Abstract
Objective
This study aimed to investigate the usefulness of bone single-positron emission tomography/computed tomography (SPECT/CT) of the hip in predicting the later occurrence of avascular necrosis (AVN) after slipped capital femoral epiphysis (SCFE) or femoral neck fracture in pediatric patients. The quantitative parameters of SPECT/CT useful in predicting AVN were identified.
Materials and Methods
Twenty-one (male:female, 10:11) consecutive patients aged < 18 years (mean age ± standard deviation [SD], 11.0 ± 2.7 years) who underwent surgery for SCFE or femoral neck fracture and postoperative bone SPECT/CT were included. The maximum standardized uptake value (SUV), mean SUV, and minimum SUV of the femoral head were measured. The ratios of the maximum SUV, mean SUV, and minimum SUV of the affected femoral head to the contralateral side were determined. Patients were followed up for > 1 year after the surgery. The SPECT/CT parameters were compared between patients who developed AVN and those who did not. The accuracy of SPECT/CT parameters for predicting AVN was assessed.
Results
Six patients developed AVN. There was a significant difference in the ratio of the mean SUV among patients who developed AVN (mean ± SD, 0.8 ± 0.3) and those who did not (1.1 ± 0.2, p = 0.018). However, there were no significant differences in the ratios of the maximum and minimum SUV between the groups (all p = 0.205). For the maximum, mean, and minimum SUVs, no significant differences were observed between the groups (p = 0.519, 0.733, and 0.470, respectively). The cutoff mean SUV ratio of 0.87 yielded a 66.7% sensitivity and 93.2% specificity for predicting AVN.
Conclusion
Quantitative bone SPECT/CT is useful for evaluating femoral head viability in pediatric patients with SCFE or femoral neck fractures. Clinicians should consider the high possibility of later AVN development in patients with a decreased mean SUV ratio.
Keywords: Avascular necrosis, Femoral head, SPECT/CT, Standardized uptake value
INTRODUCTION
Avascular necrosis (AVN) of the femoral head is the most serious complication following treatment of slipped capital femoral epiphysis (SCFE) or femoral neck fracture in pediatric patients [1]. It has a poor prognosis, and is debilitating and potentially disabling in children [2]. The incidence of AVN varies according to the severity and stability of the slip, treatment methods used for SCFE, status of fracture displacement, and the exact site of femoral neck fracture [3,4]. Revascularization procedures, such as using a vascularized fibular graft, femoral and/or pelvic osteotomy, and hip joint arthroplasty, can be considered for the treatment of AVN [5,6].
While several studies have suggested various risk factors for AVN [7,8,9,10], femoral head perfusion after surgery is one of the most important factors in assessing the risk of AVN. However, in children, the blood supply to the femoral head is age-dependent [11]. Blood supply to the femoral head is relatively low in children, whereas adults have a rich collateral supply. This is related to a higher risk of AVN in children with femoral neck fracture, with an overall rate of 20%–29% [4,12]. The risk is reduced in elderly patients because of the development of an anastomotic vessel [13].
Early prediction and detection could modify the progression of the AVN of the femoral head. Among the multiple imaging modalities, single-positron emission tomography/computed tomography (SPECT/CT) is considered effective in evaluating the development of postoperative AVN. This examination aids in the detection of AVN by supplementing other techniques, such as conventional radiography, CT, and magnetic resonance imaging.
Several studies have investigated the utility of bone SPECT/CT for the evaluation of femoral head viability in adult patients with femoral neck fractures [14,15,16,17,18,19]. However, no study has yet evaluated the usefulness of bone SPECT/CT in predicting the risk of AVN in children. Therefore, this study aimed to investigate the usefulness of bone SPECT/CT of the hip in predicting the later occurrence of AVN after SCFE or femoral neck fracture in pediatric patients, and to identify which quantitative parameters of SPECT/CT are useful in predicting AVN in these patients.
MATERIALS AND METHODS
Ethics
This retrospective study was approved by the Institutional Review Board of our hospital (IRB No. B-2105-686-102). The requirement for informed consent was waived due to the retrospective study design.
