network predictors of outcomes in tinnitus patients assigned to rTMS or TMNMT treatment. It is proposed that brain network-based measures would be more accurate than clinical metrics in predicting differential outcomes to rTMS or TMNMT tinnitus treatments. Besides, we would like to determine whether specific brain network-based predictors for treatment effect are significantly different between tinnitus group and healthy controls. We analyzed the inter-network functional connectivity using ICA. It is hypothesized that tinnitus patients with specific patterns of brain network are responding to interventions with different mechanisms for tinnitus. Specifically, the SN and AUN might involve in responding to TMNMT interventions which employs enjoyable music with the engagement of attentional and emotional elements. And consist with noise-cancellation mechanisms for tinnitus, brain network involving the medial prefrontal cortex and nucleus accumbens together with the limbic structures might responding to rTMS interventions. By assessing the brain network characteristics of patients sensitive to different types of treatment, we aimed to develop a personalized medicine approach to select the optimal tinnitus intervention for patients.
Methods
Data sources, study population, and ethics
The Medical Research Ethics Committee of Nanjing Medical University approved the study (Reference No. 2016067). Written informed consent was obtained from all subjects prior to beginning study procedures in accordance with the Declaration of Helsinki. The 86 untreated persistent tinnitus patients (all right-handed; 41 males/45 females, mean age 49.53±11.19 years; tinnitus duration > six months) were recruited into the study from the Department of Otolaryngology, Nanjing First Hospital. The patients had bilateral or central tinnitus and a score ≥ 30 on the Tinnitus Questionnaire (TQ). The tinnitus patients were assigned to one of two treatments, rTMS or TMNMT. Because the TMNMT treatment filters out the music in a half- octave band located near the pitch of the patient's tinnitus, only patients with tonal-like tinnitus with a pitch less than 8.5 kHz were included in the TMNMT arm of the study. The threshold of subjects in the TMNMT group had to be less than 70 dB HL. Forty-three tinnitus patients received rTMS of the left temporal cortex for 50 min, ten consecutive working days, while the remaining 43-tinnitus patients received TMNMT. Fifty-seven right-handed, age- and gender- matched healthy controls (HCs) without tinnitus (22 M/35 F, mean age 46.21±10.23 years) were enrolled via online advertisements. The HCs did not undergo any type of intervention throughout the study. Participants were excluded from the study if they had pulsatile tinnitus, Ménière's disease, otosclerosis, sudden deafness, MRI contraindications, history of brain diseases such as stroke, head injury confirmed by conventional MRI, or other central nervous system disorders that could potentially affect the central nervous system structure or functions. None of the included tinnitus patients reported comorbid conditions such as hypertension, depression, hyperacusis or epilepsy. The hearing threshold of both ears was measured by pure tone audiometry (PTA) and mean hearing threshold was calculated as the average of hearing thresholds at 500, 1000, 2000 and 4000 Hz.36
Treatment
Single-pulse TMS was administered to the primary auditory cortex by “8”-shaped coil (CCY-I TMS instrument, Yiruide Co., Wuhan, China). rTMS was delivered in accordance with a previous tinnitus treatment study.37 Image-guided stereotaxy with a frameless stereotactic device using structural imaging data was employed to guide TMS coil placement at the patient's primary auditory cortex. Patients sat in chairs with their forehead secured with a band against a support bar and their chin in a jaw support. The coil was held in place against the patient's head by a mechanical arm and positioned above the marked location with the handle pointing upwards using a template. To identify the coil location on subsequent treatment days, a neurosurgical marker was placed and the location of the targeted location on primary auditory cortex was marked on the scalp. On the first day of rTMS treatment, the resting motor threshold (RMT) was determined in every patient using an ascending staircase method until the lowest intensity at which five of ten consecutive pulses induced a visible twitch in the contralateral hand was reached, and the intensity was set based on the obtained motor threshold. In each of the ten consecutive days, patients received low frequency rTMS (2000 Stimuli; 1 Hz; 110% resting motor threshold) on the left temporal cortex.
TMNMT was administered for 30 min four times per day for one months through an online professional tinnitus treatment APP (https://www.soundoceans.com). The online app was used to measure PTAs in each ear and match tinnitus pitch. Tinnitus patients can choose their favorite music that was filtered online. The online filtering procedure of tailor-made notch music is as follows: (1) the spectral energy is redistributed from low to high frequency range; (2) a 1/2 octave frequency bandwidth of sound in the center of tinnitus frequency is removed (filtered out).28 Patients adjust the volume of the filtered music to a comfortable loudness. The tinnitus pitch was re-assessed to monitor changes of the tinnitus frequency during the treatment and then adapted the notch frequency to the changes. To ensure that the upper and lower frequency range of the notch can be heard by the patients, the tinnitus frequency of the included patient was less than 8.5kHz, and the hearing threshold was less than 70 dB HL.
Tinnitus severity was assessed by the Tinnitus Questionnaire (TQ) before the first and after the last tinnitus treatments.38 We calculated the ΔTQ score in all the tinnitus patients, which was defined as follows: ΔTQ score = TQ baseline - TQ treated. A “Responder” was defined as subject that showed a reduction ≥5 points from the baseline TQ score (ΔTQ score ≥ 5); others defined as “Non-Responder” (ΔTQ score < 5).38,39 On this basis, tinnitus patients were divided into an effective group (EG) of 43 “Responders” and an ineffective (IG) group of 44 “Non-Responder”. Tinnitus-related psychological symptoms were evaluated using the Self-Rating Anxiety Scale (SAS)40 and Self-Rating Depression Scale (SDS).41 Except for the tonal tinnitus inclusion criterion for the TMNMT group, there were no significant baseline differences between the TMNMT group and rTMS group in terms of age, gender, education, hearing loss, tinnitus laterality, tinnitus duration, or tinnitus severity between treatment groups (Table 1).
