Summary
Background
A few studies have reported an increased risk of birth defects (BD) with maternal exposure to nitrate in drinking water. We examined this association in a large cohort study with well-characterized exposure.
Methods
Danish singletons liveborn to Danish-born parents from 1991–2013 were identified using civil and patient registries (n=1,018,914). Exposure to nitrate was estimated using a spatial model based on national data linked with individual addresses. Odds ratios (OR) and 95% confidence intervals (95% CI) were estimated using logistic regression.
Findings
In total, 33,182 cases of BD were identified. Nitrate concentrations were generally well below US and EU standards. We observed an exposure-response relationship (p=0·004) between nitrate during pregnancy and eye BD, and increased risk in the highest exposure group (≥25 mg/L nitrate) (OR: 1·29; 95% CI: 1·00, 1·66). An interaction was observed between maternal age and continuous nitrate exposure for nervous system BD (p<0·001) indicating an increased risk among mothers <25 years-of-age (OR for 10 mg/L (OR10): 1·20; 95% CI: 1·06, 1·35). An interaction (p<0.01) with maternal age and continuous nitrate exposure was also observed for ear, face, and neck BD indicating an increased risk among babies born to mothers <25 years-of-age (OR10: 1·35; 95% CI: 1·11, 1·66). There was evidence of an inverse exposure-response relationship for any, digestive system, female genital, and urinary BD.
Interpretation
Our study is the first to report an association between nitrate and eye BD and BD of the ear, face, and neck. It also provides support to prior reports of increased risk of nervous system BD.
Funding
This study was supported by a grant from the United States National Institute of Environmental Health Sciences (R01 ES027823-01A1).
Keywords: Nitrate, Drinking Water, Birth Defects, Congenital Malformations
Research in context.
Evidence before this study
Experimental studies with mice, rats, and hamsters exposed to N-nitroso compounds, which are formed endogenously after ingestion of nitrate, have demonstrated an increase in central nervous system birth defects. Eight epidemiologic studies have examined whether an association exists between nitrate in drinking water and the risk of birth defects in humans. Of these, six case-control and one cohort study have reported an increased risk of nervous system birth defects. All studies were identified through PubMed using “birth defects,” “congenital malformations,” and “nitrate exposure” as search terms and through checking the papers cited in previous relevant publication.
Added value of this study
Most of the prior epidemiological studies identified relied on smaller study populations, crude surrogate measures of nitrate exposure, a case-control study design, and were designed to only examine one birth defect (e.g. nervous system birth defects). Our study used a cohort design to assess a wide range of birth defects. We also utilized high quality individual-level estimates of exposure to nitrate in drinking water for over 1 million births in Denmark, making this the most comprehensive and largest study to date. Our study provides evidence of an increased risk of birth defects of the eye from prenatal exposure to nitrate in drinking water. We also noted an increased risk of nervous system and ear, face, and neck birth defects, but only among children of mothers who were under 25 years old.
Implications of all the available evidence
An association between prenatal exposure to nitrate and birth defects of the eye has not been previously studied, and additional studies are thus warranted. Our effect estimates were unchanged when we restricted analyses to mothers whose drinking water met current European Union and United States nitrate standards. This, along with evidence from prior studies, suggests that the current standards may not be sufficient to prevent birth defects and other adverse birth outcomes from prenatal exposure to nitrate in drinking water.
Alt-text: Unlabelled box
Introduction
Nitrate is one of the most common contaminants in drinking water worldwide.1 Contamination results primarily from animal manure and the use of nitrogen fertilizers. It is of particular concern to communities that have intensive animal farming and extensive use of nitrogen fertilizers, including the United States (US) and Denmark. Excess nitrogen on agricultural fields may leach as water-soluble nitrate into groundwater depending on local soil and hydrogeological conditions. In 2012, approximately 2·5% of the Danish population that received drinking water from public supplies, while 37% of the population that received water from private wells had elevated concentrations of nitrate (≥25 mg/L).2
Birth defects (BD) are a leading cause of infant mortality and may be associated with substantial morbidity and long-term disability. N-nitroso compounds, which are formed endogenously after the ingestion of nitrate and nitrite, have been found to increase central nervous system (CNS) BD in mice,3 hamsters,4 and rats.5 Nitrate has also been shown to be a disruptor of gonadal steroidogenesis and thyroid function in humans.6
Several epidemiologic studies have reported an increased risk of BD from exposure to nitrate in drinking water.7, 8, 9, 10, 11, 12, 13, 14 The evidence is particularly strong for CNS BD and subcategories of CNS (i.e., neural tube defects), which has been reported in six case-control7,8,10, 11, 12,14 and one cohort study.13 An association with nitrate in drinking water has also been reported in some studies of limb deficiencies,11,13 cleft lip or palate,11 and BD of the heart.9,11,13
Common limitations of several of these previous studies relate to their use of ecologic measures of nitrate exposure and small sample size. Only one of the prior studies was a cohort study.13 Several of the case-control studies were designed to only examine the association with CNS BD,8,10,14 and one only examined heart defects.9 Thus these studies could not examine the risk for other major BD.
The objective of our study was to fill this data gap by assessing the potential association between nitrate in drinking water and the risk of specific BD in a large, population-based cohort study with well-characterized estimates of individual-level exposure to nitrate in drinking water during pregnancy.
Methods
Using the Danish Civil Registration System15 we identified all singletons liveborn in Denmark between 1991–2013 to Danish-born parents where the mother resided in Denmark during her entire pregnancy. The study began in 1991 because this was the first year in Denmark when nearly complete information was available on nitrate concentrations in public water systems. Children of non-Danish born parents were excluded to minimize the potential for confounding by diet (e.g., nitrite and antioxidants), adherence to prenatal care, and other possible life-style factors. Primary analyses excluded children of women who resided in a house with private well use during their pregnancy or who had incomplete covariate or nitrate data.
Birth defects that were diagnosed until two years-of-age were identified using the Danish National Patient Registry.17 We linked individual-level data from the Danish Medical Birth Registry16 the Danish Civil Registration System with the BD identified in the Danish National Patient Registry using the unique personal Danish identification numbers (CPR). Data from the Danish Medical Birth Registry was only available for births up to 2015. Since we included birth defects that occurred up to age 2 we only included births up to 2013 in order to allow each child to have two years of follow-up. BD were identified using the ICD-8 revision for births from 1991–1993 and the ICD-10 revision for births thereafter. BD were grouped for analysis using the 11 major BD defects categories defined in the EUROCAT guide 1·418 for ICD-10, and on a modified version of a translation to ICD-8 published by Cohen et al.19 A category for any BD was created in which cases with multiple birth defects were counted only once. The codes used to classify the BD are presented in Supplemental Table 1.
Since prior studies have reported an association with neural tube defects, analyses were conducted for subcategories of nervous system BD. Post-hoc subcategory analyses were also conducted for BD of the eye, since a strong association was observed between nitrate and this category.
Exposure assessment
Groundwater is the source of water for nearly all public water systems in Denmark. The methodology for the estimation of household levels of nitrate in drinking water has been thoroughly described in previous publications.2,20 In brief, we developed a spatial model linking drinking water quality measurements from the national monitoring database, Jupiter, at the waterworks-level with the locations of all Danish residential addresses. We have refined our spatial model by taking into further account historical changes in the water supply areas.