Patients
The inclusion criteria were as follows: 1) consecutive patients who underwent surgery for SCFE or femoral neck fracture between 2015 and 2020, 2) patients with postoperative bone SPECT/CT of the hip, 3) patients aged < 18 years, and 4) patients who were followed up for a minimum of 1 year. Patients who underwent bilateral hip surgery were excluded from the study. From the medical records, patients’ age at surgery, sex, diagnosis (SCFE or femur neck fracture), involved side, type of surgery, follow-up duration, and complications were obtained.
Surgical Procedures and Follow-Up
All surgical procedures were performed by two pediatric orthopedic surgeons. For SCFE, in situ pinning or modified Dunn osteotomy was performed according to the degree of slip. For femoral neck fractures, closed or open reduction and internal fixation using cannulated screws were performed. After surgery, the non-weight bearing was maintained for at least 8 weeks, followed by weight-bearing as tolerated. Plain hip radiographs were obtained to assess fracture healing and to periodically identify the AVN of the femoral head at the outpatient clinic. AVN of the femoral head was diagnosed when patch sclerosis and cystic changes, subchondral collapse, or flattening of the femoral head was detected on plain radiographs.
Bone SPECT/CT
SPECT/CT images were acquired 2–3 hours after Tc-99m hydroxydiphosphonate (HDP) injection, with NM/CT 670 and NM/CT 670 Pro (GE Healthcare). Dosages of Tc-99m HDP were administered according to the European Association of Nuclear Medicine dosage card (15.8 ± 6.6 mCi). Patient height, weight, and net injected activity were used to calculate the standardized uptake values (SUVs). Planar images of the hip region were acquired, followed by SPECT images. SPECT images were acquired under the following conditions: low-energy high-resolution collimation, energy window peak at 140 keV with 20% windowing (126–154 keV), scatter window at 120 keV with 10% windowing (115–125 keV), body contouring, step and shoot mode (10 s/step, 3° angle, 60 steps/detector), and zoom factor of 1.14 or 1.5. Next, CT images were acquired under the following conditions: tube voltage of 120 kVp, tube current of 60–210 mA with auto mA function, helical thickness of 2.5 mm, table speed of 37 mm/s, table feed per rotation of 18.75 mm/rot, tube rotation time of 0.5 seconds, and pitch of 0.938:1.
SPECT/CT Quantification
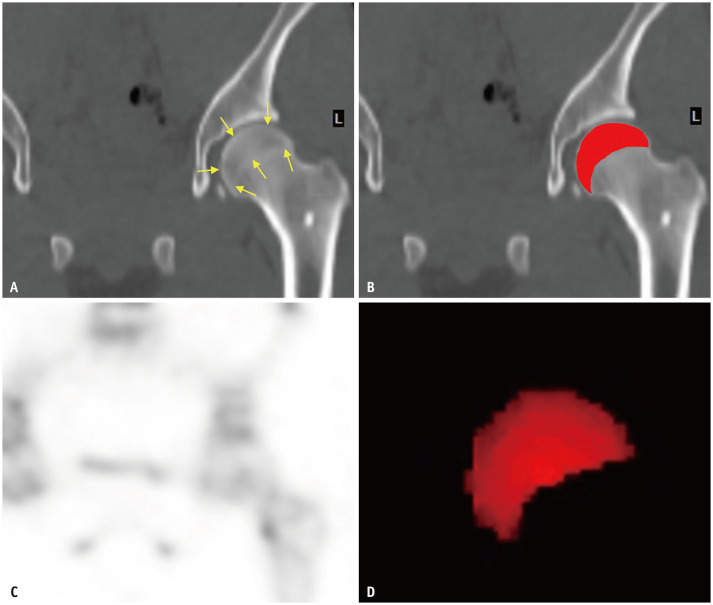
Reconstruction and SUV quantification of the femur head was performed based on previously described methods [14]. Image reconstruction was performed with dedicated software (Xeleris 4DR, GE Healthcare), using an iterative ordered subset expectation maximization method with two iterations and 10 subsets. The matrix size was 128 × 128 with a slice thickness of 2.95 mm or 3.88 mm. A Butterworth filter (frequency, 0.48) with CT-based attenuation correction, dual-energy window-based scatter correction, and resolution recovery were applied. For SUV quantification, the volumes-of-interest (VOIs) of the bilateral femur heads were determined by manually drawing regions-of-interest between the upper contour of the femur head and the lower line of the epiphysis using coronal CT images (Fig. 1). The maximum SUV, mean SUV, and minimum SUV of the VOIs were calculated using the following equations:
Fig. 1. Generation of VOIs of bilateral femur heads.