Table 1.
Baseline demographics and clinical characteristics (mean ± SD).
| rTMS (n=43) | TMNMT (n=43) | HCs (n=57) | Ρ | |
|---|---|---|---|---|
| Age(year) | 50.40±10.40 | 48.67±11.99 | 46.21±10.23 | 0.156a |
| Gender(male/female) | 18/25 | 23/20 | 22/35 | 0.313c |
| Education(year) | 11.93±2.84 | 12.86±3.49 | 13.19±2.82 | 0.116a |
| Mean HT (dB) | 15.82±2.01 | 16.02±3.08 | 16.01±2.53 | 0.922a |
| Tinnitus types (tonal/all) | 43/43 | 43/43 | - | - |
| Duration(month) | 48.42±37.96 | 40.44±29.53 | - | 0.280b |
| SAS score | 40.09±5.79 | 39.98±5.20 | - | 0.922b |
| SDS score | 40.88±6.10 | 41.33±5.00 | - | 0.714b |
| TQ score | 52.83±12.87 | 49.70±11.74 | - | 0.243b |
Abbreviations: rTMS, repetitive transcranial magnetic stimulation; TMNMT, tailor-made notch music training; HCs, healthy controls; SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; TQ, Tinnitus Questionnaire; HT, Hearing threshold. P value < 0.05 were considered statistically significant, a: one-way ANOVA, b: two-sample -tests, c: Chi-square test.
MRI scanning and data preprocessing
MRI was performed on both patients and HCs; MRI were obtained immediately before the start of treatments in the tinnitus patients. MRI was carried out using at 3.0 T MRI scanner (Ingenia, Philips Medical Systems, Netherlands) with an 8-channel receiver array head coil. The subjects were asked to remain awake, lie with their eyes closed, and avoid any thoughts during the scanning. Subjects wore earplugs (Hearos Ultimate Softness Series, USA) to attenuate scanner noise (approximately 32 dB attenuation) and foam padding placed around the head and neck were added to reduce head motion. Functional image data were acquired axially using a gradient echo-planar (EPI) imaging sequence and scanning parameters were as follows: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, slices = 36, thickness = 4 mm, flip angle (FA) = 90°, gap = 0 mm, field of view (FOV) = 240 mm × 240 mm, matrix = 64 × 64. The voxel size was 3.75 × 3.75 × 4.0 mm3. The total functional sequence lasted 8 min and 8 s. Structural images were obtained with a three-dimensional turbo fast echo (3D-TFE) T1WI sequence with high resolution as follows: TR = 8.1 ms, TE = 3.7 ms, slices = 170, thickness = 1 mm, FA = 8°, gap = 0 mm, matrix = 256 × 256, FOV = 256 mm × 256 mm. The total structural sequence lasted 5 min and 29 s. Functional data analyses were preprocessed using the Graph Theoretical Network Analysis Toolbox for Imaging Connectomics (GRETNA) (2.0.0A http://www.nitrc.org/projects/gretna/). The processing pipeline was as follows: (1) The first 10 volumes were discarded from each time series; (2) Slice-timing correction and realignment were performed for the remaining 220 consecutive images. Subjects with a head motion > 2.5 mm or a rotation in each direction > 2.5° were excluded from analysis; (3) The remaining data were spatially normalized to the Montreal Neurological Institute (MNI) template (resampling voxel size = 3 × 3 × 3 mm3) and smoothed with 6 × 6 × 6 mm3 full width at half maximum (FWHM) Gaussian kernel.
Independent component analysis
Group spatial independent component analysis (ICA) was performed using GIFT software (GIFT v4, http://mialab.mrn.org/software/gift/). The group template was used as a reference within a spatially constrained ICA algorithm to compute individual spatial maps and time-courses for all subjects. Firstly, the global mean signal per time point was removed as the standard principle component analysis (PCA) processing step during subject-level PCA reduction. Additional postprocessing steps were conducted to remove remaining noise sources, including: (1) detrending linear, quadratic, and cubic trends; (2) conducting multiple regressions of the six realignment parameters and their temporal derivatives; (3) despiking detected outliers; and (4) low-pass filtering with a cutoff frequency of 0.15 Hz. Two separates spatial ICAs were also carried out in HC and tinnitus groups to ensure that the fluctuations of components at rest in each group were similar to those found in the total group of all participants. Therefore, data were automatically decomposed into independent components by GIFT software. A modified version of the minimum description length criterion was adopted to determine the number of components from the aggregate dataset. Single participant spatial or temporally independent maps were then back-reconstructed from the aggregate mixing matrix. The group ICA was performed 100 times on all the subjects when data were automatically decomposed into 40, 50, and 60 components separately by GIFT software and 50 components was found to be the optimal number of independent components (ICs) which exhibited more gray matter rather than non-gray matter. Prior templates provided by GIFT were used to inspect all components and those whose patterns consisted above all of gray matter were selected. We then discarded components located in cerebrospinal fluid or white matter, or with low correlation to gray matter that can be of an artefactual nature through visual inspection. According to previous reports, those which could be categorized as meaningful resting-state networks (RSNs) were selected and finally 15 ICs were identified. The 10 RSNs were the (1): auditory network (AUN), (2) default mode network (DMN), (3) dorsal attention network (DAN), (4) salience network (SN), (5) executive control network (ECN), (6) right frontoparietal network (rFPN), (7) left frontoparietal network (lFPN), (8) somatomotor network (SMN), (9) visual network (VN) and (10) cerebellar network (CN) consistent with previous resting-state fMRI studies.22,42,43 Subject-specific spatial maps and time courses were also obtained using a back-reconstruction approach and the results converted to z scores.
