Abstract
Objective
To validate the performance of 3T spin-echo echo-planar imaging (SE-EPI) magnetic resonance elastography (MRE) for staging hepatic fibrosis in a large population, using surgical specimens as the reference standard.
Materials and Methods
This retrospective study initially included 310 adults (155 undergoing hepatic resection and 155 undergoing donor hepatectomy) with histopathologic results from surgical liver specimens. They underwent 3T SE-EPI MRE ≤ 3 months prior to surgery. Demographic findings, underlying liver disease, and hepatic fibrosis pathologic stage according to METAVIR were recorded. Liver stiffness (LS) was measured by two radiologists, and inter-reader reproducibility was evaluated using the intraclass correlation coefficient (ICC). The mean LS of each fibrosis stage (F0–F4) was calculated in total and for each etiologic subgroup. Comparisons among subgroups were performed using the Kruskal–Wallis test and Conover post-hoc test. The cutoff values for fibrosis staging were estimated using receiver operating characteristic (ROC) curve analysis.
Results
Inter-reader reproducibility was excellent (ICC, 0.98; 95% confidence interval, 0.97–0.99). The mean LS values were 1.91, 2.41, 3.24, and 5.41 kPa in F0–F1 (n = 171), F2 (n = 26), F3 (n = 38), and F4 (n = 72), respectively. The discriminating cutoff values for diagnosing ≥ F2, ≥ F3, and F4 were 2.18, 2.71, and 3.15 kPa, respectively, with the ROC curve areas of 0.97–0.98 (sensitivity 91.2%–95.9%, specificity 90.7%–99.0%). The mean LS was significantly higher in patients with cirrhosis (F4) of nonviral causes, such as primary biliary cirrhosis (9.56 kPa) and alcoholic liver disease (7.17 kPa) than in those with hepatitis B or C cirrhosis (4.28 and 4.92 kPa, respectively). There were no statistically significant differences in LS among the different etiologic subgroups in the F0–F3 stages.
Conclusion
The 3T SE-EPI MRE demonstrated high interobserver reproducibility, and our criteria for staging hepatic fibrosis showed high diagnostic performance. LS was significantly higher in patients with non-viral cirrhosis than in those with viral cirrhosis.
Keywords: Magnetic resonance imaging, Spin-echo echo-planar imaging, Elastography, Liver stiffness, Hepatic fibrosis
INTRODUCTION
Hepatic fibrosis is a dynamic process characterized by excessive extracellular matrix accumulation in the liver parenchyma, resulting in increased tissue stiffness [1]. Recent clinical studies have shown that liver fibrogenesis and even cirrhosis itself may be reversed in patients with viral hepatitis after seroconversion [2,3]. Thus, accurate staging of hepatic fibrosis is of paramount importance in determining both treatment options and monitoring treatment response [4]. Although liver biopsy has been the standard method for diagnosing and staging hepatic fibrosis, it has several critical limitations, including procedure-related morbidity and mortality, sampling error, interobserver variability, and poor compliance [4]. Indeed, poor patient acceptability limits its use for monitoring disease progression and treatment effects [5].
In this regard, as noninvasive alternatives to percutaneous biopsy, several elastographic techniques, including magnetic resonance elastography (MRE), have been developed to measure liver stiffness (LS) [6]. Currently, the most commonly used MRE technique is the two-dimensional (2D) gradient-recalled echo (GRE) technique, which provides high accuracy, repeatability, and inter-platform reproducibility [7]. However, the 2D GRE technique has a technical limitation in measuring shear wave velocity in patients with hepatic iron deposition, owing to its relatively high susceptibility artifacts, especially in a 3T MR system [8,9,10]. Recently, with wider adoption of the 3T system, three-dimensional (3D) spin-echo echo-planar imaging (SE-EPI)-based MRE has rapidly gained acceptance owing to its relative invulnerability to increased susceptibility artifacts and higher spatial resolution along the Z-axis compared to that of the 2D-GRE based MRE [8]. However, until now, there have been only a few studies suggesting practical criterion values for the staging of hepatic fibrosis using a 3T SE-EPI MRE system in a sufficient number of patients with available histologic results [9]. Therefore, the purpose of our study was to validate the performance of 3T SE-EPI MRE in staging hepatic fibrosis in a large population using surgical specimens as the reference standard.
MATERIALS AND METHODS
The Institutional Review Board of Seoul National University Hospital approved this retrospective study and waived the requirement for informed consent (IRB No. 2002-130-1104).