In total, we calculated annual average nitrate concentrations from 130,944 drinking water samples in 3,907 public waterworks taken between 1991–2013. Measurements of nitrate in water were taken at the exit of the waterworks, in the distribution system, and at the consumer taps which have all been found to be highly correlated.21 Approximately 11% of water supply areas had a nitrate sample from more than one water works in a given year. In these cases, we computed average concentrations, weighted by the annual drinking water production volume of each waterworks. Although nitrate levels have been declining over time in Denmark, yearly averages are adequate since no seasonal variation of nitrate concentrations in Danish public supplies has been observed and levels seldom change over a year.20
We imputed annual nitrate concentrations for years with missing information (19% of all water supply area/year dyads) using linear interpolation between two sampled years. We only imputed missing exposure when a nitrate sample was available within three years. At the tails, we used the closest observation moving forward in time for the earliest year and moving backward in time for the latest year.
Longitudinal residential address histories for each mother and child pair were obtained from the Danish Civil Registration System.15 Addresses were linked to the calendar year-specific average exposure estimates for their water system. We computed time-weighted average exposure for each mother for 90 days prior to conception, during pregnancy, and for each pregnancy trimester.
We excluded births to mothers who lived in households that had private wells anytime during their pregnancy from the main analysis because monitoring of private wells for nitrate is much less complete (∼50% of wells) and private wells have generally poorer water quality than public supplies.2
Covariates
Covariates in our main models were chosen a priori based on their known or suspected association with BD and their potential for an association with nitrate exposure. Information on birth weight (continuous), birth order (1st, 2nd, ≥3rd), maternal and paternal age (continuous), maternal smoking during pregnancy (yes/no), and pre-pregnancy maternal height and weight (continuous) were obtained from the Danish Medical Birth Registry.16 Maternal height and weight were used to calculate pre-pregnancy body mass index (BMI). Information on parental employment (employed, unemployed, not in the workforce), parental education (primary, high school, or higher), and parental income (continuous), which was normalized for inflation to 2013 using the Consumer Price Index, was obtained from the Integrated Database for Longitudinal Labour Market Research.22 Income, education, and employment data from two years prior to birth were used.
Statistical analysis
The association between the covariates with any BD and nitrate exposure was examined using contingency tables and tested using a Pearson chi-square test. Logistic regression models were used to examine the association between nitrate in maternal drinking water and BD. Models were fit using generalized estimating equations and robust standard errors to account for the clustering of children born to the same mother. Unadjusted models and models that controlled for year of birth, birth order, birth weight, sex, parental age, and maternal smoking, education, income, and employment status were fitted. The continuous covariates (birth weight, parental age, and income) were modeled using restricted cubic splines with four knots placed at 0·05, 0·35, 0·65, and 0·95 quantiles of the nitrate exposure distribution. The statistical significance of the exposure-response relationships was determined by fitting the continuous measures of exposure and computing the Wald statistic and associated p-value. Finally, for ease of interpretation, marginal standardization23 was used to derive absolute risks from models with significant exposure-response relationships.
Nitrate concentrations were modeled as both categorical and continuous variables. In the main analyses, the exposure was averaged over the pregnancy considering changes over time, and in maternal addresses. Four categories of exposure were defined a priori based on the distribution of exposure in the population and their usefulness for policy considerations. The highest category ≥25 mg/L nitrate is half of the current EU standard. The lowest category, <2 mg/L nitrate, was used as the referent.
For each major outcome category, statistical interactions between nitrate and each of the covariates were examined in models that adjusted for all of the covariates. The significance of the interactions was assessed using a likelihood ratio test comparing the model with the interaction term to a model with just the covariates and nitrate exposure variables. A priori we decided to only report interactions that were below p<0·01 due to the large number of interactions examined. All tests of statistical significance were two-sided.
Sensitivity analyses were performed to assess whether our results changed when we: 1) modeled pre-conception and trimester-specific nitrate exposure rather than the pregnancy average; 2) included children born to mothers who lived in homes with private wells during their pregnancy; 3) included additional adjustment for paternal employment, education, and income; 4) included maternal BMI in the model, which was only available from 2003 onward; 5) compared results for births before and after 2004 when Denmark initiated a BD screening program; and 6) dropped individuals who had average nitrate exposure levels greater than the EU standard of 50 mg/L nitrate to evaluate whether or not the standard is adequate to prevent an increased risk of BD.
All statistical analyses were conducted using Stata Version 16·1.
Ethical considerations
In keeping with Danish legislation, the Danish Data Protection Agency, the Danish Health Data Authority, and Statistics Denmark approved this study. This study has also been approved by the University of Illinois at Chicago's Institutional Review Board. Due to Danish legislation and the use of de-identified data, informed consent was not needed for this study.
Role of the funding source
This work was supported by funding that was provided by the National Institutes for Health/National Institute for Environmental Health Sciences grant (R01 ES027823-01A1). The funders had no role in the design, conduct, or interpretation of the study findings, and in the decision to submit this paper for publication.
Results
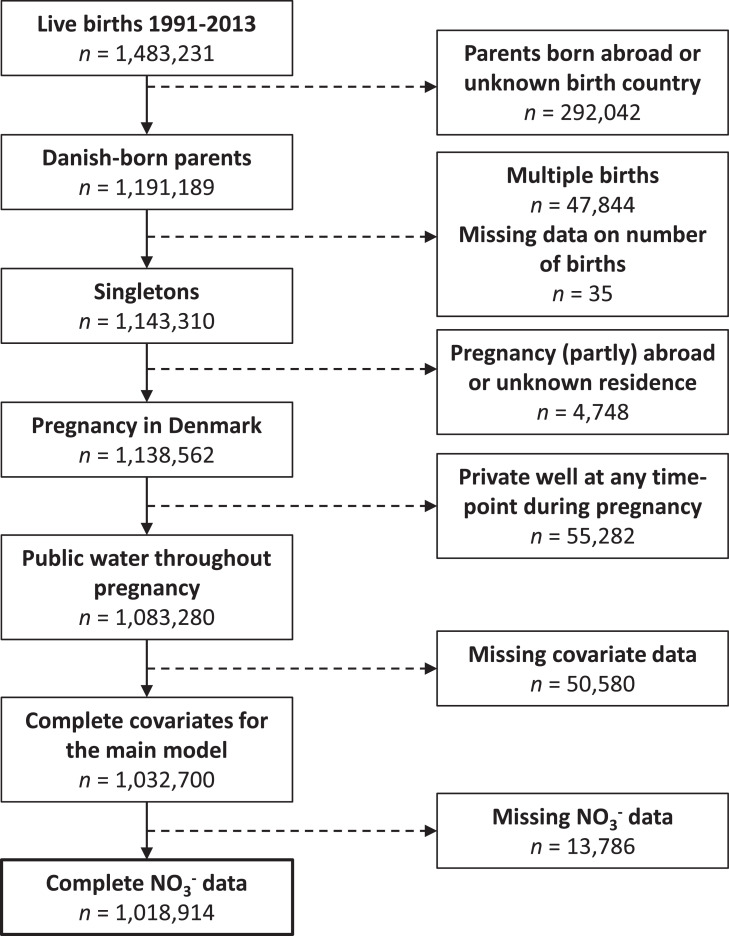
A total of 1,138,562 live-born singletons who met the study entrance criteria of having been born in Denmark between 1991–2013 to Danish-born parents and a mother who resided in Denmark during her pregnancy were identified. We excluded children of women who resided in a house with private well use during their pregnancy (n=55,282, 5%), had incomplete covariate data (n=50,580, 4%), or incomplete nitrate data (n=13,786, 1%) resulting in an analytic data set of 1,018,914 births (Figure 1). In total, 33,182 BD diagnosed within the first two years of life were identified.
Figure 1.