Static FNC analyses
The time courses of the selected RSNs were used to calculate Pearson correlation coefficient between pairs of RSNs. Each pairwise correlation coefficient represents the extent of functional network connectivity (FNC) between networks; the values were transformed to z-scores using Fisher's transformation for further analyses. Finally, 15 × 15 IC correlation matrix as well as 10 × 10 RSN correlation matrix were constructed for each subject. A group difference was estimated for each pair of ICs and evaluated using two-sample t-tests, controlling for age and gender as nuisance covariance. Multiple comparison correction was then performed with a p-value of 0.05 false discovery rate by means of FNC toolbox.
Statistical analyses
SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analyses. For demographic variables, normality of distribution was assessed using the Shapiro-Wilk test. Nonparametric tests were applied if the data were not normally distributed. Categorical variables (presented as counts) were evaluated using a chi-squared test ( test). Continuous variables (shown as the mean ± SD) were investigated with two-sample -tests and p < 0.05 was considered as statistically significant. Pearson's correlation analysis was conducted to explore relationships between changes in brain FC and clinical traits (including SAS, SDS, TQ, and duration of disease) in tinnitus patients (p < 0.05). The results of the FNC were presented using GIFT software (GIFT v4, http://mialab.mrn.org/software/gift/).
Group analyses
To test whether functional connections of RSNs identified in EG and IG were associated with treatment outcome in the groups treated with rTMS or TMNMT, a 2 × 2 analysis of variance (ANOVA) was performed with treatment outcome (EG/IG) and treatment type (rTMS/TMNMT) as variables. The interaction between outcomes and treatment type associated with functional network connections (FNCs) was evaluated. Four comparisons of interest for determining the predictive value of FNCs were generated: (1-2) Responder compared with Non-Responder within the rTMS treatment group and the TMNMT treatment group and (3-4) rTMS compared with TMNMT treatments within the EG and with the IG outcome groups. To calculate the effect sizes of the group differences, post hoc evaluations of each FNC identified from the ANOVA were conducted to evaluate the predictive validity of the imaging markers. To show whether treatment predicted the response (Y/N), two comparisons of interest for determining the predictive value of FNCs were generated: Responder compared with Non-Responder within the rTMS treatment group and the TMNMT treatment group. To determine if the identified FNCs differed between the tinnitus group and HC group at baseline, we compared the extracted FNC measures using two-sample -tests.
Subject-level analyses
Subject-level fMRI evaluations were conducted to assess the predictive value of FNCs. Receiver operating characteristic (ROC) curves were used to examine the sensitivity and specificity of various FNCs to predict which tinnitus patients would likely fall into EG or IG and to utilize FNCs to identify patients likely to be Responders versus Non-Responder for each therapy. The maximum Youden index was used to determine the FNC measures that resulted in the highest combination of sensitivity and specificity.44
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Demographic and clinical traits
Tables 1 and 2 shows the demographic and clinical characteristics of the participants. No significant differences were observed between the rTMS and TMNMT groups in terms of age, gender, education, tinnitus duration, hearing thresholds, and scores on the SAS, SDS. Besides, the threshold of subjects in the TMNMT group were all less than 25 dB HL and thus they could hear the music without hearing loss. Of the 86 tinnitus patients, 42 were classified as Responders to treatment (rTMS: N=20; TMNMT: N=22), and 44 were Non-Responder (rTMS: N=23; TMNMT: N=21). The TQ scores at baseline was not significantly different between the rTMS and TMNMT groups (rTMS: 52.83±12.87, TMNMT: 49.70±11.74, p=0.243, two-sample -tests). With regard to the treatment improvements, significant reductions in TQ scores after treatments were observed both in rTMS group (ΔTQ score 5.37±7.37, p<0.001, paired-samples -tests), and in TMNMT group (ΔTQ score 4.90±4.08, p<0.001, paired-samples -tests). Nevertheless, the mean change in TQ scores after treatments was not significantly different between rTMS and TMNMT groups (p=0.717, two-sample -tests). There were no differences in terms of the TQ scores and other baseline clinical characteristics between the responders and non-responders at baseline (p=0.723, two-sample -tests).
Table 2.
Baseline clinical characteristics between the responders and the non-responders (mean ± SD).
| Responder (n=42) | Non-Responder (n=44) | Ρ | |
|---|---|---|---|
| Age(year) | 46.95±11.41 | 52.00±10.52 | 0.036* |
| Gender(male/female) | 21/21 | 20/24 | 0.673 |
| Education(year) | 12.86±3.11 | 11.95±3.25 | 0.192 |
| Mean HT (dB) | 15.90±3.07 | 15.94±2.05 | 0.951 |
| Duration(month) | 41.05±31.80 | 47.66±36.12 | 0.371 |
| SAS score | 40.55±5.46 | 39.55±5.50 | 0.399 |
| SDS score | 41.55±5.91 | 40.68±5.22 | 0.473 |
| TQ score | 51.75±11.72 | 50.80±13.04 | 0.723 |
Abbreviations: SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; TQ, Tinnitus Questionnaire; HT, Hearing threshold.
P value < 0.05 were considered statistically significant, two-sample t-tests.
