Abstract
Background:
Whether changes in blood pressure (BP) over women’s midlife are more driven by chronological aging or the menopause transition (MT) has been debated. We sought to determine whether women can be classified into distinct trajectory groups based on pattern and level of systolic (SBP), diastolic blood pressure, pulse pressure (PP), and mean arterial pressure (MAP) over the MT, and to assess whether menopause-related factors predict the group and/or level of BP measures.
Methods:
Participants were from the Study of Women’s Health Across the Nation (SWAN). Group-based trajectory modeling was used to identify women who shared distinct BP trajectories over time relative to menopause onset and to assess associations of menopause-related factors with trajectory group and/or level of BP measures. An accelerated rise relative to menopause onset suggests a menopause contribution.
Results:
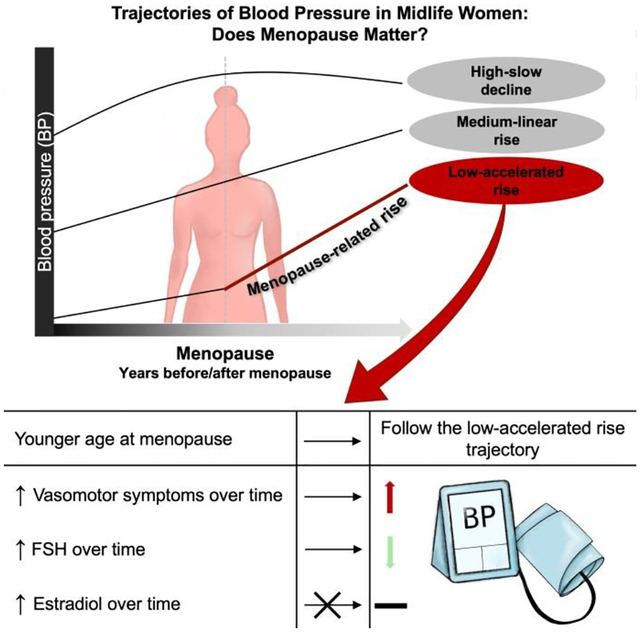
The study included 3,302 multi-racial/ethnic women with BP measures over 17 follow-up visits (baseline age[SD]: 46.3[2.7]). Women were classified into either low, medium, or high trajectory group in each BP measure. The low SBP, PP, and MAP trajectories (in 35%, 53%, and 28% of the cohort, respectively) were rising slowly before menopause but showed a significant accelerated rise 1 year after menopause, indicating a menopause contribution. The remaining BP trajectories were rising up until menopause and either continued with the same rise or declined after menopause. A younger menopause age predicted the low SBP, PP, and MAP trajectories. A greater follicle-stimulating hormone level predicted lower SBP and PP levels, while vasomotor symptoms occurrence predicted higher SBP, PP, and MAP levels over time. Estradiol did not predict trajectory or level of any BP measure.
Conclusions:
Distinct BP trajectories over the MT exist that revealed a group of women whose SBP, PP, and MAP trajectories are consistent with a menopause contribution. Our findings support frequent monitoring of BP during the MT.
Subject Terms: High Blood Pressure; Women, Sex, and Gender
Graphical Abstract
INTRODUCTION
Hypertension is an important modifiable risk factor for premature cardiovascular disease (CVD), yet 43% of adult women have hypertension.1,2 Blood pressure (BP) increases with age in both sexes, but women’s BP surpasses that of men after midlife.3 This phenomenon has generated interest in investigating the role the menopause transition (MT) may play in BP over midlife.4
After controlling for chronological aging, previous cross-sectional studies on the association between the MT and BP were inconclusive and findings were inconsistent.4 Conflicting findings may have resulted from heterogeneity among the studied populations.4 In fact, in White women of normal weight, postmenopausal women had a higher BP compared with their premenopausal counterparts.5,6 However, a similar association was not observed in overweight White women.7,8 In contrast, studies on overweight Asian populations tend to show an association between the MT and BP,9,10 while BP was similar across MT stages in studies of normal weight Asian women.11,12 Longitudinal studies of multi-ethnic populations mainly found a linear trajectory of BP over the MT with no obvious inflection at menopause, consistent with aging effect.13–15 However, the literature thus far has described changes over the MT assuming that women share a common BP trajectory. With existing racial/ethnic differences in hypertension and obesity in midlife,1 BP in women may have diverse and distinct patterns over the MT. The previous conflicting findings suggest that BP is possibly vulnerable to menopause or menopause-related factors only in women with certain characteristics.
Declines in estradiol, increases in follicle-stimulating hormone (FSH), and the occurrence of vasomotor symptoms are the hallmarks of the MT.16,17 It has been hypothesized that these menopause-related factors and age at menopause may influence BP.10,18–20 However, associations between these menopause-related factors and BP trajectories over the MT have not been evaluated thoroughly. Additionally, studies of the associations between the MT and BP measures mainly focused on systolic (SBP) but not diastolic blood pressure (DBP), pulse pressure (PP), or mean arterial pressure (MAP).4 The Study of Women’s Health across the Nation (SWAN) is a cohort study with prospective and comprehensive assessment of the MT and concurrent measurements of the associated physical and hormonal changes. Using data from SWAN, we sought to determine whether women can be classified into distinct trajectory groups based on pattern and level of SBP, DBP, PP, and MAP over time relative to the final menstrual period (FMP). A trajectory with an accelerated rise within one year of the FMP suggests a menopause contribution. We additionally sought to determine whether age at menopause, and time-varying estradiol, FSH, and vasomotor symptoms predicted the trajectory pattern and/or level of BP.
METHODS
Data Availability.
All data and materials have been made publicly available at the National Institute on Aging and can be accessed at https://agingresearchbiobank.nia.nih.gov.
Study population
SWAN is an ongoing multi-ethnic, multi-site longitudinal study designed to examine physical, biological, and psychosocial changes in women during midlife. Detailed design and methods were presented elsewhere.21 In brief, 3,302 women were recruited between 1996 and 1997 from seven sites (Detroit, MI; Boston, MA; Chicago, IL; Oakland, CA; Los Angeles, CA; Newark, NJ; and Pittsburgh, PA). To enroll in SWAN, women had to be aged 42–52 years, have an intact uterus and at least one ovary with menstrual bleeding within the past three months, not be pregnant or breast-feeding, and not have used hormone therapy in the past three months. Women self-identified as a member of one of five racial/ethnic groups: White (all sites), Black (Boston, Chicago, Detroit, Pittsburgh), Chinese/Chinese American (Oakland), Hispanic (Newark) or Japanese/Japanese American (Los Angeles). The institutional review board at each site approved the study protocol, and all participants signed informed consent before participation. Women in the current study were followed for up to 17 visits. Data from all SWAN women (n=3,302) were included in this analysis.
Time anchored to FMP.