Flowchart of the birth cohort enumeration.
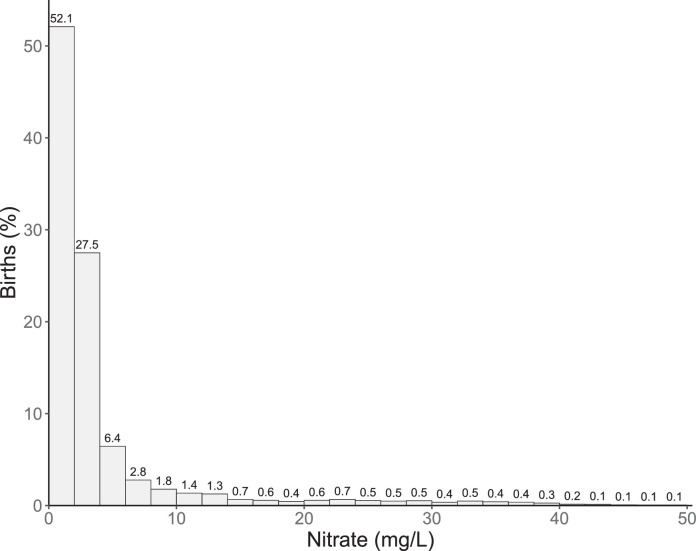
All covariates were significantly (p<0·001) associated with both case status (Table 1) and pregnancy average nitrate exposure (Supplemental Table 2), except for sex (p=0·05). Pregnancy average nitrate exposure was also significantly associated with any BD. Non-cases had a slightly larger percentage (3·8%) in the highest exposure group (≥25 mg/L nitrate) than cases (3·5%). The distribution of the nitrate exposure was highly right skewed. The mean exposure in the highest exposure group (>25 mg/L) was 34.8 mg/L (SD=8.5). Approximately 0·2% (n=2,026) of the cohort had drinking water exposures that exceeded the EU standard of 50 mg/L nitrate (Figure 2).
Table 1.
Study population characteristics by any birth defect case status.
| Non-Cases |
Cases |
|||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Birth Year | ||||
| 1991–1995 | 227 037 | 23·0 | 6 542 | 19·7 |
| 1996–2000 | 220 757 | 22·4 | 6 817 | 20·5 |
| 2001–2005 | 215 859 | 21·9 | 7 362 | 22·2 |
| 2006–2010 | 211 092 | 21·4 | 7 896 | 23·8 |
| 2010–2013 | 110 987 | 11·3 | 4 565 | 13·8 |
| Birth Order | ||||
| First | 435 906 | 44·2 | 15 426 | 46·5 |
| Second | 380 611 | 38·6 | 11 951 | 36·0 |
| ≥Third | 169 215 | 17·2 | 5 805 | 17·5 |
| Maternal Age (years) | ||||
| <25 | 134 895 | 13·7 | 4 844 | 14·6 |
| 25 to <30 | 361 475 | 36·7 | 11 559 | 34·8 |
| 30 to <35 | 340 976 | 34·6 | 11 220 | 33·8 |
| ≥35 | 148 386 | 15·1 | 5 559 | 16·8 |
| Maternal Education | ||||
| Elementary | 229 475 | 23·3 | 8 627 | 26·0 |
| High School | 464 586 | 47·1 | 15 111 | 45·5 |
| Higher Education | 291 671 | 29·6 | 9 444 | 28·5 |
| Maternal Income (Kroner/year) | ||||
| <175 000 | 239 007 | 24·2 | 8 506 | 25·6 |
| 175 000 to <250 000 | 266 041 | 27·0 | 8 761 | 26·4 |
| 250 000 to <300 000 | 195 984 | 19·9 | 6 376 | 19·2 |
| ≥300 000 | 284 700 | 28·9 | 9 539 | 28·7 |
| Maternal Employment | ||||
| Employed | 801 661 | 81·3 | 26 364 | 79·5 |
| Unemployed | 60 620 | 6·1 | 2 080 | 6·3 |
| Not in Workforce | 123 451 | 12·5 | 4 738 | 14·3 |
| Paternal Age (years) | ||||
| <25 | 67 255 | 6·8 | 2 455 | 7·4 |
| 25 to <30 | 271 851 | 27·6 | 8 870 | 26·7 |
| 30 to <35 | 363 912 | 36·9 | 11 951 | 36·0 |
| ≥35 | 282 714 | 28·7 | 9 906 | 29·9 |
| Sex | ||||
| Male | 503 519 | 51·1 | 19 831 | 59·8 |
| Female | 482 213 | 48·9 | 13 351 | 40·2 |
| Maternal Smoking | ||||
| Non-smoker | 769 506 | 78·1 | 25 633 | 77·2 |
| Smoker | 216 226 | 21·9 | 7 549 | 22·8 |
| Mean Nitrate During Pregnancy (mg/L) | ||||
| <2 | 510 195 | 51·8 | 17 264 | 52·0 |
| 2 to <5 | 310 986 | 31·5 | 10 653 | 32·1 |
| 5 to <25 | 126 645 | 12·8 | 4 092 | 12·3 |
| ≥25 | 37 906 | 3·8 | 1 173 | 3·5 |
Figure 2.
Histogram of the distribution of average pregnancy nitrate exposure.
Findings from unadjusted and adjusted models (Table 2) were similar in magnitude and direction and we therefore focused on adjusted model results. The risk of having any BD decreased with nitrate exposure in both the categorical and continuous models (p=0·02). Evidence of an inverse exposure-response relationship was also observed for most of the specific BD categories and was particularly strong for digestive system (p=0·03), female genital (p=0·002), and urinary (p=0·001) BD. BD of the eye was the only category that demonstrated a significant positive exposure-response (p=0·004) with nitrate exposure. The odds ratio (OR) for eye BD was 1.29 (95% confidence interval (95% CI): 1·00, 1·66) for the highest exposure group (≥25 mg/L nitrate) compared to the lowest exposure group (<2 mg/L nitrate) and the OR per 10 mg/L nitrate (OR10) was 1·09 (95% CI: 1·03, 1·16).
Table 2.
Results for the major birth defect categories from crude and adjusted models using categorical or continuous average estimates of prenatal exposure to nitrate.
