Abstract
Objective: To predict the mechanism of Shengmai Injection (SMI) in the acute treatment of COVID-19 by network pharmacology and molecular docking. Methods: Search the compounds in the Traditional Chinese Medicine Systems Pharmacology (TCMSP), and screen them by Drug-like properties (DL) and Oral bioavailability (OB); Using PharmMapper database and GeneCards database to collect compounds targets and COVID-19 targets, and using UniProt database to standardize the names of target genes; Using DAVID database for KEGG pathway annotation and GO bioinformatics analysis; Using Cytoscape 3.8.2 software and STRING 10.5 database to construct “Component-Target-Pathway” network and Protein-Protein Interaction network (PPI); Using molecular docking to predict the binding ability of key compounds and key proteins. Results: A total of 34 active components, 38 core targets and 180 signaling pathways were screened out. The results of molecular docking showed that Schisantherin A and Moupinamide have strong binding with EGFR and MAPK1. Conclusion: The key active compounds of SMI in the treatment of COVID-19 may be Schisantherin A and Moupinamide, and the molecular mechanism may be related to key targets such as EGFR and MAPK1, and may be involved in the PI3K-Akt signaling pathway and MAPK signaling pathway.
Keywords: network pharmacology, shengmai injection, COVID-19, molecular docking, mechanism of action, acute treatment
Introduction
Corona Virus Disease 2019 (COVID-19), which broke out in Wuhan, China, is a kind of super virus pneumonia with fast infection speed, wide infection range and strong mutation ability. COVID-19 with fever, dry cough, fatigue as the main manifestations, a small number of patients with stuffy nose, runny nose, diarrhea and other upper respiratory and digestive tract symptoms.1,2 As of Dec. 24, 2021, there have been a total of 276,753,278 confirmed cases and 5,376,631 deaths of COVID-19 worldwide, and there are 970,349 new confirmed cases and 6844 new deaths worldwide in a single day. Therefore, it is urgent to control the crazy spread of COVID-19 in time and protect human beings from it.
In recent years, the clinical application value of traditional Chinese medicine and its’ component prescription has been studied extensively and deeply by many scholars all over the world. Traditional Chinese medicine has been inherited in China for 5000 years because of it's effectiveness, security and other characteristics, and it has been gradually accepted by the authoritative medicine worldwide. According to the characteristics of the disease, different ways of administration and flexible dosage according to the symptoms have significant characteristics and advantages for the treatment of COVID-19. Shengmai Injection (SMI) is a proprietary Chinese medicine composed of Talinum paniculatum (Jacq.) Gaertn. (Hongshen), Ophiopogon japonicus (Linn. f.) Ker-Gawl. (Maidong) and Schisandra chinensis (Turcz.) Baill. (Wuweizi)3. SMI can immediately activate the cardiovascular system, improve the retraction of the heart and accelerate the heartbeat, increase the cardiac output strip, and increase the heart rate. In clinical medicine, it is used for mild to moderate cardiogenic shock, physical overdraft and low blood pressure4. In China, SMI is also a proprietary Chinese medicine for critically ill COVID-19 patients in Guidelines on the Novel Coronavirus-Infected Pneumonia Diagnosis and Treatment, and some studies have shown that ShengMai is effective in the treatment of convalescent cases of COVID-195. As a result, SMI is an effective treatment for patients with severe illness. Although SMI has a definite therapeutic effect in patients with COVID-19 in the early stages of severe disease, the mechanism of action is obscure, so the active components and mechanism of SMI in the acute treatment of COVID-19 need long-term study.
Network pharmacology is based on the theory of systems biology, through multi-platform, multi-software, multi-way analysis and exploration of drugs and diseases, the treatment of traditional Chinese medicine or auxiliary treatment of diseases of multi-component, multi-target, multi-pathway mechanism is analyzed and explained6. High-throughput molecular docking technique is used to simulate the interaction between small molecular ligands and protein receptors to predict the active sites of drugs and the binding mode and affinity between ligands and receptors. The purpose of this study is to explore the potential active components and mechanism of SMI in the acute treatment of COVID-19 by means of network pharmacology and molecular docking.
Materials and Methods
Collection and Screening of Active Components
We searched all the chemical components related to Talinum paniculatum (Jacq.) Gaertn., Ophiopogon japonicus (Linn. f.) Ker-Gawl. and Schisandra chinensis (Turcz.) Baill. on Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://old.tcmsp-e.com/tcmsp.php), and screened all the active components by Oral Bioavailability (OB) ≥ 30% and Drug-Like (DL) ≥ 0.187. For the integrity of the obtained data, we have integrated some components retrieved from other databases to supplement the components obtained from TCMSP, such as TCMID (http://www.megabionet.org/tcmid/), TCM@Taiwan (http://tcm.cmu.edu.tw/zh-tw/). After that, these active components were retrieved and verified by PubChem (https://PubChem.ncbi.nlm.nih.gov/), and their 3D structures were obtained.
