Abstract
Since numerous natural components in Eucommia ulmoides belong to phytoestrogen, its effect on hens production deserve more attention. To investigate the potential of E. ulmoides extract used as a feed additive, laying performance, egg quality, yolk cholesterol, yolk fatty acids, yolk fatty, yolk volatile components, albumen amino acids, plasma biochemical parameters, intestinal histology, and gut microbiota of hens (n = 120) were determined between basal diet (A) and dietary supplementation low (B), middle (C), and high (D) level E. ulmoides extract for 11 wk. When compared to A group, 2 percentage points elevation in laying rate was observed of D group. Significant up-regulation of immunoglobulin indexes and down-regulation of lipid related indexes in D group were also found if comparison with A group, suggesting that supplementation E. ulmoides extract at a relative high content benefited in immunity enhancing and blood-fat depressing. Meanwhile, obvious variation in albumen amino acids and yolk volatile compounds were inspected as dietary supplementation E. ulmoides extract, especially in D group, implied that the flavor of egg would change under high-level E. ulmoides extract treatment. Besides, villus height and villus height to crypt depth ratio of duodenum, jejunum, and ileum in D group were also significantly higher than that of in A group, indicating high-level E. ulmoides extract contributed to nutrient adsorption via intestinal histology changing. Moreover, the richness, diversity, and composition of gut microbiota in D group also significantly altered with a comparison of A group. These variation caused gut microbiota in D group major enriched in the KEGG pathway of insulin signing pathway, systemic lupus erythematosus, and bacterial invasion of epithelial cells, which were conducive to egg production elevation via facilitating nutrient adsorption, inflammation relieving, blood lipid amelioration, and insulin resistance alleviation. These results indicated that dietary supplementation E. ulmoides extract at high content could serve as a feed additive in the hens industry.
Key words: E. ulmoides extract, laying hens, production performance, body health, gut microbiota
INTRODUCTION
Antibiotics have been widely used in the poultry industry since the 1940s. Based on its advantages of growth-promoting and disease resistance, antibiotics are applied in numerous countries, such as India, Sweden, Finland, and Germany (Castanon, 2007; Moudgil et al., 2018). Despite the merits of antibiotics, numerous problems emerge as the times require. For example, residues of antibiotics in livestock and poultry products have gained much concern in the past several years (Kirchhelle, 2018). Antibiotics have serious negative effects on body health, such as allergic reactions, chronic toxicity, gene mutation, superbug generating, cancer-causing, and disruption of digestive system function (Chen et al., 2019; Marshall and Levy, 2011; Bacanlı and Başaran, 2019;). Therefore, residues of antibiotics in poultry products are harmful to human health. Considering the adverse effects of antibiotics, European Union (EU) has banned antibiotics used in animal industries since 2006 (European Commission, 2005). China also takes a lot of measures to reduce the application of antibiotics during the period of the 13th 5-yr plan and has prohibited commercially available antibiotics production and market circulations since July 1, 2020. Consequently, it is urgently needed to find alternatives to antibiotics with the property of efficiency and safety to poultry production.
Recently, herbal plants have been applied in the poultry and livestock industry as alternatives to antibiotics, which are major served as feed additives (Gadde et al., 2017). Herbal plants are usually regarded as traditional chinese medicine and have a long application history, which exhibits various pharmacological activities, such as antiviral, anti-inflammatory, and antioxidant (Xu et al., 2017; Yang et al., 2017; Aziz and Karboune, 2018; Abdallah et al., 2019; Zhu et al., 2019). In the poultry industry, plenty of researches have demonstrated that herbal plants can improve body health and production performance, such as dietary addition of Lycium barbarum and Glycyrrhiza glabra L. can improve growth performance, immunity, and livability of broilers (Long et al., 2020; Kalantar et al., 2017). Moreover, natural products from herbal plants are also applied in the poultry industry. For example, dietary supplementation total flavonoids from Rhizoma Drynariae and icariin from Epimedium can increase egg weight and improve bone tissue microstructure of hens (Huang et al., 2020a,b). Moreover, in the livestock industry, dietary supplementation Astragalus membranaceus can regulate body antioxidant and immunity of sheep by increasing the concentration of serum interleukin and immunoglobulin (Wang et al., 2021). Therefore, herbal plants have a huge application prospect in function as feed additives.
Eucommia ulmoides Oliv. is the unique medicinal plant of China and a total of 138 kinds of natural active compounds are separated, purified, and identified from the bark and leaves (Wang et al., 2020a), such as chlorogenic acid, geniposidic acid, and aucubin. Chlorogenic acid is the most important characteristic component of E. ulmoides. It is reported that chlorogenic acid can protect mice against Cd-induced hepatorenal injury via regulating intestinal flora balance (Ding et al., 2021). Except chlorogenic acid, geniposidic acid and aucubin are the other characteristic components with high contents in E. ulmoides (Wang et al., 2016; Yang et al., 2018). Based on the rich foundation of natural products, E. ulmoides possess multiple pharmacological activities, such as anti-inflammatory and antihyperlipidemia (Wang et al., 2019; Gu et al., 2020). Moreover, research shows that the serum levels of IgA, IgG, IgM, LDL-C, and HDL-C improvements under the function of E. ulmoides treatment, which leads to immunity enhancement and blood lipid reduction in broilers (Abaidullah et al., 2021). Besides, supplementation E. ulmoides leaf powder have been reported to increase the protein and amino acid contents of muscle tissue, so that improve the meat quality of broilers (Wang et al., 2018). However, although literatures have uncovered the effects of E. ulmoides on broilers, there is no systematic study about their effects on laying hens production. Furthermore, since numerous components belong to phytoestrogen, E. ulmoides is a rich source of estrogen receptor modulators (Ong and Tan, 2007; Wang et al., 2011) and potential has benefits in increase egg production of laying hens as its function of estrogen modulation. Therefore, the effect of E. ulmoides on production performance and body health of hens need to investigate, so that we can assess the possible of E. ulmoides extract serving as a feed additive in the hens industry.
In this study, the effects of E. ulmoides extract on laying hens were investigated. The laying performance, egg quality, plasma biochemical level, intestinal histology, and gut microbiota were analyzed under low, middle, and high-level E. ulmoides extract treatment. We hypothesis that dietary supplementation E. ulmoides extract can increase egg production and improve body health of hens based on its role of estrogen receptor modulators. Our study will provide a theoretical foundation for the application of E. ulmoides extract served as a feed additive in the hens industry.
MATERIALS AND METHODS
The experimental protocol of this study was approved by the Animal Care and Use Committee of Guangdong Academy of Sciences.
Standards and Reagents
Chlorogenic acid (CAS: 327-97-9), geniposidic acid (CAS: 27741-01-1), aucubin (CAS: 479-98-1), geniposide (CAS: 24512-63-8), pinoresinol diglucoside (CAS: 63902-38-5), and rutin (CAS: 153-18-4) standards were purchased from Yuanye Technology Co., Ltd (Shanghai, China) and had a purity ≥99%. Methanol and phosphoric acid were purchased from Merck (Darmstadt, Germany). Ultrapure water was prepared by a Milli-Q filter system (Millipore, Bedford, MA). Hexane, petroleum ether, and chloroform were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China).
Preparation of E. ulmoides Extract
The dry leaves of E. ulmoides were purchased from Hengxin Co., Ltd (Zhangjiajie, China), and extracted twice with 50% edible ethanol (liquid:solid, 1:10) under the condition of 80°C for 1 h. The extracted liquid was concentrated to 20 Brix under vacuum. Then, the obtained concentrated liquid was dried by spray drying. The inlet air temperature for spray drying was 150 to 170°C and the outlet air temperature was 90 to 95°C.
Chemical Composition of E. ulmoides Extract
Separation and quantification of chemical composition (chlorogenic acid, geniposidic acid, aucubin, geniposide, pinoresinol diglucoside, and rutin) were performed on an LC-20A HPLC system (Shimazu, Kyoto, Japan) combined with a diode array detector (DAD). Samples were separated by using Thermo BDS HYPERSIL C-18 (250 × 4.6 mm, 5 μm) analytical column. The mobile phase solutions were solvent A (0.2 % phosphoric acid in water) and solvent B (MeOH) with linear gradient elution program (5% B at 0 to 17 min; 5 to 14% B at 17 to 45 min; 14 to 35% B at 45 to 55 min; 35% to 45% B at 55 to 70 min). Aucubin and rutin were detected at 208 nm and 254 nm, respectively. Geniposidic acid, chlorogenic acid, geniposide, and pinoresinol diglucoside were monitored at 238 nm. The column temperature was kept at 30°C, the flow rate was 1 mL/min and the injection volume of the sample was 10 μL.
Animal Experiment and Treatment
Fifty-six wk old spotted-brown laying hens (n = 120) were randomly assigned to 4 groups: A group was the basal diet; B, C, and D groups were the basal diet supplementation 100, 200, and 500 mg/kg E. ulmoides extract, respectively. The basal diet was purchased from Zhengda Kangdi (Shenzhen, China) Co., Ltd. The composition and nutrient levels of basal diet are shown in Table S1. Laying hens were allowed free access to water and were fed under the constant condition of 16 h light and 8 h dark in Baishi poultry farm (Zhongshan, China). The room temperature was kept at 24 ± 1°C. The weight and number of eggs as well as feed consumption were recorded daily in the whole experimental period. The experimental period was 11 wk.