| Unadjusted |
Adjusteda |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth Defect (number of cases) | OR | 95%CI | p-value | OR | 95%CI | p-value | ||||||||||||
| Any (n=33 182) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 1·01 | 0·99 | 1·04 | 0·34 | 1·01 | 0·98 | 1·04 | 0·45 | |||||||||||
| 0·95 | 0·92 | 0·99 | 0·001 | 0·99 | 0·95 | 1·02 | 0·40 | |||||||||||
| 0·91 | 0·86 | 0·97 | 0·004 | 0·93 | 0·88 | 0·99 | 0·02 | |||||||||||
| 0·97 | 0·96 | 0·99 | 0·001 | 0·98 | 0·97 | 1·00 | 0·02 | |||||||||||
| Abdominal Wall (n=445) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 1·01 | 0·82 | 1·25 | 0·92 | 0·92 | 0·74 | 1·14 | 0·44 | |||||||||||
| 1·13 | 0·86 | 1·50 | 0·38 | 0·87 | 0·66 | 1·16 | 0·35 | |||||||||||
| 1·21 | 0·76 | 1·90 | 0·42 | 1·01 | 0·64 | 1·59 | 0·97 | |||||||||||
| 1·08 | 0·96 | 1·21 | 0·20 | 1·00 | 0·88 | 1·13 | 0·98 | |||||||||||
| Digestive System (n=2 139) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 0·98 | 0·88 | 1·10 | 0·78 | 0·96 | 0·87 | 1·06 | 0·44 | |||||||||||
| 0·93 | 0·80 | 1·08 | 0·34 | 1·02 | 0·89 | 1·16 | 0·81 | |||||||||||
| 0·75 | 0·56 | 1·00 | 0·05 | 0·70 | 0·54 | 0·92 | 0·01 | |||||||||||
| 0·90 | 0·85 | 0·97 | 0·003 | 0·93 | 0·87 | 0·99 | 0·03 | |||||||||||
| Ear, Face and Neck (n=448) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 1·22 | 0·99 | 1·50 | 0·06 | 0·96 | 0·87 | 1·06 | 0·44 | |||||||||||
| 1·04 | 0·77 | 1·39 | 0·81 | 1·02 | 0·89 | 1·16 | 0·81 | |||||||||||
| 1·26 | 0·80 | 2·00 | 0·32 | 0·70 | 0·54 | 0·92 | 0·001 | |||||||||||
| 1·02 | 0·90 | 1·15 | 0·78 | 1·03 | 0·91 | 1·17 | 0·60 | |||||||||||
| Eye (n=1 402) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 1·22 | 0·99 | 1·50 | 0·06 | 1·02 | 0·91 | 1·16 | 0·69 | |||||||||||
| 1·04 | 0·77 | 1·39 | 0·81 | 1·23 | 1·05 | 1·44 | 0·01 | |||||||||||
| 1·26 | 0·80 | 2·00 | 0·32 | 1·29 | 1·00 | 1·66 | 0·05 | |||||||||||
| 1·08 | 1·01 | 1·15 | 0·02 | 1·09 | 1·03 | 1·16 | 0·004 | |||||||||||
| Male Genital (n=3 103) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | Ref. | ||||||||||||||||
| 1·10 | 1·04 | 1·18 | 0·002 | 1·09 | 1·02 | 1·16 | 0·01 | |||||||||||
| 1·02 | 0·93 | 1·12 | 0·64 | 0·99 | 0·91 | 1·09 | 0·87 | |||||||||||
| 0·88 | 0·75 | 1·03 | 0·11 | 0·86 | 0·74 | 1·02 | 0·08 | |||||||||||
| 0·97 | 0·93 | 1·01 | 0·12 | 0·96 | 0·92 | 1·00 | 0·06 | |||||||||||
| Female Genital (n=203)c | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | Ref. | ||||||||||||||||
| 1·16 | 0·90 | 0·98 | 0·01 | 1·12 | 1·06 | 1·20 | 0·0002 | |||||||||||
| 1·11 | 0·92 | 1·04 | 0·47 | 0·99 | 0·91 | 1·08 | 0·83 | |||||||||||
| NAc | NAc | NAc | NAc | NAc | NAc | NAc | NAc | |||||||||||
| 0·97 | 0·98 | 1·03 | 0·87 | 0·94 | 0·90 | 0·98 | 0·002 | |||||||||||
| Heart (n=9 752) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 0·94 | 0·90 | 0·98 | 0·01 | 0·94 | 0·90 | 0·99 | 0·01 | |||||||||||
| 0·98 | 0·92 | 1·04 | 0·47 | 1·01 | 0·95 | 1·08 | 0·77 | |||||||||||
| 1·02 | 0·92 | 1·13 | 0·68 | 1·04 | 0·94 | 1·15 | 0·45 | |||||||||||
| 1·00 | 0·98 | 1·03 | 0·87 | 1·01 | 0·99 | 1·04 | 0·37 | |||||||||||
| Limbs (n=5 528) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 0·94 | 0·90 | 0·98 | 0·01 | 1·02 | 0·96 | 1·08 | 0·54 | |||||||||||
| 0·98 | 0·92 | 1·04 | 0·47 | 0·97 | 0·89 | 1·06 | 0·52 | |||||||||||
| 1·02 | 0·92 | 1·13 | 0·68 | 0·91 | 0·78 | 1·06 | 0·21 | |||||||||||
| 1·00 | 0·96 | 1·03 | 0·83 | 0·99 | 0·95 | 1·03 | 0·64 | |||||||||||
| Nervous System (n=1 702) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousc |
Ref. | Ref. | ||||||||||||||||
| 0·98 | 0·88 | 1·10 | 0·78 | 0·98 | 0·88 | 1·10 | 0·76 | |||||||||||
| 0·93 | 0·80 | 1·08 | 0·34 | 0·93 | 0·80 | 1·09 | 0·37 | |||||||||||
| 0·75 | 0·56 | 1·00 | 0·05 | 0·75 | 0·57 | 1·00 | 0·05 | |||||||||||
| 0·94 | 0·87 | 1·01 | 0·09 | 0·94 | 0·87 | 1·01 | 0·10 | |||||||||||
| Oro-facial Clefts (n=2154) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | Ref. | ||||||||||||||||
| 1·07 | 0·97 | 1·17 | 0·18 | 1·07 | 0·97 | 1·17 | 0·18 | |||||||||||
| 1·00 | 0·88 | 1·14 | 0·99 | 0·97 | 0·85 | 1·11 | 0·68 | |||||||||||
| 0·97 | 0·77 | 1·23 | 0·82 | 0·95 | 0·76 | 1·20 | 0·69 | |||||||||||
| 0·99 | 0·93 | 1·05 | 0·71 | 0·98 | 0·92 | 1·04 | 0·52 | |||||||||||
| Respiratory (n=1287) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | Ref. | ||||||||||||||||
| 0·97 | 0·90 | 1·05 | 0·46 | 1·22 | 1·08 | 1·38 | 0·001 | |||||||||||
| 0·85 | 0·76 | 0·95 | 0·004 | 1·15 | 0·97 | 1·37 | 0·11 | |||||||||||
| 0·66 | 0·53 | 0·81 | 0·0001 | 0·85 | 0·61 | 1·18 | 0·34 | |||||||||||
| 0·94 | 0·87 | 1·02 | 0·12 | 0·95 | 0·88 | 1·03 | 0·22 | |||||||||||
| Urinary (n=3055) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | Ref. | ||||||||||||||||
| 0·97 | 0·90 | 1·05 | 0·46 | 0·96 | 0·89 | 1·04 | 0·35 | |||||||||||
| 0·85 | 0·76 | 0·95 | 0·004 | 0·88 | 0·79 | 0·98 | 0·02 | |||||||||||
| 0·66 | 0·53 | 0·81 | 0·0001 | 0·67 | 0·54 | 0·83 | 0·0002 | |||||||||||
| 0·89 | 0·84 | 0·94 | <0·0001 | 0·90 | 0·85 | 0·95 | 0·0001 | |||||||||||
| Other (n=4244) | ||||||||||||||||||
| <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | Ref. | ||||||||||||||||
| 1·07 | 1·01 | 1·13 | 0·02 | 0·92 | 0·74 | 1·14 | 0·44 | |||||||||||
| 0·91 | 0·84 | 0·98 | 0·02 | 0·87 | 0·66 | 1·16 | 0·35 | |||||||||||
| 0·92 | 0·81 | 1·05 | 0·21 | 1·01 | 0·64 | 1·59 | 0·97 | |||||||||||
| 0·96 | 0·93 | 0·99 | 0·02 | 0·99 | 0·95 | 1·02 | 0·40 | |||||||||||
Note: Abbreviations: OR=odds ratio; 95% CI=95% confidence interval; Ref.=referent group.
Models adjusted for birth weight, birth order, birth year, sex, maternal and paternal age, and maternal smoking, income, education and employment status.
