Abstract
Currently, dental adhesives can be divided into two systems; a smear layer-removal approach with etch-and-rinse adhesives or a smear layer-modified approach with self-etching adhesives. After phosphoric acid etching, the smear layer is completely removed. More attention is, however, required when using self-etching adhesives. The smear layer is partially demineralized by the weak acidic monomer and subsequently incorporated into the hybrid layer. Therefore, the characteristics of the smear layer play an important role on the bonding performance of self-etching adhesives. Such characteristics, for instance, smear layer thickness and smear layer density, are influenced by many factors, e.g., instruments used for dentin surface preparation, cutting speed, and the abrasive particle size of the cutting instruments. This review discusses the contributing factors that affect the smear layer characteristics, and the influence of the smear layer on the bonding performance of dental adhesives. Also, the application techniques regarding how to improve the bonding performance of self-etching adhesives – the smear layer removal by using chemical agents, or the modification of the adhesive application procedures – are provided.
Keywords: Dentin, Self-etching adhesive, Smear layer, Surface preparation, TEM
1. Introduction
Adhesion to enamel and dentin can be divided into an etch-and-rinse approach and a self-etching approach. The etch-and-rinse approach requires a separate step of applying phosphoric acid to demineralize the smear layer and tooth structure. For dentin, after applying 30–40% phosphoric acid, the smear layer is completely removed, and the underlying dentin is demineralized, exposing dentin collagen [1]. The zone of exposed collagen is subsequently infiltrated with resin monomer to form a so-called hybrid layer [2,3]. In contrast, an acidic primer is used in the self-etching approach to demineralize the tooth structure and simultaneously penetrate the underlying dentin. Due to the mild pH of the acidic primer, the smear layer is partially demineralized (Fig. 1a, b). Consequently, the residual smear layer is incorporated into the hybrid layer (Fig. 1c). This structure is called the hybridized smear layer [4], or resin-smear complex [5] in some reports.
Fig. 1.
Schematic diagram of smear layer treated with self-etching adhesive. (a) Smear layer covering dentin surface after tooth preparation. (b) After being treated with self-etching adhesive, the smear layer was partially demineralized and superficial collagen was exposed. (c) After resin polymerization, the residual smear layer was incorporated into hybridized smear layer between the adhesive layer and the hybrid layer.
It has been reported that the bonding performance of self-etching adhesives is affected by the smear layer, especially with mild- and ultra-mild self-etching adhesives. This unfavorable effect of the smear layer also depends on the instruments used for surface preparation. However, in vitro studies have demonstrated that the bond strength of self-etching adhesives can be improved with several bonding modification techniques. This review discusses the smear layer characteristics and their effects on the bonding performance of dental adhesive, including application techniques to improve the clinical outcome.
2. Smear layer definition
Smear layer is a zone of tooth preparation debris found spread on the surface after tooth preparation. Some of this debris blocks the orifices of the dentinal tubules, forming smear plugs that decrease the dentin permeability by 86% [6]. However, the fluid from the dentinal tubules can permeate through the smear layer due to its micro-porous structure [7]. The smear layer is mainly composed of hydroxyapatite and collagen that is denatured by the friction and heat during tooth preparation [8]. It has been suggested that the smear layer should be removed prior to the application of the bonding agent. Complete removal and partial removal of the smear layer are observed when phosphoric acid is used in etch-and-rinse adhesives and self-etching adhesives, respectively [9,10].
3. Role of the smear layer in dentin bonding
Prior to the use of phosphoric acid, dental adhesives in the early generations bonded directly to the smear-covered surface [8,11]. The in vitro bond strengths of these adhesives have been reported to be less than 5 MPa, which could easily result in clinical debonding [8,11]. The debonded specimens were also mainly classified as cohesive failure within the smear layer because the adhesive resin was hydrophobic and, therefore, failed to penetrate through such a zone of debris [12].
3.1. Etch-and-rinse approach
3.1.1. Removing the smear layer with phosphoric acid
It is well accepted that the smear layer can be removed by applying phosphoric acid. However, the appropriate concentration of the acid needs to be carefully considered. Lower concentrations, such as 0.13% and 20%, demonstrated a less aggressive etching effect, and the smear layer has been shown not to be totally removed [9]. Similarly, the high concentration, such as 65% in Super-Bond C&B Red Activator (Sun Medical Co., Ltd., Moriyama, Japan), also demonstrated the inferior demineralization effect [13] and lower bond strength [14]. Clinically, the optimum concentration of phosphoric that is routinely used is 30-40%. With phosphoric acid etching, the smear layer does not affect etch-and-rinse adhesive systems because it is completely demineralized and rinsed away [15]. However, the long-term degradation of dentin collagen has been shown to be more pronounced with etch-and-rinse adhesive compared to that of self-etching adhesive [16]. This phenomenon is possibly due to the aggressive demineralization of phosphoric acid that expose more dentin collagen fibrils. Consequently, matrix metalloproteinases (MMPs), the endogenous enzyme responsible for the collagenolytic activity, are activated and degrade denuded exposed collagen overtime [17].
3.1.2. Alternative etchants
Due to the drawbacks of phosphoric acid, new alternative etchants have become available, e.g., Multi Etchant (Yamakin, Osaka, Japan), Shofu Enamel Conditioner (Shofu, Kyoto, Japan), and an experimental zirconium oxynitrate conditioner (Ivoclar Vivadent, Schaan, Liechtenstein) The compositions and the chemical formula of the active component of each alternative etchant are provided in Table 1. These alternative etchants demonstrated higher dentin bond strength compared with phosphoric acid [18,19]. Moreover, the inhibitory effect of the experimental zirconium oxynitrate conditioner on MMPs has also been demonstrated [19]. Additional studies are warranted to further evaluate the effects of these new etchants on different smear layer preparations.
Table 1.