Study Population
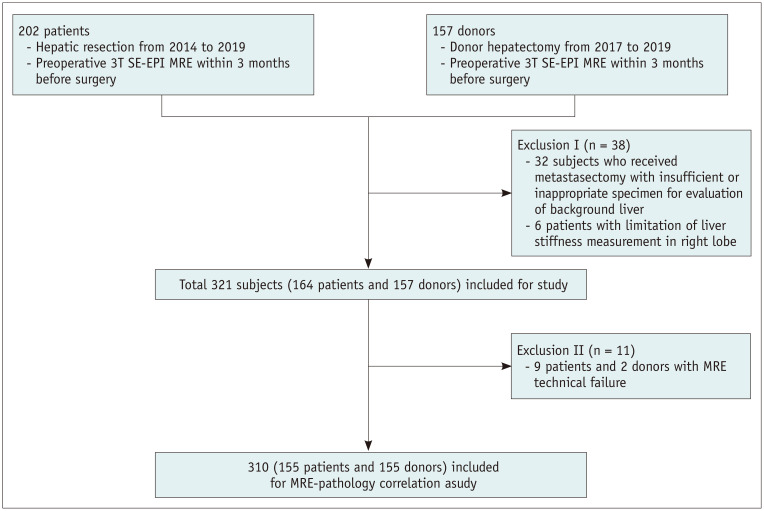
This study initially included subjects who met the following eligibility criteria: 1) patients who underwent hepatic resection for the treatment of focal lesions and liver transplantation at Seoul National University Hospital from 2014 to 2019, 2) healthy liver donors who underwent donor hepatectomy at Seoul National University Hospital from 2017 to 2019, and 3) patients who underwent preoperative 3T SE-EPI MRE ≤ 3 months prior to surgery. Subjects were excluded if they had 1) no available SE-EPI MRE at 3T, 2) insufficient or inappropriate histopathologic specimen for the evaluation of background liver, and 3) limited LS measurement in the right lobe. Figure 1 summarizes the enrollment of the study population. Age, sex, underlying etiology of chronic liver disease, and body mass index were recorded by reviewing electronic medical records.
Fig. 1. Flow chart of the study population.
MRE = MR elastography, SE-EPI = spin-echo echo-planar imaging
Acquisition of SE-EPI MRE of the Liver
Magnetic resonance imaging (MRI) of the liver, including MRE was performed on 3T scanners (MAGNETOM Skyra, n = 277, Siemens Healthineers; Discovery MR 750w, n = 27, GE Healthcare; Ingenia CX, n = 3, Philips Healthcare). Patients were requested to fast for at least 6 hours. Our routine liver MRI protocol included axial T2-weighted imaging, axial heavily T2-weighted imaging, axial T1 dual-echo in and opposed phase imaging, diffusion-weighted imaging, and dynamic imaging with gadoxetic acid (Primovist, Bayer Healthcare) using axial fat-suppressed 3D GRE T1-weighted images. Axial T2-weighted images were used as the reference anatomical image during MRE measurement (repetition time/echo time [TR/TE]: 1000/153 ms, matrix: 384 × 384, flip angle: 120°, slice thickness: 4 mm, slice interval: 4 mm, number of slices: 50).
Mechanical waves were generated by a pair of active and passive drivers (Resoundant, Mayo Foundation for Medical Education and Research, Mayo Clinic). MRE images were obtained with the patients placed in the supine position with a passive driver (18.5 cm in diameter and 3.5 cm in thickness) attached to the right anterior chest wall. The passive driver was connected to an active driver to apply 60 Hz vibrations. Axial slices containing the largest volume of the liver parenchyma were acquired using the spin-echo MRE technique with a single breath-hold during end-expiration (Magnetom Skyra, TR/TE: 1000/47 ms, matrix: 100 × 100, flip angle: 90°, slice thickness: 6 mm, slice interval: 12 mm, number of slices: 4; Discovery MR 750w, TR/TE: 1000.3/63.1 ms, matrix: 64 × 64, flip angle: 90°, slice thickness: 7 mm, slice interval: 12 mm, number of slices: 4; Ingenia CX, TR/TE: 1000/53.8 ms, matrix: 84 × 81, flip angle: 90°, slice thickness: 6 mm, slice interval: 7 mm, number of slices: 4). MR elastograms with wave images and shear stiffness maps were obtained. For each MRE stiffness map, a confidence index (ranging from 0%–100%) for stiffness measurement was estimated, and a confidence mask with a 95% threshold was automatically provided by the software.