Screening of Intersection Target Genes
Traditional Chinese medicine, including many components, to treat disease by acting on certain targets and regulating pathways. Through the combination of drug molecules and target proteins, the drugs can achieve the effect of curing diseases. If the target proteins of the disease could be identified and drugs could act on them, then the drugs can treat the disease. The obtained 3D structures were imported into SwissTargetPrediction (http://www.swisstargetprediction.ch/), and all the potential target genes of SMI were obtained by P > 08. After entering the keywords “COVID-19” and “Corona Virus Disease 2019” in GeneCards (https://www.genecards.org/), all the target genes related to the disease were obtained9. The intersection targets of disease and drug can be represented by Venn diagram, and these intersection targets can be regarded as potential targets for SMI in the acute treatment of COVID-19.
GO Function and KEGG Pathway Enrichment Analysis
The Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) v6.8 comprises a full Knowledgebase update to the sixth version of our original web-accessible programs10,11. Therefore, we used the DAVID database to annotate the Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes pathway (KEGG) of the potential targets. GO function includes three indicators, namely Biological Process (BP), Cellular Component (CC) and Molecular Function (MF). Through GO function enrichment analysis, combined with biological problems and functional annotations of genes, we can judge whether the changes of these intersection target genes have biological significance. Through KEGG pathway enrichment analysis, we could predict which signaling pathways these intersection target genes are involved in and regulate.
Protein-Protein Interaction Network
Analysis of Protein-Protein Interaction (PPI) network helps to study the molecular mechanism of disease from the perspective of the system and discover new drug targets. Then we imported the screened potential targets into STRING (https://www.string-db.org/) to obtain the connections or potential connections between protein and protein interactions, so as to find the potential target genes that interact most closely, and which are most likely to be needed12.
Network Construction
In order to visualize all the screened data to analyze them, potential components, potential targets and signaling pathways were imported into cytoscape3.8.2 software to construct a “Component-Target-Pathway” (“C-T-P”) and “Drug-Component” (“D-C”) network diagram13. Then the core components and core targets were screened by analyzing these network diagrams.
Molecular Docking
Through the analysis of PPI and network diagram, the core components and core targets were obtained. Retrieved these core targets in RCSB PDB (http://www1.rcsb.org/) to obtain their protein structures which are closely related to COVID-1914. Then we docked these protein structures with the chemical structures obtained in step 2.1 by the Discovery Studio 2019 Client software. It is generally believed that the LibDockScore ≥90 indicates that the small molecular ligand has stronger affinity with the receptor and binds more easily.
The flow chart of network pharmacology analysis is shown in Figure 1.
The abbreviations list is listed in Table 1.
Results
Collection and Screening of Active Components
By searching Talinum paniculatum (Jacq.) Gaertn., Ophiopogon japonicus (Linn. f.) Ker-Gawl. and Schisandra chinensis (Turcz.) Baill. in TCMSP and other special databases of traditional Chinese medicine, a total of 28 active components in SMI were obtained by setting threshold OB ≥ 30% and DL ≥ 0.18. Through the reports of Ophiopogon japonicus (Linn. f.) Ker-Gawl. and Schisandra chinensis (Turcz.) Baill. in the published literature, we confirmed that although their OB or DL did not reach the threshold, they were still active components. And then 34 active components were finally obtained, including 4 from Talinum paniculatum (Jacq.) Gaertn., 9 from Ophiopogon japonicus (Linn. f.) Ker-Gawl. and 21 from. Schisandra chinensis (Turcz.) Baill. Information about these chemical components is listed in Table 2.
Table 2.
Components of SMI.
| No. | Molecule name | OB(%) | DL | Pubchem CID | Source |
|---|---|---|---|---|---|
| 1 | Longikaurin A | 47.72 | 0.53 | 70698023 | Schisandra chinensis (Turcz.) Baill. |
| 2 | Schisandrin C | 46.27 | 0.84 | 443027 | |
| 3 | neokadsuranic acid A | 43.35 | 0.85 | 133561680 | |
| 4 | neokadsuranic acid B | 43.1 | 0.85 | 138111911 | |
| 5 | kadsulactone | 42.87 | 0.76 | 138112325 | |
| 6 | schisanlactone A | 42.17 | 0.86 | 44560613 | |
| 7 | schisanlactone E | 40.83 | 0.84 | 5321172 | |
| 8 | schisandronic acid | 40.45 | 0.82 | 101277401 | |
| 9 | Deoxyharringtonine | 39.27 | 0.81 | 285342 | |
| 10 | neokadsuranic acid C | 35.4 | 0.85 | 138112222 | |
| 11 | changnanic acid | 35.34 | 0.8 | 138108877 | |
| 12 | Schisandrol R | 34.84 | 0.86 | 11516888 | |
| 13 | neokadsuranin | 33.35 | 0.88 | 338282 | |
| 14 | Schisandrol G | 32.68 | 0.83 | 5317802 | |
| 15 | Angeloylgomisin O | 31.97 | 0.85 | 91864462 | |
| 16 | Interiotherin B | 31.76 | 0.77 | 20839677 | |
| 17 | Schizandrer B | 30.71 | 0.83 | 5318785 | |
| 18 | Gomisin-A | 30.69 | 0.78 | 3001662 | |
| 19 | kadsulignan B | 30.63 | 0.84 | 138112622 | |
| 20 | Kadsulignan C | 30.23 | 0.52 | 101938317 | |
| 21 | Schisantherin A | 7.56 | 0.82 | 151529 | |
| 22 | p-Coumaroyltyramine | 112.9 | 0.2 | 5372945 | Ophiopogon japonicus (Linn. f.) Ker-Gawl. |
| 23 | Moupinamide | 86.71 | 0.26 | 5280537 | |
| 24 | diosgenin | 80.88 | 0.81 | 99474 | |
| 25 | β-patchoulene | 50.69 | 0.11 | 101731 | |
| 26 | stigmasterol | 43.83 | 0.76 | 5280794 | |
| 27 | oleanic acid | 29.02 | 0.76 | 12358638 | |
| 28 | guanosine | 21.43 | 0.21 | 135398635 | |
| 29 | Adenosine | 18.32 | 0.18 | 60961 | |
| 30 | uridine | 10.49 | 0.11 | 6029 | |
| 31 | DNOP | 40.59 | 0.4 | 8346 | Talinum paniculatum (Jacq.) Gaertn. |
| 32 | beta-sitosterol | 36.91 | 0.75 | 222284 | |
| 33 | ginsenoside rh2 | 36.32 | 0.56 | 119307 | |
| 34 | Squalen | 33.55 | 0.42 | 11975273 |
Table 1.