Compositions and chemical formula of the active component of each alternative etchant.
| Product | Manufacturer | Compositions | Chemical formula of the active component |
|---|---|---|---|
| Multi Etchant | Yamakin, Osaka, Japan | Methacryloyl oxytetra ethylene glycol dihydrogen phosphate (M-TEG-P), thickener, colorant | |
| Enamel conditioner | Shofu, Kyoto, Japan | Three carboxylic acid-based organic compounds (Polyacrylic acid and other 2 smaller molecular weight organic acids), thickener, colorant | 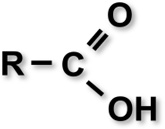 |
| Zirconium oxynitrate conditioner (experimental) | Ivoclar Vivadent, Schaan, Liechtenstein | ZrO(NO3)2, water, glycerol, fumed silica, polyethylene oxide | 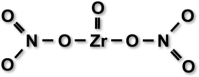 |
3.1.3. Reduced phosphoric acid etching time
Another approach to minimize the dentin collagen degradation caused by phosphoric acid etching is to reduce the etching time. Though it has been proposed that the depth of dentin demineralization was related to the etching time [20,21], no adverse effect on dentin bond strength was observed when the etching time was reduced to 5 s [22]. On the other hand, the bond strength of primary dentin was significantly improved when phosphoric acid etching time was reduced by 50% [23]. Recently, it has been demonstrated that etching with phosphoric acid to dentin for 3 s improved the bond strength and bond durability of universal adhesives [24]. Similar smear layer demineralization pattern after etching with phosphoric acid for 3 s and 15 s has also been observed. It has been stated that shortening the etching time can reduce the adverse effect of phosphoric acid on bond degradation. However, further investigation of the effect of reduced etching time on MMPs activity is needed.
3.2. Self-etching approach
3.2.1. Smear layer modified with self-etching system
When using self-etching adhesive systems, the smear layer characteristics and the etching ability of the adhesives are the two main factors that influence the bonding outcome [10,[25], [26], [27]]. It has been previously mentioned that the smear layer characteristics are critical for dentin adhesion [10]. Many investigations have evaluated the effects of the smear layer produced by different preparation instruments. However, a control group with a smear-free surface was not included for comparison in most studies. Therefore, the effects of the smear layer on dentin adhesion might be underrated. In 2000, a fractured dentin technique was introduced by Tay et al. to compare the bond strength of the self-etching adhesive to smear-free and smear-covered dentin [28]. The results demonstrated that the bond strength of a 2-step self-etching adhesive, Clearfil SE Bond, was not affected by the smear layer created by silicon carbide (SiC) paper [28]. A study by Suyama et al. compared the bond strength on fractured dentin with that on diamond bur-cut dentin [29]. They found that the diamond bur-cut smear layer interfered with the interaction of the self-etching adhesive with the underlying dentin [29]. These studies imply that the smear layers created from different instruments differentially impact the bonding with self-etching adhesives. More details regarding smear layer characteristics are discussed in the next section.
3.2.2. Classification of self-etching adhesives by pH
The etching ability of the self-etching adhesives is related to their pH, which can be classified into strong self-etching adhesives (pH ≤ 1), intermediately strong self-etching adhesives (pH 1–2), mild self-etching adhesives (pH ∼2), and ultra-mild self-etching adhesives (pH > 2.5) [25]. Typically, an adhesive with a lower pH tends to demonstrate better etching ability. However, it has been suggested to avoid using strong self-etching adhesives [25]. The etching pattern of the strong self-etching adhesives is similar to that of phosphoric acid; however, the difference is that the dissolved mineral content is not rinsed away. Therefore, the dentin bond can be weakened by the remnants of the dissolved minerals. In contrast, the mild self-etching adhesives superficially demineralize the dentin surface. The hydroxyapatite remnants are still available for chemical bonding, especially with 10-MDP monomer to form 10-MDP calcium salts [25,30]. This structure is responsible for the long-term durability of the resin-dentin interface [30]. Therefore, using mild self-etching adhesives is preferable. However, with their weak acidity, the buffering effect of the smear layer should be taken into account [26,31,32].
3.2.3. Universal adhesives
Universal adhesives can be applied either with the etch-and-rinse approach, self-etching approach, or selective enamel etching. The compositions of the universal adhesives are basically similar to the previous generation all-in-one adhesives with several improvements. The functional monomer in the universal adhesives is mainly 10-MDP, which is relatively more hydrophobic compared with other functional monomers [33,34]. Furthermore, the hydrophilic components are minimized, e.g., the concentration of HEMA in Clearfil Tri-S Bond Plus and Clearfil Universal Bond Quick is 10–35% and 2.5–10%, respectively [35,36]. Finally, the pH of the universal adhesives ranges between 1.5–3.2; thus, most of them are classified as mild and ultra-mild self-etching adhesives [37]. Therefore, the adverse effect of the smear layer should be carefully concerned when the clinicians apply universal adhesives to dentin in the self-etching approach.
4. Smear layer characteristics
4.1. Instrument used for smear layer preparation
The smear characteristics depend on the armamentarium used. The two most common instruments used for dentin surface preparation in literature are SiC paper and burs. SiC paper is used only in laboratory research to standardize the smear layer and roughen the tooth substrate before performing the bond strength test. It has been recommended to use wet sanding to polish the surface with a circular motion [38]. In some studies, dental burs, such as a diamond bur or carbide bur, were used to produce a clinically relevant smear layer. Special equipment, e.g., a CNC specimen former, was used to stabilize the handpiece during surface preparation [27]. However, free hand preparation of the smear layer using burs would be more clinically relevant. Fig. 2 demonstrates the smear layers resulting from different surface preparations.
Fig. 2.
Representative TEM images of dentin surfaces with different surface preparations. (a) No smear layer was detected in the fractured dentin specimen. (b) The smear layer prepared by a carbide bur was loosely bound to the dentin surface with approximately 300 nm-in-thickness. (c) The smear layer prepared by SiC paper was non-homogenous. (d) The smear layer prepared by a regular diamond bur was the most compact.
Surface preparation using erbium-doped:yttrium-aluminium garnet (Er:YAG) laser has been reported to be more advantageous than preparation using burs [39]. Though the process is time-consuming, Er:YAG laser causes water micro-explosions, together with the vaporization and melting of organic and inorganic components. The prepared surface is then free of smear layer where the improved bond strength should be expected [40]. However, subsurface dentin fissuring [41] and the inferior bonding performance after laser ablation has been noted [42].
Airborne-particle abrasion is also used for caries removal [43,44], tooth preparation [45], and surface roughening [46]. The aluminum oxide particle is commonly used to prepare the surface. The rough surface is beneficial to the bonding performance of adhesive by increasing surface area for resin adhesion [47]. On the other hand, Ouchi et al. [48] reported that the dentin bond strength of universal adhesives in self-etching mode significantly decreased after the surface was grit-blasted with alumina particle. They suggested that such adverse effect was caused by the compaction of the smear layer after alumina grit-blasting, which might inhibit the adhesive resin penetration. However, according to the recent systematic review and meta-analysis, it has been concluded that the airborne-particle abrasion with alumina presented no adverse effect on the dentin bond strength [49]. They recommended using the particle size larger than 30 μm with an air pressure of more than 5 bar to improve the bond strength. For the clinical application, the splattering of the particles within the operating field is inevitable. The use of rubber dam isolation with high-velocity evacuation devices is mandatory [50]. In addition, the patients and dental personnel must also be equipped with protective eyewear to prevent accidental eye irritation.