Sample Collection
Blood samples were obtained aseptically from the wing vein of 10 hens in each group after 11 wk of feeding and the plasma samples were centrifuged at 3,800 rpm for 10 min. Samples were stored at −80°C before use. The organs (liver, spleen) were collected and weighted individually. Duodenum, jejunum, and ileum were severally collected and stored at 4% paraformaldehyde until further use. The contents of the cecum were aseptically collected and preserved at −80°C for 16s rRNA gene sequencing analysis.
Laying Performance and Egg Quality
Laying rate, feed conversion rate (FCR), average egg weight, and daily feed consumption were evaluated at the end of the experimental period. Thirty eggs from each group were used to determine the egg quality. Egg-shape index and eggshell thickness were determined by an electronic digital caliper (DL91150, Deli, Ningbo, China). Moisture contents of yolk and albumen were determined in an oven. The shell strength index was measured using an eggshell strength tester (ST120H, Shengtai Ltd, Jinan, China). Yolk color and Haugh units were automatically measured using an egg analyzer (EA-01, ORKA, Ramat Hasharon, Israel). Moreover, 10 eggs were randomly selected from each group to determine the percentage of yolk. Laying rate (%) = total number of eggs/ number of hens × 100%; Average egg weight (g) = average daily total egg weight/average daily total egg number; Average daily feed consumption (g/d) = total amount of feed consumed per day/hens number; FCR = total feed consumption/total egg weight.
Determination of Yolk Cholesterol, Yolk Fatty Acids, Yolk Fatty, and Albumen Amino Acids
Ten cooked egg yolks of each group were used to detect yolk cholesterol, yolk fatty acids, yolk fatty, and whole egg amino acids. Egg yolk was prepared according to the method described by Albuquerque et al. (2016). Chromatographic analysis was performed using an HPLC system (LC-20D, Shimadzu, Kyoto,Japan). A Thermo Accucore XL C18 column (2.0 × 100 mm, 4 μm) was carried out for separation. The mobile phase consisted of A (MeOH) and the total run time of analysis was 10 min at a flow rate of 1.2 mL/min. The column temperature was kept at 30°C. The injected volume was 10 μL and the cholesterol content was determined at 210 nm.Yolk fatty acids and whole egg amino acids were carried out following GB 5009.168 and GB 5009.74, respectively.
Plasma Biochemical Parameters
Immunoglobulin A (Ig A), immunoglobulin M (Ig M), immunoglobulin G (Ig G), interleukin-1 (IL-1), interleukin-5 (IL-5), and tumor necrosis factor-α (TNF-α) were measured by using commercial Elisa kits (Beyotime Biotechnology Ltd, Shanghai, China). Total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), alkaline phosphatase (ALB), blood urea nitrogen (BUN), uric acid (UA), plasma calcium (Ca), and plasma phosphorus (P) were measured by commercial kits (Rayto Biotechnology Ltd, Shenzhen, China).
Safety Inspection of Egg Contents
The detection of Salmonella, aerobic plate count, molds, and coliforms in egg contents were carried out following GB 4789.4-2016, GB 4789.2-2016, GB 4789.15-2016, and GB 4789.3-2016, respectively. Aflatoxin B1 and heavy mental in egg contents were detected severally according to GB 5009.22-2016 and GB 5009.74-2014, respectively.
Headspace-Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS) Analysis
The yolk samples were performed on a gas chromatography-ion mobility spectrometry (GC-IMS) (FlavourSpec, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany) based on an Agilent 490 micro gas chromatograph (GC), equipped an autosampler and headspace sampling unit. The GC equipped with an MXT-5 (15 m × 0.53 mm) column was used for separation at 60°C. Yolk (2 g) was transferred into a 20 mL glass vial sealed with a silicon septum and magnetic metal crimp. The headspace vial was incubated at 90°C for 15 min. A total of 1000 μL solution was then automatically injected via heated syring at 95°C. The carrier gas followed a programmed flow: 2 mL/min for 0 to 2 min, flow ramp up to 100 mL/min in 18 min. The drift tube was kept at 45°C with a drift gas of 150 mL/min. The analytes were ionized in the IMS ionization chamber in positive mode. Each spectrum was 32 scans. SIMCA 14.1 software (Umetrics, Umeå, Sweden) was used to analyze the data. All data were processed with UV scaled before building the principal component analysis (PCA) and orthogonal partial least-squares discrimination analysis (OPLS-DA) model. PCA and OPLS-DA were used to analyze the differences in volatile components among the 4 tested groups.
Intestinal Histology Analysis
Duodenum, jejunum, and ileum were dehydrated with graded alcohol and xylene, and then embedded in paraffin. The paraffin block was cut into 4-μm sections and stained with hematoxylin and eosin. Villus height and crypt depth were determined using an automatic image analyzer (Olympus DP73 camera,Tokyo, Japan). A minimum of 6 well-oriented villus and 10 crypts were measured from different sections of each hen.
16S rRNA Gene Amplicon Sequencing and Analysis
DNA of cecum digesta was extracted by using DNA isolation kits (Qiagen NV, Hilden, Germany). Nearly specific regions were amplified from the DNA samples using the 341F/805R primers (341F primer: 5′-CCTACGGGNGGCWGCAG-3′; 805R primer: 5′-GACTACHVGGGTATCTAATCC-3′). PCR conditions were conducted as the following thermocycling program: denaturation at 98°C for 30 s, 35 cycles of 98°C for 10 s, 54°C for 30 s, and 72°C for 45 s, and a final elongation at 72°C for 10 min. After amplicons were purified and quantified, the V3-V4 region of 16S rRNA was sequenced on an Illumina HiSeq 2500 platform (Illumina, San Diego, CA). The raw FASTQ files were merged using FLASH (v1.2.7) and then demultiplexed and quality-filtered with Trimmomatic (v0.33). The chimeric sequences were removed using UCHIME (v4.2). Sequencing data analysis was conducted with QIIME 1.91 software. Sequences with ≥97% similarity were clustered into operational taxonomic units (OTUs) by open reference arithmetic. The reference OTU sequences were against the Greengenes database. The α-diversity analysis was determined by Chao 1, Shannon, Observed Species, and Simpson indexes. The unweighted UniFrac distances were used to evaluate the diversity between groups. Linear discriminant analysis effect size (LEFSe) was used to identify significantly different species and generated the graphs. The differentially abundant feature was evaluated by false discovery rates (FDR) <0.05 and linear discriminant analysis (LDA) score >2. Bacterial phenotype prediction was determined using BugBase (https://bugbase.cs.umn.edu/). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to identify microbial functions. Comparing the PICRUSt results with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, the KEGG pathway was enriched.
Statistical Analyses
All data analyses were performed on GraphPad 8.0 (San Diego, CA). All data were analyzed using one-way ANOVA. Tukey test was used to correct multiple comparisons. All results were expressed as the mean ± SD, with P < 0.05 considered statistically significance. GraphPad 8.0 software was used to draw pictures.
RESULTS
Contents of Major Components in E. ulmoides Extract
The contents of chlorogenic acid, geniposidic acid, aucubin, and rutin in E. ulmoides extract were 60.74, 63.14, 46.85, and12.15 mg/g, respectively. The extract was used to further research without otherwise specified. The chromatographic peaks of major components (chlorogenic acid, geniposidic acid, aucubin, geniposide, pinoresinol diglucoside, and rutin) standards and E. ulmoides extract are shown in Figure S1.
Laying Performance and Organ Coefficient
No mortality of hens was observed during the whole experiment. The laying rate in C and D groups were 85.31% and 85.56%, respectively, which were 2 percentage points higher than in A group (83.03%), indicating that E. ulmoides extract at a relatively high content could enhance the laying performance of hens (Table 1, Figure S2). However, the average egg weight, average daily feed consumption, FCR, and body weight revealed no significant difference among the 4 tested groups (P > 0.05). Moreover, the body weight-related organ coefficient (liver and spleen) exhibited no significant difference among the 4 tested groups either (P > 0.05, Table 1), suggesting that dietary supplementation E. ulmoides extract did not affect the weights of liver and spleen.
Table 1.
Effects of E. ulmoides extract on laying performance and organ coefficient of hens.
| Category | Item | A | B | C | D |
|---|---|---|---|---|---|
| Laying performance | Laying rate (%) | 83.03 | 83.07 | 85.31 | 85.56 |
| Average egg weight (g) | 60.44 ± 1.22 a | 60.37 ± 1.34 a | 60.75 ± 1.30 a | 60.03 ± 1.42 a | |
| Average daily feed consumption (g) | 101.62 ± 5.99 a | 96.95 ± 6.66 a | 103.43 ± 6.90 a | 103.64 ± 4.92 a | |
| Feed conversion rate (FCR) | 2.03 | 2.04 | 2.00 | 2.02 | |
| Initial body weight (kg) | 1.76 ± 0.14 a | 1.74 ± 0.15 a | 1.79 ± 0.14 a | 1.75 ± 0.15 a | |
| Final body weight (kg) | 1.88 ± 0.12 a | 1.83 ± 0.19 a | 1.86 ± 0.17 a | 1.85 ± 0.16 a | |
| Organ coefficient | Liver (%) | 0.017 ± 0.001 a | 0.019 ± 0.004 a | 0.019 ± 0.023 a | 0.019 ± 0.002 a |
| Spleen (%) | 0.0009 ± 0.0002 a | 0.0010 ± 0.0003 a | 0.0010 ± 0.0003 a | 0.0011 ± 0.0003 a |
Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
Egg Quality
The egg quality related indexes were determined and shown in Table S2. The yolk color, egg-shape index, shell strength, shell thickness, egg albumen height, Haugh unit, percentage of yolk, whole egg protein, and moisture content (yolk and albumen) displayed no significant significant difference among the 4 tested groups (P > 0.05), indicating that feeding with E. ulmoides extract could ensure egg quality remain as normal, no matter under low or high-level treatment.