Resting-state networks
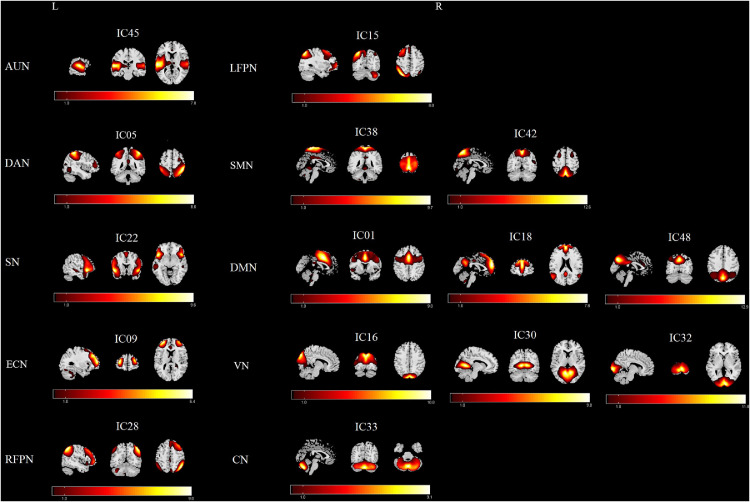
As shown in Figure 1, ten meaningful RSNs were identified and extracted using group ICA: (1) The AUN (IC45), which primarily includes bilaterally the middle and superior temporal gyrus and insula. (2) The DAN (IC05), which encompasses bilaterally the intraparietal sulcus, the intersection of precentral and superior frontal cortex with the orbital part, ventral precentral, and middle frontal gyrus. (3) The SN (IC22) that bilaterally includes the ventral anterior insula and dorsal anterior cingulate cortex. (4) The ECN (IC09, which bilaterally encompasses several medial frontal areas, including the anterior cingulate and paracingulate. (5) The RFPN (IC28) and (6) the LFPN (IC15), which included similar regions, including the middle frontal gyrus, angular gyrus, inferior and superior parietal gyri. (7) The SMN (IC38+42), which included bilaterally the precentral, postcentral, and medial frontal gyrus as well as supplementary motor areas. (8) The DMN (IC01+18+48), which primarily encompassed bilaterally the posterior cingulate/precuneus, bilateral inferior parietal gyrus, angular gyrus, superior frontal gyrus and medial frontal gyrus. (9) The VN (IC16+30+32) that bilaterally encompassed the middle and superior occipital gyrus, temporal-occipital regions, and fusiform gyrus. (10) And the CN (IC33) located in bilateral cerebellum hemispheres.
Figure 1.
RSNs extracted from the group-level ICA. The spatial maps of 15 ICs were selected and categorized as the 10 RSNs for further analysis: auditory network (AUN), dorsal attention network (DAN), salience network (SN), executive control network (ECN), right frontoparietal network (RFPN), left frontoparietal network (LFPN), somatomotor network (SMN), default mode network (DMN), visual network (VN), cerebellar network (CN). R, right; L, left.
Non-specific FNCs features of response for treatments
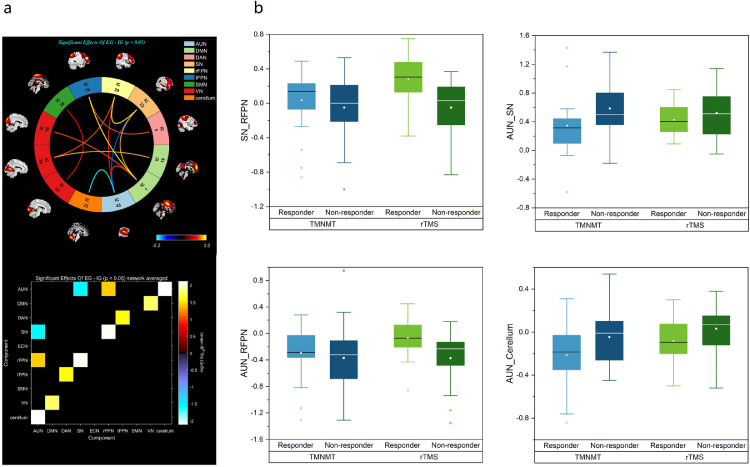
FNC analysis was used to show the differences in the brain network between all Responder and Non-Responder. As shown in Figure 2a, FNC differences were identified between the Responder and Non-Responder groups. The Responder group demonstrated significantly higher functional network connectivity in the AUN-RFPN, DMN-VN, DAN-LFPN, and SN-RFPN compared with the Non-Responder group, but lower functional network connectivity in the AUN-SN and AUN-CN. These results suggest underlying network differences between Responders and Non-Responder group. However, it is unclear if the FNCs associated with tinnitus improvement in the rTMS group are similar or different from those in the TMNMT group.
Figure 2.
FNCs differences between the responders and non-responders. (a) Functional network connectivity (FNCs) identified between the responder (n=42) and non-responder (n=44) groups: higher FNC in the AUN-RFPN, DMN-VN, DAN-LFPN, and SN-RFPN connections, lower FNC in the AUN-SN, AUN-CN connections; (b) Box plots reflect contrasts using the post hoc evaluations of each of the 4 FNCs identified from the primary ANOVA (rTMS=green; TMNMT=blue). Auditory network (AUN), default mode network (DMN), dorsal attention network (DAN), salience network (SN), right frontoparietal network (RFPN), left frontoparietal network (LFPN), somatomotor network (SMN), visual network (VN), cerebellar network (CN).
Outcome-by-treatment analysis of variance (ANOVA)
The effect sizes (Eta-squared) of FNCs identified in the primary ANOVA are summarized in Table 3. Differential outcomes to rTMS or TMNMT were associated with FNCs differences in AUN-SN, AUN-CN, AUN-RFPN and SN-RFPN. The post hoc evaluations of each of the four FNCs identified from the original primary ANOVA are shown in the boxplots (Figure 2b). In the rTMS group, Responders had more positive (higher) FNCs in the SN-RFPN than Non-Responder and less negative (higher) FNCs in the AUN-RFPN. In the TMNMT group, Responders had less positive (lower) FNCs in the AUN-SN than Non-Responder and more negative (lower) FNCs in the AUN-CN. The 4 FNCs (AUN-SN, AUN-CN, AUN-RFPN and SN-RFPN) identified in the tinnitus group as a whole (Responders plus Non-Responder) also differed from the HCs at baseline using two-sample -tests. Additional data are listed in Table S1 in the Supplement.