At each visit, women provided the date of their most recent menstrual period which enabled retrospectively assigning the final menstrual period (FMP) date as the date of the participant’s last menstrual period before 12 consecutive months of amenorrhea. However, we did not observe the FMP in 1346 (41%) women due to hormone therapy or hysterectomy. Because SWAN is a study of the menopause transition and since hormone therapy and hysterectomy are widely used treatments in the midlife women population, FMP dates for all participants is essential to characterizing menopause-related changes and to generalizing results to women represented by SWAN. Therefore, SWAN multiply imputed the unobserved FMP dates using a wealth of demographic and longitudinal characteristics known in the literature to influence age at the FMP. Our method of imputation and analysis accounted for the uncertainty in the imputation by incorporating within and between imputation variability in the estimates.22 In addition to increased sample size and precision, the advantages of reducing bias by including both women with observed and imputed FMP dates outweigh errors due to imputing the data.22 A fuller description of the imputation process is in the Supplemental Materials.
Blood pressure measurements.
BP measurements were obtained at all visits except the 14th follow-up visit. Women had not smoked or consumed any caffeinated beverage within 30 minutes of obtaining BP measurements. BP measurements were taken after at least 5 minutes of rest using the appropriate cuff size based on arm circumference. The participants were seated with feet flat on the floor (legs uncrossed) and refraining from talking during the measurements. A standard mercury sphygmomanometer was used to record SBP and DBP at the first and fifth phase Korotkoff sounds, respectively. Two sequential values were recorded with a 2-minute period between measurements. The average of the two measures was used in this analysis. To account for the effect of BP medications on BP values in visits when women reported using anti-hypertensive medications, we added 10 and 5 mmHg to the observed SBP and DBP values, respectively.3,23 PP, a BP measure reflecting arterial compliance, was calculated as: PP= SBP-DBP. MAP measures the average arterial pressure throughout one cardiac cycle and was calculated as: MAP= DBP+(PP/3). Measures of BP were treated as time-varying variables in analyses.
Blood assays.
To allow for a standardized hormonal milieu, a fasting blood sample was drawn during the early follicular phase of the menstrual cycle (day 2–5) if women were still menstruating. If a timed sample could not be obtained, because menstrual cycles became less regular over time or due to menopause, a random fasting sample was taken within 90-days of the corresponding visit. All samples were maintained at 4°C until separated and then were frozen at −80°C and shipped in dry ice to the central certified laboratory (Medical Research Laboratories, Highland Heights, Kentucky).
Endogenous sex hormones were measured at the University of Michigan Endocrine Laboratory using the Automated Chemilumisence System −180 automated analyzer (Bayer Diagnostics Corp., Norwood, MA). Estradiol was measured using a modified, off-line Automated Chemilumisence System: 180 (E2–6) at the baseline visit, the 1st–10th, 12th, and 13th follow-up visits. The lower limit of detection (LLD) was between 1 and 7 pg/mL. The inter- and intra-assay coefficients of variation were 10.6% and 6.4%, respectively.24 FSH was measured by a modification of a manual assay kit (Bayer Diagnostics) utilizing two monoclonal antibodies directed to different regions on the beta subunit at the baseline visit, the 1st–10th, 12th, 13th, and 15th follow-up visits. The LLD was between 0.4–1.0 mIU/mL. The inter-and intra-assay coefficients of variation were 11.4% and 3.8%, respectively.24 Since estradiol and FSH show dynamic changes throughout the menstrual cycle, cycle-day of blood draw (within 2–5 days of the menstrual cycle or not) was considered when analyzing these hormones. Estradiol and FSH were treated as time varying variables in analyses.
Total cholesterol was analyzed using enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN), and high-density lipoprotein cholesterol was isolated using heparin-manganese. Low-density lipoprotein cholesterol (LDL-C) was calculated using Friedewald equation.25 LDL-C was obtained at baseline, 1st, 3rd–7th, 9th, 12th,13th, and 15th follow-up visits and was treated as a time varying variable in analyses.
Study covariates.
At each visit, participants completed self and interviewer-administered questionnaires that included assessment of sociodemographic and life-style factors and medical history. Age, race/ethnicity, education, income, financial strain, smoking status, alcohol consumption, presence of hot flashes or night sweats, hormone therapy, use of anti-diabetic medications, and steroids were self-reported. BP medications were self-reported and interviewer-verified from medication container labels. Body mass index (BMI) was calculated as weight/height2. Hot flashes and night sweats were measured at all visits except the 16th follow-up visit. Women reporting either hot flashes or night sweats were considered as having vasomotor symptoms. A detailed description of diabetes definition is presented in the Supplemental Materials. Vasomotor symptoms and diabetes were treated as time-varying variables. Unless specified, other study covariates were obtained from the baseline visit. Please see the Major Resources Table in the Supplemental Materials.
Statistical analysis.
To disentangle the MT contribution to BP from chronological aging, time in years was anchored to the FMP.14 Data points 13 years before and 15 years after the FMP were dropped from the analysis due to a small number of observations at these time points. We used group-based trajectory modeling (GBTM) to detect distinct trajectories of SBP, DBP, PP, and MAP over the MT, separately. GBTM uses finite mixture modeling to identify distinct groups that share common developmental trajectories while accounting for classification uncertainty.26 To allow for flexibility in estimating the shapes of BP trajectories, we tested linear, quadratic, cubic, and quartic terms of time and retained the highest term, when significant, with the corresponding lower terms.26 We determined the number of groups based upon Bayesian Information Criterion, reasonable scientific plausibility, and whether model with an additional group identified further trajectory features. For simplicity in building this classification model, no covariate was included at this stage of the analysis. Model fit statistics are presented in the Supplemental Materials. For women with imputed FMP dates, we used the mean of the multiply imputed FMP dates for the GBTM.
Within each SBP, DBP, PP, and MAP trajectory group identified via GBTM, a piecewise-linear pattern with an inflection point and an accelerated rise within one year of the FMP was suggestive of a menopause contribution. Potential inflection points around the FMP were identified via plotting repeated measures of SBP, DBP, PP, and MAP in each trajectory group using locally weighted scatterplot smoothing (LOWESS). A piecewise-linear random coefficients model was then used to test the year of inflection initially identified via LOWESS. If no significant inflection point was identified, a linear model was utilized. The random coefficients model adjusted for the fixed effects of age at menopause, race/ethnicity, study site, and baseline BMI. Established techniques of analyzing multiply imputed data were used in the random coefficients model.22
We explored predictive associations of menopause-related factors with BP trajectories that showed a significant accelerated rise within one year of the FMP. Using a single GBTM with the same specification (number of respective BP groups and terms of time), we used multinomial logistic and multiple linear regression to estimate the odds ratio of trajectory group membership for baseline variables and beta coefficient of BP level for time-varying covariates, respectively. This model adjusted for race/ethnicity, study site, and baseline BMI, and time-varying hormone therapy, LDL-C, and diabetes. Menopause-related factors hypothesized to predict membership in a BP trajectory group were age at menopause (categorized into: <51 years, ≥51 and ≤53 years, and >53 years) and baseline estradiol, FSH, and vasomotor symptoms occurrence. Menopause-related factors hypothesized to predict BP measurement levels within each trajectory group were time-varying estradiol, FSH, and vasomotor symptoms occurrence. When we ran the model that explored predictive associations of menopause-related factors with PP trajectories, estimates of race were unstable due to small cell count and, therefore, we combined Chinese, Hispanic, and Japanese into “Other”.