Albumen Amino Acid and Yolk Fatty Acids
Seven essential amino acids (EAA) were detected in albumen (Table 2). The proportion of EAA in A group (41.45%) was slightly higher than in other groups (<39.87%). Meanwhile, Asp and Glu were the 2 dominant amino acids in albumen and their average contents reached to 1.24 and 1.74 g/100 g, respectively. The proportion of delicious amino acid (DAA) in every group was greater than 45%, indicating that the eggs were of high quality. Moreover, proportion of DAA was slightly elevated among the E. ulmoides extract added groups. Besides, the composition of bitter amino acid (BAA) significantly altered (Arg increase and His decrease, P < 0.05) as supplementation E. ulmoides extract. All of the fatty acids in yolk revealed no significant difference among the 4 tested groups (Table S3), indicating E. ulmoides extract could not induce egg fatty acids variation.
Table 2.
The contents of amino acids under E. ulmoides extract treatment.
| Item | Amino acid (g/100 g) | A | B | C | D |
|---|---|---|---|---|---|
| Delicious amino acid (DAA) | Asp | 1.24 ± 0.01 a | 1.20 ± 0.01 a | 1.24 ± 0.06 a | 1.26 ± 0.04 a |
| Glu | 1.75 ± 0.01 a | 1.69 ± 0.02 a | 1.74 ± 0.08 a | 1.78 ± 0.06 a | |
| Tyr | 0.46 ± 0.01 a | 0.45 ± 0.01 a | 0.46 ± 0.02 a | 0.46 ± 0.02 a | |
| Gly | 0.41 ± 0.01 a | 0.40 ± 0.01 a | 0.42 ± 0.02 a | 0.42 ± 0.01 a | |
| Phe | 0.64 ± 0.01 a | 0.61 ± 0.02 a | 0.63 ± 0.04 a | 0.65 ± 0.02 a | |
| Ala | 0.71 ± 0.01 a | 0.68 ± 0.01 a | 0.70 ± 0.04 a | 0.71 ± 0.02 a | |
| Sweet amino acid (SAA) | Lys | 0.90 ± 0.01 a | 0.88 ± 0.01 a | 0.89 ± 0.04 a | 0.91 ± 0.03 a |
| Pro | 0.45 ± 0.01 a | 0.45 ± 0.01 a | 0.46 ± 0.02 a | 0.47 ± 0.02 a | |
| Ser | 0.98 ± 0.01 a | 0.94 ± 0.01 a | 0.97 ± 0.04 a | 0.98 ± 0.03 a | |
| Thr | 0.59 ± 0.01 a | 0.57 ± 0.01 a | 0.59 ± 0.02 a | 0.60 ± 0.02 a | |
| Bitter amino acid (BAA) | Val | 0.71 ± 0.03 a | 0.70 ± 0.03 a | 0.71 ± 0.03 a | 0.73 ± 0.03 a |
| Leu | 0.52 ± 0.03 a | 0.52 ± 0.02 a | 0.52 ± 0.02 a | 0.53 ± 0.03 a | |
| Met | 0.36 ± 0.01 a | 0.35 ± 0.01 a | 0.36 ± 0.02 a | 0.36 ± 0.01 a | |
| Arg | 0.64 ± 0.01 b | 0.76 ± 0.02 a | 0.77 ± 0.04 a | 0.77 ± 0.04 a | |
| His | 0.46 ± 0.01 a | 0.22 ± 0.01 b | 0.22 ± 0.01 b | 0.23 ± 0.02 b | |
| Ile | 0.52 ± 0.03 a | 0.52 ± 0.02 a | 0.52 ± 0.02 a | 0.53 ± 0.03 a | |
| EAA/TAA (%) | 41.45 | 39.67 | 39.64 | 39.87 | |
| DAA/TAA (%) | 45.94 | 46.19 | 46.34 | 46.35 | |
Abbreviations: BAA, bitter amino acids; DAA, delicious amino acids; EAA, essential amino acids; SAA, sweet amino acids; TAA, total amino acids.
Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
Yolk Volatile Components
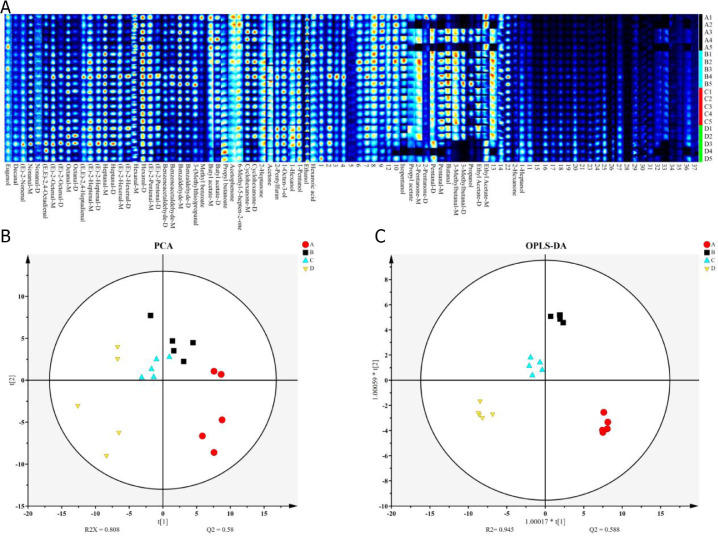
The HS-GC-IMS results showed that the composition and contents of yolk volatile components significantly changed under dietary supplementation E. ulmoides extract, regardless supplementation at low or high content (Figure 1A). The PCA and OPLS-DA analysis indicated the yolk volatile components of each group separated clearly (Figure 1B,C), certifying that E. ulmoides extract could change the flavor of eggs as dosage alteration. Fifty-six certain volatile compounds (with isomer) were determined in yolk, which were related to volatile components variation (Table S4).
Figure 1.
(A) The HS-GC-IMS results of yolk volatile components. (B) and (C) The PCA and OPLS-DA analysis of yolk volatile components of the 4 tested groups, respectively.
Safety Inspection of Egg
Microorganisms, aflatoxins, and heavy metals of eggs among the 4 tested groups were determined (Table S5). The Salmonella in egg contents was not detected. Meanwhile, aerobic plate count, moulds, and coliforms in all tested groups were lower than 100, 3, and 10 CFU/mL, respectively. Aflatoxin B1 and heavy metals (Pb, Cd) levels in egg contents of the 4 tested groups were also lower than 0.2 and 0.05 mg/kg. These results certified that E. ulmoides extract did not bring pathogenic bacteria, aflatoxins, and heavy metals to eggs, which met the safety production standard of the hens industry.
Routine Plasma Biochemical Parameters
The routine plasma biochemical parameters are listed in Table 3. The contents of ALT, ALB, and BUN revealed no significant differences among the 4 tested groups (P > 0.05). However, the AST content significantly increased from 217.81 U/L (A group) to above 255.00 U/L as dietary supplementation E. ulmoides extract (P < 0.05), no matter under low or high-level treatment. Meanwhile, the ALP in A group (313.53 U/L) were significantly higher than in B (289.79 U/L), C (280.38 U/L), and D (232.42 U/L) groups (P < 0.05). Besides, the UA contents in A group (222.91 umol/L) was also significantly lower than in B (190.61 umol/L), C (189.09 umol/L), and D (193.58 umol/L) groups. These results suggested that E. ulmoides extract had influences on liver function and purine metabolism. The plasma Ca and P contents as well as the inflammatory factor (IL-1, IL-6, TNF-α) contents also showed no significant difference among the 4 tested groups (P < 0.05), indicating that E. ulmoides extract could keep hens healthy without leading to inflammation regardless under low or high-level treatment.
Table 3.