Abbreviations List
| Abbreviation | Official Name |
|---|---|
| 3CL-Mpro | SARS-coV-23CL hydrolase |
| ACE2 | Angiotensin-converting enzyme 2 |
| AKT1 | RAC-alpha serine/threonine-protein kinase |
| AR | Androgen receptor |
| BP | Biological Process |
| BRAF | Serine/threonine-protein kinase B-raf |
| CASP3 | Caspase-3 |
| CASP8 | Caspase-8 |
| CC | Cellular Component |
| COVID-19 | Corona Virus Disease 2019 |
| C-T-P | Component-Target-Pathway |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| D-C | Drug-Component |
| DL | Drug-Like |
| EGFR | Epidermal growth factor receptor |
| GO | Gene Ontology |
| HCV | hepatitis C virus |
| IL6 | Interleukin-6 |
| JAK2 | Tyrosine-protein kinase JAK2 |
| JAK3 | Tyrosine-protein kinase JAK3 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes pathway |
| MAPK1 | Mitogen-activated protein kinase 1 |
| MF | Molecular Function |
| MTOR | Serine/threonine-protein kinase mTOR |
| NOS2 | Nitric oxide synthase, inducible |
| OB | Oral Bioavailability |
| PDB | Protein Data Bank |
| PPI | Protein-Protein Interaction |
| SMI | Shengmai Injection |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology |
| TNF | Tumor necrosis factor |
| VEGFA | Vascular endothelial growth factor A |
Screening of Intersection Target Genes
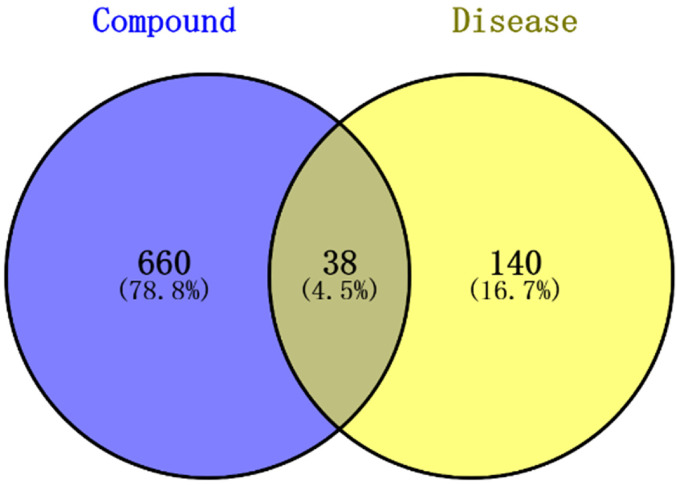
By searching the active ingredients obtained in 3.1, 698 target genes were obtained from 34 active components and 178 target genes of COVID-19 were obtained from GeneCards. The active component target genes and disease target genes were analyzed and compared by Veen diagram, as shown in Figure 2. The intersection genes of active component target genes and disease target genes can be regarded as potential targets for SMI in the acute treatment of COVID-19. These targets are shown in Table 3.
Figure 2.
Venn diagram of coincidence targets.
Table 3.
Potential Target Genes.
| Gene Official Symbol | |||||||
|---|---|---|---|---|---|---|---|
| ACE | ADA | AKT1 | AR | BRAF | CASP3 | CASP6 | CASP8 |
| CFTR | CXCL8 | DPP4 | EGFR | ELANE | F2 | FGFR1 | FGFR2 |
| FLT3 | IL2 | IL6 | JAK2 | JAK3 | KIT | MAPK1 | MCL1 |
| MMP9 | MTOR | NOS2 | PTPN11 | REN | SERPINE1 | SHH | STAT1 |
| STAT3 | TERT | TLR4 | TNF | TTR | VEGFA | ||
Figure 1.
Flow chart of network pharmacology analysis.