We compared baseline characteristics between women with observed and imputed FMP dates and conducted sensitivity analyses restricted to women with an observed FMP. We also ran models without adding 10 and 5 mmHg to the observed SBP and DBP values, respectively, when women reported using anti-hypertensive medications. Additionally, as sensitivity analyses, we adjusted the classification and the random coefficients model for baseline age and respective BP measure. All analyses were performed with SAS 9.4 (SAS Institute, Cary NC) with a significance level set at 0.05.
RESULTS
Distinct blood pressure trajectories.
Using 32,967 observations of data spanning 28 years across the MT with a median follow-up of 19.1 years, women were classified into three distinct trajectory groups in SBP, DBP, PP, and MAP (figure 1).
Figure 1. Trajectories of blood pressure over the menopause transition (n=3302).
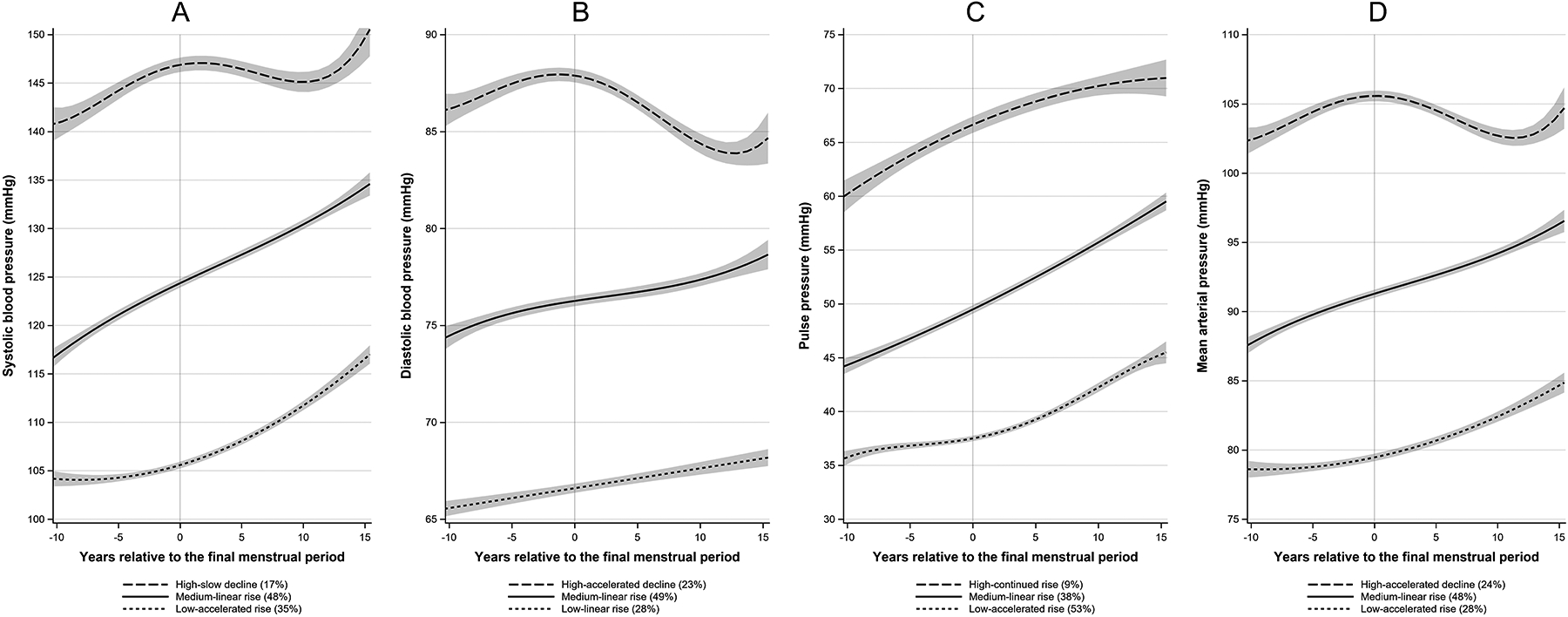
Blood pressure represents the predicted mean at each time point within each trajectory group from group-based trajectory modelling. No factors were included in the model. Bands represent 95% CI. Panel A: systolic blood pressure; Panel B: diastolic blood pressure; Panel C: pulse pressure; Panel D: mean arterial pressure.
The low-accelerated rise SBP, PP, and MAP trajectories (in 35%, 53%, and 28% of the cohort, respectively) were rising slowly before the FMP but showed an accelerated rise 1 year after the FMP. The low-linear rise DBP trajectory (28%), all the medium-linear rise trajectories (SBP: 48%, DBP: 49%, PP: 38%, and MAP: 48%), and the high-continued rise PP trajectory (9%) showed a monotonic rise over the MT. The high-slow decline SBP (17%) and the high-accelerated decline DBP (23%) and MAP (24%) trajectories were rising before and showed a slow and an accelerated decline after the FMP, respectively.
White, Chinese, and Japanese women were more likely to follow the low-accelerated rise SBP, PP and MAP trajectories. Hispanic women were more likely to follow the medium-linear rise SBP, PP, and MAP trajectories. Black women were more likely to follow the high-slow decline SBP, the high-continued rise PP, and the high-accelerated decline MAP trajectories. Women in the low-accelerated rise SBP, PP, and MAP trajectories had a borderline normal weight with a BMI of 24.6, 25.9, and 24.6 kg/m2, respectively. Table 1 and tables S1–S3 show baseline characteristics by SBP, DBP, PP, and MAP trajectory groups, respectively.
Table 1.