4.2. Smear layer thickness
The smear layer thickness typically depends on the size of the abrasive particles used. The bigger the abrasive particle size is, the thicker smear layer will be formed [9,26,51]. Tani and Finger reported the thickness of the smear layer ranging from 0.9 to 2.6 μm for the SiC-ground smear layer and 1.0 to 2.8 μm for diamond bur-cut smear layer [51]. Oliveira et al. demonstrated that the thickness of the carbide bur-prepared smear layer was 1.8 μm, which was similar to those prepared by SiC papers and diamond burs, except for the 240-grit SiC paper where the 3.0-μm-thick smear layer was observed [9]. It might be difficult to compare the thickness of the smear layer from different instruments between literatures. However, when comparing between the most commonly used instruments (SiC paper and diamond burs) with corresponding abrasive particle sizes, the SiC-ground smear layers have been shown to be significantly thicker than those prepared by diamond burs [9,26].
4.3. Smear layer density
The smear layer density or denseness is the degree of the smear compaction which is also influenced by the preparation instruments [9,27]. A dense smear layer was observed when prepared using diamond burs, whereas a loosely organized smear layer was detected when prepared using SiC paper [9,27,52]. Although both instruments are used with a similar abrade motion, the higher cutting speed of a diamond bur could compress the preparation debris into a more compact smear layer [9,10,27]. Mine et al. demonstrated that the ultra-mild self-etching adhesive could not completely demineralize and infiltrate to the diamond bur-cut smear layer [5]. In contrast, the smear layer produced by carbide burs is thin and loosely bound. This might be due to the different cutting motion of the carbide bur because a new surface is produced when the cutting blade scrapes the dentin surface [27].
The cutting speed also has influence on the smear layer density and bonding performance of self-etching adhesives. The fissure steel bur with low-speed cutting (2000 rpm) demonstrated similar smear layer characteristics when compared to those prepared by 600-grit SiC paper [53,54]. The etching patterns of self-etching primer on those surfaces were also similar. On the contrary, the coarse diamond bur with high-speed cutting (100,000–120,000 rpm) produced a thick smear layer, which was subsequently more difficult to be removed by self-etching primers [53,54].
The smear layer density was indirectly evaluated by counting the number of occluded dentinal tubules [9] and the reaction of the smear layer after applying acidic agents, e.g., phosphoric acid or self-etching adhesives, using a scanning electron microscope (SEM) [27,55]. A recent study demonstrated the characteristics of the smear layer using transmission electron microscopy (TEM) in a longitudinal direction [10]. With this technique, the density and the reaction of the smear layer to various acidic agents can be evaluated directly, as presented in Fig. 3 [10,56].
Fig. 3.
TEM observation of dentin surface prepared by superfine diamond bur in longitudinal view (a). The dentin surface was etched with 35% phosphoric acid solution (b) and treated with Clearfil SE2 primer (Kuraray Noritake; Tokyo, Japan) (c).
The density of the smear layer is more critical than its thickness, especially for mild- and ultra-mild self-etching adhesives [5,10,26,29,31]. Acidic monomers are less effective in interacting with a dense smear layer for the dissolution. Therefore, resin penetration is hindered, resulting in low bond strength. In contrast, it is easier for mild acidic monomers to remove the loosely-organized SiC-ground smear layer and penetrate into the underlying dentin, forming a uniform hybrid layer and resin tags [50,57].
5. Application techniques to improve the bonding performance of self-etching adhesives
As mentioned above, most of the universal adhesives currently available are mild and ultra-mild self-etching adhesives. The weak acidity of these adhesives can be buffered by the smear layer, especially one created using a diamond bur [5,26,31,32]. Therefore, bonding modifications either with chemical agents or adhesive application techniques might be optional to improve the bonding performance of these adhesives.
5.1. Chemical agents
There are several chemical agents that can be used to modify the smear layer. The mechanisms of these agents depend on the compositions of the smear layer targeted. The organic components of the smear layer can be dissolved using deproteinizing agents [58,59], while the inorganic component can be bound using chelating agents [60,61].
Sodium hypochlorite (NaOCl) and hypochlorous acid (HOCl) are the recommended deproteinizing agents for modifying the smear layer [[62], [63], [64]]. The collagen fibrils in the smear layer can form hydrogen bonds with water. Consequently, the hydrated collagen fibrils inhibit resin penetration and polymerization. Therefore, it has been recommended to remove the collagen before applying the adhesive [65,66]. However, residual oxygen species can be generated after irrigating with deproteinizing solutions [67]. The residual oxygen species also inhibit resin polymerization and affect the resin-dentin bond strength [[67], [68], [69]]. It is then suggested to reverse the bond strength of the deproteinized dentin by using antioxidants or reducing agents. Antioxidants scavenge the oxygen, and many of them can be used to reverse the bond strength of the deproteinized dentin. The effectiveness of antioxidants depends on the number of hydroxyl (−OH) groups in each molecule [70]. A previous study reported that the dentin bond strength of NaOCl-treated dentin could be reversed by irrigating with 10% ascorbic acid for 1 min [71]. Another investigation also demonstrated a promising effect of rosmarinic acid and p-toluenesulfinic acid sodium salt solution (Accel; Sun Medical, Kyoto, Japan) on deproteinized dentin by rinsing for 5 s prior to the adhesive application [72]. The combination of a deproteinizing agent and an antioxidant is currently the best option to dissolve the organic phase of the smear layer with no adverse effect on polymerization.
Ethylenediaminetetraacetic acid (EDTA) is used to selectively remove the inorganic component from the smear layer due to its ability to bind to hydroxyapatite [73]. The effect of EDTA depends on the concentration used and time of application [[73], [74], [75]], where the irrigation with 17% EDTA for 1 min has been recommended [76]. Partial removal of the smear layer without altering the morphology of the underlying dentin has been observed after using EDTA [77,78]. It has also been advised to pre-treat the dentin with EDTA instead of using phosphoric acid [73]. Although the chelating effect produced by EDTA was milder than the etching potential of phosphoric acid [79], the resistance to resin-dentin bond degradation was improved with EDTA conditioning [80,81]. However, the bond strengths of self-etching adhesives to EDTA-treated dentin are inconsistent. No benefit from EDTA has been observed when the smear layer was treated with 0.5 M EDTA (pH 7) for 2 min before bonding with self-etching adhesives [82]. On the contrary, the bond strength of Clearfil SE Bond significantly increased after dentin conditioning with 0.5 M EDTA at pH 7.2 for 30 s [83]. Another study also demonstrated improved dentin bond strength with two self-etching adhesives after applying 24% EDTA gel (pH 7.0) for 1 min [84]. Nevertheless, these studies have evaluated the bond strength to SiC-ground dentin. Further investigations determining the effect of EDTA pretreatment on bur-cut dentin are needed.