GO Function and KEGG Pathway Enrichment Analysis
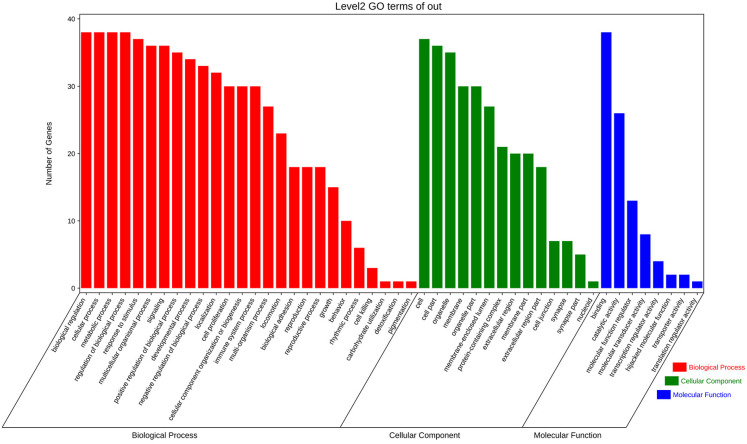
The intersection target genes were imported into the DAVID database, then the GO function and KEGG pathway can be analyzed by these intersection target genes. The results are shown in Figure 3 and Figure 4. Through the GO function enrichment analysis, 346 items were finally obtained. In Figure 3, it is clear that these target proteins are involved in biological functions such as biological regulation, cellular process, metabolic process and more.
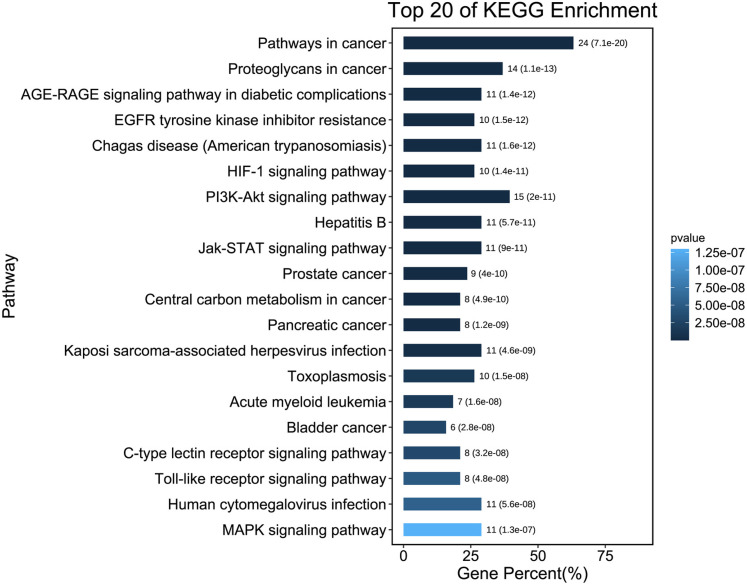
Figure 3.
Bubble chart of the results of KEGG pathway enrichment.
Figure 4.
Bubble chart of the results of GO function enrichment.
Finally, according to statistical data, the smaller the P value, the pathway is related to the disease, and the top 20 signaling pathways were obtained. In Figure 4, these target proteins can be analyzed to participate in the regulation of cancer, immune correlation, and infectious diseases pathways. Among them, AGE-RAGE signaling pathway in diabetic complications involves 10 genes, like MAPK1, IL6, VEGFA, AKT1. PI3K-Akt signaling pathway involves 15 genes, like MAPK1, EGFR, IL6, VEGFA, AKT1. Jak-STAT signaling pathway involves 11 genes, like AKT1, EGFR, IL6, STAT3. MAPK signaling pathway involves 11 genes, like MAPK1, EGFR, VEGFA, AKT1. According to the results, we speculated that SMI may act on key genes such as EGFR, MAPK1, IL6, VEGFA, AKT1, participate in the regulation of some cancer and immune-related pathways, and thus play a role in the acute treatment of COVID-19.