Measurement of LS
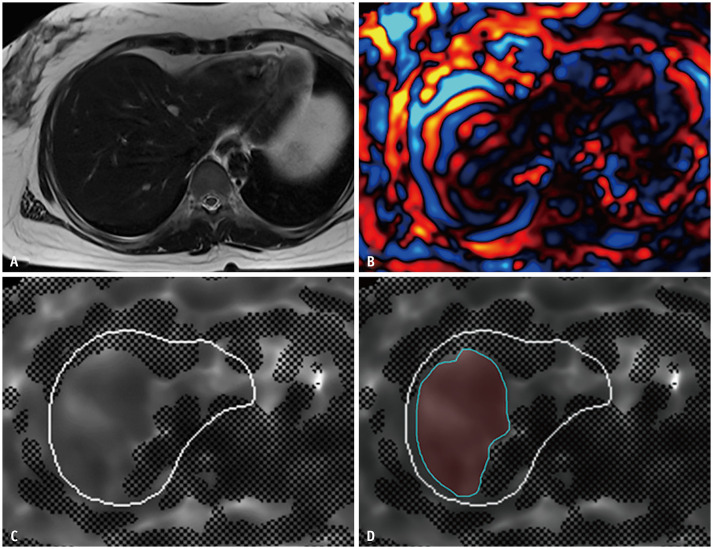
One radiologist (with 5 years of experience in liver MRI) measured LS [10] by drawing freehand regions of interest (ROIs) on the stiffness maps, only on valid areas of 95% confidence maps so as to include the largest part of the liver parenchyma of the right lobe and left medial segment (S4), while excluding hilar vessels, liver edges, and focal lesions (e.g., mass, coagulation necrosis, etc.) (Fig. 2). The mean LS values (in kPa) and area (mm2) of each ROI were recorded. To minimize sampling error, the measurement of a slice was discarded if the area of the ROI was less than 1000 mm2 [10]. The arithmetic mean of the measured stiffness values (in kPa) from all available ROIs (maximum of four ROIs in each subject) was determined as the representative LS of a subject. Technical failure of MRE was defined as the absence of visualized wave propagation on the wave images and/or no pixel value with a confidence index higher than 95% on the confidence map according to a previous study [11].
Fig. 2. An example of liver stiffnes measurement.
A. Anatomic images (axial T2-weighted image) of a 35-year-old healthy female donor with no fibrosis (F0). B. Wave image. C. Confidence map of an elastogram. D. A free-hand drawn region of interest on the unmasked area of the confidence map in the right lobe and segment 4, excluding large vessels and liver edges.
For the estimation of interobserver variability, 60 MRE examinations were selected among 310 exams using the randomization function of a commercially available spreadsheet software program (Microsoft Excel, Version 2013, Microsoft). Thereafter, another radiologist (with 5 years of experience in liver MRI) who was blinded to the histopathologic results of the subjects constructed their own ROIs.
Correlation with Histopathologic Results
Routine pathological reports were recorded and used for analysis. Some reports written in unstandardized terminology or with ambiguous pathologic diagnosis of background liver were retrospectively reviewed by an expert liver pathologist (with 15 years of clinical experience in the interpretation of pathologic results from the liver). All pathologic reports described the grade of the fibrosis stage and necroinflammatory activity in the patients using standardized guidelines published by the Korean Study Group for the Pathology of Digestive Diseases and according to the METAVIR scoring system [12,13].
Statistical Analysis
The technical success rate of the MRE is presented as a percentage. Interobserver reproducibility for LS measurement between the two radiologists was assessed by calculating the intraclass correlation coefficient (ICC). Spearman’s coefficient of rank correlation (rho) was calculated to evaluate the correlation between the fibrosis score and LS. The mean LS values of each fibrosis stage (F0–F1, F2, F3, and F4) in each etiologic subgroup (e.g., chronic hepatitis B [CHB], chronic hepatitis C [CHC], alcoholic liver disease, primary biliary cirrhosis [PBC], and others) were evaluated and compared using the Kruskal-Wallis and Conover post hoc tests.
Receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic performance of LS values in the staging of liver fibrosis. The cutoff values for hepatic fibrosis staging were determined using the highest Youden index. Since the METAVIR scoring system is not a binary reference, the Obuchowski measure was used as a performance parameter to correct bias [14,15]. For cross-validation of cutoffs, the study population was further divided into two groups chronologically (n = 144 and 166). Donors were randomly allocated to each subgroup, considering the group sizes. Cross-validation of cutoffs was performed in both groups. All statistical analyses were performed using a commercially available software program (MedCalc, Version 19.5.2, MedCalc Software) and a free software program (R version 4.0.0; R Package for Statistical Computing, www.r-project.org). Differences were considered statistically significant at p values less than 0.05, except for the post hoc Conover test (p < 0.0083 [0.05/6] based on Bonferroni correction).
