Abstract
This paper aims to highlight the pharmacological aspects of listed herbal skincare products used for the treatment of various disorders caused due to ultraviolet radiation. The pharmacological aspects include safety and efficacy validation as per regulatory guidelines following internationally accepted scientific principles for their development of skincare products. Herbal products have always been used traditionally for the treatment of various skin ailments and have become more prevalent because of their safety and high efficacy benefits. The incorporation of synthetic molecules and chemical substances in the different medicinal and pharmaceutical formulations is the leading cause of the dermal toxicity. Therefore, the developments of herbal skincare products containing scientifically validated herbal ingredients have better acceptance, respect, and belief in the society. The listed herbal products in this review can help take forward the commercial development of skincare products for therapeutic as well as beauty care purposes from such plants.
Keywords: Ultraviolet radiation, Skin disorders, Traditional plants, Herbal products, Skincare products
Highlights
-
•
Reviewed studies related to skin beneficial effects of herbals against UV radiation–induced skin disorders.
-
•
Includes more than 100 publications on pharmacological information and experimental models used in skin biology.
-
•
Review summarizes signalling pathways involved in skin photodamage and role of traditionally used herbal products.
-
•
Discusses the serious side effects of inorganic filters based commercially available sunscreens.
-
•
The skin care products can be developed from these herbal product/s for therapeutic/beauty care purposes.
1. Introduction
The human skin plays a significant role in physiological functions of sensation, protection, thermoregulation, defense system, and metabolic mechanisms to help in maintaining homeostasis [1,2]. It is mostly exposed to different environmental factors such as harmful radiation, toxic chemicals, and pathogens [3]. The ultraviolet (UV) portion of sunlight is responsible for various skin disorders [4]. Continuous exposure to UVB leads to various adverse effects on the skin [5]. The increase in awareness about the photoaging and carcinogenic effects of UV radiation (UVR) resulted in tremendous increase in the demand for herbal skincare products. The most frequently used inorganic filters in the market possess titanium dioxide (TiO2) and zinc oxide (ZnO). These UV filters are suggested to be the most frequent cause of dermal toxicity and contact allergy, which is caused by a wide range of chemicals present in sunscreens [6]. The herbal products exhibit various therapeutic properties and have always been used for centuries in the treatment of many skin disorders [7]. Several herbal products have been uncovered for their therapeutic potential and are gaining considerable attention in the market as skincare products [8]. Internationally, numerous studies have been conducted on many herbal products to reveal their therapeutic potential via various in vivo and in vitro models. However, future long-term studies and new approaches are required in this area [9].
2. Methodology
The literature published between 2003 and 2018 was searched on Pubmed and Google Scholar databases and as included in this review. The main keywords used were “skin”, “ultraviolet radiation”, “UVB”, “skin disorders”, “photodamage”, “photoaging”, “skin cancer”, “natural products”, “herbal products”, “photo-protection”, “sunscreens” and “skincare products”. The present review summarizes the published data over a hundred scientifically validated herbal products that include their biological and pharmacological information and various experimental models used to study skin photodamage.
3. Ultraviolet radiation (UVR)
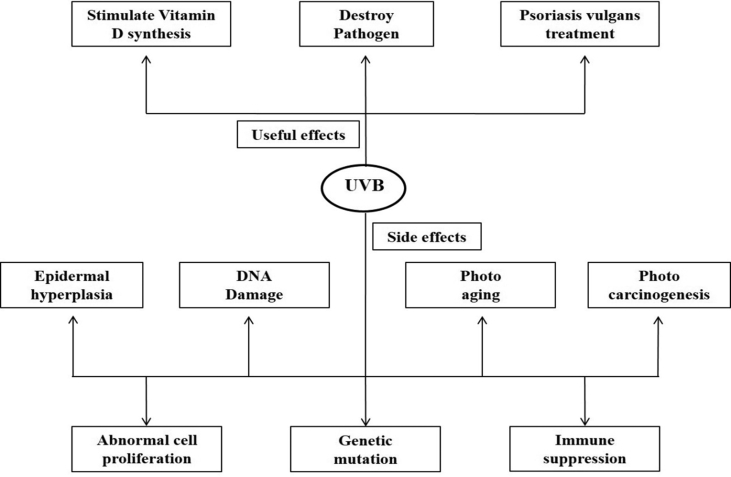
Ultraviolet radiation is divided into three subtypes as UVA, UVB, and UVC as based on wavelengths and biological effects which are accountable for various skin disorders [4]. UVA has wavelengths between 320 and 400 nm and termed as long-wave radiation is absorbed by the deep dermis. UVB, also known as mid-UV, has wavelengths between 290 and 320 nm and is mostly consumed or dissipated within the epidermis and is significantly responsible for skin photodamage. UVB is a complete mutagen and more efficient than UVA in contributing most of the hazardous effects associated with sun exposure [10,11]. UVC, known as shorter UV, has wavelengths between 200 and 290 nm and is also absorbed within the epidermis and has germicidal property [12]. UVR can be both beneficial and harmful to human skin. The extent of UVR reaching into the earth's surface depends on latitude, altitude, season, daytime, cloudiness, and the ozone layer. The beneficial effects include Vitamin D production, killing different pathogens, and treating certain skin diseases like Psoriasis vulgaris and vitiligo. The damaging effects mostly include degradation of the extracellular matrix and all the significant risk factors associated with sunlight include sunburn, tanning, photoaging, and skin cancer development [[13], [14], [15]] (Fig. 1).