A. ROIs were manually drawn between the upper contour of the femur head and the lower line of the epiphysis (arrows) using coronal CT images. B. ROIs superimposed to the CT image. C. Accordant SPECT images were visually inspected simultaneously. D. VOIs for the femoral head. ROI = region-of-interest, VOI = volume-of-interest
The converting factors for counts of radioactivity were 151.8 counts/min for NM/CT 670 and 152.8 counts/min for NM/CT 670 Pro. The ratios of the maximum SUV, mean SUV, and minimum SUV of the affected femoral head to the contralateral femoral head were also used for analyses.
Statistical Analyses
Descriptive statistics, such as the mean and standard deviation, were used to summarize patient demographics. A comparison of the SPECT/CT parameters between those who developed and did not develop AVN was performed using the Mann-Whitney U test. A receiver operating characteristic (ROC) curve was used to define the optimal cutoff values of the quantitative parameters for the prediction of AVN, and the sensitivity and specificity were obtained. All statistical analyses were performed using SPSS software for Windows (Version 25.0; IBM Corp.) and MedCalc software for Windows (Version 18.11; MedCalc®). All statistical tests were two-tailed, and a p value of < 0.05, was considered significant.
RESULTS
Our clinical data warehouse included 25 patients who underwent postoperative bone SPECT/CT after surgery for SCFE or femoral neck fracture. Among the 25 patients, three were excluded due to inadequate follow-up duration, and one was excluded due to bilateral hip involvement. Therefore, 21 patients were included in this study. The mean age of the patients and the mean follow-up duration were 11.0 ± 2.7 years and 3.3 ± 1.4 years, respectively. Of the 21 patients, six developed AVN (four patients with SCFE, two with femur neck fracture) (Fig. 2). Of these six patients, four underwent femoral valgization osteotomy, and one underwent both femoral varization osteotomy and Chiari osteotomy (Table 1).
Fig. 2. Representative cases of patients with and without AVN.
A, B. Planar bone scan (A) and SPECT/CT (B) images of a 3-year-old patient who developed AVN of the left femur head 2 months after open reduction and internal fixation of the femur neck fracture. Planar scan and SPECT/CT showed a reduced photon count on the left femur head. The ratio of the mean SUV was 0.22. C, D. Planar bone scan (C) and SPECT/CT (D) images of an 11-year-old patient who underwent in situ pinning of the right slipped capital femoral epiphysis. The ratio of the mean SUV was 1.09. The patient did not develop AVN of the femur head. AVN = avascular necrosis, SUV = standardized uptake value
Table 1. Summary of Patients Demographics.
| Sex (male:female) | 10:11 |
| Age at surgery, year | 11.0 ± 2.6 (3.7 to 15.8) |
| Follow-up duration, years | 3.3 ± 1.3 (1.0 to 6.3) |
| Side of limbs (right, left) | 9, 12 |
| Diagnosis (SCFE, femur neck fracture) | 15, 6 |
| Avascular necrosis (yes, no) | 6, 15 |
Data are mean ± standard deviation (range). Otherwise, the data are number of patients. SCFE = slipped capital femoral epiphysis
Bone SPECT/CT of the hip was performed after surgery following a mean interval of 52.3 days (range, 34–83 days). The mean volume of the VOIs was 21.7 ± 8.8 mL for the affected femur head, and 20.8 ± 7.5 mL for the contralateral femur head. There was a significant difference in the ratio of the mean SUV among patients who developed AVN (0.8 ± 0.3) and those who did not (1.1 ± 0.2, p = 0.018). However, there were no significant differences in the ratios of the maximum and minimum SUV between the two groups (all p = 0.205). For the maximum, mean, and minimum SUV, no significant difference was observed between patients who developed AVN and those who did not (p = 0.519, 0.733, and 0.470, respectively) (Table 2).