Baseline characteristics of study participants by SBP trajectory group
| Variable* | Total, n=3302 | Low-accelerated rise 1172 (35%) |
Medium-linear rise 1574 (48%) |
High-slow decline 556 (17%) |
P-value† |
|---|---|---|---|---|---|
| Age (years) | 46.34 ± 2.69 | 46.04 ± 2.60 | 46.39 ± 2.67 | 46.82 ± 2.84 | 6.67×10−8 |
| Race/ethnicity, N (%) | 1.00×10−30 | ||||
| White | 1551 (47.0) | 709 (60.5) | 710 (45.1) | 132 (23.7) | |
| Black | 934 (28.3) | 150 (12.8) | 450 (28.6) | 334 (60.1) | |
| Chinese | 250 (7.6) | 134 (11.4) | 96 (6.1) | 20 (3.6) | |
| Hispanic | 286 (8.7) | 25 (2.1) | 209 (13.3) | 52 (9.4) | |
| Japanese | 281 (8.5) | 154 (13.1) | 109 (6.9) | 18 (3.2) | |
| Education, N (%) | 2.60×10−29 | ||||
| Less than high school | 1551 (47.0) | 709 (60.5) | 710 (45.1) | 132 (23.7) | |
| Some College | 934 (28.3) | 150 (12.8) | 450 (28.6) | 334 (60.1) | |
| College Degree or higher | 250 (7.6) | 134 (11.4) | 96 (6.1) | 20 (3.6) | |
| Income, N (%) | 6.34×10−27 | ||||
| <$35,000 | 1004 (31.3) | 239 (20.9) | 522 (34.1) | 243 (45.1) | |
| $35,000-$75,000 | 1308 (40.7) | 491 (43.0) | 615 (40.1) | 202 (37.5) | |
| >$75,000 | 900 (28.0) | 411 (36.0) | 395 (25.8) | 94 (17.4) | |
| Financial Strain, N (%) | 1312 (40.0) | 356 (30.5) | 669 (42.9) | 287 (51.8) | 2.00×10−18 |
| Smoking Status, N (%) | 2.82×10−8 | ||||
| Never | 1890 (57.3) | 702 (60.0) | 907 (57.6) | 281 (50.5) | |
| Past | 830 (25.2) | 309 (26.4) | 391 (24.8) | 130 (23.4) | |
| Current | 580 (17.6) | 159 (13.6) | 276 (17.5) | 145 (26.1) | |
| Alcohol consumption (drinks per week), N (%) | 0.00049 | ||||
| None | 1553 (49.6) | 524 (47.1) | 727 (48.4) | 302 (58.1) | |
| <1 drinks | 326 (10.4) | 106 (9.5) | 172 (11.5) | 48 (9.2) | |
| 1–7 drinks | 813 (25.9) | 304 (27.3) | 401 (26.7) | 108 (20.8) | |
| >7 drinks | 441 (14.1) | 178 (16.0) | 201 (13.4) | 62 (11.9) | |
| Systolic blood pressure (mmHg) | 117.85 ± 17.03 | 104.72 ± 8.49 | 119.37 ± 10.96 | 141.22 ± 17.73 | 1.00×10−30 |
| Diastolic blood pressure (mmHg) | 75.48 ± 10.52 | 68.89 ± 7.63 | 76.87 ± 8.77 | 85.38 ± 11.07 | 1.00×10−30 |
| Pulse pressure (mmHg) | 42.38 ± 12.07 | 35.83 ± 7.23 | 42.50 ± 9.68 | 55.82 ± 14.79 | 1.00×10−30 |
| Mean arterial pressure (mmHg) | 89.60 ± 11.75 | 80.84 ± 7.15 | 91.03 ± 8.40 | 103.99 ± 11.75 | 1.00×10−30 |
| Blood pressure medication, N (%) | 471 (14.3) | 29 (2.5) | 184 (11.7) | 258 (46.4) | 1.00×10−30 |
| Body mass index (kg/m2) | 28.26 ± 7.21 | 24.63 ± 4.92 | 29.24 ± 6.94 | 33.24 ± 8.15 | 1.00×10−30 |
| Diabetes, N (%) | 152 (4.6) | 10 (0.9) | 74 (4.7) | 68 (12.2) | 7.07×10−25 |
| Low-density lipoprotein cholesterol (mg/dl) | 116.11 ± 31.01 | 111.47 ± 29.10 | 118.35 ± 30.76 | 119.81 ± 34.41 | 2.10×10−9 |
| Age at FMP (years) | 52.15 ± 2.89 | 51.88 ± 2.71 | 52.34 ± 2.95 | 52.18 ± 3.05 | 0.00024 |
| Vasomotor symptoms, N (%) | 1287 (39.2) | 377 (32.4) | 631 (40.3) | 279 (50.5) | 4.18×10−12 |
| Estradiol (pg/mL), Median (Q1, Q3) | 55.2 (33.0, 88.7) | 57.6 (34.2, 89.6) | 55.2 (32.8, 88.4) | 50.3 (31.8, 85.2) | 0.15 |
| Follicle-stimulating hormone (mIU/mL), Median (Q1, Q3) | 15.9 (10.8, 26.4) | 16.2 (10.9, 27.2) | 15.7 (10.7, 26.1) | 16.2 (10.9, 25.5) | 0.23 |
| Hormone therapy, N (%) | 6 (0.2) | 1 (0.1) | 4 (0.3) | 1 (0.2) | 0.59 |
Mean ± SD is presented unless otherwise specified
Analysis of variance (ANOVA) was used to compare means for all continuous normally distributed variables. Kruskal-Wallis test was used to compare medians for estradiol and follicle-stimulating hormone. Chi-square test was used to compare frequency distributions for categorical variables
BP group-specific pattern: linear vs piecewise-linear change.
The low-accelerated rise SBP, PP, and MAP trajectory groups showed a piecewise-linear pattern with a significant inflection point and an accelerated rise 1 year after the FMP, consistent with an MT contribution (table 2). However, the other two SBP, PP, and MAP trajectories and the three DBP trajectories were not MT-related as they showed a linear pattern or a piecewise-linear pattern with a decline close to the FMP.
Table 2.
Annual Changes in Blood Pressure measures (mmHg) in Time Segments, by Trajectory Groups, (n=3302) *
| Blood pressure measure | Trajectory Group | n | Inflection point | Change in blood pressure (95% CI) | P-Value for difference | |
|---|---|---|---|---|---|---|
| Before inflection | After inflection | |||||
| Systolic | High-slow decline | 556 | FMP | 0.50 (0.24, 0.76) | −0.12 (−0.32, 0.08) | 0.0010 |
| Medium-linear rise | 1574 | N/A | 0.67 (0.63, 0.72) | N/A | ||
| Low-accelerated rise | 1172 | 1 year after FMP | 0.23 (0.16, 0.29) | 0.77 (0.71, 0.84) | 5.43×10−24 | |
| Diastolic | High-accelerated decline | 770 | FMP | 0.10 (−0.02, 0.21) | −0.35 (−0.42, −0.27) | 4.97×10−8 |
| Medium-linear rise | 1613 | N/A | 0.13 (0.10, 0.16) | N/A | ||
| Low-linear rise | 919 | N/A | 0.13 (0.10, 0.16) | N/A | ||
| Pulse pressure | High-continued rise | 303 | N/A | 0.34 (0.18, 0.50) | N/A | |
| Medium-linear rise | 1250 | N/A | 0.59 (0.55, 0.63) | N/A | ||
| Low-accelerated rise | 1749 | 1 year after FMP | 0.22 (0.17, 0.26) | 0.54 (0.49, 0.58) | 1.36×10−17 | |
| Mean arterial pressure | High-accelerated decline | 781 | FMP | 0.24 (0.10, 0.38) | −0.25 (−0.34, −0.16) | 5.35×10−7 |
| Medium-linear rise | 1593 | N/A | 0.32 (0.29, 0.35) | N/A | ||
| Low-accelerated rise | 928 | 1 year after FMP | 0.12 (0.06, 0.17) | 0.39 (0.34, 0.43) | 3.08×10−10 | |
Models adjusted for age at the FMP, site, race/ethnicity, and SWAN baseline body-mass index
Menopause-related predictors of SBP, PP, and MAP trajectories.