Fig. 1.
Effects of UVR on the skin [12].
4. Environmental skin disorders
Photodamage is a type of skin disorder thatarises from the exposure of the skin to solar light or UVR which includes erythema, edema, hypermelanogenesis, oxidative stress, inflammation, immunosuppression, and further development of cutaneous malignancies.
4.1. Erythema (sunburn)
Erythema is an acute inflammatory response to continuous UVR exposure. The severity of erythema depends upon the rate and extent of skin exposure to UVR [16].
4.2. Hypermelanogenesis
UVR exposure to the skin may result in immediate pigmentation or delayed pigmentation response. UVR exposure to the skin elevates melanin synthesis, which increases number and activity of melanocytes, leading to the deposition of melanin granules in the upper layers of the epidermis [16].
4.3. UVR -induced epigenetic alterations
Epigenetics is the modification in gene expression, function, and generation of a heritable phenotype irrespective of change in DNA sequence. UVR-induced histone acetylation contributes to the UVR transcriptional response [17]. Epigenetic alterations are reversible, and therefore dietary food or bioactive compounds may have the ability to reverse, inhibit or delay the epigenetic modifications for the prevention of the disease [18].
4.4. UVR–induced microRNAs regulationec
MicroRNAs (miRNAs) are small non-coding RNAs which act as a regulator in post-transcriptional modifications. Recent studies show that miRNAs have been explored as a therapeutic target against UVR-induced cellular responses [19]. The diversity of miRNA targets determines their involvement in various cellular networks. miRNAs have shown to regulate many biological processes such as cell differentiation, proliferation, and apoptosis [20].
4.5. UVR-induced DNA damage and repair
Reactive oxygen species (ROS) generated by UVR irradiation can indirectly damage DNA bases which lead to dimerization and is considered as the biomarker of oxidative DNA damage [21]. The injury persists beyond repair leading to cell death by induction of the apoptosis pathway. If the DNA damage continues in the S-phase of the cell cycle, there is an increase in proliferation, which ultimately leads to skin carcinogenesis [22].
4.6. Immunological responses
UV radiation also affects the immune system and its exposure results in the generation of T-suppressor cells. UVR passes on suppressive effects on many immune parameters. The decrease in cellular immunity is responsible for various pathogens and loss of immune surveillance against tumors [23].
4.7. Photoaging
Aging is a natural and complicated process of progressive deterioration in the functioning of all organs in the body, including the skin. Intrinsic aging is a genetic phenomenon that occurs with time [24,25]. The onset of aging and its severity is exacerbated by exposure of skin to environmental factors. UVR is the most harmful factor which induces aging-like skin changes termed photoaging. Intrinsic photoaging is a time-dependent phenomenon that mainly depends on the extent of sun exposure and skin color [26,27]. Photoaging has more profound effects than an intrinsic one, accounting for about 90% of all the visible aging changes [28].