5.2. Modified techniques of the adhesive application
The adhesive application can be modified mechanically to improve the smear layer removal. The suggested modified techniques are the active application and the multiple application of the adhesive.
Active application is to rub the adhesive with force on the tooth substrate during application. Using this method, the smear layer dissolution is enhanced by force applied and the stirring effect (Fig. 4) [85]. However, no significant difference in bond strength was detected when various forces were used [86]. There are also many benefits of active application technique in addition to the improvement of smear layer removal, such as promoting the chemical interaction with the 10-MDP monomer [87], enhancing the solvent evaporation [88], ameliorating the bond durability [89], increasing resin penetration and reducing nanoleakage [90].
Fig. 4.
Representative TEM images of superfine diamond bur-prepared smear layers treated with Clearfil SE2 primer (Kuraray Noritake; Tokyo, Japan) with the inactive application (a) or active application (b).
Ultrasonic treatment is also used to remove the smear layer. Nakabayashi and Saimi removed the smear layer by polishing the dentin surface with hydroxyapatite paste and immersing it in an ultrasonic cleaner [91]. The technique was modified by Saikaew et al. to remove the smear layer with an ultrasonic cleaner followed by brushing with an ultra-soft toothbrush [26]. However, these techniques are not clinically relevant. Many studies have investigated the modification of using an ultrasonic device to clinically remove the smear layer. One study attached a custom-made brush to the tip of an ultrasonic scaler to remove the smear layer prior to applying the adhesive. With this technique, the smear layer thickness decreased, and a higher bonding performance was achieved after ultrasonic brushing for 30 s [92]. An ultrasonic device was also used in another study to agitate the adhesive during application. The benefit of this technique was, however, material-dependent [93].
The adhesive application time has been indicated by the manufacturer to warrant the optimal bond strength. Basically, applying the adhesive at the time according to the manufacturer’s instructions is a minimum requirement to ensure the sufficient interaction between resin monomer and underlying dentin [[94], [95], [96]]. Notably, some manufacturers claimed that their universal adhesives, i.e., G-Premio Bond and Clearfil Universal Bond Quick, can be applied using less time without compromising the bond strength [97,98]. It has been demonstrated that less application time resulted in inferior bonding performances [[94], [95], [96],99]. However, in several studies, applying the universal adhesives with less time demonstrated a stable bond after 1 year [95] and 2 years of water storage [100].
Increasing the adhesive application time is also recommended to improve the bond strength. A significant effect was found after extending the application time to 90 s and 150 s for acetone-based and ethanol-based adhesive, respectively [101]. The results from another study, however, demonstrated no significant effect from doubling the application time [102]. A plausible explanation might be that the prolonged application time increases solvent evaporation rather than enhances the smear layer dissolution.
Multiple adhesive application has been proposed by Frankenberger et al. to ensure that the entire dentin surface is coated with the adhesive [103]. A study demonstrated the benefits of applying the adhesive twice, both with and without light-curing between each coat [104]. These two techniques demonstrated significantly higher bond strength compared with a single application. However, the mechanisms for the improvement of bonding performance are different. By applying the adhesive twice without light-curing between each coat, the smear layer dissolution is enhanced due to the higher concentration of the acidic monomer (Fig. 5). This technique also allows more time for demineralization and interaction to the underlying dentin with the resin monomers. Thus, the smear layer removal and the resin penetration can be improved [56,104]. In contrast, the improved bonding performance after double application with light-curing between each coat is mainly due to the thicker adhesive layer and better mechanical properties of the resin-dentin interface, rather than enhancing the smear layer interaction.
Fig. 5.
Representative TEM images of superfine diamond bur-prepared dentin treated with Scotchbond Universal adhesive (3 M Oral Care; St. Paul, MN, USA) with single application (a) or double application (b).
6. Conclusion
The smear layer characteristics differently affect the bonding performances of self-etching adhesives. As most of the so-called universal adhesives can be classified as mild- and ultra-mild self-etching adhesives, the bonding performances can also be influenced by the smear layer when applied in self-etching approach. Their adhesion potential can be improved by enhancing the smear layer removal, both chemically and mechanically. Chemical agents such as NaOCl or EDTA, can be used prior to the adhesive application to interact with the organic component and inorganic component of the smear layer, respectively. Whereas, mechanical smear layer removal can also be improved by modifying the adhesive application. From this review article, we recommend the clinicians to use the adhesive according to the manufacturer’s recommended application time with active application and/or double adhesive application. We urge that future investigations should focus on the long-term results when bonded to a clinically relevant smear layer.
Role of the funding source
None.
Conflict of interest
The author confirms that there is no conflict of interest to be declared.