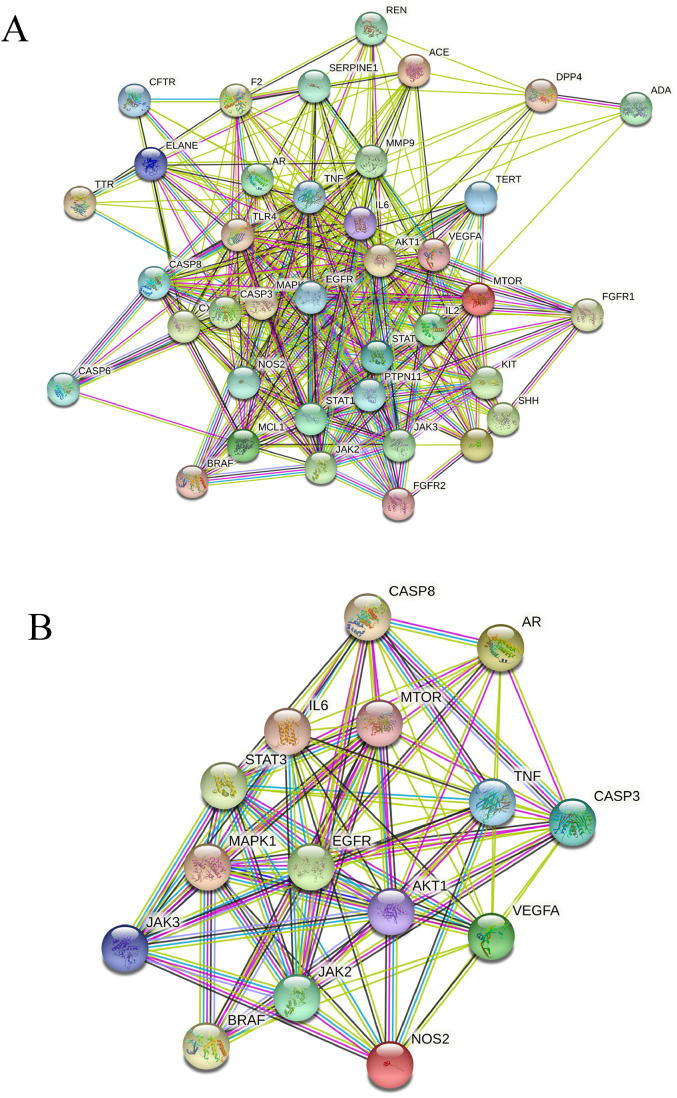
Protein-Protein Interaction Network
Imported the potential targets into the STRING database, generated an interactive network diagram, and analyzed it by Cytoscape3.8.2 software, as shown in Figure 5A. Obviously, these proteins have high scores in PPI network, such as EGFR, IL6, MAPK1, VEGFA and AKT1. We also analyzed the top 15 target genes with high scores in the PPI network diagram (Figure 5B), and further found that target genes such as EGFR and MAPK1 occupied the core position in the “C-T-P” topology and PPI network. Therefore, we predicted that the mechanism of SMI in the acute treatment of COVID-19 may be related to the regulation of key targets and related co-expression genes.
Figure 5.
PPI interaction network. A: Components and Disease Related Targets PPI. B: Interaction of Top 15 Targets in the “Component-Target-Pathway” Network.
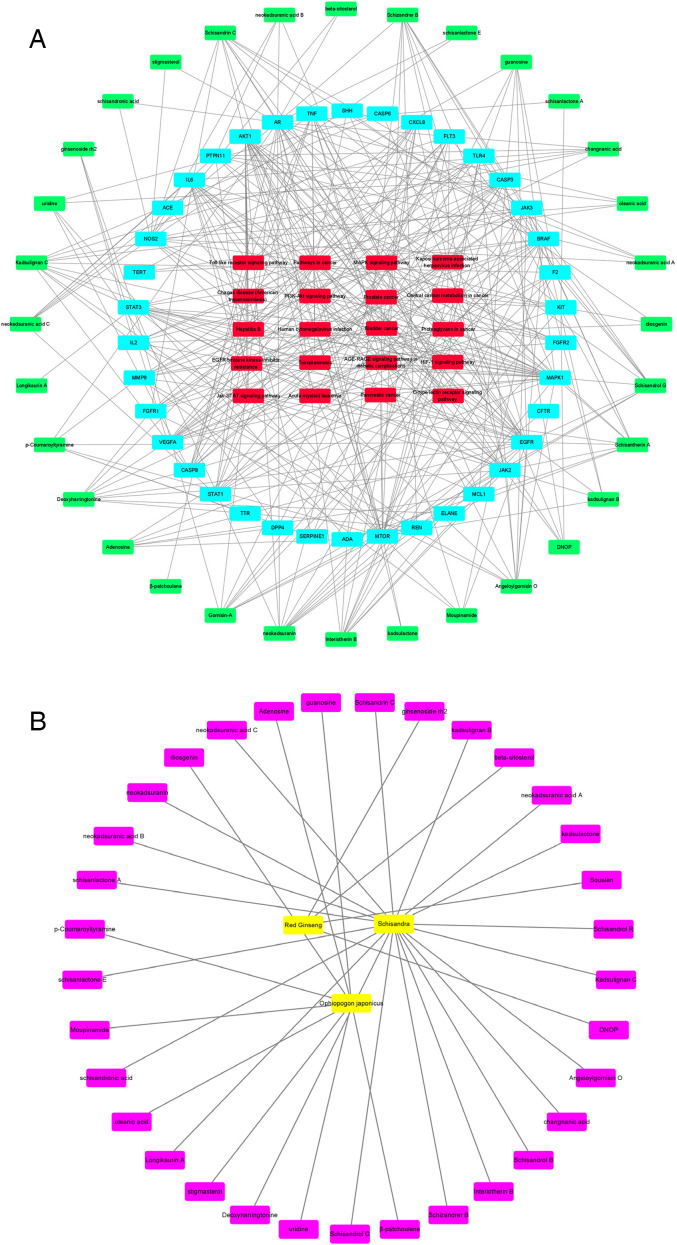
Network Construction
34 active components, 38 potential targets and the top 20 signaling pathways were imported into cytoscape3.8.2 software to construct “C-T-P” and “D-C” network diagram, as shown in Figure 6A and Figure 6B. In Figure 6A, there are 92 nodes, including 34 green active component nodes, 38 blue potential target nodes, 20 red signal pathway nodes, with 396 edges. In Figure 6B, there are 37 nodes, including 3 yellow drug nodes, 34 purple active ingredient nodes, with 34 edges. The network diagram showed the interaction between edges. The higher the correlation is, the more concentrated the convergence of these edges will be, meanwhile the greater the Degree score of the node will be. At the same time, different components interact with the same gene, which is very similar to the mechanism of multi-gene interaction of multi-component of traditional Chinese medicine. According to the critical degree between the components and the genes, the top 15 key genes were screened, which were EGFR, MAPK1, AR, MTOR, AKT1, JAK2, STAT3, BRAF, NOS2, IL6, JAK3, VEGFA, TNF, CASP8, CASP3. The top 15 components and genes are shown in Table 4. As can be seen from the network diagram, SMI acts on multi-gene through multi-components, coordinates and regulates through multi-pathway, and has the characteristics of restorative treating diseases.