4.8. Photocarcinogenesis
Photocarcinogenesis is the unlimited growth of cells in the skin and is categorized into basal cell carcinomas (BCC), squamous cells carcinoma (SCC) (both specifically called non-melanocytic carcinomas), and cutaneous malignant melanomas. Cancer prevailing in basal layers of the epidermis is called basal cell carcinoma. It grows slowly and rarely spreads to other parts. SCC occurs in the middle layer of the epidermis and is considered more aggressive than BCC. Melanomas arise in the melanocytes; the cells are involved in melanin production and are responsible for 75% of all skin-related deaths. The survey from the past several years has found that there has been an increase in the prevalence of skin cancer. It has been assessed that approximately 1 in 5 Americans will develop skin cancer. It has been surveyed that over 2–3 million new cases of non-melanoma skin cancer (NMSC) appear per year globally, the highest rate among the Caucasian population [[29], [30], [31]].
Various natural products with their detailed molecular mechanism have been explored for their cellular photoprotective effects against UVR by utilizing different experimental models. Various studies revealed that topical application of almond oil has been found capable of preventing the structural damage caused by UV irradiation [32]. The inhibitory effect of encapsulated curcumin on UV-induced photoaging in mice has been examined [33]. Amla (Emblica officinalis) has been studied for its protective effect against UVB-induced photoaging [34]. Curcumin prevented UVR-induced transcriptional response in human keratinocytes [17]. A recent study showed the decreased expression of miRNAs in epigallocatechin gallate-mediated UVB protection in human dermal fibroblasts [35]. Glycyrrhizic acid prevented the oxidative stress-mediated DNA damage response through modulation of autophagy in UVB-irradiated human primary dermal fibroblasts [36].
5. Molecular signaling pathways induced by UVR
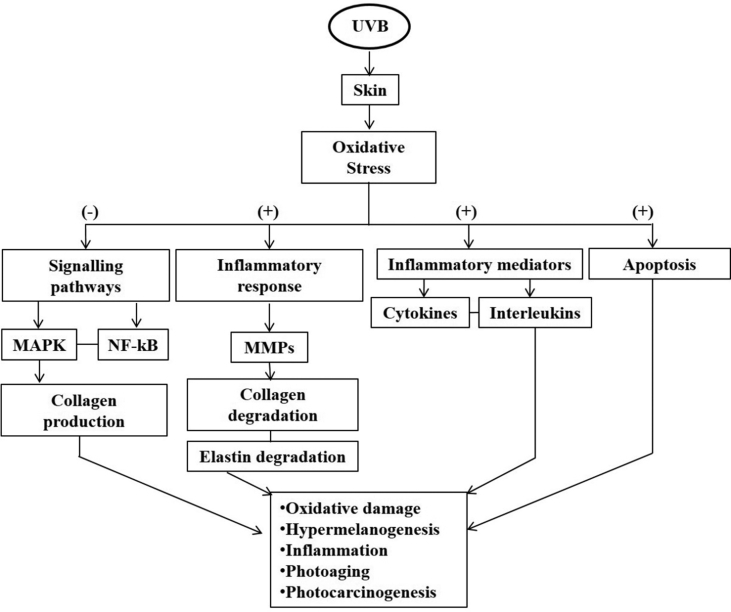
UVR exposure stimulates the production of ROS which overwhelms the defense mechanism of the skin, making endogenous cytoprotectants insufficient and causes alterations in inflammatory mediators, vascularity, and infiltration of inflammatory cells [7]. ROS stimulates various intracellular signaling pathways including mitogen-activated protein kinases (MAPK) and alters primarily the mitochondrial pathway, leading to cell death [37,38]. The generation of ROS by UVR acts as a mediator to activate several signaling cascades for various cellular responses. Therefore, the inhibition of ROS-induced signaling pathways can help to protect skin cells from UVR [39,40]. Moreover, the altered expression of various components (MMPs, cytokines) leads to various pathophysiological conditions. This will provide an ideal framework for more specific targets for the assessment of risk factors, diagnostic biomarkers, and effective therapeutic alternatives for studying various molecular signalling pathways induced by UVR [41] (Fig. 2).
Fig. 2.
Mechanism of UVB induced skin photodamage and central pathways affected by chronic exposure of the skin to UVB radiation [40].
6. Traditionally used plants for skin disorders
Plant-derived products have been used for medicinal purposes for centuries and the earliest records from 2900–2600 BC document the use of approximately 1000 plants, such as oil of Cedrus species (cedar), Commiphora myrrha (myrrh), Cupressus sempervirens (cypress), Glycyrrhiza glabra (Liquorice), and Papaver somniferum (opium) [42]. Various natural phytoingredients (curcumin, silymarin, ginkgo biloba) have been exploited for their cellular protective effects with the detailed mechanisms in different experimental models [41]. Herbal products from the plant's sources for cosmetic or skin pathological states have always been popular because of several advantages such as fewer side-effects, better patient tolerance, being cheaper and acceptable due to the long history of use. These include aloe vera, grapevine, ginseng, green tea, tea tree oil, rosemary, lemon, soybean, papaya, garlic, ginkgo, olive oil, and Ocimum [43] (Table 1).