References
- 1.Perdigão J., Lambrechts P., Van Meerbeek B., Tomé Â.R., Vanherle G., Lopes A.B. Morphological field emission-SEM study of the effect of six phosphoric acid etching agents on human dentin. Dent Mater. 1996;12:262–271. doi: 10.1016/s0109-5641(96)80033-9. [DOI] [PubMed] [Google Scholar]
- 2.Nakabayashi N., Nakamura M., Yasuda N. Hybrid layer as a dentin-bonding mechanism. J Esthet Dent. 1991;3:133–138. doi: 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakabayashi N., Ashizawa M., Nakamura M. Identification of a resin-dentin hybrid layer in vital human dentin created in vivo: durable bonding to vital dentin. Quintessence Int. 1992;23:135–141. [PubMed] [Google Scholar]
- 4.Tay F.R., Sano H., Carvalho R., Pashley E.L., Pashley D.H. An ultrastructural study of the influence of acidity of self-etching primers and smear layer thickness on bonding to intact dentin. J Adhes Dent. 2000;2:83–98. [PubMed] [Google Scholar]
- 5.Mine A., De Munck J., Cardoso M.V., Van Landuyt K.L., Poitevin A., Van Ende A., et al. Dentin-smear remains at self-etch adhesive interface. Dent Mater. 2014;30:1147–1153. doi: 10.1016/j.dental.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Pashley D.H., Livingston M.J., Greenhill J.D. Regional resistances to fluid flow in human dentine in vitro. Arch Oral Biol. 1978;23:807–810. doi: 10.1016/0003-9969(78)90159-0. [DOI] [PubMed] [Google Scholar]
- 7.Pashley D.H. The effects of acid etching on the pulpodentin complex. Oper Dent. 1992;17:229–242. [PubMed] [Google Scholar]
- 8.Eick J.D., Cobb C.M., Chappell R.P., Spencer P., Robinson S.J. The dentinal surface: its influence on dentinal adhesion. Part I. Quintessence Int. 1991;22:967–977. [PubMed] [Google Scholar]
- 9.Oliveira S.S.A., Pugach M.K., Hilton J.F., Watanabe L.G., Marshall S.J., Marshall Jr G.W. The influence of the dentin smear layer on adhesion: a self-etching primer vs. a total-etch system. Dent Mater. 2003;19:758–767. doi: 10.1016/s0109-5641(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 10.Saikaew P., Matsumoto M., Sattabanasuk V., Harnirattisai C., Carvalho R.M., Sano H. Ultra-morphological characteristics of dentin surfaces after different preparations and treatments. Eur J Oral Sci. 2020;128:246–254. doi: 10.1111/eos.12698. [DOI] [PubMed] [Google Scholar]
- 11.Retief D.H., Denys F.R. Adhesion to enamel and dentin. Am J Dent. 1989;2:133–144. [PubMed] [Google Scholar]
- 12.Eick J.D. Smear layer—materials surface. Proc Finn Dent Soc. 1992;88(Suppl 1):225–242. [PubMed] [Google Scholar]
- 13.Nogawa H., Koizumi H., Saiki O., Hiraba H., Nakamura M., Matsumura H. Effect of a self-etching primer and phosphoric acid etching on the bond strength of 4-META/MMA-TBB resin to human enamel. Dent Mater J. 2015;34:219–226. doi: 10.4012/dmj.2014-227. [DOI] [PubMed] [Google Scholar]
- 14.Takagaki T., Nikaido T., Tsuchiya S., Ikeda M., Foxton R.M., Tagami J. Effect of hybridization on bond strength and adhesive interface after acid-base challenge using 4-META/MMA-TBB resin. Dent Mater J. 2009;28:185–193. doi: 10.4012/dmj.28.185. [DOI] [PubMed] [Google Scholar]
- 15.Pashley D.H., Tay F.R., Breschi L., Tjaderhane L., Carvalho R.M., Carrilho M., et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto M., Ohno H., Sano H., Kaga M., Oguchi H. In vitro degradation of resin–dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 17.Mazzoni A., Scaffa P., Carrilho M., Tjäderhane L., Di Lenarda R., Polimeni A., et al. Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J Dent Res. 2013;92:82–86. doi: 10.1177/0022034512467034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T., Takagaki T., Baba Y., Vicheva M., Matsui N., Hiraishi N., et al. Effects of different tooth conditioners on the bonding of universal self-etching adhesive to dentin. J Adhes Dent. 2019;21:77–85. doi: 10.3290/j.jad.a41917. [DOI] [PubMed] [Google Scholar]
- 19.Mancuso E., Comba A., Mazzitelli C., Maravic T., Josic U., Del Bianco F., et al. Bonding to dentin using an experimental zirconium oxynitrate etchant. J Dent. 2021;108 doi: 10.1016/j.jdent.2021.103641. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Spencer P. Effect of acid etching time and technique on interfacial characteristics of the adhesive–dentin bond using differential staining. Eur J Oral Sci. 2004;112:293–299. doi: 10.1111/j.1600-0722.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 21.Perdigão J., Lopes M. The effect of etching time on dentin demineralization. Quintessence Int. 2001;32:19–26. [Google Scholar]
- 22.Abu-Hanna A., Gordan V.V. Evaluation of etching time on dentin bond strength using single bottle bonding systems. J Adhes Dent. 2004;6:105–110. [PubMed] [Google Scholar]
- 23.Sardella T.N., de Castro F.L., Sanabe M.E., Hebling J. Shortening of primary dentin etching time and its implication on bond strength. J Dent. 2005;33:355–362. doi: 10.1016/j.jdent.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Takamizawa T., Barkmeier W.W., Tsujimoto A., Suzuki T., Scheidel D.D., Erickson R.L., et al. Influence of different pre-etching times on fatigue strength of self-etch adhesives to dentin. Eur J Oral Sci. 2016;124:210–218. doi: 10.1111/eos.12253. [DOI] [PubMed] [Google Scholar]
- 25.Van Meerbeek B., Yoshihara K., Yoshida Y., Mine A., De Munck J., Van Landuyt K. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Saikaew P., Senawongse P., Chowdhury A.A., Sano H., Harnirattisai C. Effect of smear layer and surface roughness on resin–dentin bond strength of self-etching adhesives. Dent Mater J. 2018 doi: 10.4012/dmj.2017-349. [DOI] [PubMed] [Google Scholar]
- 27.Sattabanasuk V., Vachiramon V., Qian F., Armstrong S.R. Resin–dentin bond strength as related to different surface preparation methods. J Dent. 2007;35:467–475. doi: 10.1016/j.jdent.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Tay F.R., Carvalho R., Sano H., Pashley D.H. Effect of smear layers on the bonding of a self-etching primer to dentin. J Adhes Dent. 2000;2:99–116. [PubMed] [Google Scholar]
- 29.Suyama Y., Luhrs A.K., De Munck J., Mine A., Poitevin A., Yamada T., et al. Potential smear layer interference with bonding of self-etching adhesives to dentin. J Adhes Dent. 2013;15:317–324. doi: 10.3290/j.jad.a29554. [DOI] [PubMed] [Google Scholar]
- 30.Inoue S., Koshiro K., Yoshida Y., De Munck J., Nagakane K., Suzuki K., et al. Hydrolytic stability of self-etch adhesives bonded to dentin. J Dent Res. 2005;84:1160–1164. doi: 10.1177/154405910508401213. [DOI] [PubMed] [Google Scholar]
- 31.Ermis R.B., De Munck J., Cardoso M.V., Coutinho E., Van Landuyt K.L., Poitevin A., et al. Bond strength of self-etch adhesives to dentin prepared with three different diamond burs. Dent Mater. 2008;24:978–985. doi: 10.1016/j.dental.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Inoue H., Inoue S., Uno S., Takahashi A., Koase K., Sano H. Microtensile bond strength of two single-step adhesive systems to bur-prepared dentin. J Adhes Dent. 2001;3:129–136. [PubMed] [Google Scholar]
- 33.Feitosa V.P., Sauro S., Ogliari F.A., Ogliari A.O., Yoshihara K., Zanchi C.H., et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent Mater. 2014;30:e317–e323. doi: 10.1016/j.dental.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Hass V., Abuna G., Pinheiro Feitosa V., Martini E.C., Sinhoreti M.A., Furtado Carvalho R., et al. Self-etching enamel bonding using cidic functional monomers with different-length carbon chains and hydrophilicity. J Adhes Dent. 2017;19:497–505. doi: 10.3290/j.jad.a39565. [DOI] [PubMed] [Google Scholar]
- 35.Kuraray Noriteke Dental. Safety data sheet: CLEARFIL TRI-S BOND PLUS. Available from: https://kuraraydental.com/wp-content/uploads/sds/chairside/usa/clearfil-s3-bond-plus-clearfil-tri-s-bond-plus-unit-dose-sds-usa.pdf.