Figure 6.
A. “Component-Target-Pathway” Network Figure 6B. “Drug-Component” Network.
Table 4.
The Top 15 Active Components and Core Targets.
| Component | Degree | Gene | ENSG ID | Degree |
|---|---|---|---|---|
| neokadsuranin | 11 | EGFR | ENSG00000146648 | 27 |
| Kadsulignan C | 11 | MAPK1 | ENSG00000100030 | 27 |
| Schisantherin A | 10 | AR | ENSG00000169083 | 24 |
| Schisandrin C | 10 | MTOR | ENSG00000198793 | 24 |
| Schizandrer B | 10 | AKT1 | ENSG00000142208 | 22 |
| Angeloylgomisin O | 10 | JAK2 | ENSG00000096968 | 18 |
| Interiotherin B | 10 | STAT3 | ENSG00000168610 | 16 |
| Schisandrol G | 9 | BRAF | ENSG00000157764 | 15 |
| Gomisin-A | 9 | NOS2 | ENSG00000007171 | 15 |
| Deoxyharringtonine | 9 | IL6 | ENSG00000136244 | 14 |
| guanosine | 7 | JAK3 | ENSG00000105639 | 14 |
| p-Coumaroyltyramine | 6 | VEGFA | ENSG00000112715 | 12 |
| Adenosine | 6 | TNF | ENSG00000232810 | 12 |
| changnanic acid | 6 | CASP8 | ENSG00000064012 | 11 |
| Moupinamide | 5 | CASP3 | ENSG00000164305 | 11 |
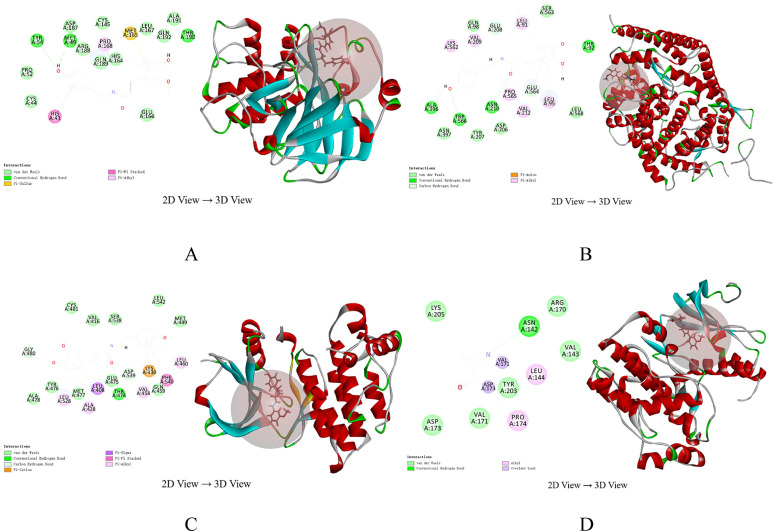
Molecular Docking
It is generally believed that the lower binding between the small molecule ligands and the receptors is, the higher the LibDock score, the larger the interaction, the stronger the potential activity of the component. According to PPI and network analysis results, we chose three components with higher scores Schisantherin A, Gomisin-a, moupinamide, and two currently recognized targets 3CL and ACE2 related to COVID-19 for molecular docking15. Then we let these three components dock with core gene EGFR, MAPK1. The docking results were analyzed as a screening criterion in LibDock score, and showed that the LibDock score of the selected target genes and components were greater than the threshold 90, showing good binding activity. This means that these three components play an important role in the process of SMI in the acute treatment of COVID-19. The docking results are shown in Table 5 and Figure 7A, 7B, 7C, 7D.
Table 5.
Results of Molecular Docking.
| Component | Source | LibDock score | |||
|---|---|---|---|---|---|
| 3CL(6lu7) | ACE2(1r42) | EGFR(6di9) | MAPK1(4zzn) | ||
| Schisantherin A | Schisandra | 116.367 | 99.028 | 112.891 | 103.013 |
| Gomisin-A | Schisandra | 114.871 | 94.7242 | 109.247 | 100.198 |
| Moupinamide | Ophiopogon japonicus | 126.928 | 117.485 | 113.348 | 109.374 |
Figure 7.