Table 1.
Represents traditionally used plants for various skin disorders.
| S. No. | Plant binomial name | Common name | Family | Therapeutic use | Reference |
|---|---|---|---|---|---|
| 1. | Aloe barbadensis | Aloe vera | Asphodelaceae | Cuts, burns and eczema | [44] |
| 2. | Azadirachta indica | Neem | Meliaceae | Immuno-modulator, anti-inflammatory, anti-malarial, anti-fungal, anti-bacterial, anti-viral, anti-oxidant, anti-mutagenic and anti-carcinogenic. | [45] |
| 3. | Carica papaya L. | Papaya | Caricaceae | Eczema, dermatitis and psoriasis | [46] |
| 4. | Curcuma longa | Turmeric | Zingiberaceae | Scleroderma and anti-inflammatory | [47] |
| 5. | Emblica officinalis | Amla | Phyllanthaceae | Anti-aging and skin disorders | [48] |
| 6. | Gingko biloba | Gingko | Ginkgoaceae | Wound healing | [49] |
| 7. | Glycyrrhiza glabra | Liquorice | Fabaceae | Anti-inflammatory, anti-microbial, anti-oxidative, anti-cancer and immune-modulator | [50] |
| 8. | Ocimum sanctum | Tulsi | Labiatae | Anti-microbial, immune-modulator, anti-stress and anti-inflammatory | [51] |
| 9. | Psoralea corylifolia | Babchi | Leguminosae | Psoriasis, leukoderma, and leprosy | [52] |
| 10. | Santalum album L. | Sandalwood | Santalaceae | Acne, eczema, atopic dermatitis, psoriasis, anti-inflammatory, anti-microbial and anti-proliferative | [41] |
7. Pros and cons of commercially available sunscreens
Currently, cosmaceutics, wherein biocative ingredients are used, are more preferred over synthetic molecules or chemical substances mainly due to safety issues in long-term treatment, which leads to dermal toxicity. The increase in awareness about the carcinogenic and photoaging effects of UVR and socio-economic upliftment of the society has resulted in a tremendous increase in the demand of herbal skincare products, especially sunscreen products by the society. The sunscreens mostly employ inorganic UV filters and show protection against solar radiation, which involves physical phenomena, i.e., scattering and reflection of UVR. The most frequently used inorganic filters are titanium dioxide (TiO2) and zinc oxide (ZnO) which reflects the UV light, and tend to be opaque and white on the skin, and consequently is unacceptable for cosmetic use. The other oxides used are alumina, ceria, and zirconia. These oxides are formulated as nanoparticles, which decreases the reflection of visible radiation and, thereby, produces less white coloration when applied to the skin, and confers additional beneficial and efficacious photoprotection. According to Balogh et al., although sunscreens report several advantages, they also have numerous safety challenges that need to be overcome [53,54].
These inorganic UV filters are suggested to be the most frequent cause of dermal toxicity, such as photo-contact allergy which is caused by a wide range of synthetic molecules or chemical substances present in sunscreens [6]. The photo-toxicity of TiO2 nanoparticles has been a severe concern [55]. Benzophenone-3 is one of the major causes of allergic reactions, especially contact dermatitis. The research on the use of herbal products is steadily increasing to overcome the occurrence of harmful effects associated with sunscreen products [56].
The use of safe cosmetic agents is essential for the prevention and treatment of UVR-induced skin disorders. There is a need to develop new herbal formulations that are safe and efficient. The history of the use of herbal products for the treatment of various dermatological agents for skincare purposes is as old as human civilization. Therefore, there is a wide scope for the development of new herbal formulations based on herbal ingredients which are efficient and scientifically validated in various in vivo and in vitro systemss.
8. Herbal skincare products as a therapeutic alternative
Herbal skincare products are now-a-days more preferred and chosen by people because of their fewer side-effects and cost-effectiveness [57]. Traditionally, herbal products are applied to a particular area or a part of the skin for the treatment of various skin ailments. The topical formulations can be classified into creams, gels, ointments, lotions, and foams [58,59].