RESULTS
We initially identified 359 subjects (202 patients and 157 donors). Of these, 38 were excluded because of inappropriate or insufficient specimens (all metastasectomy) for the evaluation of background liver (n = 32), unevaluable LS in the right lobe (huge mass replacing the right lobe [n = 5], and previous right hemihepatectomy [n = 1]). Among the remaining 321 subjects (164 patients and 157 donors), 11 (9 patients and 2 donors) were deemed to have technical failure. The technical success rate was 96.6% (310/321). Those with technical failure of MRE were excluded from further analyses; thus, 310 subjects finally comprised our study population (Fig. 1). The baseline demographic and clinicopathologic characteristics of the 310 study subjects are summarized in Table 1. Histopathologic examination showed that 96.8% (150/155) of healthy donors had no or mild (stage F0 or F1) liver fibrosis, while 86.5% (134/155) of patients had significant (≥ stage F2) fibrosis. The overall proportion of subjects with significant necroinflammatory activity (≥ stage A2) was 6.5% (20/310).
Table 1. Baseline Demographic, Clinicopathologic, and Imaging Characteristics.
| Patients (n = 155) | Healthy Donors (n = 155) | ||
|---|---|---|---|
| Age, years | 60.1 ± 11.4 | 35.7 ± 11.0 | |
| Sex, male:female | 110:45 | 95:60 | |
| MRI scanners | |||
| Skyra | 124 | 155 | |
| Discovery MR750w | 28 | - | |
| Ingenia CX | 3 | - | |
| BMI, kg/m2 | 24.2 ± 3.5 | 23.8 ± 3.3 | |
| Etiology of CLD | |||
| Hepatitis B virus | 92 | ||
| Hepatitis C virus | 11 | - | |
| Alcohol | 12 | - | |
| Primary biliary cirrhosis | 5 | - | |
| Others* | 17 | - | |
| No CLD | 18 | 155 | |
| Surgical indication† | |||
| Primary liver cancer | 114 (84, 17, 4) | - | |
| Metastatic cancer | 4 (4, 0, 0) | - | |
| Benign mass | 4 (4, 0, 0) | - | |
| Intrahepatic duct stone | 2 (2, 0, 0) | - | |
| Liver cirrhosis | 31 (0, 24, 7) | - | |
| Donor hepatectomy | - | 155 | |
| Sum of measured area, mm2 | 15899.2 ± 10299.4 | 15590.9 ± 5932.9 | |
| METAVIR fibrosis stage | |||
| F0 | 9 | 93 | |
| F1 | 12 | 57 | |
| F2 | 21 | 5 | |
| F3 | 40 | - | |
| F4 | 73 | - | |
| METAVIR necroinflammatory activity | |||
| A0 | 16 | 60 | |
| A1 | 120 | 94 | |
| A2 | 12 | 1 | |
| A3 | 7 | ||
Data are mean ± standard deviation or number of patients. *Others include secondary biliary cirrhosis (n = 2), autoimmune hepatitis (n = 2), Wilson disease (n = 2), non-alcoholic fatty liver disease (n = 1), combined hepatitis B virus and alcoholic liver disease (n = 3), and CLD of uncertain etiology (n = 7), †Number in parenthesis are number of patients who received (hepatic resection, living donor liver transplantation, deceased donor liver transplantation). BMI = body mass index, CLD = chronic liver disease
Relationships between LS and Hepatic Fibrosis Stages
The numbers of subjects with F0, F1, F2, F3, and F4 fibrosis were 102, 69, 26, 38, and 72, respectively. The mean LS values were 1.91, 2.41, 3.24, and 5.41 kPa in F0–F1, F2, F3, and F4, respectively. The LS values were demonstrated to have a significantly positive correlation with fibrosis stage (rho = 0.76; 95% confidence interval [CI] 0.67–0.83; p < 0.001).
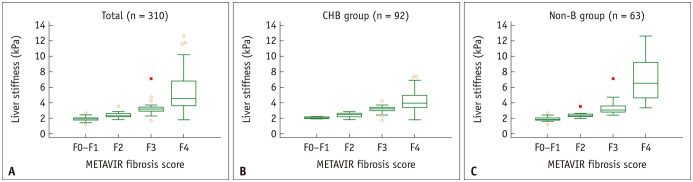
The results of the subgroup analyses are summarized in Table 2. In the subgroup with CHB (n = 92), the mean LS in the F0–F1, F2, F3, and F4 stage populations were 2.07, 2.42, 3.13, and 4.28 kPa, respectively. The LS values in the CHB subgroup also showed a positive correlation with the fibrosis stage (0.76 [95% CI 0.66–0.84], p < 0.001). Concerning LS values of subgroups according to etiology, patients with F4 had significantly different LS values depending on their underlying diseases: CHB with cirrhosis (n = 42) showed significantly lower LS value (mean ± standard deviation, 4.28 ± 1.31 kPa) than alcoholic liver disease (n = 10) or PBC (n = 5) (7.17 ± 3.44, and 9.56 ± 1.41 kPa, respectively, p values < 0.0083 [0.05/6]). Patients with CHC (n = 6) also showed lower LS than those with PBC (4.92 ± 1.37 vs. 9.56 ± 1.41 kPa, p < 0.0083 [0.05/6]). However, no subgroup with different etiologies showed significant differences in LS values in F0–F3 stages. Figure 3 shows the distribution plot of LS values according to the METAVIR fibrosis stage in the total population, patients with CHB, and patients without CHB.
