Abstract
We report a case of group C streptococcal meningitis in a woman with a history of close animal contact as well as head trauma as a result of a kick by a horse. Blood and cerebrospinal fluid cultures grew Streptococcus equi subsp. zooepidemicus, as did a throat culture taken from the colt that had kicked her 2 weeks prior to admission.
CASE REPORT
A 49-year-old female was admitted to the hospital following the rapid onset of headache, nausea, vomiting, fever, and neck pain. She cared for eight horses and a donkey. All of the animals had been well with the exception of a new colt (Billy), which had signs of a respiratory tract infection with cough and thick, yellow nasal discharge. As a result of the care required for the horse she had had frequent contact with his oral and nasal secretions. Two weeks prior to her admission, Billy had kicked her in the face with his hind knee. She suffered no loss of consciousness or break in the skin. Two days later, she developed a sore throat, myalgia, and neck swelling, which persisted until the day of admission.
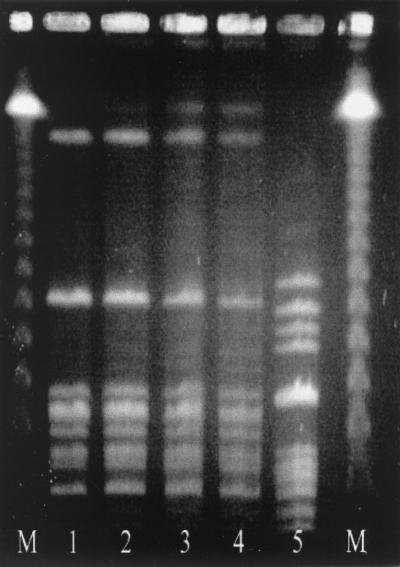
On admission, she was noted to appear toxic, was photophobic, and had a decreased level of consciousness. Relevant findings included a temperature of 38.0°C, bruising of the left periorbital area, cervical lymphadenopathy, and a white blood cell count of 19.8 × 109/liter, with 85% neutrophils and 8% band forms. A lumbar puncture yielded cloudy cerebrospinal fluid (CSF) containing 3,011 × 106 neutrophils per liter. The CSF was processed for staining by the cytocentrifuge method (4). A Gram stain of the slide revealed numerous pus cells but no organisms. The CSF protein concentration was 1.21 g/liter, and the glucose concentration was 3.3 mM, with a serum glucose concentration of 8.8 mM. Streptococcus equi subsp. zooepidemicus (Lancefield group C) was isolated from blood and CSF cultures (Fig. 1, lanes 1 and 2, respectively). She was initially treated empirically with intravenous ceftriaxone for 2 days and then changed to 4 × 106 U of intravenous penicillin G every 4 h for 8 more days once the pathogen was identified. She defervesced within 24 h and was discharged after 11 days with diplopia.
FIG. 1.
Pulsed-field gel electrophoresis of isolates of S. equi subsp. zooepidemicus (see the text). Lanes M, molecular weight markers.
Oropharyngeal swabs taken from her husband and her two children were negative for S. equi subsp. zooepidemicus; however, two of the eight horses (Billy and Rusty) and her donkey (Archie) tested positive (Fig. 1, lanes 3 to 5 respectively). Briefly, oropharyngeal swabs were inoculated directly onto two Columbia agar plates containing 5% sheep blood and then selectively enriched for streptococci in Todd-Hewitt broth containing 8 μg of gentamicin and 15 μg of nalidixic acid (MedOx, Nepean, Ontario, Canada) per ml. The blood agar plates were streaked for isolated colonies and incubated for up to 48 h at 35°C; one was placed in anaerobic atmospheric conditions and the other was placed in 5% CO2. After overnight incubation at 35°C, the swabs from the Todd-Hewitt broth were inoculated onto two further blood agar plates that were incubated as previously stated. Growth of colonies of gram-positive cocci that were catalase negative, pyrrolidonyl arylamidase negative, and beta-hemolytic on the blood agar plates was identified using standard procedures (9). Colonies were then agglutinated with streptococcal grouping reagents A, B, C, and G (Prolab Diagnostics, Richmond Hill, Ontario, Canada). Those isolates that agglutinated in the group C reagent were identified using API 20 STREP strips (BioMerieux, Marcy l'Etoile, France). The original colonies isolated on blood agar appeared nonmucoid. Molecular characterization of the isolates by standard pulsed-field gel electrophoresis, using the restriction endonuclease SmaI, revealed that the isolates from the horses were identical to the patient's isolates and different from the donkey's (Fig. 1) (10).