Results from the continuous model for an increase in exposure of 10 mg/L.
To preserve confidentiality and because models were based on less than five cases results are not available.
There was no evidence of an association between prenatal nitrate exposure and any of the subcategories of nervous system BD (Table 3). An association was observed between prenatal nitrate exposure and congenital cataracts in the second to highest exposure category (5–25 mg/L nitrate) (OR: 1·41, 95% CI: 1·05, 1·90). Evidence of an exposure-response relationship (p=0.0005) was observed for the other eye BD (Table 4).
Table 3.
Results for the subcategories of nervous system birth defects from adjusted modelsa using categorical or continuous estimates of prenatal exposure to nitrate in household drinking water.
| Birth Defect (number of cases) | Nitrate (mg/L) | OR | 95%CI | p-value | |
|---|---|---|---|---|---|
| Neural Tube (n=424) | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 0·89 | 0·72 | 1·10 | 0·29 | ||
| 0·72 | 0·52 | 1·00 | 0·05 | ||
| 0·88 | 0·53 | 1·46 | 0·62 | ||
| 0·95 | 0·82 | 1·10 | 0·48 | ||
| Anencephaly (n=12)c | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| NAc | NAc | NAc | NAc | ||
| NAc | NAc | NAc | NAc | ||
| NAc | NAc | NAc | NAc | ||
| 1·19 | 0·70 | 2·01 | 0·52 | ||
| Encephalocele (n=62) | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 0·77 | 0·43 | 1·37 | 0·37 | ||
| 0·49 | 0·19 | 1·24 | 0·13 | ||
| NAc | NAc | NAc | NAc | ||
| 0·92 | 0·60 | 1·41 | 0·70 | ||
| Hydrocephalus (n=501) | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 1·06 | 0·87 | 1·29 | 0·55 | ||
| 1·08 | 0·83 | 1·40 | 0·59 | ||
| 0·63 | 0·35 | 1·12 | 0·12 | ||
| 0·92 | 0·81 | 1·04 | 0·18 | ||
| Microcephalus (n=395) | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 1·00 | 0·80 | 1·26 | 0·97 | ||
| 0·87 | 0·62 | 1·20 | 0·39 | ||
| 0·69 | 0·38 | 1·26 | 0·22 | ||
| 0·88 | 0·75 | 1·03 | 0·10 | ||
| Spina Bifida (n=356) | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 0·90 | 0·71 | 1·13 | 0·36 | ||
| 0·78 | 0·55 | 1·09 | 0·15 | ||
| 0·80 | 0·44 | 1·42 | 0·44 | ||
| 0·94 | 0·80 | 1·09 | 0·41 | ||
| Other Nervous System (n=513) | <2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 0·93 | 0·76 | 1·13 | 0·47 | ||
| 1·06 | 0·81 | 1·39 | 0·66 | ||
| 0·72 | 0·42 | 1·23 | 0·23 | ||
| 0·97 | 0·84 | 1·12 | 0·67 | ||
Note: Abbreviations: OR=odds ratio; 95% CI= 95% confidence interval; Ref.=referent group.
Models adjusted for birth weight, birth order, birth year, sex, maternal and paternal age, and maternal smoking, income, education and employment status.
Results from the continuous model for an increase in exposure of 10 mg/L.
To preserve confidentiality and because models were based on less than five cases results are not available.
Table 4.
Results for the subcategories of eye BD from adjusteda models using categorical or continuous estimates of prenatal exposure to nitrate.
| Birth Defect (number of cases) | Nitrate | OR | 95%CI | p-value | |
|---|---|---|---|---|---|
|
Anophtalmos (n=17)b |
<2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
NAc | NAc | NAc | NAc |
| NAc | NAc | NAc | NAc | ||
| NAc | NAc | NAc | NAc | ||
| NAc | NAc | NAc | NAc | ||
| 0·60 | 0·24 | 1·49 | 0·27 | ||
|
Anophtalmos/Microthalmos (n=135) |
<2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 1·18 | 0·81 | 1·73 | 0·39 | ||
| 1·06 | 0·62 | 1·80 | 0·84 | ||
| 1·23 | 0·53 | 2·83 | 0·63 | ||
| 1·03 | 0·84 | 1·27 | 0·76 | ||
|
Congenital Cataracts (n=362) |
<2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 1·03 | 0·81 | 1·31 | 0·83 | ||
| 1·41 | 1·05 | 1·90 | 0·02 | ||
| 1·01 | 0·57 | 1·78 | 0·97 | ||
| 1·03 | 0·91 | 1·16 | 0·65 | ||
|
Micropthalmos (n=127) |
<2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 1·20 | 0·81 | 1·78 | 0·37 | ||
| 1·15 | 0·67 | 1·96 | 0·62 | ||
| 1·33 | 0·58 | 3·07 | 0·50 | ||
| 1·07 | 0·88 | 1·30 | 0·52 | ||
|
Other Eye (n=930) |
<2 mg/L 2 to <5 mg/L 5 to <25 mg/L ≥25 mg/L Continuousb |
Ref. | |||
| 0·99 | 0·86 | 1·15 | 0·93 | ||
| 1·19 | 0·98 | 1·45 | 0·08 | ||
| 1·43 | 1·06 | 1·92 | 0·02 | ||
| 1·13 | 1·06 | 1·21 | 0·0005 | ||
Note: Abbreviations: OR=odds ratio; 95%CI=95% confidence interval; Ref.=referent group.
Models adjusted for birth weight, birth order, birth year, sex, maternal and paternal age, and maternal smoking, income, education and employment status.
Results from the continuous model for an increase in exposure of 10 mg/L.
To preserve confidentiality and because models were based on less than five cases results are not available.
Strong evidence of an interaction with maternal age was observed for nervous system BD (p<0.001). The risk of nervous system BD increased with exposure for mothers <25 years-of-age (OR10: 1·20, 95% CI: 1·06, 1·35) and decreased with exposure in the other age groups. We also observed evidence (p<0·01) of an interaction between maternal age and nitrate exposure for ear, face, and neck BD. The pattern of the interaction was similar with an increased risk for mothers <25 years-of-age (OR10: 1·35, 95% CI: 1·11, 1.66; p=0.003) and no increased risk in the other age categories. There was no evidence of an interaction between maternal age and nitrate for other BD or with other covariates (Table 5).
Table 5.
Results from adjusted continuous modelsab that include an interaction term between maternal age and nitrate exposure.