Table 3.
Imaging features differentiating responder and non-responder by treatment type.
| FNC | Responder: non-Responder with rTMS |
Responder: non-Responder with TMNMT |
||
|---|---|---|---|---|
| η2 | P | η2 | P | |
| AUN_SN | 0.009 | 0.382 | 0.058 | 0.027* |
| AUN_RFPN | 0.071 | 0.014* | 0.004 | 0.556 |
| AUN_CERELLUM | 0.028 | 0.129 | 0.058 | 0.027* |
| DMN_VN | 0.043 | 0.059 | 0.027 | 0.139 |
| DAN_IFPN | 0.026 | 0.139 | 0.044 | 0.054 |
| SN_RFPN | 0.112 | 0.002* | 0.008 | 0.421 |
TMNMT: tailor made notch music training; rTMS: repetitive transcranial magnetic stimulation.
P value < 0.05 were considered statistically significant, analysis of variance (ANOVA).
Subject-level prediction of outcomes
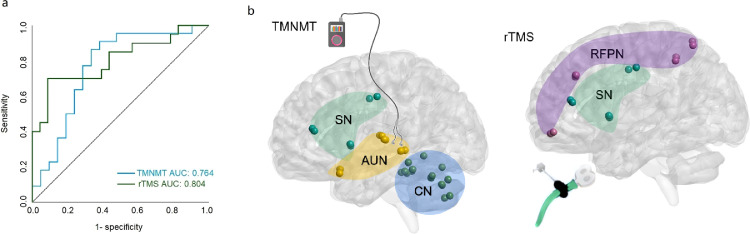
A binary logistic regression model was used for classifying EG vs. IG for rTMS and TMNMT treatments. Accuracy statistics (sensitivity, specificity) are reported for the models. The receiver operating characteristic (ROC) curves were used to characterize the overall predictive value of the FNCs measures for outcomes in the two treatments (Figure 3a). Each FNC measure identified from the original primary ANOVA had significant predictive value for Responders in rTMS or TMNMT, but the area under the curve (AUC) for the ROC curve values was highest for FNC in the SN-RFPN for the rTMS treatment (0.804, 95% CI 0.668-0.940). And for Responders in the TMNMT, the AUC was highest for the combination of FNCs for AUN-SN and AUN-CN (0.764, 95% CI 0.614-0.914). We evaluated the prognoses and screened patients with a sensitivity of 70.0% and specificity of 91.3% using the FNC in the SN-RFPN for rTMS, and a sensitivity of 86.4% and specificity of 66.7% using the combination of FNCs in the AUN-SN and AUN-CN for TMNMT, respectively. Based on these promising results, only the SN-RFPN FNC in the rTMS group and the AUN-SN and AUN-CN FCs in the TMNMT group were used to guide more in further analyses (Figure 3b). Predictive accuracies were higher in models using the identified FNCs measures compared to models using demographic/clinical measures alone. Additional data are listed in Tables S2 and S3 in the Supplement.
Figure 3.
Results of subject-level prediction analysis. (a) Receiver operating characteristic curves for treatments response, showing optimal FNCs features for classifying outcomes for each treatment (rTMS=green, n=43; TMNMT=blue, n=43). (b) Optimal FNCs features with the highest area under the curve (AUC) for each treatment: the FNC in the SN-RFPN for rTMS, the combination of FNCs in the AUN-SN and AUN-CN for TMNMT, respectively.
Correlation between imaging biomarkers and clinical traits
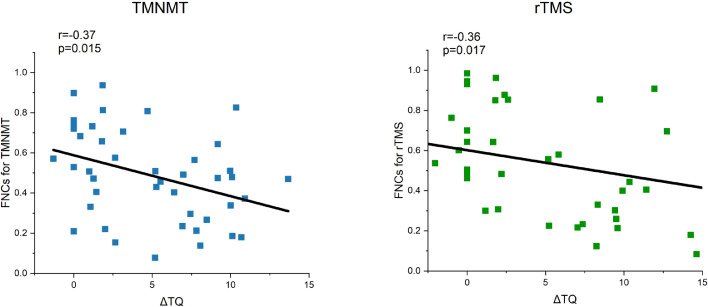
As shown in Figure 4, the Pearson's correlations between FNCs obtained from subject-level prediction and the individual ΔTQ scores for all subjects (N=86) were significant for both rTMS group (r=-0.36, p=0.017) and TMNMT group (r=0.37, p=0.015). Associations between the clinical traits listed in Table 1 with the FNC measures were analyzed to evaluate whether the imaging biomarkers were simply correlated with clinical or demographic characteristics or truly independent predictive variables. There were no significant correlations between FNCs and the clinical/demographic variable.
Figure 4.
Correlation between the FNC coefficient obtained from subject-level prediction and the individual ΔTQ scores across all 86 patients. The right panel with green symbols shows the data for rTMS; The left panel with blue symbols shows the data for TMNMT. The Pearson's correlations between the FNCs obtained from subject-level prediction and ΔTQ scores were significant for both rTMS group (r=-0.36, p=0.017) and TMNMT group (r=0.37, p=0.015). TQ, Tinnitus questionnaire, ΔTQ score = TQ baseline - TQ treated.