Effects of E. ulmoides extract on plasma biochemical parameters.
| Category | Item | A | B | C | D |
|---|---|---|---|---|---|
| Liver function | ALT (U/L) | 10.33 ± 0.53 a | 9.89 ± 1.17 a | 9.09 ± 0.79 a | 9.33 ± 1.08 a |
| AST (U/L) | 217.81 ± 4.38 b | 263.02 ± 5. 00 a | 259.08 ± 5.21 a | 255.43 ± 5.99 a | |
| ALB (g/L) | 31.19 ± 1.42 a | 30.92 ± 0.73 a | 31.57 ± 1.12 a | 31.27 ± 1.27 a | |
| ALP (U/L) | 313.53 ± 9.15 a | 289.79 ± 8.81 b | 280.38 ± 7.23 b | 232.42 ± 6.94 c | |
| Nitrogen metabolism | BUN (mmol/L) | 0.41 ± 0.02 a | 0.40 ± 0.01 a | 0.39 ± 0.02 a | 0.38 ± 0.02 a |
| UA (umol/L) | 222.91 ± 10.93 a | 190.61 ± 9.62 b | 189.09 ± 7.51 b | 193.58 ± 5.90 b | |
| Shell strength-related element | Ca (mmol/L) | 3.09 ± 0.06 a | 2.98 ± 0.08 a | 3.07 ± 0.07 a | 3.00 ± 0.06 a |
| P (mmol/L) | 4.85 ± 0.55 a | 4.43 ± 0.60 a | 4.65 ± 0.61 a | 4.41 ± 0.46 a | |
| Inflammatory factory | TNF-α (ng/L) | 49.08 ± 1.80 a | 49.08 ± 3.67 a | 48.62 ± 3.29 a | 50.37 ± 3.28 a |
| IL-1 (ng/L) | 228.50 ± 6.70 a | 234.84 ± 9.93 a | 231.99 ± 12.58 a | 232.42 ± 12.51 a | |
| IL-6 (ng/L) | 30.38 ± 0.54 a | 30.04 ± 1.59 a | 30.52 ± 1.79 a | 31.59 ± 2.29 a |
Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
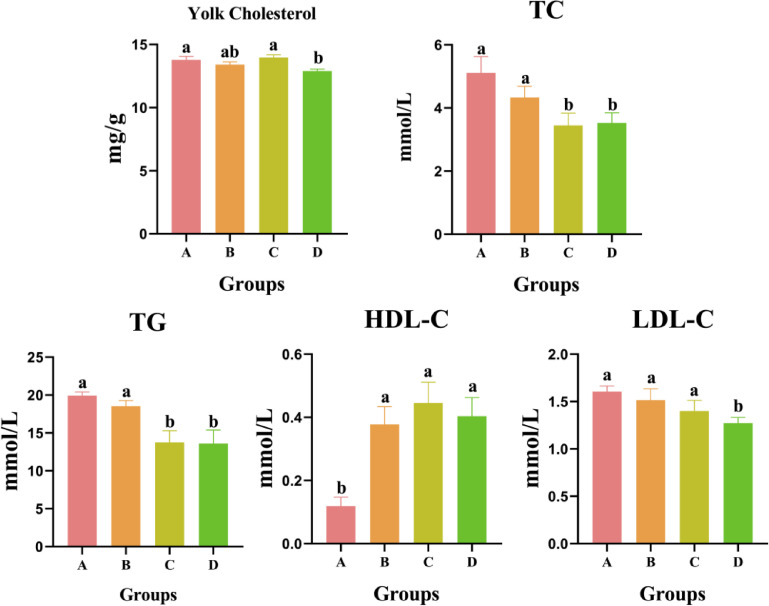
Lipid-Related Indexes in Yolk and Plasma
The lipid-related indexes in yolk and plasma are shown Figure 2. The yolk cholesterol content in D group was 12.90 mg/g and was significantly lower than in A (13.78 mg/g) and C (13.96 mg/g) groups (P < 0.05). Meanwhile, the plasma TG and TC contents in C and D groups were also significantly lower than in A and B groups (P < 0.05). Moreover, when compared with A group (1.61 mmol/L), plasma LDL-C content in D group decreased to 1.19 mmol/L, with significant difference (P < 0.05). Exception the down-regulation indexes, the plasma HDL-C content in B, C, and D groups increased to 0.38, 0.45, and 0.40 mmol/L, which were 3.2, 3.8, and 3.3 times higher than in A group (0.12 mmol/L, P < 0.05).
Figure 2.
Effects of E. ulmoides extract on lipid related indexes in yolk and plasma. Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
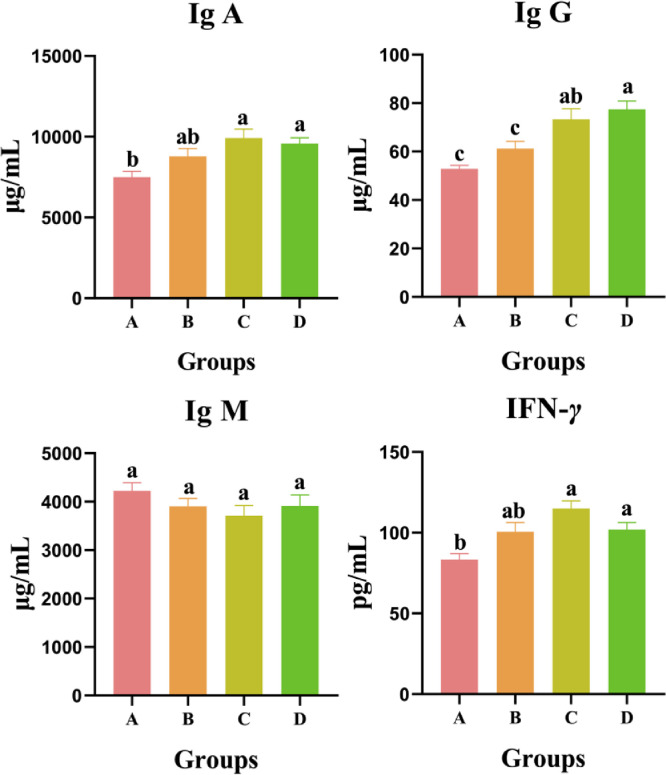
Plasma Immunoglobulin Indexes
Plasma immunoglobulin indexes are shown in Figure 3. The plasma IgA in A group were 7,498.87 μg/mL, which was significantly lower than in C (9,921.27 μg/mL) and D (9,584.16 μg/mL) groups (P < 0.05). Similar situation was observed for plasma IgG and IFN-γ, where the plasma IgG and IFN-γ in C and D groups were also significantly higher than in A group (P < 0.05). Meanwhile, plasma Ig G in D group (77.48 μg/mL) was significantly higher than in B group (61.20 μg/mL, P < 0.05). However, no significant difference of plasma IgM was detected among the 4 tested groups (P > 0.05).
Figure 3.
Effects of E. ulmoides extract on plasma immunoglobulin indexes. Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
Intestinal Histology
The villus height of duodenum, jejunum, and ileum in A group were 1,235, 1,141, and 890 μm, respectively, and were significantly lower than in D (1,435, 1,358, and 1,050 μm) group (P < 0.05, Figure 4A–D). Down-regulation in crypt depth of duodenum, jejunum, and ileum were observed under different E. ulmoides extract treatments. Meanwhile, the crypt depth of jejunum and ileum in A group were significantly higher than in D group (P < 0.05, Figure 4E). Based on the alteration of villus height and crypt depth, the villus height to crypt depth ratio of duodenum, jejunum, and ileum changed too. The ratios in A and B groups were significantly lower than in D group (P < 0.05). Moreover, the ratios of duodenum and jejunum in C group were also significantly higher than in A group (P < 0.05). These results implied that dietary supplementation E. ulmoides extract was beneficial to intestinal morphology improvement, especially supplementation at a relative high content.
Figure 4.
Effect of E. ulmoides extract on intestinal morphology. (A–D) Representative photomicrographs (5x) of villus in the duodenum, jejunum, and ileum of the 4 tested groups. (E) Villus height, crypt depth, and villus height to crypt depth ratio of duodenum, jejunum, and ileum in the 4 tested groups. Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
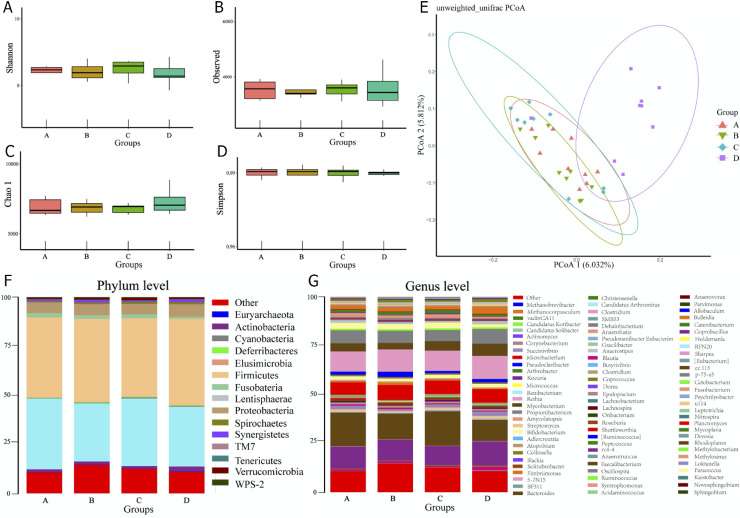
Gut Microbiota
Alpha-diversity indexes of Shannon, Observed Species, Chao 1, and Simpson exhibited no significant differences among the 4 tested groups (P < 0.05, Figure 5A–D), whereas the result of β-diversity by principal coordinate analysis (PCoA) showed the gut microbiota communities in D group distinguished from the A, B, and C groups clearly (Figure 5E). Moreover, the composition of gut microbiota among the 4 tested groups were observably changed both at phylum and genus levels (Figure 5F,G). The above-mentioned results revealed that the gut microbiota communities in the cecum could alter under the treatment of E. ulmoides extract, especially under high-level treatment.
Figure 5.