Results of moupinamide molecular docking. A: 3CL-Moupinamide. B: ACE2-Moupinamide. C: EGFR-Moupinamide. D: MAPK1-Moupinamide.
Discussion
In more than 5000 years of application, traditional Chinese medicine has fully proved it's effectiveness and security. Since 2003, traditional Chinese medicine has played an important role in the prevention and control of major epidemic such as SARS, H1N1. The clinical treatment of COVID-19 proves that traditional Chinese medicine still plays an irreplaceable role16–18. Therefore, screening the effective compound of anti-COVID-19 based on clinical practice is of great significance for the prevention and treatment of the epidemic situation.
SMI is a traditional Chinese patent medicine composed of Talinum paniculatum (Jacq.) Gaertn., Ophiopogon japonicus (Linn. f.) Ker-Gawl. and Schisandra chinensis (Turcz.) Baill. It is an effective drug for the treatment of acute diseases such as septic shock and heart failure19–21. Based on the theory of systems biology, this study constructed “C-T-P” and “D-C” topology networks through network pharmacology to explore the active components, potential targets and signaling pathways of COVID-19 in the acute treatment of SMI, in order to predict the mechanism of action.
Through data screening and analysis, we obtained 34 active components, 38 potential targets and 20 signaling pathways that may be the information for SMI in the acute treatment of COVID-19. Through the analysis of “C-T-P” and “D-C” topology network, we found that Schisantherin A, Gomisin-A, Moupinamide occupy the core position in the network diagram. There are findings indicated that schisantherin A exerted potent anti-inflammatory properties in LPS-induced mouse ARDS, possibly through blocking the activation of NF-κB and mitogen activated protein kinases (MAPKs) signaling pathways22. Gomisin-A may exert neuroprotective effects by attenuating the microglia-mediated neuroinflammatory response via inhibiting the TLR4-mediated NF-κB and MAPKs signaling pathways23. It has been reported that the anti-inflammatory effects of Moupinamide might be attributed to downregulation of COX-2 and iNOS via suppression of AP-1 and the JNK signaling pathway in RAW 264.7 macrophages24. Then we docked these components with SARS-coV-23CL hydrolase (3CL-Mpro) and Angiotensin-converting enzyme 2 (ACE2), and found that they have good docking effect and strong binding ability, indicating that these active components are the core components of SMI in the acute treatment of COVID-19.
Through the analysis of PPI and “C-T-P”, we found that the potential target Epidermal growth factor receptor (EGFR), Mitogen-activated protein kinase 1 (MAPK1) not only has a high Degree score, but also has a correlation with COVID-19. EGFR Acts as a receptor for hepatitis C virus (HCV) in hepatocytes and facilitates cell entry. Mediates HCV entry by promoting the formation of the CD81-CLDN1 receptor complexes that are essential for HCV entry and by enhancing membrane fusion of cells expressing HCV envelope glycoproteins25. Depending on the cellular context, the MAPK/ERK cascade mediates diverse biological functions such as cell growth, adhesion, survival and differentiation through the regulation of transcription, translation, cytoskeletal rearrangements26.
According to the results of enrichment analysis of GO function and KEGG signaling pathway, most of the 38 potential targets are involved in biological regulation, cellular process, metabolic process and other biological processes. The above core targets are closely related to AGE-RAGE signaling pathway in diabetic complications, PI3K-Akt signaling pathway and MAPK signaling pathway, and these pathways are related to oxidative stress, cell growth, transcription, translation, cell proliferation, cell movement and glycogen metabolism27,28.
To sum up, based on the results of network pharmacology and molecular docking, we speculated that Schisantherin A, Gomisin-A and Moupinamide in SMI may act on 3CL, ACE2, EGFR, MAPK1 and other targets through AGE-RAGE signaling pathway in diabetic complications, PI3K-Akt signaling pathway, MAPK signaling pathway and other pathways, so as to exert the effects of anti-inflammation, anti-shock, immune regulation and more. Therefore, SMI through the multi-component, multi-gene, multi-pathway of the joint action of acute treatment of COVID-19.
Conclusion
In summary, in this study, through network pharmacology, molecular docking and previous literature research, the key active compounds of SMI in the treatment of COVID-19 may be Schisantherin A and Moupinamide, and the molecular mechanism may be related to key targets such as EGFR and MAPK1, and may be involved in the PI3K-Akt signaling pathway and MAPK signaling pathway. This study provides a valuable scientific basis for further acute treatment of COVID-19 with SMI and lays a theoretical foundation for follow-up clinical trials.
Acknowledgments
This study was supported by Dr Yang.
Data Availability: For reasonable requirements, the data related to this study can be requested from the corresponding author.