The herbal products exhibit various therapeutic properties such as anti-oxidant, anti-inflammatory, anti-viral, cardio-protective, neuroprotective, and hepato-protective properties [7]. Many plants-derived natural molecules such as alkaloids, flavonoids, isoflavones, proanthocyanidins, phenolic compounds, and essential oils are associated with the treatment of many dermatological disorders. Several herbal products have been uncovered for their therapeutic potential against UV irradiation and are gaining considerable attention [8]. The photoprotective properties of such herbal products regarding safety and efficacy need to be validated as per the regulatory guidelines which follows internationally accepted scientific principles. It is requisite for the development of such herbal skincare products possessing strong therapeutic potential for the treatment of UVB-induced skin disorders and a better quality of life [60]. The combination of different herbal products may result in their synergistic effect. This will further help in pre-clinical and clinical investigations in the area of dermatology.
9. Scientific investigation of herbal products against UVR-induced skin disorders
UVR has many deleterious effects on human skin. Among these results, photoaging and skin cancer are of great concern [56]. Due to changes in lifestyle and awareness about the dangers of the UVR, there has been a significant upsurge in research in the area of the use of herbal products for the prevention and treatment of UVR-induced skin disorders. There is a need to understand novel therapeutic targets which are involved in the progression of UVR–induced skin disorders [61]. These herbal products are mostly rich in polyphenols, flavonoids, and have a noticeable activity that can impede the skin from the deleterious effects of UVR. These herbal products should be incorporated for the development of herbal skincare products [62]. Internationally, many herbal products have been studied which had shown novel therapeutic potential for the prevention and treatment of UVR-induced skin disorders (Refer to supplementary table 2).
However, scanty reports are available on the skin beneficial effects of herbal products on skin photodamage, photoaging, and photo-carcinogenesis. These findings help in understanding the fact that there is a tremendous need to study various herbal products for their skin beneficial effects. There is a need for the development of herbal formulation based on scientifically validated herbal products for therapeutic as well as beautification purposes.
10. Discussion
Skin is the largest organ and continuous exposure to UVR leads to different pathological and dermatological conditions. UVR acts as a mediator to activate several signaling cascades for various cellular responses. The increase in consciousness about the carcinogenic and photoaging effects of UVR and socio-economic improvement of the society has resulted in the increased demand for herbal skincare products, especially sunscreen products. Currently, cosmaceutics, are more preferred over synthetic molecule or chemical substances due to safety issues related to dermal toxicity. The research on the use of herbal products is steadily increasing to overcome the occurrence of harmful effects associated with sunscreen products using inorganic filters. Herbal products have always been used traditionally for the treatment of various skin ailments. There is a need to develop new herbal formulations that are safe and efficient. The listed herbal products in this paper have shown skin beneficial effects against UVR-induced skin disorders in various experimental models which recapitulate the same in humans. It is requisite for the development of such herbal skincare products possessing strong therapeutic potential for the treatment of UVB-induced skin disorders and a better quality of life. A lot has been done in the last decade for the safety and efficacy of herbal products for discussing more active natural products for skincare.
11. Conclusion
This review provides the summarized information of listed herbal skincare products used for the treatment of various disorders caused due to UVR. These products were scientifically validated as per the regulatory guidelines which follow internationally accepted scientific principles. The data of these listed herbal products including biological, pharmacological information, and experimental models have been summarized and transformed into tabular forms. These herbal products can be taken forward for formulation and development purposes as skincare products for therapeutic as well as beauty care uses.
Source(s) of funding
Department of Science and Technology (DST), New Delhi For Senior Research Fellowship to RRS (IF180691) and Council of Scientific and Industrial Research (CSIR), New Delhi For Research Grant (HCP-007) are acknowledged for financial assistance.
Conflict of interest
None.
Author contributions
RRS: Contributed to the literature review, writing of MS, and its formatting.
AD: Contributed to the writing of MS.