Relative to the medium-linear rise group (aging-related trajectory) and women with age at menopause between 51 and 53 years (a range encompassing the mean of 52.15 years), women with a younger age at menopause and White women were more likely to follow the low-accelerated rise SBP, PP, and MAP trajectories (table 3, table S4, and table 4). Vasomotor symptoms occurrence predicted a higher BP level over time within the low-accelerated rise and medium-linear rise SBP and PP trajectories and in all MAP trajectories. Greater FSH predicted a lower BP level over time within the medium-linear rise SBP and in all PP trajectories. Baseline or time-varying estradiol did not predict BP trajectory groups or levels, respectively.
Table 3.
Predictors of SBP trajectory group membership and level across the menopause transition, (n=3302) *
| Variables‡ | SBP trajectory group, n (%) | ||
|---|---|---|---|
| Low-accelerated rise 1172 (35%) |
Medium-linear rise 1574 (48%) |
High-slow decline 556 (17%) |
|
| Predictors of SBP trajectory group membership, OR (95% CI) § | |||
| Race/ethnicity | |||
| White | 1 [ref] | [ref] | 1 [ref] |
| Black | 0.30 (0.22, 0.42) | [ref] | 3.08 (2.28, 4.17) |
| Hispanic | 0.29 (0.08, 1.13) | [ref] | 3.33 (1.11, 10.01) |
| Japanese | 0.44 (0.27, 0.70) | [ref] | 1.63 (0.69, 3.86) |
| Chinese | 0.37 (0.23, 0.61) | [ref] | 2.84 (1.03, 7.84) |
| Baseline BMI (per 1-SD increase) | 0.33 (0.28, 0.39) | [ref] | 1.50 (1.33, 1.70) |
| Age at menopause | |||
| Before 51 years | 1.73 (1.31, 2.28) | [ref] | 1.06 (0.78, 1.45) |
| Between 51 and 53 years | 1 [ref] | [ref] | 1 [ref] |
| After 53 years | 0.75 (0.58, 0.98) | [ref] | 1.34 (1.00, 1.80) |
| Baseline estradiol† (per 1-SD increase) | 1.01 (0.89, 1.14) | [ref] | 1.11 (0.96, 1.28) |
| Baseline FSH† (per 1-SD increase) | 1.00 (0.87, 1.14) | [ref] | 1.10 (0.95, 1.26) |
| Baseline vasomotor symptoms | 0.80 (0.64, 1.00) | [ref] | 1.21 (0.95, 1.54) |
| Predictors of SBP level over time within each trajectory group, beta (SE) § | |||
| Estradiol† (per 1-SD increase) | 0.11 (0.12) | −0.12 (0.13) | 0.14 (0.23) |
| FSH† (per 1-SD increase) | −0.16 (0.10) | −0.39 (0.12) | −0.06 (0.22) |
| Vasomotor symptoms | 0.92 (0.30) | 1.17 (0.30) | 0.96 (0.52) |
| Hormone therapy use | 0.65 (0.43) | 0.57 (0.42) | −1.57 (0.84) |
| LDL-C (per 1-SD increase) | 0.39 (0.16) | 0.30 (0.16) | 0.42 (0.24) |
| Diabetes | 4.93 (0.93) | 5.36 (0.56) | 4.92 (0.80) |
Bold indicates P-value <0.05. Actual p-values are provided in table S12
Log-transformed
Model further adjusted for site and cycle-day of blood draw. There were 107 (3%) women excluded from this model due to missing covariate values (BMI [42], baseline estradiol [8], baseline FSH [1], baseline vasomotor symptoms [18], LDL-C [38])
Estimates of SBP trajectory group membership and level are from the same model
LDL-C: low-density lipoprotein cholesterol, SBP: systolic blood pressure
Table 4.
Predictors of MAP trajectory group membership and level across the menopause transition, (n=3302) *
| Variables‡ | MAP trajectory group, n (%) | ||
|---|---|---|---|
| Low-accelerated rise 928 (28%) |
Medium-linear rise 1593 (48%) |
High-accelerated decline 781 (24%) |
|
| Predictors of MAP trajectory group membership, OR (95% CI) § | |||
| Race/ethnicity | |||
| White | 1 [ref] | [ref] | 1 [ref] |
| Black | 0.36 (0.25, 0.50) | [ref] | 3.26 (2.44, 4.36) |
| Hispanic | 0.28 (0.05, 1.66) | [ref] | 2.99 (1.29, 6.91) |
| Japanese | 0.52 (0.33, 0.83) | [ref] | 2.06 (0.97, 4.38) |
| Chinese | 0.50 (0.31, 0.81) | [ref] | 5.23 (2.11, 12.95) |
| Baseline BMI (per 1-SD increase) | 0.36 (0.31, 0.43) | [ref] | 1.40 (1.25, 1.57) |
| Age at menopause | |||
| Before 51 years | 1.37 (1.04, 1.80) | [ref] | 0.91 (0.68, 1.21) |
| Between 51 and 53 years | 1 [ref] | [ref] | 1 [ref] |
| After 53 years | 0.36 (0.25, 0.50) | [ref] | 1.10 (0.84, 1.43) |
| Baseline estradiol† (per 1-SD increase) | 0.28 (0.05, 1.66) | [ref] | 1.07 (0.94, 1.21) |
| Baseline FSH† (per 1-SD increase) | 0.52 (0.33, 0.83) | [ref] | 0.97 (0.85, 1.11) |
| Baseline vasomotor symptoms | 0.50 (0.31, 0.81) | [ref] | 1.17 (0.94, 1.47) |
| Predictors of MAP level over time within each trajectory group, beta (SE) § | |||
| Estradiol† (per 1-SD increase) | 0.03 (0.09) | −0.04 (0.09) | 0.13 (0.14) |
| FSH† (per 1-SD increase) | −0.04 (0.07) | −0.09 (0.08) | 0.08 (0.13) |
| Vasomotor symptoms | 0.86 (0.22) | 0.84 (0.20) | 1.29 (0.31) |
| Hormone therapy use | 0.63 (0.31) | 0.13 (0.29) | −1.15 (0.48) |
| LDL-C (per 1-SD increase) | 0.35 (0.11) | 0.44 (0.10) | 0.49 (0.15) |
| Diabetes | 3.85 (0.57) | 2.70 (0.39) | 2.41 (0.49) |
Bold indicates P-value <0.05. Actual p-values are provided in table S12
Log-transformed
Model further adjusted for site and cycle-day of blood draw. There were 107 (3%) women excluded from this model due to missing covariate values (BMI [42], baseline estradiol [8], baseline FSH [1], baseline vasomotor symptoms [18], LDL-C [38])
Estimates of pulse pressure trajectory group membership and level are from the same model
LDL-C: low-density lipoprotein cholesterol, MAP: mean arterial pressure
Sensitivity analyses.