Large-colony Lancefield group C streptococci (GCS) consist of S. dysgalactiae subsp. equisimilis, S. dysgalactiae subsp. dysgalactiae, S. equi subsp. equi, and S. equi subsp. zooepidemicus. S. equi subsp. zooepidemicus is a normal commensal of the skin and upper respiratory mucosa of horses (11). It is by far the most common cause of wound infections and causes respiratory tract infections of foals and young horses, including purulent nasal discharge and abscesses of submandibular lymph nodes in some cases (strangles). GCS are rarely implicated in serious human illness. When isolated from humans, GCS are usually found either as part of the commensal flora of the skin, nasopharynx, oropharynx, intestinal tract, or vagina (7) or as a cause of mild upper respiratory tract infection or skin and soft tissue infection (1). A study of 150,000 blood cultures obtained at the Mayo Clinic over 9 years found only eight cases of GCS bacteremia (6); a more recent review of 192 cases of beta-hemolytic streptococcal bacteremia identified only 6 (3.0%) cases caused by GCS (3), and a review of 789 cases of bacterial meningitis did not identify a single case caused by GCS (8). Only 14 adult cases of GCS meningitis have been reported in the literature, with a case fatality rate of 57% and with most having no obvious source of the infection (2).
The present scenario resembles an earlier case of apparent direct transmission of S. equi subsp. zooepidemicus from a pet horse to its owner (5). The additional history of head trauma in this case raises the possibility of this being a portal of entry for the organism. Horse trainers have considerable close contact with their horses, including their mucous secretions. This could readily explain the transmission of GCS resulting in colonization and/or infection and could show how trauma could lead to subsequent infection. This case is a reminder of the important potential of zoonotic infections due to GCS.
REFERENCES
- 1.Barnham M, Kerby J, Chandler R S, Millar M R. Group C streptococci in human infection: a study of 308 isolates with clinical correlations. Epidemiol Infect. 1989;102:379–390. doi: 10.1017/s0950268800030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman A C, Ramsay A D, Pallett A P. Fatal infection associated with group C streptococci. J Clin Pathol. 1993;46:965–967. doi: 10.1136/jcp.46.10.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeli Y, Ruoff K L. Report of cases of and taxonomic considerations for large-colony-forming Lancefield group C streptococcal bacteremia. J Clin Microbiol. 1995;33:2114–2117. doi: 10.1128/jcm.33.8.2114-2117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapin K. Clinical microscopy. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; 1995. pp. 33–51. [Google Scholar]
- 5.Low D E, Young M R, Harding G K. Group C streptococcal meningitis in an adult. Probable acquisition from a horse. Arch Intern Med. 1980;140:977–978. [PubMed] [Google Scholar]
- 6.Mohr D N, Feist D J, Washington J A, Hermans P E. Infections due to group C streptococci in man. Am J Med. 1979;66:450–456. doi: 10.1016/0002-9343(79)91067-2. [DOI] [PubMed] [Google Scholar]
- 7.Ortel T L, Kallianos J, Gallis H A. Group C streptococcal arthritis: case report and review. Rev Infect Dis. 1990;12:829–837. doi: 10.1093/clinids/12.5.829. [DOI] [PubMed] [Google Scholar]
- 8.Qadri S M, Berotte J M, Wende R D. Incidence and etiology of septic meningitis in a metropolitan county hospital. Am J Clin Pathol. 1976;65:550–556. doi: 10.1093/ajcp/65.4.550. [DOI] [PubMed] [Google Scholar]
- 9.Ruoff K L. Streptococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 299–307. [Google Scholar]
- 10.Takai S, Anzai T, Yashiro H, Ishii C, Tsubaki S, Wada R, Timoney J F. Detection of DNA restriction fragment polymorphisms in Streptococcus equi. Vet Rec. 2000;146:159–161. doi: 10.1136/vr.146.6.159. [DOI] [PubMed] [Google Scholar]
- 11.Timoney J F, Gillespie J H, Scott F W, Barlough J E. Hagan and Bruner's microbiology and infectious diseases of domestic animals. London, United Kingdom: Comstock Publishing Associates; 1988. pp. 186–187. [Google Scholar]