- 36.Kuraray Noritake Dental. Safety dara sheet: Clearfil Universal Bond Quick. Available from: https://kuraraydental.com/wp-content/uploads/sds/chairside/usa/clearfil-universal-bond-quick-sds-usa.pdf.
- 37.Nagarkar S., Theis-Mahon N., Perdigão J. Universal dental adhesives: current status, laboratory testing, and clinical performance. J Biomed Mater Res B Appl Biomater. 2019;107:2121–2131. doi: 10.1002/jbm.b.34305. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong S., Breschi L., Ozcan M., Pfefferkorn F., Ferrari M., Van Meerbeek B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (mμTBS) approach. Dent Mater. 2017;33:133–143. doi: 10.1016/j.dental.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Visuri S.R., Gilbert J.L., Wright D.D., Wigdor H.A., Walsh J.T., Jr Shear strength of composite bonded to Er:YAG laser-prepared dentin. J Dent Res. 1996;75:599–605. doi: 10.1177/00220345960750011401. [DOI] [PubMed] [Google Scholar]
- 40.Aoki A., Ishikawa I., Yamada T., Otsuki M., Watanabe H., Tagami J., et al. Comparison between Er:YAG laser and conventional technique for root caries treatment in vitro. J Dent Res. 1998;77:1404–1414. doi: 10.1177/00220345980770060501. [DOI] [PubMed] [Google Scholar]
- 41.Dunn W.J., Davis J.T., Bush A.C. Shear bond strength and SEM evaluation of composite bonded to Er:YAG laser-prepared dentin and enamel. Dent Mater. 2005;21:616–624. doi: 10.1016/j.dental.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Ceballos L., Toledano M., Osorio R., Tay F.R., Marshall G.W. Bonding to Er-YAG-laser-treated dentin. J Dent Res. 2002;81:119–122. [PubMed] [Google Scholar]
- 43.Yazici A.R., Ozgünaltay G., Dayangaç B. A scanning electron microscopic study of different caries removal techniques on human dentin. Oper Dent. 2002;27:360–366. [PubMed] [Google Scholar]
- 44.Goldstein R.E., Parkins F.M. Air-abrasive technology: its new role in restorative dentistry. J Am Dent Assoc. 1994;125:551–557. doi: 10.14219/jada.archive.1994.0077. [DOI] [PubMed] [Google Scholar]
- 45.Laurell K.A., Hess J.A. Scanning electron micrographic effects of air-abrasion cavity preparation on human enamel and dentin. Quintessence Int. 1995;26:139–144. [PubMed] [Google Scholar]
- 46.Heikkinen T.T., Lassila L.V., Matinlinna J.P., Vallittu P.K. Effect of operating air pressure on tribochemical silica-coating. Acta Odontol Scand. 2007;65:241–248. doi: 10.1080/00016350701459753. [DOI] [PubMed] [Google Scholar]
- 47.Chaiyabutr Y., Kois J.C. The effects of tooth preparation cleansing protocols on the bond strength of self-adhesive resin luting cement to contaminated dentin. Oper Dent. 2008;33:556–563. doi: 10.2341/07-141. [DOI] [PubMed] [Google Scholar]
- 48.Ouchi H., Takamizawa T., Tsubota K., Tsujimoto A., Imai A., Barkmeier W.W., et al. The effects of aluminablasting on bond durability between universal adhesives and tooth substrate. Oper Dent. 2020;45:196–208. doi: 10.2341/18-170-L. [DOI] [PubMed] [Google Scholar]
- 49.Lima V.P., Soares K., Caldeira V.S., Faria E.S.A.L., Loomans B., Moraes R.R. Airborne-particle abrasion and dentin bonding: systematic review and meta-analysis. Oper Dent. 2021;46:E21–e33. doi: 10.2341/19-216-L. [DOI] [PubMed] [Google Scholar]
- 50.Rafique S., Fiske J., Banerjee A. Clinical trial of an air-abrasion/chemomechanical operative procedure for the restorative treatment of dental patients. Caries Res. 2003;37:360–364. doi: 10.1159/000072168. [DOI] [PubMed] [Google Scholar]
- 51.Tani C., Finger W.J. Effect of smear layer thickness on bond strength mediated by three all-in-one self-etching priming adhesives. J Adhes Dent. 2002;4:283–289. [PubMed] [Google Scholar]
- 52.Sano H., Chowdhury A.F.M.A., Saikaew P., Matsumoto M., Hoshika S., Yamauti M. The microtensile bond strength test: its historical background and application to bond testing. Jpn Dent Sci Rev. 2020;56:24–31. doi: 10.1016/j.jdsr.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogata M., Harada N., Yamaguchi S., Nakajima M., Pereira P.N., Tagami J. Effects of different burs on dentin bond strengths of self-etching primer bonding systems. Oper Dent. 2001;26:375–382. [PubMed] [Google Scholar]
- 54.Ogata M., Harada N., Yamaguchi S., Nakajima M., Tagami J. Effect of self-etching primer vs phosphoric acid etchant on bonding to bur-prepared dentin. Oper Dent. 2002;27:447–454. [PubMed] [Google Scholar]
- 55.Semeraro S., Mezzanzanica D., Spreafico D., Gagliani M., Re D., Tanaka T., et al. Effect of different bur grinding on the bond strength of self-etching adhesives. Oper Dent. 2006;31:317–323. doi: 10.2341/04-171. [DOI] [PubMed] [Google Scholar]
- 56.Saikaew P., Chowdhury A., Matsumoto M., Carvalho R.M., Sano H. Effects of double application of a resin cement primer and different diamond burs on cement-dentin bond strength. J Adhes Dent. 2020;22:311–320. doi: 10.3290/j.jad.a44554. [DOI] [PubMed] [Google Scholar]
- 57.Kenshima S., Reis A., Uceda-Gomez N., Tancredo Lde L., Filho L.E., Nogueira F.N., et al. Effect of smear layer thickness and pH of self-etching adhesive systems on the bond strength and gap formation to dentin. J Adhes Dent. 2005;7:117–126. [PubMed] [Google Scholar]
- 58.Mountouris G., Silikas N., Eliades G. Effect of sodium hypochlorite treatment on the molecular composition and morphology of human coronal dentin. J Adhes Dent. 2004;6:175–182. [PubMed] [Google Scholar]