Women with imputed and observed FMP showed small differences in baseline demographic variables, smoking and BP variables (table S5). Analyses restricted to women with observed FMP resulted in similar conclusions (figure S1 and tables S6–S8). Not adding 10 and 5 mmHg to the observed SBP and DBP values, respectively, when women reported using anti-hypertensive medications resulted in similar overall conclusions (figure S2 and tables S9–S11). Our classification and random coefficients models showed comparable findings after adjusting for baseline age and respective BP measure (data not shown).
DISCUSSION
Using data from SWAN, one of the largest and longest study-to-date of the menopause transition in the US, we identified three distinct groups of women based on their common trajectories of SBP, DBP, PP, and MAP. We identified a group of women, the low-accelerated rise SBP, PP, and MAP trajectory group, whose trajectory showed a piecewise-linear increase with an inflection point 1 year after the FMP consistent with an MT increase. These findings are in line with previous studies of the MT that showed progressive decrements in arterial compliance between the pre- and the peri-menopausal periods and accelerated increases in arterial stiffness 1 year before the FMP.27,28 In women who followed a linear trajectory, however, BP was mainly consistent with a chronological aging effect. Menopause-related factors showed significant associations with BP trajectories, such that age at menopause before 51 years predicted the low-accelerated rise BP trajectories, greater FSH predicted a lower SBP and PP levels, and vasomotor symptoms occurrence predicted a higher SBP, PP, and MAP levels during the MT. Contrary to previous studies that found a linear BP trajectory over the MT consistent with aging effect, the current findings suggest that women cluster in different BP trajectories that differ in level, pattern, and relationship with menopause and its related factors. Our findings highlight the period of the MT when SBP, PP, and MAP are likely to show accelerated increases. Therefore, frequent and timely monitoring of CVD risk factors in women transitioning through menopause represents a window for counselling about lifestyle changes.29,30
Although the low-accelerated rise SBP, PP, and MAP trajectories are consistent with a menopause contribution, that does not directly indicate that women following these trajectories are at high risk of elevated SBP or MAP or PP widening complications. However, previous studies have shown a graded log-linear association between SBP and CVD death down to a SBP of 115 mmHg without a clear threshold.31 Additionally, among healthy midlife men and women without previous histories of hypertension or other traditional CVD risk factors, the risk of incident CVD showed a stepwise increase with increasing SBP starting as low as 90 mmHg.32 Interestingly, sex-specific analysis of 27,542 community-based participants (54% women) showed that SBP of 100 to 109 mmHg relative to SBP <100 mmHg was associated with a greater CVD risk in women (hazard ratio 1.25) but not men, in whom the same risk was seen at SBP 130 to 139 mmHg.33 The increased CVD risk associated with SBP increases also holds across a broad age spectrum, starting as young as 30 years old.34 Whether the acceleration in SBP and MAP or widening of PP one year after the FMP in women following the low-accelerated rise trajectory predicts future clinical complications remains to be explored.
Women following the SBP high-slow decline and the DBP and MAP high-accelerated decline trajectories showed interesting phenomena of plateau and decrease after the FMP, respectively. We hypothesized that these women may have received more aggressive antihypertensive therapy after menopause, which may explain this plateau/decrease in BP measures. However, exploratory analyses including adjusting for aggressive antihypertensive therapy did not confirm our hypothesis. A previous analysis from the National Health and Nutrition Examination Survey noticed a similar SBP plateauing in Black and Mexican American women and a similar DBP decrease in White, Black, and Mexican American women around the age 60.35 Our study may extends previous observations into a longitudinal context by showing that Black women with high SBP, who tended to follow the high-slow decline trajectory, may experience a plateau in SBP after menopause. Although requiring further investigation, this plateau in the high-slow decline SBP trajectory appears to be a different phenomenon from the late-life SBP decrease which greatly steeps around age 80.36
In other work, greater serum levels of endogenous estradiol during the menstrual cycle or pregnancy are inversely related to SBP.37 Additionally, surgically induced menopause increases SBP within a few weeks.38 Thus, it is possible that estradiol depletion during the MT would be associated with a higher SBP. However, our results did not support this hypothesis. In fact, previous studies found no link between endogenous estradiol and SBP level or risk of incident hypertension.39,40 It is hypothesized that the vasodilatory and cardio-protective actions of endogenous estradiol are mediated in part through its metabolites.41 2-methoxyestradiol is an estradiol metabolite that has estrogen receptor-independent growth inhibitory effects on smooth muscle and endothelial cells.41 Interestingly, estradiol metabolites, but not estradiol, were associated with a lower SBP in postmenopausal women.42 These results highlight the complexity of the MT and future studies should compare estradiol metabolite levels and its potential effects on vascular function across the stages of the MT.
Age at menopause showed no,11 or even inverse,10 associations with BP levels in previous cross-sectional studies. In one longitudinal study, the rate of change in SBP over the MT did not differ among women with different ages at menopause.43 However, our results did not align with previous studies, as we showed that age at menopause has a direct association with SBP, PP, and MAP trajectory groups, such that a younger age at menopause predicted the low-accelerated rise SBP, PP, and MAP group. This disagreement across the studies may have resulted from the potential recall bias of age at menopause in cross-sectional studies or from our different analytic approach that assumed the presence of distinct BP trajectories over the MT.
Women transitioning through menopause frequently report vasomotor symptoms.44 A previous analysis in SWAN showed that premenopausal vasomotor symptoms predicted a higher risk of incident hypertension.18 Although the exact mechanism linking vasomotor symptoms with BP is not completely understood, evidence of greater sympathetic nervous system activation in women reporting vasomotor symptoms may be one plausible mechanism.45 Our findings extend the previous SWAN analysis by showing vasomotor symptoms predicting a higher SBP, PP, and MAP over time regardless of the trajectory.18
In women, FSH is known for its gonadal functions of follicular growth initiation and maturation. It is hypothesized that FSH has extragonadal functions after finding FSH receptors being expressed on blood vessels,46 adipose tissues,47 and the liver.48 In fact, greater FSH predicted a lower SBP in a cross-sectional analysis among Chinese women.19 We showed a similar inverse association between FSH and SBP and PP over time. It is hypothesized that FSH has an angiogenesis property that is mediated through increasing vascular endothelial growth factor (VEGF).49 In turn, VEGF showed an inverted U-shaped association with CVD events with the lowest risk experienced at the lower and upper end of the distribution.50 However, it is not understood how FSH may reduce SBP and PP; the underlying pathological mechanisms remain to be elucidated in future studies.
This work includes some limitations. We did not observe the FMP in 41% of women. Additionally, for women without an observed FMP, GBTM does not offer the option of including uncertainty of the imputation in determining trajectories of BP. However, a sensitivity analysis excluding women without observed FMP did not alter our conclusions. By follow-up visit 14, >99% of SWAN women had reached their FMP, and thus we defined attrition as women lost to follow-up before visit 14. We had a 28% attrition rate and BP trajectory groups were associated with attrition. However, relaxing the assumption that attrition is independent of the trajectories by building the missing data mechanism into the GBTM resulted in a similar group sizes and shapes. As suggested by Haviland et al.51, when trajectory groups are well separated, as in our case, attrition will effectively be at random, and building the missing data structure into the model is not needed. Conclusions related to the model that explored predictive associations of menopause-related factors with PP trajectories were limited after combining Chinese, Hispanic, and Japanese into “Others” due to small cell count in the high-continued rise PP group. Findings of temporal associations between the MT and BP measures do not imply causal relationships. Additionally, our findings are considered hypothesis generating and are susceptible to type-1 error. Future confirmatory studies should adjust for multiple testing.