Table 2. Measured Liver Stiffness according to Etiologic Causes and Fibrosis Stage.
| F0–F1 | F2 | F3 | F4 | |
|---|---|---|---|---|
| Total (n = 310) | 1.91 ± 0.21 (171) | 2.41 ± 0.34 (26) | 3.24 ± 0.82 (40) | 5.41 ± 2.39 (73) |
| Healthy donors (n = 155) | 1.90 ± 0.19 (150) | 2.29 ± 0.17 (5) | ||
| CHB (n = 92) | 2.07 ± 0.12 (9) | 2.42 ± 0.30 (13) | 3.13 ± 0.50 (28) | 4.28 ± 1.31 (42) |
| Alcoholic (n = 12) | - | 2.22 (1) | 2.76 (1) | 7.17 ± 3.44 (10) |
| CHC (n = 11) | - | 3.54 (1) | 3.56 ± 0.83 (4) | 4.92 ± 1.37 (6) |
| PBC (n = 5) | - | - | - | 9.56 ± 1.41 (5) |
| Miscellaneous (n = 35)* | 1.91 ± 0.37 (12) | 2.33 ± 0.21 (6) | 3.58 ± 1.61 (7) | 6.66 ± 1.85 (10) |
| p value for Kruskal Wallis test† | - | 0.227 | 0.402 | < 0.001 |
Data are mean ± standard deviation (when applicable). Data in parenthesis are the number of patients. *Miscellaneous include secondary biliary cirrhosis (n = 2), autoimmune hepatitis (n = 2), Wilson disease (n = 2), non-alcoholic fatty liver disease (n = 1), combined hepatitis B virus and alcoholic liver disease (n = 3), chronic liver disease of uncertain etiology (n = 7), and no underlying chronic liver disease (n = 17), †Kruskal Wallis test was done among different etiologies (CHB, CHC, alcoholic liver disease, and PBC). CHB = chronic hepatitis B, CHC = chronic hepatitis C, PBC = primary biliary cirrhosis
Fig. 3. Box-and-whisker plot depicting liver stiffness values according to METAVIR fibrosis scores in (A) total study subjects, (B) CHB patient group, and (C) Non-B patient group.
CHB = chronic hepatitis B, Non-B = chronic liver disease other than chronic hepatitis B
Diagnostic Performance of LS in Staging Liver Fibrosis
The diagnostic performance of LS in staging hepatic fibrosis was evaluated using ROC curve analysis, with reported areas under the ROC curves of 0.97–0.98 (sensitivity 91.2% to 95.9%, specificity 90.7% to 99.0%). The highest discriminating cutoff values for diagnosing significant fibrosis (≥ stage F2), advanced fibrosis (≥ stage F3), and cirrhosis (stage F4) were 2.18, 2.71, and 3.15 kPa, respectively. Obuchowski measures were 0.89 ± 0.01. The determined cutoff values for staging hepatic fibrosis were applied to the subgroup of patients with CHB (n = 92). The reported ranges of sensitivities and specificities within the subgroup for staging hepatic fibrosis were 88.6%–94.0% and 68.0%–95.5%, respectively.
Cross-validation was performed by splitting the study population into Group I (72 patients who underwent MRE between 2014 and 2017 and 72 randomly allocated donors) and Group II (83 patients who underwent MRE between 2018 and 2019 and 83 randomly allocated donors). The cross-validated sensitivity and specificity used cutoff values derived from the other groups of the study subjects. The cross-validated sensitivity and specificity for diagnosing hepatic fibrosis in Group I were 85.5%–90.9% and 91.7%–98.2%, respectively. and in Group II were 97.1%–98.3% and 89.2%–90.4%, respectively. The results of the ROC curve analysis, subgroup analysis, and cross-validation are summarized in Table 3.