Overall structure and composition of microbiota in cecum digesta of the 4 tested groups. (A–D) Shannon, Observed species, Chao 1, and Simpson indexes of the gut microbiota, respectively. (E) Unweighted Unifrac PCoA estimates for the gut microbiota. (F, G) The relative abundance of bacterial at phyla and genus levels, respectively. * P < 0.05, ** P < 0.01 compared to A group.
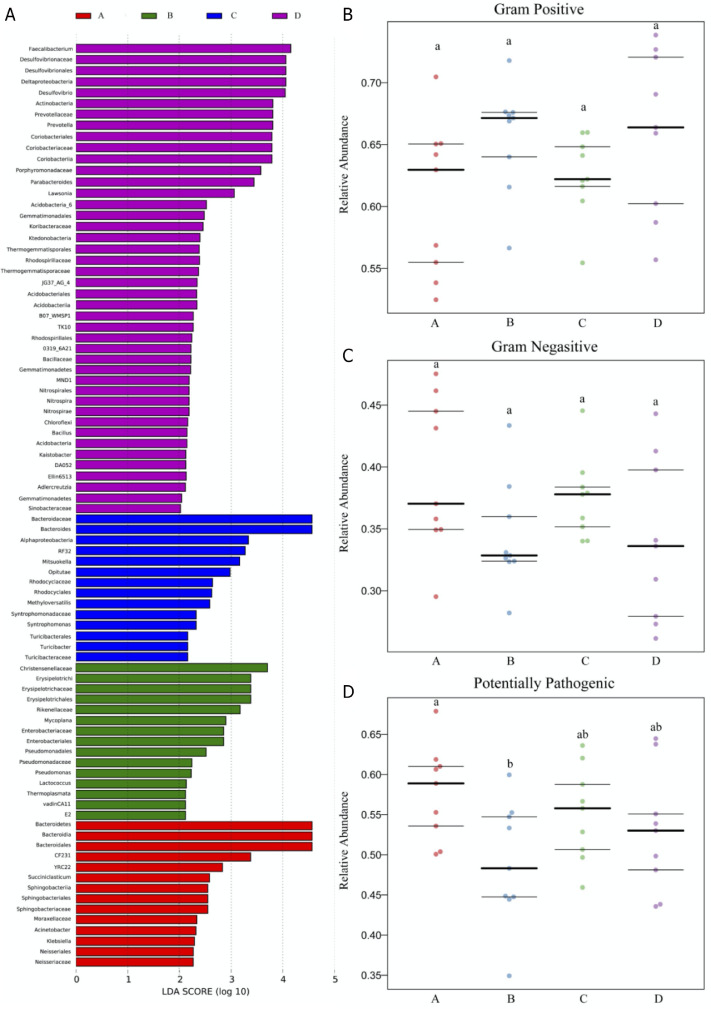
Discriminative LEFSe Analysis and Bacterial Phenotype Prediction
The LDA score (log 10 > 2) indicated 86 certain genus revealed significant difference among the 4 tested groups, with 14, 15, 14, and 43 in A to D group (Figure 6A), certifying that supplementation of E. ulmoides extract at a relative high content contributed to gut microbiota communities alteration. When compared with A group, up-regulation and down-regulation of gram positive and gram negative were found in B and D groups, without significance (P > 0.05, Figure 6B,C), indicating that gut microbiota communities would be affected by E. ulmoides extract. Meanwhile, down-regulation of potentially pathogenic was observed as supplementation E. ulmoides extract and significant difference between A and B groups were determined (P < 0.05), suggesting that E. ulmoides extract could reduce pathogen invasion.
Figure 6.
LEFSe analysis and bacterial phenotype prediction result of microbiota composition in cecum digesta of the 4 tested groups. (A) Gut microbiota difference at the genus level among the 4 tested groups based on LDA score (log > 2); (B–D) The relative abundance of gram-positive, gram-negative, and pathogenic potential, respectively. Different small letters indicated significant differences at P < 0.05 level of Tukey test under different treatments.
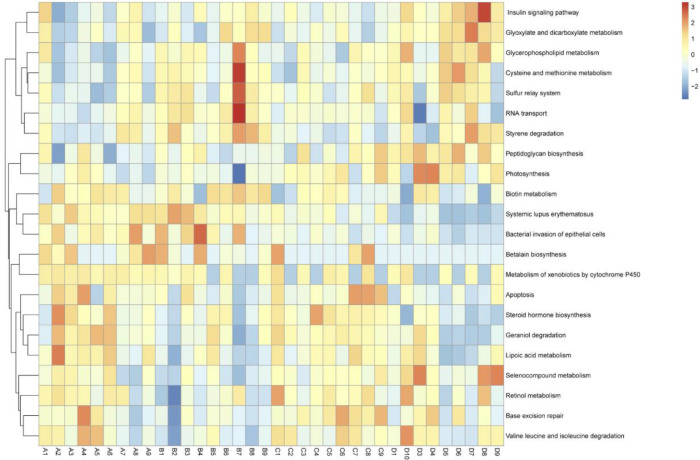
KEGG Pathway Based on PICRUSt Gene Prediction Information
Twenty-two significant enriched KEGG pathways among the 4 tested groups were predicted by PICRUSt analysis (P < 0.05, Figure 7), and these pathways in D group revealed apparently difference with them in A group. These enrichment pathways might involvement with lipid metabolism and immunity variation, such as insulin signaling pathway, systemic lupus erythematosus, and bacterial invasion of epithelial cells pathways.
Figure 7.
Heat map of significant enrichment KEGG pathway of the 4 tested groups.
DISCUSSION
Laying Performance Enhancing by Dietary Supplementation E. ulmoides Extract
Numerous studies had reported that egg production (laying rate) increased in hens under dietary supplementation medical plant extract, such as Tribulus terrestris extract (increase 8.8%, Abad et al., 2020), ginger extract (increase 2.2%, An et al., 2019), allium extract (increase 6.3%, Akbari and Torki, 2016), and mulberry leaf extract (increase 0.9%, Lin et al., 2017). However, the effects of E. ulmoides extract on laying hens production were still limited. In this study, 2 percentage points elevation in laying rate was observed by adding 0.2% and 0.5% E. ulmoides extract, indicating that supplementation E. ulmoides extract at a relative high content could enhance laying performance of hens. Comparison with the above-mentioned plants, although the nutritional compounds and clinical applications of E. ulmoides are not exactly the same with the them, these results might a corroborative evidence of that E. ulmoides extract can heighten laying performance of hens based on some similar active ingredients, such as flavonoids and polyphenols. Differ from the laying performance elevation, the body weight of hens did not increase with a comparison of control group, which was consistent with our previous reports that the body weight of broilers did not alter under different E. ulmoides leaf powder treatments (Wang et al., 2018), indicating the growth performance of hens were not well associated with E. ulmoides treatment. Therefore, the effects of E. ulmoides extract on increase egg production might ascribe to numerous natural components in E. ulmoides belong to phytoestrogen, for example, aucubin and wogonin (Wang et al., 2011), which potential function as estrogen receptor modulators in hens and contribute to egg production.
Since E. ulmoides is rich in polysaccharides and possess a variety of nutrients, the extracts is suitable for microbial reproduction and is easily be contaminated when store. As the long-term experiment period, the extract potential encounter microbial pollution. Simultaneously, presence of food microorganisms, aflatoxins B1, and heavy metals will cause the growth rate, egg production, and egg quality decrease (Jones et al., 1995; Fouad et al., 2019; Ma et al., 2020). Consideration of the these points, although the extract used in this experiment met the safety standards, we also detected these indexes of egg contents and none of them were detected in eggs, indicating that dietary supplementation E. ulmoides extract did not affect laying performance through bringing pathogenic bacteria, aflatoxins, and heavy metals to eggs. Our findings confirmed that supplementation E. ulmoides extract at a relative high content could enhance laying performance, which was green and safety for the hens industry.
Flavor of Egg Changed by Dietary Supplementation E. ulmoides Extract
Research evidences have demonstrated that dietary inclusion of plant extract cause changes in amino acid metabolism and fatty acid composition in mature laying hens, so that the flavor of egg will alter (Kaya et al., 2013). In this study, the composition of amino acid and fatty acid were determined. The proportion of EAA descending and DAA rising were observed, and composition of BAA changed too (His increase and Arg decrease) as dietary supplementation E. ulmoides extract, no matter under low or high-level treatment. These results indicated the composition of amino acid would alter to copy with feeding materials variation, which were consistent with our previous findings that supplementation E. ulmoides leaf powder changed the meat amino acid contents of broilers (Wang et al., 2018). However, although changes in yolk amino acid were observed, we did not find any variation in fatty acid among the 4 tested groups. This might own to the low fatty acid content in E. ulmoides extract (An and Guo, 1998), which could not make fatty acid accumulation in eggs. Besides, our previous studies have established the fingerprint of flavor compounds in E. ulmoides bark and leaf by HS-GC-IMS and PLS-DA methods and further certify the volatile components from E. ulmoides are significantly changed by different microbial strains through biofermentation technology, thus resulting in flavor substance variation (Wang et al., 2020b,c). Hence, the volatile components of eggs under E. ulmoides extract treatment were therefore detected. The HS-GC-IMS results also showed that the composition and contents of yolk volatile components significantly varied as supplementation E. ulmoides extract. Consequently, the yolk flavor components under E. ulmoides extract treatment might be induced alteration via gut microbiota mediating, which cocertified the changes happened in flavor of eggs. Together with amino acid contents, all of these alterations manifested the flavor of eggs would significantly change as dietary supplementation E. ulmoides extract. Moreover, because we major focused on the effects of E. ulmoides on laying performance and body health, we neglected to investigate the meat quality of hens. In order to evaluate the whole effects of E. ulmoides on laying hens, the meat quality will be analyzed and a conjoint analysis with egg production will be conducted in our next work.