Major strengths of our study include the careful prospective characterization of the timing of the FMP, concurrent measurements of endogenous sex hormones and menopause-related factors, the multiethnic composition of SWAN, and the analytic technique that allowed linking menopause related factors with distinct SBP, PP, and MAP trajectories.
Conclusions.
Distinct patterns of BP trajectories over the MT exist that revealed a group of women whose SBP, PP, and MAP trajectories are consistent with a menopause contribution to BP over midlife. Our findings support frequent monitoring of BP during the MT. Additionally, menopause-related factors including age at menopause, vasomotor symptoms, and FSH predicted SBP, PP, and MAP pattern and level over time. Future work should investigate potential mechanisms by which the MT may accelerate SBP, PP, and MAP increase.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Hypertension contributes to cardiovascular disease (CVD) and 43% of adult women are known to have hypertension.
After midlife, blood pressure (BP) in women exceeds that of men, suggesting a contribution of the menopause transition.
Declines in estradiol, increases in follicle-stimulating hormone (FSH), and the occurrence of vasomotor symptoms are the hallmarks of the menopause transition and are hypothesized to influence BP.
However, the literature thus far has described global BP changes over the menopause transition assuming that women share a common BP trajectory.
What New Information Does This Article Contribute?
Based on multiple years of BP measurements, women were classified based on the level and shape of their BP trajectory into three distinct groups: low, medium, and high.
Only the low trajectories demonstrate a contribution of the menopause transition with a significant accelerated rise occuring 1 year after menopause.
The other BP trajectories were rising before the menopause transition and either continued with same rise or declined after menopause.
Age at menopause, vasomotor symptoms, and FSH, but not estradiol, predict SBP, PP, and MAP level and shape.
The literature on the contribution of the menopause transition to BP changes in midlife is not consistent. Some studies show that postmenopausal women had a higher BP compared with their premenopausal counterparts, while others showed comparable BP measures before and after menopause. Heterogeneity among the previously studied populations may have resulted in the conflicting findings. To study this question, women were clustered into different trajectories based on level and shape of their BP measures. Our study revealed a group of midlife women whose trajectory is consistent with a menopause contribution (the low trajectory), associated with an accelerated rise after menopause in SBP, PP, and MAP measurements. Women with earlier menopause are more likely to experience the low trajectory pattern. Both greater frequency of vasomotor symptoms and lower levels of follicle stimulating hormones are related to increases in BP levels over time. Our results support the timely monitoring of CVD risk factors prior to the menopause transition and the critical need to adopt heart healthy lifestyle as early as possible.
ACKNOWLEDGMENT
Clinical Centers: University of Michigan, Ann Arbor - Carrie Karvonen-Gutierrez, PI 2021 - present, Siobán Harlow, PI 2011 – 2021, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA - Sherri-Ann Burnett-Bowie, PI 2020 - Present; Joel Finkelstein, PI 1999 – 2020; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL - Imke Janssen, PI 2020 - Present; Howard Kravitz, PI 2009 – 2020; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser - Elaine Waetjen and Monique Hedderson, PIs 2020 - Present; Ellen Gold, PI 1994 – 2020; University of California, Los Angeles - Arun Karlamangla, PI 2020 - Present; Gail Greendale, PI 1994 – 2020; Albert Einstein College of Medicine, Bronx, NY - Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry - New Jersey Medical School, Newark - Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA - Rebecca Thurston, PI 2020 - Present; Karen Matthews, PI 1994 – 2020.
NIH Program Office: National Institute on Aging, Bethesda, MD – Rosaly Correa-de-Araujo 2020 - present; Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair
The authors thank the study staff at each site and all the women who participated in SWAN.
The authors thank Safaa Bokhari for creating the graphical abstract.
SOURCES OF FUNDING
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
This manuscript was also supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
Nonstandard Abbreviations and Acronyms
- BP
blood pressure
- FMP
final menstrual period
- FSH
follicle-stimulating hormone
- GBTM
group-based trajectory modeling
- LDL-C
low-density lipoprotein cholesterol
- LLD
lower limit of detection
- LOWESS
locally weighted scatterplot smoothing
- MAP
mean arterial pressure
- MT
menopause transition
- PP
pulse pressure
- SWAN
Study of Women’s Health across the Nation
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This article is published in its accepted form. It has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation Research involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version.
DISCLOSURES
Saad Samargandy: None
Karen A. Matthews: None
Maria M. Brooks: No conflict of interest for this paper. As a disclosure, serving as DSMB member for Cerus Corporation
Emma Barinas-Mitchell: None
Jared W. Magnani: None
Rebecca C. Thurston: Astellas, Pfizer, Procter and Gamble, Virtue Health
Samar R. El Khoudary: None
SUPPLEMENTAL MATERIALS
Figures S1 and S2
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update. Circulation. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Stu. The Lancet. 2018;392(10159):1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji H, Kim A, Ebinger JE, et al. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA cardiology. 2020;5(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51(4):952–959. [DOI] [PubMed] [Google Scholar]
- 5.Bonithon-Kopp C, Scarabin PY, Darne B, Malmejac A, Guize L. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. International journal of epidemiology. 1990;19(1):42–48. [DOI] [PubMed] [Google Scholar]
- 6.Tremollieres FA, Pouilles JM, Cauneille C, Ribot C. Coronary heart disease risk factors and menopause: a study in 1684 French women. Atherosclerosis. 1999;142(2):415–423. [DOI] [PubMed] [Google Scholar]
- 7.De Kat AC, Dam V, Onland-Moret NC, Eijkemans MJC, Broekmans FJM, Van Der Schouw YT. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC medicine. 2017;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuh D, Langenberg C, Hardy R, et al. Cardiovascular risk at age 53 years in relation to the menopause transition and use of hormone replacement therapy: a prospective British birth cohort study. BJOG: An International Journal of Obstetrics and Gynaecology. 2005;112(4):476–485. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Zhou X, Guo X, et al. Prevalence and risk factors of hypertension among pre- and post-menopausal women: a cross-sectional study in a rural area of northeast China. Maturitas. 2015;80(3):282–287. [DOI] [PubMed] [Google Scholar]
- 10.Izumi Y, Matsumoto K, Ozawa Y, et al. Effect of age at menopause on blood pressure in postmenopausal women. American journal of hypertension. 2007;20(10):1045–1050. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Hayashi K, Mishra G, Yasui T, Kubota T, Mizunuma H. Independent association between age at natural menopause and hypercholesterolemia, hypertension, and diabetes mellitus: Japan nurses’ health study. Journal of atherosclerosis and thrombosis. 2013;20(2):161–169. [DOI] [PubMed] [Google Scholar]
- 12.Son MK, Lim NK, Lim JY, et al. Difference in blood pressure between early and late menopausal transition was significant in healthy Korean women. BMC women’s health. 2015;15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646. [DOI] [PubMed] [Google Scholar]
- 14.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? Journal of the American College of Cardiology. 2009;54(25):2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luoto R, Sharrett AR, Schreiner P, Sorlie PD, Arnett D, Ephross S. Blood pressure and menopausal transition: the Atherosclerosis Risk in Communities study (1987–95). Journal of hypertension. 2000;18(1):27–33. [DOI] [PubMed] [Google Scholar]
- 16.Tepper PG, Brooks MM, Randolph JF Jr., et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause (New York, NY). 2016;23(10):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tepper PG, Randolph JF Jr., McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). The Journal of clinical endocrinology and metabolism. 2012;97(8):2872–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson EA, El Khoudary SR, Crawford SL, et al. Hot Flash Frequency and Blood Pressure: Data from the Study of Women’s Health Across the Nation. J Womens Health (Larchmt). 2016;25(12):1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N, Shao H, Chen Y, et al. Follicle-Stimulating Hormone, Its Association with Cardiometabolic Risk Factors, and 10-Year Risk of Cardiovascular Disease in Postmenopausal Women. Journal of the American Heart Association. 2017;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Current hypertension reports. 2006;8(5):368–376. [DOI] [PubMed] [Google Scholar]
- 21.Sowers MFR, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. 2000.