Table 3. Performance of MR Elastography in Staging Hepatic Fibrosis.
| ≥ F2 Stage | ≥ F3 Stage | F4 Stage | |||
|---|---|---|---|---|---|
| Apparent validation (internal), all patients (n = 310) | |||||
| AUC (95% CI) | 0.98 (0.95–0.99) | 0.98 (0.96–0.99) | 0.97 (0.94–0.98) | ||
| Cutoff for sensitivity and specificity, kPa | 2.18 | 2.71 | 3.15 | ||
| Sensitivity, % | 95.0 (132/139) | 91.2 (103/113) | 95.9 (70/73) | ||
| Specificity, % | 92.4 (158/171) | 99.0 (195/197) | 90.7 (215/237) | ||
| Obuchowski measure | 0.891 ± 0.011 | ||||
| Apparent validation (internal), CHB subgroup (n = 92) | |||||
| Sensitivity, % | 94.0 (78/83) | 88.6 (62/70) | 92.9 (39/42) | ||
| Specificity, % | 77.8 (7/9) | 95.5 (21/22) | 68.0 (34/50) | ||
| Cross validation (internal)* | |||||
| Group I (n = 144) | |||||
| Cutoff for sensitivity and specificity, kPa | 2.19 | 2.36 | 3.20 | ||
| Sensitivity, % | 97.1 (68/70) | 98.3 (57/58) | 97.5 (39/40) | ||
| Specificity, % | 89.2 (66/74) | 89.5 (77/86) | 90.4 (94/104) | ||
| Group II (n = 166) | |||||
| Cutoff for sensitivity and specificity, kPa | 2.28 | 2.71 | 3.15 | ||
| Sensitivity, % | 88.4 (61/69) | 85.5 (47/55) | 90.9 (30/33) | ||
| Specificity, % | 96.9 (94/97) | 98.2 (109/111) | 91.7 (122/133) | ||
*For the cross validation, the cutoff for calculating sensitivity and specificity was derived from the other group of the study subjects.
AUC = areas under the curve, CHB = chronic hepatitis B, CI = confidence interval
Interobserver Reproducibility
The inter-reader reproducibility between the two readers was excellent (ICC, 0.98; 95% CI 0.97–0.99).
DISCUSSION
The 3T SE-EPI MRE system demonstrated high technical success (96.6%, 310/321), high interobserver reproducibility (ICC, 0.98; 95% CI 0.97–0.99), and reliable performance in the staging of liver fibrosis. The cutoff values for diagnosing significant fibrosis (≥ stage F2), advanced fibrosis (≥ stage F3), and cirrhosis (stage F4) were determined to be 2.18 kPa, 2.71 kPa, and 3.15 kPa, respectively in ROC curve analysis. These cutoff values are slightly lower than the values suggested by other previous studies. Chou et al. [16] reported cutoff values of 3.00, 3.24, and 3.90 kPa for diagnosing ≥ F2, ≥ F3, and ≥ F4 fibrosis, respectively, while Batheja et al. [17] reported a cutoff value of 3.0 kPa for diagnosing ≥ F3 fibrosis. We believe that the discrepant results between our study and previous studies may be mainly attributed to the differences in the study populations (the majority of our study population had hepatitis B virus-related liver diseases), rather than to different MRI field strengths (3T vs. 1.5T) or MRI sequences [18].
Our study results also revealed that for clinically significant hepatic fibrosis (≥ F2, F3, and F4), the mean LS values of each stage of the CHB group were generally lower than those of the patients without CHB. Furthermore, the cirrhosis groups were shown to have different LS values depending on their etiology; patients with PBC had the highest mean LS values, followed by those with alcoholic cirrhosis, CHC cirrhosis, and CHB cirrhosis. Our study results are in good agreement with previous study results, in which it was suggested that the LS values in patients with viral hepatitis were lower than those in patients with non-viral chronic liver disease [10,19,20]. The different pathophysiologies of hepatic inflammation and fibrosis, as well as different patterns of fibrosis formation (macronodular and heterogeneous cirrhosis in hepatitis B infection) may explain the differences in observed LS values [10]. Considering that LS measurements correlate with the degree of fibrosis, the varying fibrosis burden among the patients with the same fibrosis stage from different etiologies could result in different mean LS measurements between the two groups [21]. Previous studies regarding transient elastography also suggested etiology-specific cutoff values for diagnosing hepatic fibrosis [20,22]. The differences in LS observed among different etiologic subgroups in our study suggest that such etiology-specific criteria may also be needed for MRE. However, since the number of patients in the non-hepatitis B subgroup was too small, caution must be exercised in interpreting our study results. Moreover, the indication for surgery in patients with hepatitis B-related cirrhosis was mostly for the treatment of liver tumors (78.6%, 33/42), it was indicated for the treatment of liver failure in most cases of cirrhosis without hepatitis B (67.7%, 21/31), adding more bias in comparison of LS among different etiologic subgroups. Thus, further studies with larger, less biased populations are warranted.