| Birth Defect (number of cases) | Maternal age (years) | OR10 | 95%CI | p-value | p for interaction | |
|---|---|---|---|---|---|---|
|
Any (n=33182) |
<25 25 to <30 30 to <35 ≥35 |
1·00 | 0·96 | 1·03 | 0·83 | 0·71 |
| 0·97 | 0·95 | 1·00 | 0·04 | |||
| 0·98 | 0·95 | 1·01 | 0·13 | |||
| 0·99 | 0·95 | 1·04 | 0·77 | |||
|
Abdominal (n=445) |
<25 25 to <30 30 to <35 ≥35 |
1·01 | 0·83 | 1·25 | 0·89 | 0·33 |
| 0·90 | 0·73 | 1·13 | 0·37 | |||
| 0·98 | 0·76 | 1·26 | 0·88 | |||
| 1·30 | 0·98 | 1·72 | 0·07 | |||
|
Digestive System (n=2139) |
<25 25 to <30 30 to <35 ≥35 |
0·90 | 0·77 | 1·07 | 0·24 | 0·09 |
| 0·92 | 0·83 | 1·02 | 0·13 | |||
| 0·86 | 0·76 | 0·98 | 0·02 | |||
| 1·09 | 0·96 | 1·25 | 0·19 | |||
|
Ear, Face and Neck (n=448) |
<25 25 to <30 30 to <35 ≥35 |
1·35 | 1·11 | 1·66 | 0·003 | 0·0095 |
| 1·08 | 0·90 | 1·30 | 0·39 | |||
| 0·84 | 0·64 | 1·12 | 0·23 | |||
| 0·65 | 0·36 | 1·17 | 0·16 | |||
|
Eye (n=1402) |
<25 25 to <30 30 to <35 ≥35 |
1·13 | 0·97 | 1·31 | 0·13 | 0·57 |
| 1·06 | 0·95 | 1·18 | 0·29 | |||
| 1·15 | 1·04 | 1·26 | 0·005 | |||
| 1·02 | 0·84 | 1·22 | 0·86 | |||
|
Genital (Female; n=203) |
<25 25 to <30 30 to <35 ≥35 |
0.90 | 0·49 | 1·65 | 0·73 | 0·06 |
| 0·60 | 0·35 | 1·04 | 0·07 | |||
| 0.97 | 0·67 | 1·39 | 0·86 | |||
| 0·94 | 0·01 | 1·12 | 0·06 | |||
|
Genital (Male; n=3103) |
<25 25 to <30 30 to <35 ≥35 |
1·02 | 0·90 | 1·14 | 0·80 | 0·82 |
| 0·96 | 0·89 | 1·04 | 0·35 | |||
| 1·01 | 0·93 | 1·10 | 0·80 | |||
| 0·98 | 0·85 | 1·12 | 0·74 | |||
|
Heart (n=9752) |
<25 25 to <30 30 to <35 ≥35 |
0·99 | 0·93 | 1·06 | 0·80 | 0·61 |
| 1·00 | 0·95 | 1·04 | 0·89 | |||
| 1·03 | 0·98 | 1·08 | 0·26 | |||
| 1·04 | 0·97 | 1·11 | 0·26 | |||
|
Limb (n=5528) |
<25 25 to <30 30 to <35 ≥35 |
0·95 | 0·87 | 1·05 | 0·33 | 0·58 |
| 1·02 | 0·97 | 1·08 | 0·46 | |||
| 0·98 | 0·91 | 1·04 | 0·47 | |||
| 0·98 | 0·89 | 1·08 | 0·73 | |||
|
Nervous System (n=1702) |
<25 25 to <30 30 to <35 ≥35 |
1·20 | 1·06 | 1·35 | 0·003 | 0.0005 |
| 0·88 | 0·78 | 0·99 | 0·03 | |||
| 0·89 | 0·78 | 1·03 | 0·11 | |||
| 0·76 | 0·59 | 0·98 | 0·03 | |||
|
Oral (n=2154) |
<25 25 to <30 30 to <35 ≥35 |
0·96 | 0·83 | 1·11 | 0·58 | 0·74 |
| 1·02 | 0·93 | 1·12 | 0·65 | |||
| 0·95 | 0·86 | 1·06 | 0·40 | |||
| 0·95 | 0·80 | 1·12 | 0·52 | |||
|
Respiratory (n=1287) |
<25 25 to <30 30 to <35 ≥35 |
0·97 | 0·80 | 1·17 | 0·72 | 0·91 |
| 0·93 | 0·81 | 1·06 | 0·32 | |||
| 0·94 | 0·82 | 1·09 | 0·43 | |||
| 1·02 | 0·84 | 1·23 | 0·87 | |||
|
Urinary (n=3055) |
<25 25 to <30 30 to <35 ≥35 |
0·83 | 0·71 | 0·98 | 0·03 | 0·52 |
| 0·92 | 0·84 | 1·01 | 0·07 | |||
| 0·86 | 0·77 | 0·95 | 0·004 | |||
| 0·94 | 0·81 | 1·08 | 0·35 | |||
|
Other (n=4244) |
<25 25 to <30 30 to <35 ≥35 |
0·99 | 0·89 | 1·11 | 0·92 | 0·21 |
| 0·92 | 0·85 | 1·00 | 0·04 | |||
| 1·02 | 0·95 | 1·09 | 0·58 | |||
| 1·03 | 0·93 | 1·14 | 0·63 | |||
Note: Abbreviations: OR=odds ratio; 95%CI=95% confidence interval; Ref.=referent group.
Models adjusted for birth weight, birth order, birth year, sex, maternal and paternal age, and maternal smoking, income, education and employment status.
Results from the continuous model for an increase in exposure of 10 mg/L.
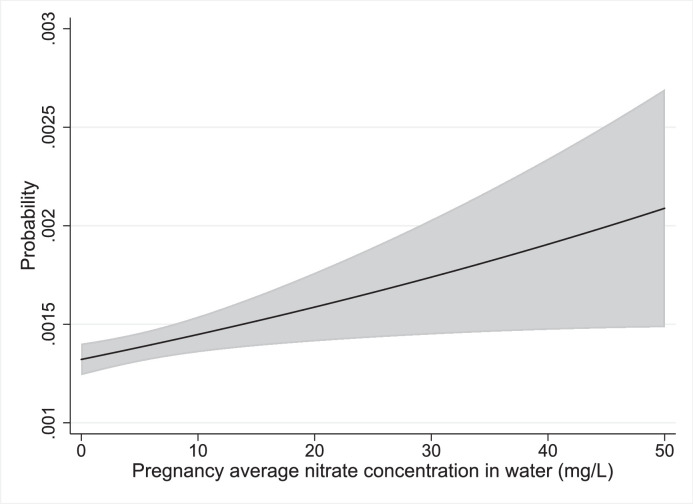
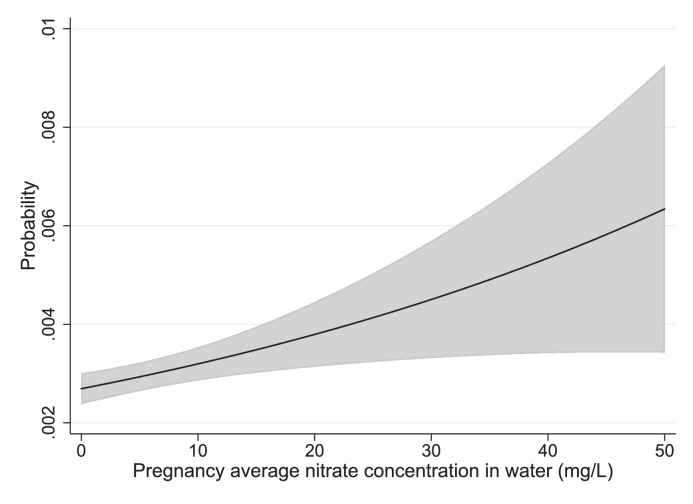
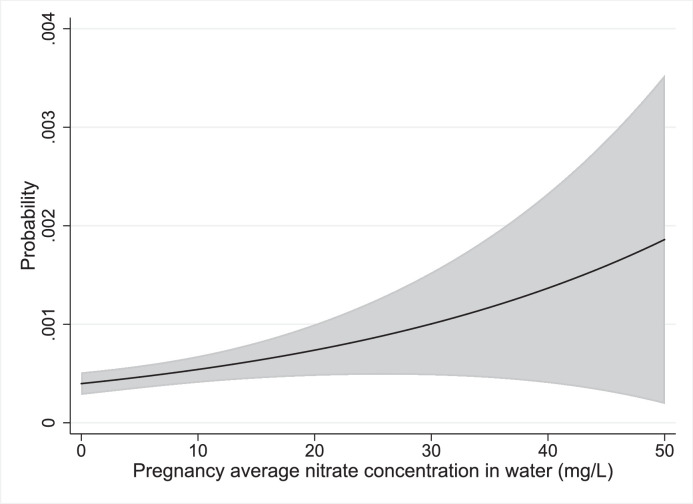
The predicted absolute risks from marginal standardized models for eye neoplasms are illustrated in Figure 3. Results for children of mothers < 25 years old for nervous system and ear, face and neck BD are illustrated in Figure 4, Figure 5.