- 22.Little RJ, Rubin DB. Statistical analysis with missing data. Vol 793: John Wiley & Sons; 2019. [Google Scholar]
- 23.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–2935. [DOI] [PubMed] [Google Scholar]
- 24.El Khoudary SR, Santoro N, Chen HY, et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. European journal of preventive cardiology. 2016;23(7):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Nagin DS, NAGIN D. Group-based modeling of development. Harvard University Press; 2005. [Google Scholar]
- 27.Samargandy S, Matthews KA, Brooks MM, et al. Arterial Stiffness Accelerates Within 1 Year of the Final Menstrual Period: The SWAN Heart Study. Arteriosclerosis, thrombosis, and vascular biology. 2020;40(4):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause (New York, NY). 2014;21(6):624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137(24):e843–e852. [DOI] [PubMed] [Google Scholar]
- 30.El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet (London, England). 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 32.Whelton SP, McEvoy JW, Shaw L, et al. Association of Normal Systolic Blood Pressure Level With Cardiovascular Disease in the Absence of Risk Factors. JAMA cardiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji H, Niiranen TJ, Rader F, et al. Sex Differences in Blood Pressure Associations With Cardiovascular Outcomes. Circulation. 2021;143(7):761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet (London, England). 2014;383(9932):1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of Hypertension in the US Adult Population. Hypertension. 1995;25(3):305–313. [DOI] [PubMed] [Google Scholar]
- 36.Delgado J, Bowman K, Ble A, et al. Blood Pressure Trajectories in the 20 Years Before Death. JAMA Internal Medicine. 2018;178(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barton M, Meyer MR. Postmenopausal Hypertension. Hypertension. 2009;54(1):11–18. [DOI] [PubMed] [Google Scholar]
- 38.Mercuro G, Zoncu S, Saiu F, Mascia M, Melis GB, Rosano GM. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas. 2004;47(2):131–138. [DOI] [PubMed] [Google Scholar]
- 39.Shelley JM, Green A, Smith AM, et al. Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Annals of epidemiology. 1998;8(1):39–45. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224(1):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubey RK, Jackson EK. Potential vascular actions of 2-methoxyestradiol. Trends in Endocrinology & Metabolism. 2009;20(8):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masi CM, Hawkley LC, Xu X, Veenstra TD, Cacioppo JT. Serum estrogen metabolites and systolic blood pressure among middle-aged and older women and men. American journal of hypertension. 2009;22(11):1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akahoshi M, Soda M, Nakashima E, et al. Effects of age at menopause on serum cholesterol, body mass index, and blood pressure. Atherosclerosis. 2001;156(1):157–163. [DOI] [PubMed] [Google Scholar]
- 44.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. The American journal of medicine. 2005;118 Suppl 12B:14–24. [DOI] [PubMed] [Google Scholar]
- 45.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertility and sterility. 1998;70(2):332–337. [DOI] [PubMed] [Google Scholar]
- 46.Stilley JAW, Christensen DE, Dahlem KB, et al. FSH Receptor (FSHR) Expression in Human Extragonadal Reproductive Tissues and the Developing Placenta, and the Impact of Its Deletion on Pregnancy in Mice1. Biology of Reproduction. 2014;91(3):74–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui H, Zhao G, Liu R, Zheng M, Chen J, Wen J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. Journal of lipid research. 2012;53(5):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Y, Wang ES, Xing LL, et al. Follicle-Stimulating Hormone Induces Postmenopausal Dyslipidemia Through Inhibiting Hepatic Cholesterol Metabolism. The Journal of clinical endocrinology and metabolism. 2016;101(1):254–263. [DOI] [PubMed] [Google Scholar]
- 49.Sun D, Bai M, Jiang Y, et al. Roles of follicle stimulating hormone and its receptor in human metabolic diseases and cancer. Am J Transl Res. 2020;12(7):3116–3132. [PMC free article] [PubMed] [Google Scholar]
- 50.Kaess BM, Preis SR, Beiser A, et al. Circulating vascular endothelial growth factor and the risk of cardiovascular events. Heart (British Cardiac Society). 2016;102(23):1898–1901. [DOI] [PubMed] [Google Scholar]
- 51.Haviland AM, Jones BL, Nagin DS. Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociological Methods & Research. 2011;40(2):367–390. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials have been made publicly available at the National Institute on Aging and can be accessed at https://agingresearchbiobank.nia.nih.gov.
Study population
SWAN is an ongoing multi-ethnic, multi-site longitudinal study designed to examine physical, biological, and psychosocial changes in women during midlife. Detailed design and methods were presented elsewhere.21 In brief, 3,302 women were recruited between 1996 and 1997 from seven sites (Detroit, MI; Boston, MA; Chicago, IL; Oakland, CA; Los Angeles, CA; Newark, NJ; and Pittsburgh, PA). To enroll in SWAN, women had to be aged 42–52 years, have an intact uterus and at least one ovary with menstrual bleeding within the past three months, not be pregnant or breast-feeding, and not have used hormone therapy in the past three months. Women self-identified as a member of one of five racial/ethnic groups: White (all sites), Black (Boston, Chicago, Detroit, Pittsburgh), Chinese/Chinese American (Oakland), Hispanic (Newark) or Japanese/Japanese American (Los Angeles). The institutional review board at each site approved the study protocol, and all participants signed informed consent before participation. Women in the current study were followed for up to 17 visits. Data from all SWAN women (n=3,302) were included in this analysis.