Table 2. Comparison of SPECT/CT Parameters of Femoral Head between AVN and No-AVN Groups.
| SPECT/CT Parameters | AVN Group (n = 6) | No-AVN Group (n = 15) | P |
|---|---|---|---|
| Mean SUV, g/mL | 8.1 ± 3.5 | 9.6 ± 4.2 | 0.519 |
| Ratio of the mean SUV | 0.8 ± 0.3 | 1.1 ± 0.2 | 0.018 |
| Maximum SUV, g/mL | 24.1 ± 19.5 | 21.8 ± 9.7 | 0.733 |
| Ratio of the maximum SUV | 0.8 ± 0.4 | 1.0 ± 0.2 | 0.205 |
| Minimum SUV, g/mL | 1.7 ± 0.9 | 2.2 ± 1.0 | 0.470 |
| Ratio of the minimum SUV | 0.9 ± 0.5 | 3.0 ± 4.2 | 0.205 |
Data are mean ± standard deviation. AVN = avascular necrosis, SUV = standardized uptake value
The ratio of the mean SUV was a useful factor for the prediction of AVN after SCFE or femoral neck fracture in pediatric patients; thus, we performed ROC curve analysis using the ratio of the mean SUV. The area under the ROC curve was 0.833 (95% confidence interval, 0.608–0.958; p = 0.001). The cutoff value of the mean SUV ratio of 0.87 yielded a 66.7% sensitivity and a 93.2% specificity for predicting AVN (Fig. 3).
Fig. 3. The receiver operating characteristic curve of the ratio of the mean SUV for predicting avascular necrosis.

The area under the curve was 0.833 (95% confidence interval, 0.608–0.958). The cutoff mean SUV ratio of 0.87 yielded 66.7% sensitivity and 93.2% specificity. SUV = standardized uptake value
DISCUSSION
A previous study evaluated femoral head viability in pediatric patients by visually grading and analyzing bone scan uptakes by experts [20]. Postoperative bone scan was proven to be a valuable technique for assessing the risk of AVN following femoral neck fracture, SFCE, or Legg-Calve-Perthes disease. However, visual grading and interpretations have the probability of poor inter-rater reliability, which can be overcome by using quantitative parameters. A recent study investigated the utility of quantitative bone SPECT/CT in assessing femoral head viability in adults with femoral neck fractures [14]. The minimum SUV of the affected hip was found to be a novel quantitative parameter.
DosSantos et al. [21] evaluated the role of SPECT/CT in detecting the viability of the femoral head in pediatric patients with SCFE. However, only two patients were included for a qualitative SPECT/CT analysis. To the best of our knowledge, our study is the first to evaluate the usefulness of SPECT/CT in quantitatively assessing femoral head viability in children. We analyzed not only the individual SUV values of the affected side, but also the ratio of the SUV values of the affected femoral head to the contralateral femur head. It was observed that only the ratio of the mean SUV of the affected femoral head to the contralateral femoral head was significantly different between the two groups. The normal reference range of bone uptake varies according to patient age due to continuous femoral growth in children. The femoral epiphysis ossifies and fuses to the femoral physis until the age of 14–16 years. Therefore, based on the findings of this study, it may be reasonable to conclude that the ratio of the mean SUV is the only valuable quantitative parameter for the prediction of AVN.
Traumatic AVN in children commonly results in femoral head collapse with secondary degenerative changes, resulting in poor clinical outcomes [22]. However, to date, no clear guidelines exist for the successful treatment of AVN in pediatric patients. Intravenous bisphosphonate therapy, closed bone graft epiphysiodesis, and core decompression combining implantation of bone marrow-derived cells with recombinant human bone morphogenetic protein-2 [22,23,24] have been suggested as effective therapeutic options for early stage AVN in pediatric patients. We opine that early prediction of AVN using quantitative bone SPECT/CT and intervention may alter the course of AVN. If a postoperative bone SPECT/CT shows a decrease in the ratio of the mean SUV, clinicians should consider a prolonged period of non-weight-bearing and early surgical intervention.