Discussion
There has been a growing interest in developing various therapies to treat patients with debilitating tinnitus; however, the success of different interventions has been variable possibly because the therapeutic intervention selected is ineffective in treating the aberrant neural activity responsible for the patient's tinnitus.26, 27, 28, 29 To address this issue, we tested the hypothesis that neuroimaging metrics could be used to predict if TMNMT or rTMS could successfully treat a person's tinnitus. We found that significant differences in the pretreatment brain networks predicted the clinical outcomes of rTMS or TMNMT for tinnitus patients. FNC signatures were more significantly more accurate than clinical metrics in predicting differential outcomes to rTMS or TMNMT tinnitus treatments. These results suggest that neuroimaging may serve as promising for selecting the optimal neuromodulation intervention for precision treatments of tinnitus and other neurological treatments by identifying brain networks connections. Furthermore, the FNC signatures combined with positive clinical outcome could provide insights into the network-level connectivity involved in different forms tinnitus.16,19,45
The FNCs associated with tinnitus efficacy in the rTMS group differed from those in the TMNMT group. The optimal FNCs signatures were significantly associate with the individual ΔTQ scores for rTMS group and TMNMT group, but not with the baseline TQ score and other clinical traits. These results indicate that: (1) FNCs signals could be used to decide which tinnitus patients would be most likely to respond to TMS or TMNMT treatment and (2) to recommend that patients with higher FNCs in the SN-RFPN be assigned to rTMS while lower FNCs in the AUN-SN and AUN-CN receive TMNMT.
The present findings are broadly in line with previous neuroimaging studies of tinnitus. Tinnitus has been consistently linked to AUN abnormalities, typically hyperactivity and enhanced functional connectivity in the central auditory pathway together with other brain regions outside the classical auditory pathway. Enhanced functional connectivity between the AUN and subdivisions of the cerebellum in tinnitus.4 The cerebellum has traditionally been thought to be involved in motor control and coordination, but some cerebellar regions such as parts of the vermis and parafloccular lobe receive inputs from auditory network,46 respond to sound47 and may be involved in gating tinnitus.48 Increased activation of the SN, which is linked to the salience and attention, is believed to prevent habituation of phantom sounds resulting in the persistent perception of tinnitus.25 TMNMT has been reported to reduce tinnitus loudness and distress with inhibition-induced long-term plasticity in the auditory cortex.27 Because TMNMT employs enjoyable music, the engagement of attentional and emotional elements of the SN combined with AUN could impart positive sentiments that make the phantom sound less aversive leading to a positive therapeutic outcome. Thus, the functional connectivity between the AUN and SN together with the AUN and CN may therefore determine whether a tinnitus patient will respond positively to TMNMT.
It is well known that rTMS can disrupt dysfunctional cortical networks by creating electric currents in the brain.49 We found that enhanced FNC in the SN-RFPN predicted that rTMS on the left temporal cortex would lead to tinnitus improvement. This suggest that therapies targeting non-auditory areas in one hemisphere might be more effective in suppressing tinnitus than bilateral treatment of auditory areas. As a lateralized and quasi-independent network, the FPN plays a key role in decision-making and cognitive control.50 Tinnitus-related changes have previously been observed in the RFPN.51 It has been proposed that noise-cancellation mechanisms19 involving the medial prefrontal cortex and nucleus accumbens together with projections from the limbic structures cancel tinnitus signal at the thalamus. This fronto-limbic-striatal sound-inhibitory system filters out aberrant tinnitus signals in the ascending auditory pathway. When this front-limbic-striatal network is disrupted, the “noise cancellation” circuit fails to block the aberrant neural signaling resulting in chronic tinnitus. Our results indicate that tinnitus patients with stronger SN-RFPN connections, presumably with higher prefrontal control over emotion-generating limbic systems, would benefit more from rTMS intervention than those with weak SN-RFPN FNC.
Although the rTMS parameters in the present study were considered possibly optimised based on evidences from previously tinnitus treatment study with respect to observed effects,29 it is unclear how effective rTMS delivered to different brain regions or durations would be since no extra group was set for comparisons of rTMS parameters. And the changes in brain networks could not be evaluated due to lack of MRI data after treatment. As for TMNMT treatment, time course is various from 5 subsequent days to one year in previous studies. It has been reported that both the short-term and intensive (5 subsequent days, 6 h/day) TMNMT and long-term (3 months, 2 h/day) TMNMT could take effect in patients with tinnitus in tinnitus loudness and tinnitus-related neural activity.52, 53, 54, 55 But the induced changes for short-term TMNMT were not persistent, which encourages a longer-term training of the TMNMT. In our study, TMNMT was administered for 30 min four times per day for one months and the changes after one-month TMNMT treatment might be not so remarkable (mean change in TQ scores 4.90±4.08). It might need to explore if TMNMT treatment of a longer duration such as one year would induce larger improvements in tinnitus symptoms.
There were some limitations in this study. It is an early proof of concept type study that needs to be replicated in a less select, larger group of patients. Moreover, the validity and generality of these findings need to be evaluated in a larger and more diverse group of subjects with different degrees of hearing loss, wider age range, and more varied tinnitus and psychological profiles. The utility of using FNC patterns to select the most effective tinnitus intervention could be evaluated with other therapeutic approaches (e.g., cognitive behavioral therapy) or combination therapies (e.g., counseling plus sound therapy). To validate the current findings, prospective studies should be conducted in which rTMS or TMNMT is assigned based on the FNC patterns observed in individual subjects. The results in the present study might need to be further validated as a component of multivariable treatment prediction models, and their predictive capacity for suggesting which treatment should be used also need to be validated in larger sample sizes.
Declaration of interests
The authors declare that there are no conflicts of interests.
Acknowledgments
Contributors
Liping Lan, Yu-Chen Chen, and Yuexin Cai designed research and verified the underlying data; Yin Liu, Yuanqing Wu, Zhen-Gui Xu, and Jin-Jing Xu collected the data; Yu-Chen Chen did the statistical analysis; Liping Lan analyzed data and wrote the initial draft; Jae-Jin Song, Richard Salvi, Xindao Yin, Yu-Chen Chen, and Yuexin Cai drafted and revised the manuscript for content, and all authors contributed to, read, and approved the final manuscript.