Body Health Improvement by Dietary Supplementation E. ulmoides Extract
Literatures related to hyperlipidemia amelioration often announce that natural products have benefits in lipid metabolism, such as DMY from Ampelopsis grossedentata can significantly reduce TG, TC, and LDL-C levels and increase HDL-C contents in mice and high-fat diet hamsters (Liu et al., 2017; Fan et al., 2020). Meanwhile, E. ulmoides is also reported to take function in blood lipids improving based on its multiple pharmacological components (Wang et al., 2019). Compared with A group, plasma TG, TC, and LDL-C contents significant decreasing and HDL-C contents significant increasing were determined in D group, indicating dietary supplementation E. ulmoides extract contributed to lower blood lipid of hens. Previous researches have showed that E. ulmoides can ameliorate hypertriglyceridaemia in rats by up-regulating genes involved in hepatic α-, β-, and ω-oxidation (Kobayashi et al, 2012) and the active ingredients of E. ulmoides leaves (flavonoids and phenolics) participate in regulating blood lipid metabolism via mediating PPARγ expression (Gong et al, 2022). Consequently, the mechanism of E. ulmoides on lipid-decreasing must be a complex network based on its numerous active ingredients and the specific mechanisms need deeper research. Meanwhile, since most of the yolk cholesterol was synthesized in liver and related to lipid metabolism of hens (Naber, 1976), the yolk cholesterol was also analyzed in this study. Evidence have shown that feeding plant extract can reduce yolk and blood cholesterol levels of hens, such as sumac extract and ginger extract (Gurbuz and Salih, 2017). Consistently, our results also showed the yolk cholesterol content in D group significant descended if comparison with A group. Therefore, it could easily conclude that high level E. ulmoides extract contributed to ameliorate hyperlipidemia of hens. Ig A and Ig M are the main classes of Ig and play a critical role in immune regulation in poultry (Moon et al., 2021). Interestingly, plasma Ig A, Ig G, and IFN-γ contents also increased in D group, indicating that supplementation E. ulmoides extract at a relative high content could enhance immunity of hens. High concentration of chlorogenic acid from E. ulmoides had been certified to stimulate immune response of chickens (Abaidullah et al., 2021), which was in accordance with our results that high level E. ulmoides extract benefited in hens immunity elevation. Exception lipid metabolism and immune regulation, E. ulmoides extract further exhibited the ability of UA-lowering. Significant decrease of UA content in B, C, and D groups were determined, which was consistent with the antihyperuricemia effect of E. ulmoides in hyperuricemic mice (Fang et al., 2019). Comprehensive the above-mentioned points, it was therefore concluded that high level E. ulmoides extract revealed stronger lipid metabolism and immune regulation abilities, and further exhibited huge property in hyperuricemia therapy.
Gut Microbiota Well Associated with Hens Production Elevation via E. ulmoides Extract Mediating
According to intestinal histomorphology, a higher villus height to crypt depth ratio and villus height facilitate nutrients absorption (Ege et al., 2019; Chen et al., 2021). In this study, we observed that the villus height and the villus height to crypt depth ratio in D group significantly increased with a comparison of A group, suggesting that supplementation E. ulmoides extract at a relative high content was beneficial for intestinal histology changing and nutrients absorption. Meanwhile, plenty of studies have showed that gut microbiota is closely related to nutrient absorption (Zhao et al., 2019). In our research, the gut microbiota communities significantly altered at phylum and genus levels. Moreover, half of the certain microbiota with significant difference at genus level highly enriched in D group, such as amino acid metabolism related beneficial bacterium Prevotella (Huang et al., 2020c), which was in accordance with amino acid alteration in eggs. As former results had indicated that the weight of hens and eggs did not alter under E. ulmoides extract treatment and the plasma Ca and P contents (closely related to egg quality, Xiao et al., 2019) kept at the equilibrium level between control and E. ulmoides extract treatment, we thus concluded that dietary supplementation E. ulmoides extract could enhance laying performance on the basis of maintaining egg quality as normal. The reason of E. ulmoides to maintain egg quality was major attributable to E. ulmoides extract facilitate nutrients absorption via intestinal histomorphology changing and gut microbiota communities variation, which contributed to materials conversion of hens. Since the experiment hens were aged, the growth performance of hens did not be induced improving. Therefore, the nutrients absorption enhancement mainly promoted the egg production elevation without affecting the weight and the quality of eggs. Similar situation have been observed by dietary supplementation Mentha arvensis and Geranium thunbergii, which improve egg production and keep egg quality as normal (Dilawar et al., 2021). Furthermore, based on numerous components belong to phytoestrogen, E. ulmoides is a rich source of estrogen receptor modulators (Yao et al., 2007). Therefore, E. ulmoides extract increase the estrogen level of aged hens, which participate in increase egg production without affecting egg quality. However, the accurate mechanism of E. ulmoides to maintain egg quality isn't that simple and need further study.
Gut microbiota is necessary for immune homeostasis, and a healthy gut microbiota is important for the regulation of the host immune responses (Belkaid and Harrison, 2017). In this study, certain microbiota with high abundance at genus level in D group are involvement with immune response, such as Faecalibacterium (Yeoh et al., 2021), Desulfovibrionaceae (Su et al., 2021), and Prevotella (Larsen, 2017). It is reported that genus Prevotella could promote Ig A and Ig G response in sera and synovial fluids of arthritis patients (Moen et al., 2003), and genus Faecalibacterium and Desulfovibrionaceae can take function in inflammation regulation (Schneeberger et al., 2015; Chang et al., 2019). Accordance with certain microbiota high abundance in D group, higher contents of plasma Ig A and Ig G in D group were also detected. Besides, the plasma IFN-γ level also rose in D group when compared with A group. These results verified that dietary supplementation E. ulmoides extract at high level could simulate immune response via mediating gut microbiota communities alteration. Except for these immune response related microbiota, the pathogenic bacterium in the gastrointestinal tract also affect immunity regulation, and most of pathogenic bacterium belong to gram negative bacteria (Xiao et al., 2014). In our research, the bacterial phenotype prediction result showed that the gram negative bacteria in D group significantly decreased if comparison with A group, implying the high level E. ulmoides extract treatment could alleviate inflammation as gram negative bacteria down-regulation. Meanwhile, PICRUSt functional prediction analysis revealed that the inhibition of systemic lupus erythematosus and bacterial invasion of epithelial cells pathways were enriched in D group, which was consistent with gram negative bacteria descending in D group. Former study have announced that inflammation had impacts on production performance of agricultural animals (Mani et al., 2012). Hence, these results confirmed that high level E. ulmoides extract could enhance immune response and reduce pathogen invasion, which contributed to improving production performance and body health of hens through inflammation relieving.
Numerous studies have indicated that poultry has higher blood glucose levels and insulin resistance, despite the level of endogenous hyperactive insulin circulating at normal levels (Simon et al., 2011;Simon et al., 2012). Moreover, insulin can significantly participate in regulation of hepatic lipid metabolism and the development of steatosis during in insulin resistance rodent models and humans (Titchenell et al., 2017). In this study, bacteria functional prediction revealed that the insulin signaling pathway was significantly enriched in D group. As the insulin signaling pathway was related to insulin metabolism and resulted in lipid homeostasis, the higher abundance of gut microbiota in D group contributed to lipid metabolism. Moreover, the enrichment pathway and profiles of gut microbiota alteration were in agreement with the lipid related indexes variation in D group (plasma TC, TG, LDL-C levels decrease and HDL-C increase). It is reported that insulin resistance is closely associated with poor egg production, high mortality, and abnormal ovarian morphology (Walzem and Chen, 2014; Chuang et al., 2020). Based on the insulin metabolism heightened in D group, the effect of insulin resistance would alleviate. Thus, the egg production, mortality, and abnormal ovarian morphology would therefore improve. Hence, it could conclude that gut microbiota enrichment in insulin signaling pathway benefited in blood lipid amelioration and insulin resistance alleviation in hens, and further manifested that dietary supplementation E. ulmoides extract at a high content could therefore enhance production performance and improve body health.
Comprehensive the above-mentioned points, the egg production elevation under E. ulmoides extract treatment might ascribe to 3 aspects: 1) E. ulmoides extract facilitate nutrients absorption via intestinal histomorphology changing and gut microbiota communities variation; 2) E. ulmoides extract stimulate immune response and reduce pathogen invasion, so that contribute to production performance elevation through inflammation relieving; 3) E. ulmoides extract promote egg production, mortality, and abnormal ovarian morphology of hens improvements via regulating gut microbiota participation in insulin signaling pathway, which benefit in blood lipid amelioration and insulin resistance alleviation.