Regarding the MRE sequence, we used 3D SE-EPI MRE on various 3T MR systems. According to the literature, the most common cause of technical failure of MRE is iron overload in the liver, as severe iron overload reduces the signal intensity of the parenchyma such that the amplitude of the signal of propagating shear waves is very low [1,23]. Although the most widely used clinical MRE sequence is the 2D GRE MRE sequence, the technical failure rate of 2D GRE MRE has been reported to be significantly higher at 3T than at 1.5T [11]. Iron deposition in the liver is a characteristic of hemochromatosis and is often a coexisting finding in many chronic liver diseases, which may limit the use of 2D GRE MRE at 3T for fibrosis staging [1,23,24]. In a recent meta-analysis, the technical failure rates of GRE MRE and SE-EPI MRE techniques were reported to be 4.5% and 1.3%–3.4%, respectively [25]. In our study, the technical failure rate of SE-EPI MRE was 3.4%, which is consistent with that reported in previous studies. The interobserver reproducibility in our study was also consistent with a previous study involving a larger population [26].
Our study had several limitations. First, the study had inherent selection bias owing to its retrospective nature. To minimize bias, we consecutively enrolled patients who met our inclusion criteria. Second, the heterogeneous nature of the MR scanning protocol and machines used in our study may have caused additional bias. However, as a previous meta-analysis has shown, MRE measurements are quite reproducible among examinations conducted using systems from different vendors [27]. Moreover, the resultant cutoff values from our study might be more vendor-neutral than those in other single-vendor studies. Third, the skewed composition of our patient group was another limitation. In our study, since the eligibility criteria were surgical resection or explantation, our patient population typically had advanced hepatic fibrosis. To overcome this bias stemming from skewness, we included the donor population in our study. In real-world practice, which includes both surgical candidates and patients undergoing medical management, this performance is expected to improve. Lastly, the heterogeneous nature of the study population and the small size of non-hepatitis B etiologic subgroups are the major drawbacks of our study. Care must be taken for generalized utilization of our results, including cutoff values.
In conclusion, 3T SE-EPI MRE demonstrated high technical success and interobserver reproducibility. Our cutoff values for staging hepatic fibrosis showed high diagnostic performance. Furthermore, the LS values in patients with non-viral cirrhosis were substantially higher than those in patients with cirrhosis caused by viral hepatitis.
Acknowledgments
We appreciate the statistical consultation provided by the Medical Research Collaborating Center at the Seoul National University Hospital.
Footnotes
Conflicts of Interest: Dr. Jeong Min Lee reports grants from Bayer Healthcare, Canon Healthcare, Philips Heathcare, GE Healthcare, CMS, Guerbet, Samsung Medison, Bracco, personal fees from Bayer Healthcare, Siemens Healthineer, Samsung Medison, Guerbet, Philips, outside the submitted work.
Jeong Min Lee and Ijin Joo who is on the editorial board of the Korean Journal of Radiology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
- Conceptualization: Se Woo Kim, Jeong Min Lee, Won Chang.
- Data curation: Se Woo Kim, Sungeun Park, Haeryoung Kim.
- Formal analysis: Se Woo Kim, Jeong Min Lee, Ijin Joo, Jeong Hee Yoon.
- Methodology: Se Woo Kim, Jeong Min Lee, Won Chang.
- Supervision: Jeong Min Lee.
- Validation: Sungeun Park.
- Writing—original draft: Se Woo Kim.
- Writing—review & editing: Se Woo Kim, Jeong Min Lee, Ijin Joo, Jeong Hee Yoon.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Hoodeshenas S, Yin M, Venkatesh SK. Magnetic resonance elastography of liver: current update. Top Magn Reson Imaging. 2018;27:319–333. doi: 10.1097/RMR.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacke F, Weiskirchen R. An update on the recent advances in antifibrotic therapy. Expert Rev Gastroenterol Hepatol. 2018;12:1143–1152. doi: 10.1080/17474124.2018.1530110. [DOI] [PubMed] [Google Scholar]
- 3.Zoubek ME, Trautwein C, Strnad P. Reversal of liver fibrosis: from fiction to reality. Best Pract Res Clin Gastroenterol. 2017;31:129–141. doi: 10.1016/j.bpg.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol. 2005;42:S22–S36. doi: 10.1016/j.jhep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz JM, Venkatesh SK, Ehman RL, Jhaveri K, Kamath P, Ohliger MA, et al. Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY) 2017;42:2037–2053. doi: 10.1007/s00261-017-1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DH, Lee JM, Han JK, Choi BI. MR elastography of healthy liver parenchyma: normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013;38:1215–1223. doi: 10.1002/jmri.23958. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Parthasarathy S, Goyal P, McCarthy RJ, Larson AC, Miller FH. Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdom Imaging. 2015;40:818–834. doi: 10.1007/s00261-014-0137-6. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451.e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang W, Lee JM, Yoon JH, Han JK, Choi BI, Yoon JH, et al. Liver fibrosis staging with MR elastography: comparison of diagnostic performance between patients with chronic hepatitis B and those with other etiologic causes. Radiology. 2016;280:88–97. doi: 10.1148/radiol.2016150397. [DOI] [PubMed] [Google Scholar]