Acknowledgments
This work was funded by Key R&D Program of Guangdong Province, China (Grant No. 2018B030339001), National Natural Science Foundation of China (82071062), Natural Science Foundation of Guangdong province (2021A1515012038), the Fundamental Research Funds for the Central Universities (20ykpy91), Sun Yat-Sen Clinical Research Cultivating Program (SYS-Q-201903), Medical Science and Technology Development Foundation of Nanjing Department of Health (No. ZKX20037), and Natural Science Foundation of Jiangsu Province (No. BK20211008).
Data sharing statement
The dataset that used and analyzed in this study is available on reasonable request from the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103862.
Contributor Information
Yu-Chen Chen, Email: cycxwq@njmu.edu.cn.
Yuexin Cai, Email: caiyx25@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Piccirillo J.F., Rodebaugh T.L., Lenze E.J. Tinnitus. JAMA. 2020;323(15):1497–1498. doi: 10.1001/jama.2020.0697. [DOI] [PubMed] [Google Scholar]
- 2.Shargorodsky J., Curhan G.C., Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Stockdale D., McFerran D., Brazier P., et al. An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Serv Res. 2017;17(1):577. doi: 10.1186/s12913-017-2527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y.C., Li X., Liu L., et al. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife. 2015;4:e06576. doi: 10.7554/eLife.06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanneste S., Alsalman O., De Ridder D. Top-down and bottom-up regulated auditory phantom perception. J Neurosci. 2019;39(2):364–378. doi: 10.1523/JNEUROSCI.0966-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits M., Kovacs S., de Ridder D., Peeters R.R., van Hecke P., Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49(8):669–679. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 7.van der Loo E., Gais S., Congedo M., et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;4(10):e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llinas R.R., Ribary U., Jeanmonod D., Kronberg E., Mitra P.P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifford B. The bewitched ear: state of the art genomics research on tinnitus. EBioMedicine. 2021;(67) doi: 10.1016/j.ebiom.2021.103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elgoyhen A.B., Langguth B., De Ridder D., Vanneste S. Tinnitus: perspectives from human neuroimaging. Nat Rev Neurosci. 2015;16(10):632–642. doi: 10.1038/nrn4003. [DOI] [PubMed] [Google Scholar]
- 11.Moazami-Goudarzi M., Michels L., Weisz N., Jeanmonod D. Temporo-insular enhancement of EEG low and high frequencies in patients with chronic tinnitus. QEEG study of chronic tinnitus patients. BMC Neurosci. 2010;11:40. doi: 10.1186/1471-2202-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J.J., Vanneste S., Schlee W., Van de Heyning P., De Ridder D. Onset-related differences in neural substrates of tinnitus-related distress: the anterior cingulate cortex in late-onset tinnitus, and the frontal cortex in early-onset tinnitus. Brain Struct Funct. 2015;220(1):571–584. doi: 10.1007/s00429-013-0648-x. [DOI] [PubMed] [Google Scholar]
- 13.Vanneste S., De Ridder D. Deafferentation-based pathophysiological differences in phantom sound: tinnitus with and without hearing loss. Neuroimage. 2016;129:80–94. doi: 10.1016/j.neuroimage.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y.C., Bo F., Xia W., et al. Amygdala functional disconnection with the prefrontal-cingulate-temporal circuit in chronic tinnitus patients with depressive mood. Progressn Neuro Psychopharmacol Biol Psychiatry. 2017;79(Pt B):249–257. doi: 10.1016/j.pnpbp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lan L., Li J., Chen Y., et al. Alterations of brain activity and functional connectivity in transition from acute to chronic tinnitus. Hum Brain Mapp. 2021;42(2):485–494. doi: 10.1002/hbm.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De R.D., Vanneste S., Langguth B., Llinas R. Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front Neurol. 2015;6:124. doi: 10.3389/fneur.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts L.E., Eggermont J.J., Caspary D.M., Shore S.E., Melcher J.R., Kaltenbach J.A. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30(45):14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes S.H., Schormans A.L., Sigel G., Beh K., Herrmann B., Allman B.L. Uncovering the contribution of enhanced central gain and altered cortical oscillations to tinnitus generation. Prog Neurobiol. 2021;196 doi: 10.1016/j.pneurobio.2020.101893. [DOI] [PubMed] [Google Scholar]
- 19.Rauschecker J.P., May E.S., Maudoux A., Ploner M. Frontostriatal gating of tinnitus and chronic pain. Trends Cogn Sci. 2015;19(10):567–578. doi: 10.1016/j.tics.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minen M.T., Camprodon J., Nehme R., Chemali Z. The neuropsychiatry of tinnitus: a circuit-based approach to the causes and treatments available. J Neurol Neurosurg Psychiatry. 2014;85(10):1138–1144. doi: 10.1136/jnnp-2013-307339. [DOI] [PubMed] [Google Scholar]
- 21.Jafari Z., Kolb B.E., Mohajerani M.H. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56 doi: 10.1016/j.arr.2019.100963. [DOI] [PubMed] [Google Scholar]
- 22.Smith S.M., Fox P.T., Miller K.L., et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldassarre A., Metcalf N.V., Shulman G.L., Corbetta M. Brain networks' functional connectivity separates aphasic deficits in stroke. Neurology. 2019;92(2):e125-e35.. doi: 10.1212/WNL.0000000000006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Ridder D., Vanneste S., Weisz N., et al. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev. 2014;44(Sp. Iss. SI):16–32. doi: 10.1016/j.neubiorev.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 25.De Ridder D., Elgoyhen A.B., Romo R., Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011;108(20):8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deklerck A.N., Marechal C., Perez F.A.M., Keppler H., Van Roost D., Dhooge I.J.M. Invasive neuromodulation as a treatment for tinnitus: a systematic review. Neuromodulation. 2020;23(4):451–462. doi: 10.1111/ner.13042. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto H., Stracke H., Stoll W., Pantev C. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci U S A. 2010;107(3):1207–1210. doi: 10.1073/pnas.0911268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein A., Engell A., Junghoefer M., et al. Inhibition-induced plasticity in tinnitus patients after repetitive exposure to tailor-made notched music. Clin Neurophysiol. 2015;126(5):1007–1015. doi: 10.1016/j.clinph.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Londero A., Bonfils P., Lefaucheur J.P. Transcranial magnetic stimulation and subjective tinnitus. A review of the literature, 2014-2016. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1):51–58. doi: 10.1016/j.anorl.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Han L., Na Z., Chunli L., et al. Baseline functional connectivity features of neural network nodes can predict improvement after sound therapy through adjusted narrow band noise in tinnitus patients. Front Neurosci. 2019;13:614. doi: 10.3389/fnins.2019.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lv H., Liu C., Wang Z., et al. Altered functional connectivity of the thalamus in tinnitus patients is correlated with symptom alleviation after sound therapy. Brain Imaging Behav. 2020;14(6):2668–2678. doi: 10.1007/s11682-019-00218-0. [DOI] [PubMed] [Google Scholar]
- 32.Wei X., Lv H., Wang Z., et al. Neuroanatomical alterations in patients with tinnitus before and after sound therapy: a voxel-based morphometry study. Front Neurosci. 2020;14:911. doi: 10.3389/fnins.2020.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q., Lv H., Wang Z., et al. Outcomes at 6 months are related to brain structural and white matter microstructural reorganization in idiopathic tinnitus patients treated with sound therapy. Hum Brain Mapp. 2021;42:753–765. doi: 10.1002/hbm.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeown M.J., Makeig S., Brown G.G., Tzyy㏄ing J., Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 2015;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smitha K., Raja K.A., Arun K., et al. Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017 doi: 10.1177/1971400917697342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bing D., Ying J., Miao J., et al. Predicting the hearing outcome in sudden sensorineural hearing loss via machine learning models. Clin Otolaryngol. 2018;43(3):868–874. doi: 10.1111/coa.13068. [DOI] [PubMed] [Google Scholar]
- 37.Poeppl T.B., Langguth B., Lehner A., et al. Brain stimulation-induced neuroplasticity underlying therapeutic response in phantom sounds. Hum Brain Mapp. 2018;39(1):554–562. doi: 10.1002/hbm.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goebel G., Hiller W. [The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire] HNO. 1994;42(3):166–172. [PubMed] [Google Scholar]
- 39.Adamchic I., Tass P.A., Langguth B., et al. Linking the Tinnitus Questionnaire and the subjective Clinical Global Impression: which differences are clinically important? Health Qual Life Outcomes. 2012;10(1):79. doi: 10.1186/1477-7525-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zung W.W. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 41.Zung W.W.K. In: Assessment of depression Berlin. Sartorius N., Ban T.A., editors. Springer; 1986. Zung self-rating depression scale and depression status inventory; pp. 221–231. [Google Scholar]
- 42.Du Y., Pearlson G.D., Liu J., et al. A group ICA based framework for evaluating resting fMRI markers when disease categories are unclear: application to schizophrenia, bipolar, and schizoaffective disorders. Neuroimage. 2015;122:272–280. doi: 10.1016/j.neuroimage.2015.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kam T.E., Zhang H., Jiao Z., Shen D. Deep learning of static and dynamic brain functional networks for early MCI detection. IEEE Trans Med Imaging. 2020;39(2):478–487. doi: 10.1109/TMI.2019.2928790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Sedley W., Friston K.J., Gander P.E., Kumar S., Griffiths TD. An integrative tinnitus model based on sensory precision. Trends Neurosci. 2016;39(12):799–812. doi: 10.1016/j.tins.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petacchi A., Laird A.R., Fox P.T., Bower J.M. Cerebellum and auditory function: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):118–128. doi: 10.1002/hbm.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockwood A.H., Salvi R.J., Coad M.L., Arnold S.A., Wack D.S., Murphy B.W., et al. The functional anatomy of the normal human auditory system: responses to 0.5 and 4.0 kHz tones at varied intensities. Cereb Cortex. 1999;9(1):65–76. doi: 10.1093/cercor/9.1.65. (New York, NY: 1991) [DOI] [PubMed] [Google Scholar]
- 48.Bauer C.A., Kurt W., Sybert L.T., Brozoski T.J. The cerebellum as a novel tinnitus generator. Hear Res. 2013;295:130–139. doi: 10.1016/j.heares.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folmer R.L. Repetitive transcranial magnetic stimulation for tinnitus. Arch Otolaryngol Head Neck Surg. 2011;137(7):730. doi: 10.1001/archoto.2011.107. author reply -2. [DOI] [PubMed] [Google Scholar]
- 50.Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q., Lv H., Wang Z., et al. Brain structural and functional reorganization in tinnitus patients without hearing loss after sound therapy: a preliminary longitudinal study. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.573858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teismann H., Okamoto H., Pantev C. Short and intense tailor-made notched music training against tinnitus: the tinnitus frequency matters. PLoS One. 2011;6(9):e24685. doi: 10.1371/journal.pone.0024685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pantev C., Rudack C., Stein A., et al. Study protocol: munster tinnitus randomized controlled clinical trial-2013 based on tailor-made notched music training (TMNMT) BMC Neurol. 2014;14:40. doi: 10.1186/1471-2377-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wunderlich R., Lau P., Stein A., et al. Impact of spectral notch width on neurophysiological plasticity and clinical effectiveness of the tailor-made notched music training. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein A., Wunderlich R., Lau P., et al. Clinical trial on tonal tinnitus with tailor-made notched music training. BMC Neurol. 2016;16(1):38. doi: 10.1186/s12883-016-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.