CONCLUSION
In the present study, the effects of E. ulmoides extract on laying performance and body health of hens are investigated. First of all, egg production elevation as well as egg quality maintaining as normal are observed as supplementation E. ulmoides extract at a relative high content. Second, variations in albumen amino acid and yolk volatile components suggest flavor of egg will change by dietary supplementation with E. ulmoides extract. Thirdly, high level E. ulmoides extract exhibit huge property in lipid-decreasing, immune enhancing, and uric acid dropping, which benefits in body health improving of hens. At last, E. ulmoides extract increase egg production is tightly involvement with gut microbiota communities variation, which participate in nutrients absorption, inflammation relieving, blood lipid amelioration, and insulin-resistance alleviation. These results can deepen our understanding of E. ulmoides extract application in hens production and provided a scientific basis for E. ulmoides extract used as a feed additive in the hens industry.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 42107020), the GDAS’ Project of Science and Technology Development (2019GDASYL-0502003), the Science and Technology Project of Hunan Province (2020SK2028), the Traditional Chinese Medicine of Hunan Province (2021238), and Guangdong Zhongkangyuan Biotechnology Co., Ltd.
DISCLOSURES
The authors declare that no potential conflicts of interest are disclosed.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101650.
Appendix. Supplementary materials
REFERENCES
- Abad P., Arroyo-Manzanares N., Ariza J.J., Baños A., García-Campaña A.M. Effect of Allium extract supplementation on egg quality, productivity, and intestinal microbiota of laying hens. Animals. 2020;11:41. doi: 10.3390/ani11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaidullah M., Peng S., Song X., Zou Y., Li L., Jia R., Yin Z. Chlorogenic acid is a positive regulator of MDA5, TLR7 and NF-κB signaling pathways mediated antiviral responses against Gammacoronavirus infection. Int. Immunopharmacol. 2021;7 doi: 10.1016/j.intimp.2021.107671. [DOI] [PubMed] [Google Scholar]
- Abdallah A., Zhang P., Zhong Q., Sun Z. Application of traditional chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug. Metab. 2019;20:54–64. doi: 10.2174/1389200219666180523102920. [DOI] [PubMed] [Google Scholar]
- Akbari M., Torki M. Effects of adding aqueous extract of Tribulus terrestris to diet on productive performance, egg quality characteristics, and blood biochemical parameters of laying hens reared under low ambient temperature (6.8 ± 3°C) Int. J. Biometeorol. 2016;60:867–871. doi: 10.1007/s00484-015-1079-6. [DOI] [PubMed] [Google Scholar]
- Albuquerque T.G., Oliveira M.B., SanchesSilva A., Costa H.S. Cholesterol determination in foods: comparison between high performance and ultrahigh performance liquid chromatography. Food. Chem. 2016;193:18–25. doi: 10.1016/j.foodchem.2014.09.109. [DOI] [PubMed] [Google Scholar]
- An Q., Guo Z. GC-MS analysis of fatty acid compounds in Eucommia ulmoides leaves. J. Hebei Univ. (Natural Science Edition) 1998;18:372–374. [Google Scholar]
- An S., Liu G., Guo X., An Y., Wang R. Ginger extract enhances antioxidant ability and immunity of layers. Anim. Nutr. 2019;5:407–409. doi: 10.1016/j.aninu.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M., Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: a review. Crit. Rev. Food. Sci. Nutr. 2018;58:486–511. doi: 10.1080/10408398.2016.1194256. [DOI] [PubMed] [Google Scholar]
- Bacanlı M., Başaran N. Importance of antibiotic residues in animal food. Food. Chem. Toxicol. 2019;125:462–466. doi: 10.1016/j.fct.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Harrison O.J. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chang C.J., Lin T.L., Tsai Y.L., Wu T.R., Lai W.F., Lu C.C., Lai H.C. Next generation probiotics in disease amelioration. J. Food Drug. Anal. 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhang H., Du E., Jin F., Zheng C., Fan Q., Zhao N., Guo W., Zhang W., Huang S., Wei J. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poult. Sci. 2021;100:835–843. doi: 10.1016/j.psj.2020.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ying G.G., Deng W.J. Antibiotic residues in food: extraction, analysis, and human health concerns. J. Agric. Food. Chem. 2019;67:7569–7586. doi: 10.1021/acs.jafc.9b01334. [DOI] [PubMed] [Google Scholar]
- Chuang W.Y., Hsieh Y.C., Chen L.W., Lee T.T. Evaluation of the relationship between adipose metabolism patterns and secretion of appetite-related endocrines on chicken. Animals. 2020;10:1282. doi: 10.3390/ani10081282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilawar M.A., Mun H.S., Rathnayake D., Yang E.J., Seo Y.S., Park H.S., Yang C.J. Egg quality parameters, production performance and immunity of laying hens supplemented with plant extracts. Animals. 2021;11:975. doi: 10.3390/ani11040975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Li X., Liu Y., Wang S., Cheng D. Protection mechanisms underlying oral administration of chlorogenic acid against cadmium-induced hepatorenal injury related to regulating intestinal flora balance. J. Agric. Food Chem. 2021;69:1675–1683. doi: 10.1021/acs.jafc.0c06698. [DOI] [PubMed] [Google Scholar]
- Ege G., Bozkurt M., Koçer B., Tüzün A.E., Uygun M., Alkan G. Influence of feed particle size and feed form on productive performance, egg quality, gastrointestinal tract traits, digestive enzymes, intestinal morphology, and nutrient digestibility of laying hens reared in enriched cages. Poult. Sci. 2019;98:3787–3801. doi: 10.3382/ps/pez082. [DOI] [PubMed] [Google Scholar]
- European Commission. 2005. Ban on antibiotics as growth promoters in animal feed enters into effect. Press Release Database.
- Fan L., Qu X., Yi T., Peng Y., Jiang M., Miao J., Xiao P. Metabolomics of the protective effect of Ampelopsis grossedentata and its major active compound dihydromyricetin on the liver of high-fat diet hamster. Evid. Based. Complement. Alternat. Med. 2020;35:1–15. doi: 10.1155/2020/3472578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C., Chen L., He M., Luo Y., Zhou M., Zhang N., Yuan J., Wang H., Xie Y. Molecular mechanistic insight into the anti-hyperuricemic effect of Eucommia ulmoides in mice and rats. Pharm. Biol. 2019;57:112–119. doi: 10.1080/13880209.2019.1568510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A.M., Ruan D., El-Senousey H.K., Chen W., Jiang S., Zheng C. Harmful effects and control strategies of aflatoxin B1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry: review. Toxins. 2019;11:176. doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health. Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gong M., Su C., Fan M., Wang P., Cui B., Guo Z., Liang S., Yang L., Liu X., Dai L., Wang Z. Mechanism by which Eucommia ulmoides leaves regulate nonalcoholic fatty liver disease based on system pharmacology. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114603. [DOI] [PubMed] [Google Scholar]
- Gu T., Li G., Wu X., Zeng T., Xu Q., Li L., Vladyslav S., Chen G., Lu L. Effects of immunopotentiators on biochemical parameters, proinflammatory cytokine, and nonspecific immune responses in Shaoxing ducklings. Poult. Sci. 2020;99:5461–5471. doi: 10.1016/j.psj.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuz Y., Salih Y.G. Influence of sumac (Rhus Coriaria L.) and ginger (Zingiber fficinale) on egg yolk fatty acid, cholesterol and blood parameters in laying hens. J. Anim. Physiol. Anim. Nutr. 2017;101:1316–1323. doi: 10.1111/jpn.12652. [DOI] [PubMed] [Google Scholar]
- Huang J., Tong X.F., Yu Z.W., Hu Y.P., Zhang L., Liu Y., Zhou Z.X. Dietary supplementation of total flavonoids from Rhizoma Drynariae improves bone health in older caged laying hens. Poult. Sci. 2020;99:5047–5054. doi: 10.1016/j.psj.2020.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Hu Y., Tong X., Zhang L., Yu Z., Zhou Z. Untargeted metabolomics revealed therapeutic mechanisms of icariin on low bone mineral density in older caged laying hens. Food Funct. 2020;11:3201–3212. doi: 10.1039/c9fo02882j. [DOI] [PubMed] [Google Scholar]
- Huang S., Ji S., Yan H., Hao Y., Zhang J., Wang Y., Cao Z., Li S. The day-to-day stability of the ruminal and fecal microbiota in lactating dairy cows. Microbiol. Open. 2020;9:e990. doi: 10.1002/mbo3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F.T., Rives D.V., Carey J.B. Salmonella contamination in commercial eggs and an egg production facility. Poult. Sci. 1995;74:753–757. doi: 10.3382/ps.0740753. [DOI] [PubMed] [Google Scholar]
- Kalantar M., Hosseini S.M., Yang L., Raza S.H.A., Gui L., Rezaie M., Syed S.F., Gui L., Rezaie M., Khojastekey M. Performance, immune, and carcass characteristics of broiler chickens as affected by thyme and licorice or enzyme supplemented diets. Open J. Anim. Sci. 2017;7:105–109. [Google Scholar]