- 11.Wagner M, Corcuera-Solano I, Lo G, Esses S, Liao J, Besa C, et al. Technical failure of MR elastography examinations of the liver: experience from a large single-center study. Radiology. 2017;284:401–412. doi: 10.1148/radiol.2016160863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Yu E. Histologic grading and staging of chronic hepatitis: on the basis of standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Diseases. Korean J Hepatol. 2003;9:42–46. [PubMed] [Google Scholar]
- 14.Lambert J, Halfon P, Penaranda G, Bedossa P, Cacoub P, Carrat F. How to measure the diagnostic accuracy of noninvasive liver fibrosis indices: the area under the ROC curve revisited. Clin Chem. 2008;54:1372–1378. doi: 10.1373/clinchem.2007.097923. [DOI] [PubMed] [Google Scholar]
- 15.Obuchowski NA. Estimating and comparing diagnostic tests’ accuracy when the gold standard is not binary. Acad Radiol. 2005;12:1198–1204. doi: 10.1016/j.acra.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Chou CT, Chen RC, Wu WP, Lin PY, Chen YL. Prospective comparison of the diagnostic performance of magnetic resonance elastography with acoustic radiation force impulse elastography for pre-operative staging of hepatic fibrosis in patients with hepatocellular carcinoma. Ultrasound Med Biol. 2017;43:2783–2790. doi: 10.1016/j.ultrasmedbio.2017.08.1879. [DOI] [PubMed] [Google Scholar]
- 17.Batheja M, Vargas H, Silva AM, Walker F, Chang YH, De Petris G, et al. Magnetic resonance elastography (MRE) in assessing hepatic fibrosis: performance in a cohort of patients with histological data. Abdom Imaging. 2015;40:760–765. doi: 10.1007/s00261-014-0321-8. [DOI] [PubMed] [Google Scholar]
- 18.Morisaka H, Motosugi U, Glaser KJ, Ichikawa S, Ehman RL, Sano K, et al. Comparison of diagnostic accuracies of two- and three-dimensional MR elastography of the liver. J Magn Reson Imaging. 2017;45:1163–1170. doi: 10.1002/jmri.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obara N, Ueno Y, Fukushima K, Nakagome Y, Kakazu E, Kimura O, et al. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J Gastroenterol. 2008;43:720–728. doi: 10.1007/s00535-008-2225-2. [DOI] [PubMed] [Google Scholar]
- 20.Behairy BEL-S, Sira MM, Zalata KR, Salama EL-SE, Abd-Allah MA. Transient elastography compared to liver biopsy and morphometry for predicting fibrosis in pediatric chronic liver disease: does etiology matter? World J Gastroenterol. 2016;22:4238–4249. doi: 10.3748/wjg.v22.i16.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh SK, Xu S, Tai D, Yu H, Wee A. Correlation of MR elastography with morphometric quantification of liver fibrosis (Fibro-C-Index) in chronic hepatitis B. Magn Reson Med. 2014;72:1123–1129. doi: 10.1002/mrm.25002. [DOI] [PubMed] [Google Scholar]
- 22.Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol. 2010;25:1726–1731. doi: 10.1111/j.1440-1746.2010.06437.x. [DOI] [PubMed] [Google Scholar]
- 23.Besa C, Wagner M, Lo G, Gordic S, Chatterji M, Kennedy P, et al. Detection of liver fibrosis using qualitative and quantitative MR elastography compared to liver surface nodularity measurement, gadoxetic acid uptake, and serum markers. J Magn Reson Imaging. 2018;47:1552–1561. doi: 10.1002/jmri.25911. [DOI] [PubMed] [Google Scholar]
- 24.Wagner M, Besa C, Bou Ayache J, Yasar TK, Bane O, Fung M, et al. Magnetic resonance elastography of the liver: qualitative and quantitative comparison of gradient echo and spin echo echoplanar imaging sequences. Invest Radiol. 2016;51:575–581. doi: 10.1097/RLI.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DW, Kim SY, Yoon HM, Kim KW, Byun JH. Comparison of technical failure of MR elastography for measuring liver stiffness between gradient-recalled echo and spin-echo echo-planar imaging: a systematic review and meta-analysis. J Magn Reson Imaging. 2020;51:1086–1102. doi: 10.1002/jmri.26918. [DOI] [PubMed] [Google Scholar]
- 26.Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations. Radiology. 2016;278:114–124. doi: 10.1148/radiol.2015142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trout AT, Serai S, Mahley AD, Wang H, Zhang Y, Zhang B, et al. Liver stiffness measurements with MR elastography: agreement and repeatability across imaging systems, field strengths, and pulse sequences. Radiology. 2016;281:793–804. doi: 10.1148/radiol.2016160209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.