There were some limitations to this study. First, our sample size was small because of the rarity of the patient population. A large cohort multicenter study is required to support our findings. Second, the time interval between surgery and postoperative bone SPECT/CT varied from 5 to 12 weeks due to the retrospective design of this study, although the timing of the scans might have affected the results. Therefore, a prospective study with the same interval of postoperative bone SPECT/CT is needed. Third, adjacent uptakes to the femur head, such as the uptake by the postoperative healing process, femur neck fracture, and growth plate may be attributed to the unwanted spill-over effects. However, on visual inspection of SPECT/CT images, there were no significant interfering uptakes adjacent to the VOIs of the femoral head. While it may be difficult to precisely demarcate the true femoral head activity free from adjacent activities, the ratio of mean SUV values is less likely to be affected by unwanted adjacent uptakes than the maximum SUVs.
In conclusion, we demonstrated that quantitative bone SPECT/CT is useful for evaluating femoral head viability in pediatric patients with SCFE or femoral neck fractures. Clinicians should consider the high possibility of later development of AVN in patients with a decreased mean SUV ratio.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoo Sung Song, Ki Hyuk Sung, Won Woo Lee.
- Data curation: Yoo Sung Song, Ki Hyuk Sung, Nak Tscheol Kim.
- Formal analysis: Ki Hyuk Sung, Nak Tscheol Kim.
- Investigation: Yoo Sung Song, Ki Hyuk Sung, Nak Tscheol Kim.
- Methodology: Yoo Sung Song, Won Woo Lee.
- Project administration: Yoo Sung Song, Ki Hyuk Sung.
- Resources: Ki Hyuk Sung, Moon Seok Park.
- Software: Yoo Sung Song, Won Woo Lee.
- Supervision: Won Woo Lee, Moon Seok Park.
- Validation: Yoo Sung Song, Ki Hyuk Sung.
- Visualization: Yoo Sung Song, Nak Tscheol Kim.
- Writing—original draft: Yoo Sung Song, Ki Hyuk Sung.
- Writing—review & editing: all authors.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Zaltz I, Baca G, Clohisy JC. Unstable SCFE: review of treatment modalities and prevalence of osteonecrosis. Clin Orthop Relat Res. 2013;471:2192–2198. doi: 10.1007/s11999-012-2765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krahn TH, Canale ST, Beaty JH, Warner WC, Lourenço P. Long-term follow-up of patients with avascular necrosis after treatment of slipped capital femoral epiphysis. J Pediatr Orthop. 1993;13:154–158. [PubMed] [Google Scholar]
- 3.Naseem H, Chatterji S, Tsang K, Hakimi M, Chytas A, Alshryda S. Treatment of stable slipped capital femoral epiphysis: systematic review and exploratory patient level analysis. J Orthop Traumatol. 2017;18:379–394. doi: 10.1007/s10195-017-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spence D, DiMauro JP, Miller PE, Glotzbecker MP, Hedequist DJ, Shore BJ. Osteonecrosis after femoral neck fractures in children and adolescents: analysis of risk factors. J Pediatr Orthop. 2016;36:111–116. doi: 10.1097/BPO.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand T, Urbaniak JR, Lark RK. Vascularized fibular grafts for avascular necrosis after slipped capital femoral epiphysis: is hip preservation possible? Clin Orthop Relat Res. 2013;471:2206–2211. doi: 10.1007/s11999-012-2781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins MM, Sood M, Hashemi-Nejad A, Catterall A. The management of avascular necrosis after slipped capital femoral epiphysis. J Bone Joint Surg Br. 2005;87:1669–1674. doi: 10.1302/0301-620X.87B12.16665. [DOI] [PubMed] [Google Scholar]
- 7.Pape HC, Krettek C, Friedrich A, Pohlemann T, Simon R, Tscherne H. Long-term outcome in children with fractures of the proximal femur after high-energy trauma. J Trauma. 1999;46:58–64. doi: 10.1097/00005373-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A. Fractures of the femoral neck in children: long-term follow-up in 62 hip fractures. Injury. 2006;37:90. doi: 10.1016/j.injury.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Togrul E, Bayram H, Gulsen M, Kalaci A, Ozbarlas S. Fractures of the femoral neck in children: long-term follow-up in 62 hip fractures. Injury. 2005;36:123–130. doi: 10.1016/j.injury.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Bagatur AE, Zorer G. Complications associated with surgically treated hip fractures in children. J Pediatr Orthop B. 2002;11:219–228. doi: 10.1097/00009957-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br. 1957;39-B:358–394. doi: 10.1302/0301-620X.39B2.358. [DOI] [PubMed] [Google Scholar]