Ethical Approval: Ethical Approval is not applicable for this article.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iDs: Chen Wang https://orcid.org/0000-0001-5291-3694
Yan-fang Yang https://orcid.org/0000-0001-5214-7758
References
- 1.Habas K, Nganwuchu C, Shahzad F, et al. Resolution of coronavirus disease 2019 (COVID-19). Expert Rev Anti Infect Ther. 2020;18(12):1201–1211. doi: 10.1080/14787210.2020.1797487 [DOI] [PubMed] [Google Scholar]
- 2.Tarique M, Ahmad S, Malik A, et al. Novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) and other coronaviruses: a genome-wide comparative annotation and analysis. Mol Cell Biochem. 2021;476(5):2203‐2217. doi: 10.1007/s11010-020-04027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Liu C, Duan L, Bai J, Mao Q, Jie W. Shengmai injection combined with conventional therapy in treating Adriamycin-related cardiotoxicity: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(45):e23084. doi: 10.1097/MD.0000000000023084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Zhou X, Chen X, et al. Efficacy and safety of shengmai injection for chronic heart failure: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2020;2020(1):9571627. Published 2020 Jun 20. doi: 10.1155/2020/9571627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Q, Zhang QJ. Clinical efficacy analysis of shengmai San in the treatment of Qi-Yin deficiency syndrome in convalescent stage of COVID-19[J]. ZhongYiYao XueBao. 2021;49([263[03]]):84‐86. doi: 10.19664/j.cnki.1002-2392.210069 [DOI] [Google Scholar]
- 6.Wang X, Wang ZY, Zheng JH, Li S. TCM Network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19(1):1‐11. doi: 10.1016/S1875-5364(21)60001-8 [DOI] [PubMed] [Google Scholar]
- 7.Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(13). Published 2014 Apr 16. doi: 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daina A, Michielin O, Zoete V. Swisstargetprediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357‐W364. doi: 10.1093/nar/gkz382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54(1):1.30.1‐1.30.33. Published 2016 Jun 20. doi: 10.1002/cpbi.5 [DOI] [PubMed] [Google Scholar]
- 10.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1‐13. doi: 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44‐57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 12.Szklarczyk D, Gable AL, Lyon D, et al. STRING V11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607‐D613. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498‐2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman H, Henrick K, Nakamura H. Announcing the worldwide protein data bank. Nat Struct Biol. 2003;10(12):980. doi: 10.1038/nsb1203-980 [DOI] [PubMed] [Google Scholar]
- 15.Ionescu MI. An overview of the crystallized structures of the SARS-CoV-2. Protein J. 2020;39(6):600‐618. doi: 10.1007/s10930-020-09933-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Zhang Y. Traditional Chinese medicine treatment of COVID-19. Complement Ther Clin Pract. 2020;39(1):101165. doi: 10.1016/j.ctcp.2020.101165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Yu J, Zhou Y, Shen M, Sun L. Becoming a faithful defender: traditional Chinese medicine against coronavirus disease 2019 (COVID-19). Am J Chin Med. 2020;48(4):763‐777. doi: 10.1142/S0192415X2050038X [DOI] [PubMed] [Google Scholar]
- 18.Zhuang W, Fan Z, Chu Y, et al. Chinese Patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front Pharmacol. 2020;11(1):1066. Published 2020 Jul 17. doi: 10.3389/fphar.2020.01066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Ruan X, Xu X, et al. Shengmai injection suppresses angiotensin II-induced cardiomyocyte hypertrophy and apoptosis via activation of the AMPK signaling pathway through energy-dependent mechanisms. Front Pharmacol. 2019;10(1):1095. Published 2019 Sep 20. doi: 10.3389/fphar.2019.01095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Duan X, Wang K, Wu J, Zhang X. Shengmai injection as an adjunctive therapy for the treatment of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Med. 2019;43(1):140‐147. doi: 10.1016/j.ctim.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Ye Q, Xu S, et al. Shengmai injection alleviates H2O2-induced oxidative stress through activation of AKT and inhibition of ERK pathways in neonatal rat cardiomyocytes. J Ethnopharmacol. 2019;239(1):111677. doi: 10.1016/j.jep.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 22.Zhou E, Li Y, Wei Z, et al. Schisantherin A protects lipopolysaccharide-induced acute respiratory distress syndrome in mice through inhibiting NF-κB and MAPKs signaling pathways. Int Immunopharmacol. 2014;22(1):133‐140. doi: 10.1016/j.intimp.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Hu D, Zhang L, et al. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway. Food Chem Toxicol. 2014;63(1):119‐127. doi: 10.1016/j.fct.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Yu L, Wang MH. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: involvement of AP-1 and MAP kinase signaling pathways. Chem Biol Interact. 2015;235(1):56‐62. doi: 10.1016/j.cbi.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 25.Lupberger J, Zeisel MB, Xiao F, et al. EGFR And EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17(5):589‐595. doi: 10.1038/nm.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21‐44. doi: 10.1080/02699050500284218 [DOI] [PubMed] [Google Scholar]
- 27.Kay AM, Simpson CL, Stewart JA, Jr.. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res. 2016;2016(1):6809703. doi: 10.1155/2016/6809703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y, Shi X, Sheng K, et al. PI3 K/Akt Signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (review). Mol Med Rep. 2019;19(2):783‐791. doi: 10.3892/mmr.2018.9713 [DOI] [PMC free article] [PubMed] [Google Scholar]