- 59.Di Renzo M., Ellis T.H., Sacher E., Stangel I. A photoacoustic FTIRS study of the chemical modifications of human dentin surfaces: II. Deproteination. Biomaterials. 2001;22:793–797. doi: 10.1016/s0142-9612(00)00239-8. [DOI] [PubMed] [Google Scholar]
- 60.O’Connell M.S., Morgan L.A., Beeler W.J., Baumgartner J.C. A comparative study of smear layer removal using different salts of EDTA. J Endod. 2000;26:739–743. doi: 10.1097/00004770-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 61.Aktener B.O., Bilkay U. Smear layer removal with different concentrations of EDTA-ethylenediamine mixtures. J Endod. 1993;19:228–231. doi: 10.1016/S0099-2399(06)81296-3. [DOI] [PubMed] [Google Scholar]
- 62.Thanatvarakorn O., Nakajima M., Prasansuttiporn T., Ichinose S., Foxton R.M., Tagami J. Effect of smear layer deproteinizing on resin–dentine interface with self-etch adhesive. J Dent. 2014;42:298–304. doi: 10.1016/j.jdent.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Thanatvarakorn O., Prasansuttiporn T., Thittaweerat S., Foxton R.M., Ichinose S., Tagami J., et al. Smear layer-deproteinizing improves bonding of one-step self-etch adhesives to dentin. Dent Mater. 2018;34:434–441. doi: 10.1016/j.dental.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 64.Prasansuttiporn T., Nakajima M., Kunawarote S., Foxton R.M., Tagami J. Effect of reducing agents on bond strength to NaOCl-treated dentin. Dent Mater. 2011;27:229–234. doi: 10.1016/j.dental.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida E., Hashimoto M., Hori M., Kaga M., Sano H., Oguchi H. Deproteinizing effects on resin-tooth bond structures. J Biomed Mater Res B Appl Biomater. 2004;68:29–35. doi: 10.1002/jbm.b.10080. [DOI] [PubMed] [Google Scholar]
- 66.Mohammadi Z. Sodium hypochlorite in endodontics: an update review. Int Dent J. 2008;58:329–341. doi: 10.1111/j.1875-595x.2008.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 67.Ishizuka T., Kataoka H., Yoshioka T., Suda H., Iwasaki N., Takahashi H., et al. Effect of NaClO treatment on bonding to root canal dentin using a new evaluation method. Dent Mater J. 2001;20:24–33. doi: 10.4012/dmj.20.24. [DOI] [PubMed] [Google Scholar]
- 68.Nikaido T., Takano Y., Sasafuchi Y., Burrow M.F., Tagami J. Bond strengths to endodontically-treated teeth. Am J Dent. 1999;12:177–180. [PubMed] [Google Scholar]
- 69.Vongphan N., Senawongse P., Somsiri W., Harnirattisai C. Effects of sodium ascorbate on microtensile bond strength of total-etching adhesive system to NaOCl treated dentine. J Dent. 2005;33:689–695. doi: 10.1016/j.jdent.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Bendary E., Francis R.R., Ali H.M.G., Sarwat M.I., El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci. 2013;58:173–181. [Google Scholar]
- 71.Weston C.H., Ito S., Wadgaonkar B., Pashley D.H. Effects of time and concentration of sodium ascorbate on reversal of NaOCl-induced reduction in bond strengths. J Endod. 2007;33:879–881. doi: 10.1016/j.joen.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Prasansuttiporn T., Thanatvarakorn O., Tagami J., Foxton R.M., Nakajima M. Bonding durability of a self-etch adhesive to normal versus smear-layer deproteinized dentin: effect of a reducing agent and plant-extract antioxidant. J Adhes Dent. 2017;19:253–258. doi: 10.3290/j.jad.a38409. [DOI] [PubMed] [Google Scholar]
- 73.Blomlöf J., Blomlöf L., Lindskog S. Effect of different concentrations of EDTA on smear removal and collagen exposure in periodontitis-affected root surfaces. J Clin Periodontol. 1997;24:534–537. doi: 10.1111/j.1600-051x.1997.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 74.Brännström M., Nordenvall K.J., Glantz P.O. The effect of EDTA-containing surface-active solutions on the morphology of prepared dentin: an in vivo study. J Dent Res. 1980;59:1127–1131. doi: 10.1177/00220345800590070501. [DOI] [PubMed] [Google Scholar]
- 75.Berry E.A., 3rd, von der Lehr W.N., Herrin H.K. Dentin surface treatments for the removal of the smear layer: an SEM study. J Am Dent Assoc. 1987;115:65–67. doi: 10.14219/jada.archive.1987.0202. [DOI] [PubMed] [Google Scholar]
- 76.Calt S., Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002;28:17–19. doi: 10.1097/00004770-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Cehreli Z.C., Altay N. Etching effect of 17% EDTA and a non-rinse conditioner (NRC) on primary enamel and dentin. Am J Dent. 2000;13:64–68. [PubMed] [Google Scholar]
- 78.Spencer P., Wang Y., Walker M.P., Swafford J.R. Molecular structure of acid-etched dentin smear layers-in situ study. J Dent Res. 2001;80:1802–1807. doi: 10.1177/00220345010800090601. [DOI] [PubMed] [Google Scholar]
- 79.Kusunoki M., Itoh K., Oikawa M., Hisamitsu H. Measurement of shear bond strength to intact dentin. Dent Mater J. 2010;29:199–205. doi: 10.4012/dmj.2009-040. [DOI] [PubMed] [Google Scholar]
- 80.Osorio R., Erhardt M.C., Pimenta L.A., Osorio E., Toledano M. EDTA treatment improves resin-dentin bonds’ resistance to degradation. J Dent Res. 2005;84:736–740. doi: 10.1177/154405910508400810. [DOI] [PubMed] [Google Scholar]
- 81.Kim D.S., Park S.H., Choi G.W., Choi K.K., Kim S.Y. Effect of EDTA treatment on the hybrid layer durability in total-etch dentin adhesives. Dent Mater J. 2011;30:717–722. doi: 10.4012/dmj.2011-056. [DOI] [PubMed] [Google Scholar]
- 82.Chaves P., Giannini M., Ambrosano G.M. Influence of smear layer pretreatments on bond strength to dentin. J Adhes Dent. 2002;4:191–196. [PubMed] [Google Scholar]