- 12.Wang WT, Li YQ, Guo YM, Li M, Mei HB, Shao JF, et al. Risk factors for the development of avascular necrosis after femoral neck fractures in children: a review of 239 cases. Bone Joint J. 2019;101-B:1160–1167. doi: 10.1302/0301-620X.101B9.BJJ-2019-0275.R1. [DOI] [PubMed] [Google Scholar]
- 13.Palocaren T. Femoral neck fractures in children: a review. Indian J Orthop. 2018;52:501–506. doi: 10.4103/ortho.IJOrtho_404_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryoo HG, Lee WW, Kim JY, Kong E, Choi WH, Yoon JK K-SPECT Group. Minimum standardized uptake value from quantitative bone single-photon emission computed tomography/computed tomography for evaluation of femoral head viability in patients with femoral neck fracture. Nucl Med Mol Imaging. 2019;53:287–295. doi: 10.1007/s13139-019-00600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Oh M, Yoon S, Kim J, Kim JW, Chang JS, et al. Risk stratification for avascular necrosis of the femoral head after internal fixation of femoral neck fractures by post-operative bone SPECT/CT. Nucl Med Mol Imaging. 2017;51:49–57. doi: 10.1007/s13139-016-0443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan W, Zhu L, Chen J, Guo C, Yan Z. Identifying patients who will most benefit from single photon emission computerized tomography and computerized tomography after femoral neck fracture. Med Sci Monit. 2017;23:5669–5674. doi: 10.12659/MSM.904026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan HF, Shen F, Zhang J, Shi HC, Gu YS, Yan ZQ. Predictive value of single photon emission computerized tomography and computerized tomography in osteonecrosis after femoral neck fracture: a prospective study. Int Orthop. 2015;39:1417–1422. doi: 10.1007/s00264-015-2709-7. [DOI] [PubMed] [Google Scholar]
- 18.Han YH, Jeong HJ, Sohn MH, Yoon SJ, Lim ST. Incidence and severity of femoral head avascularity after femoral neck or intertrochanteric fractures on preoperative bone single photon emission computed tomography/computed tomography: preliminary study. Nucl Med Commun. 2019;40:199–205. doi: 10.1097/MNM.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 19.Park SJ, Ko BS, Moon KH, Lee M. Prediction value of SPECT/CT in avascular necrosis of femoral head after femur neck fracture. Geriatr Orthop Surg Rehabil. 2019;10:2151459319872943. doi: 10.1177/2151459319872943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh AK, Washington ER, 4th, Bobbey AJ, Spottswood SE. Evaluation of femoral head viability via bone scintigraphy in the postoperative pediatric patient. Pediatr Radiol. 2018;48:350–358. doi: 10.1007/s00247-017-4030-7. [DOI] [PubMed] [Google Scholar]
- 21.DosSantos A, Macalintal N, Bouchareb Y, Joel E, Jan H, Haroon A. Incremental value of SPECT/CT in the detection of femoral head viability in slipped capital femoral epiphysis. J Nucl Med Technol. 2018;46:153–154. doi: 10.2967/jnmt.117.202283. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am. 2007;89:1727–1734. doi: 10.2106/JBJS.F.00964. [DOI] [PubMed] [Google Scholar]
- 23.Gao F, Sun W, Guo W, Wang B, Cheng L, Li Z. Combined with bone marrow-derived cells and rhbmp-2 for osteonecrosis after femoral neck fractures in children and adolescents: a case series. Sci Rep. 2016;6:30730. doi: 10.1038/srep30730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napora JK, Gilmore A, Son-Hing JP, Grimberg DC, Thompson GH, Liu RW. Early MRI detection and closed bone graft epiphysiodesis may alter the course of avascular necrosis following unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2018;38:202–207. doi: 10.1097/BPO.0000000000000786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.