Figure 3.
Marginally standardized plot of the predicted probability of eye birth defects as a function of nitrate exposure.
Figure 4.
Marginally standardized plot of the probability of nervous system BD as a function of nitrate exposure for children with mothers age< 25.
Figure 5.
Marginally standardized plot of the probability of ear, face and neck BD as a function of nitrate exposure for children with mothers age<25.
Sensitivity analyses
The results were generally unchanged when pre-conception (Supplemental Table 3) or trimester-specific (Supplemental Table 4) estimates of exposure were used rather than the pregnancy average. This is largely explained by the fact the preconception and trimester specific nitrate exposure estimates were highly correlated (R2=0·96, 0·99) with the pregnancy average. The exposure-response relationship for eye BD was somewhat stronger for third trimester (OR10: 1·10, 95% CI: 1·04, 1·17), than for the first (OR10: 1·07, 95% CI: 1·01, 1·14) or second trimester (OR10: 1·09, 95% CI: 1·03, 1·16). The results were largely unchanged when we included children whose mothers lived in homes with private wells sometime during their pregnancy (Supplemental Table 5) or when paternal socioeconomic status variables (income, education, and employment) were included (Supplemental Table 6). The addition of BMI to the model, which was only available from 2003 onward, resulted in a slightly stronger exposure-response relationship for eye BD (OR10: 1·14, 95% CI: 1·04, 1·25) (Supplemental Table 7) than in the main analysis (OR10: 1·09, 95% CI: 1·03, 1·16) (Table 2).
Stratifying the analysis by pre- and post-2004, which is the year that ultrasound screening during gestational week 18–20 was widely implemented in Denmark, revealed stronger evidence of an exposure-response relationship for eye BD in the 2004–2015 time period (OR10: 1·14, 95% CI: 1·03, 1·25; p=0.01) than in 1991–2003 (OR10: 1·07, 95% CI: 0·98, 1·15; p=0.12) (Supplemental Table 8). Finally, our findings were unchanged when children of mothers who had average nitrate exposure drinking water concentrations greater than the EU standard of 50 mg/L nitrate were excluded from the analysis (Supplemental Table 9).
Discussion
We observed strong evidence of an exposure-response relationship (p=0.004) between nitrate and BD of the eye (OR10: 1·09, 95% CI: 1·03, 1·16) in our study. Ours is the first study to report findings of an association between nitrate exposure from residential tap water and BD of the eye. The register-based retrospective cohort design and large sample size of our study permitted us to examine eye and other BD that were not examined in prior studies. An association between the use of nitrosatable drugs during pregnancy and the risk of eye BD was reported in a study by Olshan and Faustman.24 Nitrosatable drugs may react with exogenous and endogenous nitrate and nitrite to form N-nitroso compounds, which are known to be teratogenic in experimental animals.3, 4, 5 Nitrate has also been found to be highly concentrated in eye tissues where it plays an important role in nitrous oxide regulation.25
Prior epidemiologic studies have most consistently reported an association between nitrate in drinking water and the risk of nervous system BD, particularly neural tube defects or spina bifida.7,8,10,11,13,14 N-nitroso compounds, which are formed endogenously have been found to increase central nervous system BD in several animal species as well. 3, 4, 5
Overall, we did not observe an association between nervous system BD and nitrate in our study. However, we did observe strong evidence (p=0.0005) of an interaction between maternal age and nitrate exposure for nervous system BD, which revealed a strong exposure-response relationship for women <25 years-of-age. We also observed weaker evidence (p<0.01) of an interaction between nitrate and ear, face and neck BD among children of mothers <25 years-of-age. An association between nitrate and BD of the ear, face, and neck BD has not been examined in prior studies.
It is unclear why there was an interaction with maternal age for these specific BD sites. One possibility is that women <25 years-of-age are more likely to have unplanned pregnancies than older women and thus less likely to take folate supplements during preconception and early pregnancy. Neural tube malformations are believed to occur early in pregnancy when the neural tube is formed and before women may know they are pregnant.26 Folate supplementation is well recognized to reduce neural tube defects and knowledge of the importance of folate supplementation during pregnancy has been reported to be low in Danish women <30 years-of-age.27 Young women may also be more likely to reside in the larger cities as they may be students and thereby be co-exposed to ambient air pollution and other urban stressors. They may also be more likely to smoke and have more other unhealthy behaviors than women of older ages that have planned to become pregnant. Chance is also a possible explanation for these findings due to the many interactions tested in this study.
In addition to its size and cohort design, our study has several strengths over prior studies which mostly used surrogates of exposure such as private well use,8 ecologic measures of exposure,13 or exposures at birth.7,14 In contrast, our study used detailed estimates of nitrate exposure at the household level and address histories that considered maternal changes of residence. Only one prior study had information on bottled water use,13 which may be an important source of exposure misclassification in prior studies. We did not have information on bottled water use, but the use of bottled water in Denmark is low with only 20.5 L consumed per capita annually in 2014 and much less for earlier years.28
Our study has a few limitations which future studies should address. We lacked information on dietary sources of nitrate and nitrite, intake of antioxidants that might modify the effect of nitrate exposure on the risk of birth defects, and on the use of nitrosatable drugs that may promote the formation of N-nitroso compounds.13 We also lacked information on the amount of water consumption at home and outside of the home. The effect of this misclassification of exposure is most likely to bias our findings towards the null assuming these are random errors that are non-differential with respect to BD. Although we did not have information on co-contaminants in drinking water, public water in Denmark is recognized to be high-quality and generally complies with legal guideline values.29 The nitrate exposures in our study were quite low, which is both a limitation due to reduced statistical power, and a strength since we were able to examine risks at nitrate concentrations below the current standards.
Another limitation is our lack of access to BD in pregnancies that resulted in fetal loss. It is noteworthy that we observed a significant inverse exposure-response relationship for diagnosis of any BD (p=0.02) and for several of the specific BD categories. This seems to reflect a bias in our study, as nitrate is unlikely to prevent BD. A probable explanation for this is live birth bias, which may occur when the exposure causes fetal mortality from severe BD and thus prevents selection of these births into the study.30 Use of nitrosatable prescription drugs has been reported to be associated with an increased risk of fetal loss31 and it is possible that nitrate may potentiate the effects of nitrosatable drugs. This bias might also explain the lack of evidence in our study for an increased risk of limb deficiencies and heart defects, which have been reported in some prior studies.13,14 It may also have negatively biased the results for other BD and particularly for anencephaly and other nervous system BD that have high fetal mortality.