STA: Contributed to the design of MS, Literature review, Writing, Editing, and Formatting of the MS.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2021.07.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lai-Cheong J.E., McGrath J.A. Structure and function of skin, hair and nails. Medicine. 2009;37(5):223–226. [Google Scholar]
- 2.Wysocki A., Mustoe T., Schultz G. Molecular cell biology of skin. Rev Cell Biol Mol Med. 2006:217–250. [Google Scholar]
- 3.Celleno L., Tamburi F. Structure and function of the skin. Nutr Cosmetics: Beauty from Within. 2009:3–45. [Google Scholar]
- 4.Venus M., Waterman J., McNab I. Basic physiology of the skin. Surgery. 2010;29(10):471–474. [Google Scholar]
- 5.Im A.R., Song J.H., Lee M.Y., Chae S. Magnolol reduces UVB-induced photodamage by regulating matrix metalloproteinase activity. Environ Toxicol Pharmacol. 2015;39(1):417–423. doi: 10.1016/j.etap.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson I., Hillerstro¨m L., Stenfeldt A.-L., Mårtensson J., Bo¨rje A. Photodegradation of dibenzoylmethanes: potential cause of photocontact allergy to sunscreens. Chem Res Toxicol. 2009;22(11):1881–1892. doi: 10.1021/tx900284e. [DOI] [PubMed] [Google Scholar]
- 7.Afnan Q., Kaiser P.J., Rafiq R.A., Nazir L.A., Bhushan S., Bhardwaj S.C., et al. Glycyrrhizic acid prevents ultraviolet-B-induced photodamage: a role for mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp Dermatol. 2016;25(6):440–446. doi: 10.1111/exd.12964. [DOI] [PubMed] [Google Scholar]
- 8.Afaq F., K Katiyar S. Polyphenols: skin photoprotection and inhibition of photocarcinogenesis. Mini Rev Med Chem. 2011;11(14):1200–1215. doi: 10.2174/13895575111091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katiyar S.K., Matsui M.S., Elmets C.A., Mukhtar H. Polyphenolic antioxidant (-)-Epigallocatechin-3-Gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69(2):148–153. [PubMed] [Google Scholar]
- 10.Ramachandran S., Prasad N.R., Karthikeyan S. Sesamol inhibits UVB-induced ROS generation and subsequent oxidative damage in cultured human skin dermal fibroblasts. Arch Dermatol Res. 2010;302(10):733–744. doi: 10.1007/s00403-010-1072-1. [DOI] [PubMed] [Google Scholar]
- 11.Johnson S., Frogley J. 2010. Brighter than sunshine; p. 2010. [Google Scholar]
- 12.Kaidbey K.H., Kligman A.M. The acute effects of long-wave ultraviolet radiation on human skin. J Invest Dermatol. 1979;72(5):253–256. doi: 10.1111/1523-1747.ep12531710. [DOI] [PubMed] [Google Scholar]
- 13.Korać R.R., Khambholja K.M. Potential of herbs in skin protection from ultraviolet radiation. Phcog Rev. 2011;5(10):164. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nghiem D.X., Kazimi N., Clydesdale G., Ananthaswamy H.N., Kripke M.L., Ullrich S.E. Ultraviolet A radiation suppresses an established immune response: implications for sunscreen design. J Invest Dermatol. 2001;117(5):1193–1199. doi: 10.1046/j.0022-202x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura Y., Ananthaswamy H.N. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expet Rev Mol Med. 2002;4(26):1–22. doi: 10.1017/S146239940200532X. [DOI] [PubMed] [Google Scholar]
- 16.Hönigsmann H. Erythema and pigmentation. Photodermatol Photoimmunol Photomed. 2002;18(2):75–81. doi: 10.1034/j.1600-0781.2002.180204.x. [DOI] [PubMed] [Google Scholar]
- 17.Pollack B.P., Sapkota B., Boss J.M. Ultraviolet radiation-induced transcription is associated with gene-specific histone acetylation. Photochem Photobiol. 2009;85(3):652–662. doi: 10.1111/j.1751-1097.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 18.Katiyar S.K., Singh T., Prasad R., Sun Q., Vaid M. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: interaction of bioactive dietary components on epigenetic targets. Photochem Photobiol. 2012;88(5):1066–1074. doi: 10.1111/j.1751-1097.2011.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.N Syed D., Imran Khan M., Shabbir M., Mukhtar H. MicroRNAs in skin response to UV radiation. Curr Drug Targets. 2013;14(10):1128–1134. doi: 10.2174/13894501113149990184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee D., Shin C. MicroRNA–target interactions: new insights from genome-wide approaches. Ann N Y Acad Sci. 2012;1271(1):118. doi: 10.1111/j.1749-6632.2012.06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadet J., Douki T., Pouget J.-P., Ravanat J.-L. Singlet oxygen DNA damage products: formation and measurement. Methods Enzymol. 1999;319:143–153. doi: 10.1016/s0076-6879(00)19016-0. [DOI] [PubMed] [Google Scholar]