- Kaya A., Kaya H., Macit M., Çelebi Ş., Esenbuga N., Yoruk M.A., Karaoglu M. Effects of dietary inclusion of plant extract mixture and copper into layer diets on egg yield and quality, yolk cholesterol and fatty acid composition. Kafkas Univ. Vet. Fak. Derg. 2013;19:673–679. [Google Scholar]
- Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017) Palgrave. Commun. 2018;4:96. [Google Scholar]
- Kobayashi Y., Hiroi T., Araki M., Hirokawa T., Miyazawa M., Aoki N., Kojima T., Ohsawa T. Facilitative effects of Eucommia ulmoides on fatty acid oxidation in hypertriglyceridaemic rats. J. Sci. Food Agric. 2012;92:358–365. doi: 10.1002/jsfa.4586. [DOI] [PubMed] [Google Scholar]
- Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.C., Lee M.T., Chang S.C., Chang Y.L., Shih C.H., Yu B., Lee T.T. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult. Sci. 2017;96:1191–1203. doi: 10.3382/ps/pew350. [DOI] [PubMed] [Google Scholar]
- Liu T.T., Zeng Y., Tang K., Chen X., Zhang W., Xu X.L. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis. 2017;262:39–50. doi: 10.1016/j.atherosclerosis.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Long L.N., Kang B.J., Jiang Q., Chen J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Shi Y.Z., Wu Q.J., Wang Y.Q., Wang J.P., Liu Z.H. Effects of varying dietary intoxication with lead on the performance and ovaries of laying hens. Poult. Sci. 2020;99:4505–4513. doi: 10.1016/j.psj.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V., Weber T.E., Baumgard L.H., Gabler N.K. Growth and development symposium: endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 2012;90:1452–1465. doi: 10.2527/jas.2011-4627. [DOI] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen K., Brun J.G., Madland T.M., Tynning T., Jonsson R. Immunoglobulin G and A antibody responses to Bacteroides forsythus and Prevotella intermedia in sera and synovial fluids of arthritis patients. Clin. Diagn. Lab. Immunol. 2003;10:1043–1050. doi: 10.1128/CDLI.10.6.1043-1050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.G., Lee S.K., Lee W.D., Oh J.S., Kothari D., Kim S.K. Effect of dietary supplementation of a phytogenic blend containing Schisandra chinensis, Pinus densiflora, and Allium tuberosum on productivity, egg quality, and health parameters in laying hens. Anim. Biosci. 2021;34:285–294. doi: 10.5713/ajas.20.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil P., Bedi J., Moudgil A.D., Gill J., Aulakh R. Emerging issue of antibiotic resistance from food-producing animals in India: perspective and legal framework. Food Rev. Int. 2018;34:447–462. [Google Scholar]
- Naber E.C. The cholesterol problem. the egg and lipid metabolism in the laying hen. Poult. Sci. 1976;55:14–30. doi: 10.3382/ps.0550014. [DOI] [PubMed] [Google Scholar]
- Ong V.Y., Tan B.K. Novel phytoandrogens and lipidic augmenters from Eucommia ulmoides. BMC Complement Altern. Med. 2007;7:3. doi: 10.1186/1472-6882-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M., Everard A., Gómez-Valadés A.G., Matamoros S., Ramírez S., Delzenne N.M., Gomis R., Claret M., Cani P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J., Milenkovic D., Godet E., Cabau C., Collin A., Metayer-Coustard S., Rideau N., Tesseraud S., Derouet M., Crochet S., Cailleau-Audouin E., Hennequet-Antier C., Gespach C., Porter T.E., Duclos M.J., Dupont J., Cogburn L.A. Insulin immuno-neutralization in fed chickens, effects on liver and muscle transcriptome. Physiol. Genom. 2012;44:283–292. doi: 10.1152/physiolgenomics.00057.2011. [DOI] [PubMed] [Google Scholar]
- Simon J., Rideau N., Taouis M., Dupont J. Plasma insulin levels are rather similar in chicken and rat. Gen. Comp. Endocrinol. 2011;171:267–268. doi: 10.1016/j.ygcen.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Su L., Li D., Su J., Zhang E., Chen S., Zheng C., Luo T., Li M., Chen X., Huang G., Xie Y., Li S. Polysaccharides of sporoderm-broken spore of Ganoderma lucidum modulate adaptive immune function via gut microbiota regulation. Evid. Based Complement. Alternat. Med. 2021;23 doi: 10.1155/2021/8842062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titchenell P.M., Lazar M.A., Birnbaum M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol. Metab. 2017;28:497–505. doi: 10.1016/j.tem.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzem R.L., Chen S.E. Obesity-induced dysfunctions in female reproduction: lessons from birds and mammals. Adv. Nutr. 2014;5:199–206. doi: 10.3945/an.113.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.Y., Tang L., He J.W., Li J., Wang Y.Z. Ethnobotany, phytochemistry and pharmacological properties of Eucommia ulmoides: a review. Am. J. Chin. Med. 2019;47:259–300. doi: 10.1142/S0192415X19500137. [DOI] [PubMed] [Google Scholar]
- Wang H., Li M.C., Yan J., Yang D., Su Y.F., Fan G.W., Zhu Y., Gao X.M., Paoletti R. Estrogenic properties of six compounds derived from Eucommia ulmoides Oliv. and their differing biological activity through estrogen receptors α and β. Food Chem. 2011;129:408–416. doi: 10.1016/j.foodchem.2011.04.092. [DOI] [PubMed] [Google Scholar]
- Wang X.J., Ding L.M., Wei H.Y., Jiang C.X., Yan Q., Hu C.S., Jia G.X., Zhou Y.Q., Henkin Z., Degen A.A. Astragalus membranaceus root supplementation improves average daily gain, rumen fermentation, serum immunity and antioxidant indices of Tibetan sheep. Animal. 2021;15 doi: 10.1016/j.animal.2020.100061. [DOI] [PubMed] [Google Scholar]
- Wang X., Peng S., Wang Z.H., Peng M.J., Zhang M.L., Yang Q.L. Effects of Eucommia ulmoides leaf powder in diet on growth performance and meat quality of luhua chicken. Hans J. Food Nutr. Sci. 2018;7:288–296. [Google Scholar]
- Wang Y., Liao D., Qin M., Li X. Simultaneous determination of catalpol, aucubin, and geniposidic acid in different developmental stages of rehmannia glutinosa leaves by high performance liquid chromatography. J. Anal. Methods. Chem. 2016;2016 doi: 10.1155/2016/4956589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Peng S., Peng M., She Z., Yang Q., Huang T. Adsorption and desorption characteristics of polyphenols from Eucommia ulmoides Oliv. leaves with macroporous resin and its inhibitory effect on α-amylase and α-glucosidase. Ann. Transl. Med. 2020;8:1004. doi: 10.21037/atm-20-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Peng M., She Z., Qiu Y., Yang Q., Zhang M., Huang T., Shi S., Zhang C. Determination of compounds in Eucommia ulmoides Oliv. bark and its fermentation products via headspace gas chromatography-ion mobility spectrometry. BioResource. 2020;15:6941–6959. [Google Scholar]
- Wang Z., Peng M., She Z., Zhang M., Yang Q. Development of a flavor fingerprint by gas chromatography ion mobility spectrometry with principal component analysis for volatile compounds from Eucommia ulmoides Oliv. leaves and its fermentation products. BioResources. 2020;15:9180–9196. [Google Scholar]
- Xiao S., Fei N., Pang X., Shen J., Wang L., Zhang B., Zhang M., Zhang X., Zhang C., Li M., Sun L., Xue Z., Wang J., Feng J., Yan F., Zhao N., Liu J., Long W., Zhao L. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol. Ecol. 2014;87:357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.Q., Shao D., Sheng Z.W., Wang Q., Shi S.R. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, brown laying hens. Poult. Sci. 2019;98:3298–3303. doi: 10.3382/ps/pez178. [DOI] [PubMed] [Google Scholar]
- Xu J., Chen H.B., Li S.L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 2017;37:1140–1185. doi: 10.1002/med.21431. [DOI] [PubMed] [Google Scholar]
- Yang X., Wei M., Tian H., Liu T., Yang L. Enrichment and purification of aucubin from Eucommia ulmoides ionic liquid extract using macroporous resins. Materials. 2018;11:1758. doi: 10.3390/ma11091758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Li Y., Wang J., Sun K., Tao W., Wang Z., Xiao W., Pan Y., Zhang S., Wang Y. Systematic investigation of Ginkgo Biloba leaves for treating cardio-cerebrovascular diseases in an animal model. ACS Chem. Biol. 2017;12:1363–1372. doi: 10.1021/acschembio.6b00762. [DOI] [PubMed] [Google Scholar]
- Yao J., Zhang J., Hou J.F. Effects of ipriflavone on caged layer bone metabolism in vitro and in vivo. Poult Sci. 2007;86:503–507. doi: 10.1093/ps/86.3.503. [DOI] [PubMed] [Google Scholar]
- Yeoh Y.K., Zuo T., Lui G.C.Y., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., Chan V., Ling L., Joynt G., Hui D.S.C., Chow K.M., Ng S.S.S., Li T.C.M., Ng R.W., Yip T.C., Wong G.L.H., Chan F.K., Wong C.K., Chan P.K., Ng S.C. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhang X., Liu H., Brown M.A., Qiao S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2019;20:145–154. doi: 10.2174/1389203719666180514145437. [DOI] [PubMed] [Google Scholar]
- Zhu L., Gu P., Shen H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019;68:242–251. doi: 10.1016/j.intimp.2018.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.