- 83.Jacques P., Hebling J. Effect of dentin conditioners on the microtensile bond strength of a conventional and a self-etching primer adhesive system. Dent Mater. 2005;21:103–109. doi: 10.1016/j.dental.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Soares C.J., Castro C.G., Santos Filho P.C., da Mota A.S. Effect of previous treatments on bond strength of two self-etching adhesive systems to dental substrate. J Adhes Dent. 2007;9:291–296. [PubMed] [Google Scholar]
- 85.Saikaew P., Chowdhury A., Sattabanasuk V., Srimaneekarn N., Teanchai C., Carvalho R.M., et al. Bonding performance of self-etching adhesives to bur-cut dentin with active application mode. J Adhes Dent. 2021;23:357–365. doi: 10.3290/j.jad.b1645379. [DOI] [PubMed] [Google Scholar]
- 86.Irmak Ö, Yaman B.C., Orhan E.O., Ozer F., Blatz M.B. Effect of rubbing force magnitude on bond strength of universal adhesives applied in self-etch mode. Dent Mater J. 2018;37:139–145. doi: 10.4012/dmj.2017-018. [DOI] [PubMed] [Google Scholar]
- 87.Yoshihara K., Yoshida Y., Hayakawa S., Nagaoka N., Irie M., Ogawa T., et al. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011;7:3187–3195. doi: 10.1016/j.actbio.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 88.Loguercio A.D., Muñoz M.A., Luque-Martinez I., Hass V., Reis A., Perdigão J. Does active application of universal adhesives to enamel in self-etch mode improve their performance? J Dent. 2015;43:1060–1070. doi: 10.1016/j.jdent.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 89.Moritake N., Takamizawa T., Ishii R., Tsujimoto A., Barkmeier W., Latta M., et al. Effect of active application on bond durability of universal adhesives. Oper Dent. 2019;44:188–199. doi: 10.2341/17-384-L. [DOI] [PubMed] [Google Scholar]
- 90.Thanatvarakorn O., Prasansuttiporn T., Takahashi M., Thittaweerat S., Foxton R.M., Ichinose S., et al. Effect of scrubbing technique with mild self-etching adhesives on dentin bond strengths and nanoleakage expression. J Adhes Dent. 2016;18:197–204. doi: 10.3290/j.jad.a36033. [DOI] [PubMed] [Google Scholar]
- 91.Nakabayashi N., Saimi Y. Bonding to intact dentin. J Dent Res. 1996;75:1706–1715. doi: 10.1177/00220345960750091401. [DOI] [PubMed] [Google Scholar]
- 92.Niyomsujarit N., Senawongse P., Harnirattisai C. Bond strength of self-etching adhesives to dentin surface after smear layer removal with ultrasonic brushing. Dent Mater J. 2019;38:287–294. doi: 10.4012/dmj.2017-333. [DOI] [PubMed] [Google Scholar]
- 93.Bagis B., Turkaslan S., Vallittu P.K., Lassila L.V. Effect of high frequency ultrasonic agitation on the bond strength of self-etching adhesives. J Adhes Dent. 2009;11:369–374. [PubMed] [Google Scholar]
- 94.Saikaew P., Chowdhury A.F., Fukuyama M., Kakuda S., Carvalho R.M., Sano H. The effect of dentine surface preparation and reduced application time of adhesive on bonding strength. J Dent. 2016;47:63–70. doi: 10.1016/j.jdent.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Saikaew P., Matsumoto M., Chowdhury A., Carvalho R.M., Sano H. Does shortened application time affect long-term bond strength of universal adhesives to dentin? Oper Dent. 2018;43:549–558. doi: 10.2341/17-205-L. [DOI] [PubMed] [Google Scholar]
- 96.Huang X.Q., Pucci C.R., Luo T., Breschi L., Pashley D.H., Niu L.N., et al. No-waiting dentine self-etch concept-Merit or hype. J Dent. 2017;62:54–63. doi: 10.1016/j.jdent.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 97.GC. G-Premio Bond from GC. Available from: https://cdn.gceurope.com/v1/PID/gpremiobond/leaflet/LFL_G-Premio_BOND_en.pdf.
- 98.Kuraray Noriteke Dental. Safety data sheet: CLEARFIL Universal Bond Quick. Available from: https://kuraraydental.com/wp-content/uploads/2018/12/clearfil-universal-bond-quick-new-cap-brochure-sm.pdf.
- 99.Ahmed M.H., Yoshihara K., Mercelis B., Van Landuyt K., Peumans M., Van Meerbeek B. Quick bonding using a universal adhesive. Clin Oral Investig. 2020;24:2837–2851. doi: 10.1007/s00784-019-03149-8. [DOI] [PubMed] [Google Scholar]
- 100.Karadas M. Influence of reduced application time on bonding durability of universal adhesives to demineralized enamel. Clin Oral Investig. Available online 30 April 2021 from 10.1007/s00784-021-03972-y. [DOI] [PubMed]
- 101.Cardoso Pde C., Loguercio A.D., Vieira L.C., Baratieri L.N., Reis A. Effect of prolonged application times on resin-dentin bond strengths. J Adhes Dent. 2005;7:143–149. [PubMed] [Google Scholar]
- 102.Erhardt M.C., Osorio R., Pisani-Proenca J., Aguilera F.S., Osorio E., Breschi L., et al. Effect of double layering and prolonged application time on MTBS of water/ethanol-based self-etch adhesives to dentin. Oper Dent. 2009;34:571–577. doi: 10.2341/08-060-L. [DOI] [PubMed] [Google Scholar]
- 103.Frankenberger R., Perdigão J., Rosa B.T., Lopes M. "No-bottle" vs "multi-bottle" dentin adhesives—a microtensile bond strength and morphological study. Dent Mater. 2001;17:373–380. doi: 10.1016/s0109-5641(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 104.Chowdhury A.F.M.A., Saikaew P., Alam A., Sun J., Carvalho R.M., Sano H. Effects of double application of contemporary self-etch adhesives on their bonding performance to dentin with clinically relevant smear layers. J Adhes Dent. 2019;21:59–66. doi: 10.3290/j.jad.a41986. [DOI] [PubMed] [Google Scholar]