- 22.Trautinger F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin Exp Dermatol. 2001;26(7):573–577. doi: 10.1046/j.1365-2230.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- 23.Simon J., Tigelaar R., Bergstresser P., Edelbaum D., Cruz P. Ultraviolet B radiation converts Langerhans cells from immunogenic to tolerogenic antigen-presenting cells. Induction of specific clonal anergy in CD4+ T helper 1 cells. J Immunol. 1991;146(2):485–491. [PubMed] [Google Scholar]
- 24.Gilchrest B.A. CRC Press; 1984. Skin and aging processes. [Google Scholar]
- 25.Langton A., Sherratt M., Griffiths C., Watson R. Review Article: a new wrinkle on old skin: the role of elastic fibres in skin ageing. Int J Cosmet Sci. 2010;32(5):330–339. doi: 10.1111/j.1468-2494.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- 26.Thurstan S.A., Gibbs N.K., Langton A.K., Griffiths C.E., Watson R.E., Sherratt M.J. Chemical consequences of cutaneous photoageing. Chem Cent J. 2012;6(34):273–274. doi: 10.1186/1752-153X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandel R., Poljšak B., Godic A., Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013 doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herschthal J., Kaufman J. 2007. Cutaneous aging: a review of the process and topical therapies. [Google Scholar]
- 29.Diepgen T., Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(s61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 30.Simões M., Sousa J., Pais A. Skin cancer and new treatment perspectives: a review. Cancer Lett. 2015;357(1):8–42. doi: 10.1016/j.canlet.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 31.De Gruijl F. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35(14):2003–2009. doi: 10.1016/s0959-8049(99)00283-x. [DOI] [PubMed] [Google Scholar]
- 32.Sultana Y., Kohli K., Athar M., Khar R., Aqil M. Effect of pre-treatment of almond oil on ultraviolet B–induced cutaneous photoaging in mice. J Cosmet Dermatol. 2007;6(1):14–19. doi: 10.1111/j.1473-2165.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal R., Kaur I.P. Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice. Rejuvenation Res. 2010;13(4):397–410. doi: 10.1089/rej.2009.0906. [DOI] [PubMed] [Google Scholar]
- 34.Adil M.D., Kaiser P., Satti N.K., Zargar A.M., Vishwakarma R.A., Tasduq S.A. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. J Ethnopharmacol. 2010;132(1):109–114. doi: 10.1016/j.jep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 35.An I.-S., An S., Park S., Lee S.N., Bae S. Involvement of microRNAs in epigallocatechin gallate-mediated UVB protection in human dermal fibroblasts. Oncol Rep. 2013;29(1):253–259. doi: 10.3892/or.2012.2083. [DOI] [PubMed] [Google Scholar]
- 36.Umar S.A., Tanveer M.A., Nazir L.A., Divya G., Vishwakarma R.A., Tasduq S.A. Glycyrrhizic acid prevents oxidative stress mediated DNA damage response through modulation of autophagy in ultraviolet-B-irradiated human primary dermal fibroblasts. Cell Physiol Biochem. 2019;53(1):242–257. doi: 10.33594/000000133. [DOI] [PubMed] [Google Scholar]
- 37.Afaq F., Ahmad N., Mukhtar H. Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene. 2003;22(58):9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- 38.Assefa Z., Van Laethem A., Garmyn M., Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta Rev Cancer. 2005;1755(2):90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Kang S., Chung J.H., Lee J.H., Fisher G.J., Wan Y.S., Duell E.A., et al. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol. 2003;120(5):835–841. doi: 10.1046/j.1523-1747.2003.12122.x. [DOI] [PubMed] [Google Scholar]
- 40.Cooper S., Bowden G. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets. 2007;7(4):325. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad A., Ahmad R. Understanding the mechanism of hepatic fibrosis and potential therapeutic approaches. Saudi J Gastroenterol: Off J Saudi Gastroenterol Assoc. 2012;18(3):155. doi: 10.4103/1319-3767.96445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borchardt J.K. The beginnings of drug therapy: ancient mesopotamian medicine. Drug News Perspect. 2002;15(3):187–192. doi: 10.1358/dnp.2002.15.3.840015. [DOI] [PubMed] [Google Scholar]
- 43.Pazyar N., Yaghoobi R., Rafiee E., Mehrabian A., Feily A. Skin wound healing and phytomedicine: a review. Skin Pharmacol Physiol. 2014;27(6):303–310. doi: 10.1159/000357477. [DOI] [PubMed] [Google Scholar]
- 44.Rajeswari R., Umadevi M., Rahale C.S., Pushpa R., Selvavenkadesh S., Kumar K.S., et al. Aloe vera: the miracle plant its medicinal and traditional uses in India. J Pharmacogn Phytochem. 2012;1(4):118–124. [Google Scholar]
- 45.Subapriya R., Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anti Cancer Agents. 2005;5(2):149–156. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen T.T., Shaw P.N., Parat M.O., Hewavitharana A.K. Anticancer activity of C arica papaya: a review. Mol Nutr Food Res. 2013;57(1):153–164. doi: 10.1002/mnfr.201200388. [DOI] [PubMed] [Google Scholar]
- 47.Thangapazham R.L., Sharma A., Maheshwari R.K. The molecular targets and therapeutic uses of curcumin in health and disease. Springer; 2007. Beneficial role of curcumin in skin diseases; pp. 343–357. [DOI] [PubMed] [Google Scholar]
- 48.Kumar K.S., Bhowmik D., Dutta A., Yadav A.P., Paswan S., Srivastava S., et al. Recent trends in potential traditional Indian herbs Emblica officinalis and its medicinal importance. J Pharmacogn Phytochem. 2012;1(1):18–28. [Google Scholar]
- 49.Bairy K., Rao C. Wound healing profiles of Ginkgo biloba. J Nat Remedies. 2001;1(1):25–27. [Google Scholar]
- 50.Anilkumar D., Joshi H., Nishteswar K. Review of glycyrrhiza glabra (yastimadhu)-a broad spectrum herbal drug. Pharma Sci Monit. 2012;3(4) [Google Scholar]
- 51.Mondal S., Mirdha B.R., Mahapatra S.C. The science behind sacredness of Tulsi (Ocimum sanctum Linn.) Indian J Physiol Pharmacol. 2009;53(4):291–306. [PubMed] [Google Scholar]
- 52.Khushboo P., Jadhav V., Kadam V., Sathe N. Psoralea corylifolia Linn.—“Kushtanashini”. Phcog Rev. 2010;4(7):69. doi: 10.4103/0973-7847.65331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manaia E.B., Kaminski R.C.K., Corrêa M.A., Chiavacci L.A. Inorganic UV filters. Braz J Pharmaceut Sci. 2013;49(2):201–209. [Google Scholar]
- 54.Afaq F., Adhami V.M., Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res Fund Mol Mech Mutagen. 2005;571(1–2):153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Horie M., Sugino S., Kato H., Tabei Y., Nakamura A., Yoshida Y. Does photocatalytic activity of TiO2 nanoparticles correspond to photo-cytotoxicity? Cellular uptake of TiO2 nanoparticles is important in their photo-cytotoxicity. Toxicol Mech Methods. 2016;26(4):284–294. doi: 10.1080/15376516.2016.1175530. [DOI] [PubMed] [Google Scholar]
- 56.Lautenschlager S., Wulf H.C., Pittelkow M.R. Photoprotection. Lancet. 2007;370(9586):528–537. doi: 10.1016/S0140-6736(07)60638-2. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee P.K., Maity N., Nema N.K., Sarkar B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19(1):64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Jamadar M.J., Shaikh R.H. Preparation and evaluation of herbal gel formulation. J Pharmaceut Edu Res. 2017;1(2):201–224. [Google Scholar]
- 59.Mishra U., Murthy P.N., Pasa G., Nayak R.K. Formulation and evaluation of herbal gel containing methanolic extract of ziziphus xylopyrus. Asian J Biochem Pharmaceut Res. 2011;4(1) [Google Scholar]
- 60.Kraft J.N., Lynde C.W. Moisturizers: what they are and a practical approach to product selection. Skin Therapy Lett. 2005;10(5):1–8. [PubMed] [Google Scholar]
- 61.Datta H.S., Paramesh R. Trends in aging and skin care: ayurvedic concepts. J Ayurveda Integr Med. 2010;1(2):110. doi: 10.4103/0975-9476.65081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinnell S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48(1):1–22. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.