Reporting of BD in Denmark is mandatory by law and we would expect compliance to be very high. Of course, the reporting may be more accurate and complete for some anatomical sites than for others. For example, in a Danish study it was reported that only 4·5% (95% CI: 1·2-7·8) of major heart BD were detected at birth.32 In contrast, a European study of clubfoot reported that 100% of reported cases in Denmark were confirmed after review of the medical records.33
In general, the nitrate concentrations in Danish groundwater have been decreasing since the 1980’s.34 This is due to implementation of national mitigation measures such as for the handling of animal manure, nitrogen quotas for specific crops, and growing catch crops after harvesting of the main crop. However, there is still a large regional and local variation in the content of nitrate in groundwater in Denmark mainly due to variation in animal density, nitrate leaching from fields, and vulnerability of the groundwater aquifers. The Danish drinking water nitrate trends are also affected by infrastructural changes as for example the location and abstraction depth of drinking water because waterworks are trying to avoid the upper nitrate containing groundwater.35 Denmark is not yet meeting the demands of both national and EU legislations, and more locally targeted mitigation measures are being developed to further lower the nitrate content in groundwater. 36
Far higher levels of water contamination by nitrate may exist in other countries that are highly agricultural and do not use strict mitigation strategies. For example, concentrations of up to 1,500 mg/L were found in an agricultural area of India.37 If causal, our findings raise serious concerns about the potential for an increased risk in BD in areas of the world with higher nitrate levels than Denmark.
In conclusion, we found strong evidence of an increased risk of BD of the eye. To our knowledge this association has not been examined in prior studies of nitrate contaminated drinking water. We also observed strong evidence of interaction by maternal age, indicating an increased risk of nervous system BD and ear, face, and neck BD among children whose mothers were <25 years-of-age at the time of birth. Our findings were unchanged when we restricted our analyses to children of mothers who had average exposures that were below the current EU standard of 50 mg/L nitrate, which is nearly equivalent to the US Environmental Protection Agency standard of 44 mg/L.1 Our findings add to mounting evidence that the current nitrate in drinking water standards may not be sufficient to prevent an increased risk of BD and other adverse birth outcomes.
Contributors
Leslie Stayner: conceptualization, funding acquisition, methodology, formal data analysis, writing original draft, project administration
Anja Søndergaard Jensen: methodology, data analysis, writing-review, editing
Jörg Schullehner: methodology, writing-review, editing, data acquisition
Vanessa Coffman: methodology, writing-review, editing
Betina Trabjerg: methodology, writing-review, editing
Jørn Olsen: methodology writing-review, editing
Birgitte Hansen: writing-review and editing, data acquisition
Marie Pedersen: funding acquisition, methodology, writing-review, editing
Carsten Pedersen: methodology, writing-review, editing
Torben Sigsgaard: conceptualization, funding acquisition, methodology, writing-review, editing
Declaration of interests
The authors have no conflicts of interest to declare.
Footnotes
The U.S. EPA's MCL for nitrate in drinking water is 10 mg/L measured as nitrogen (NO3--N). The EU standard is based on measurement of the whole nitrate ion (NO3-). Concentrations of NO3--N can be converted to NO3- by multiplying by 4.42. Thus the U.S. MCL is 44.2 mg/L as NO3-, roughly equivalent to the EU standard of 50 mg/L.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100286.
Appendix. Supplementary materials
Data availability statement
Due to privacy concerns, the data from this study are not available to outside researchers unless they have approval from the Danish Data Protection Agency.
References
- 1.Shukla Saurabh, Saxena Abhishek. Global status of nitrate contamination in groundwater: its occurrence, health impacts, and mitigation measures. Handbook of Environmental Materials Management. 2018:869–888. [Google Scholar]
- 2.Schullehner J., Hansen B. Nitrate exposure from drinking water in Denmark over the past 35 years. Environmental Research Letters. 2014;9 [Google Scholar]
- 3.Platzek T., Bochert G., Rahm U. Embryotoxicity induced by alkylating agents. Teratogenicity of acetoxymethyl-methylnitrosamine: dose-response relationship, application route dependency and phase specificity. Arch Toxicol. 1983;52:45–69. doi: 10.1007/BF00317981. [DOI] [PubMed] [Google Scholar]
- 4.Givelber H.M., DiPaolo J.A. Teratogenic effects of N-ethyl-N-nitrosourea in the Syrian hamster. Cancer Res. 1969;29:1151–1155. [PubMed] [Google Scholar]
- 5.Koyama T., Handa J., Handa H., Matsumoto S. Methylnitrosourea-induced malformations of brain in SD-JCL rat. Arch Neurol. 1970;22:342–347. doi: 10.1001/archneur.1970.00480220056008. [DOI] [PubMed] [Google Scholar]
- 6.Guillette L.J., Jr. Endocrine disrupting contaminants–beyond the dogma. Environ Health Perspect. 2006;114:9–12. doi: 10.1289/ehp.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsch M.M., Scragg R.K., McMichael A.J., Baghurst P.A., Dyer K.F. Congenital malformations and maternal drinking water supply in rural South Australia: a case-control study. Am J Epidemiol. 1984;119:473–486. doi: 10.1093/oxfordjournals.aje.a113764. [DOI] [PubMed] [Google Scholar]
- 8.Arbuckle T.E., Sherman G.J., Corey P.N., Walters D., Lo B. Water nitrates and CNS birth defects: a population based case-control study. Arch Environ Health. 1988;43:162–167. doi: 10.1080/00039896.1988.9935846. [DOI] [PubMed] [Google Scholar]
- 9.Cedergren M.I., Selbing A.J., Löfman O., Källen B.A. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environ Res. 2002;89:124–130. doi: 10.1006/enrs.2001.4362. [DOI] [PubMed] [Google Scholar]
- 10.Croen L.A., Todoroff K., Shaw G.M. Maternal exposure to nitrate from drinking water and diet and risk for neural tube defects. Am J Epidemiol. 2001;153:325–331. doi: 10.1093/aje/153.4.325. [DOI] [PubMed] [Google Scholar]
- 11.Brender J.D., Weyer P.J., Romitti P.A., et al. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ Health Perspect. 2013;121:1083–1089. doi: 10.1289/ehp.1206249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtby C.E., Guernsey J.R., Allen A.C., Vanleeuwen J.A., Allen V.M., Gordon R.J. A population-based case-control study of drinking-water nitrate and congenital anomalies using Geographic Information Systems (GIS) to develop individual-level exposure estimates. Int J Environ Res Public Health. 2014;11:1803–1823. doi: 10.3390/ijerph110201803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaisdell J., Turyk M.E., Almberg K.S., Jones R.M., Stayner L.T. Prenatal exposure to nitrate in drinking water and the risk of congenital anomalies. Environ Res. 2019;176 doi: 10.1016/j.envres.2019.108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brender J.D., Olive J.M., Felkner M., Suarez L., Marckwardt W., Hendricks K.A. Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology. 2004;15:330–336. doi: 10.1097/01.ede.0000121381.79831.7b. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen C.B. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen L., Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- 17.Lynge E., Sandegaard J.L., Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 18.EUROCAT . EUROCAT Central Registry, University of Ulster; 2018. EUROCAT Guide 1.4, Version 28/12/2018: Instruction for the registration of congenital anomalies. [Google Scholar]
- 19.Cohen E., Horváth-Puhó E., Ray J.G., et al. Association between the birth of an infant with major congenital anomalies and subsequent risk of mortality in their mothers. JAMA. 2016;316:2515–2524. doi: 10.1001/jama.2016.18425. [DOI] [PubMed] [Google Scholar]
- 20.Schullehner J., Jensen N.L., Thygesen M., Hansen B., Sigsgaard T. Drinking water quality estimation at household level for the use in Danish population-based long-term epidemiologic studies using nitrate as example. Journal of Geochemical Exploration. 2017;283:178–186. [Google Scholar]
- 21.Schullehner J., Stayner L., Hansen B. Nitrate, Nitrite, and Ammonium Variability in Drinking Water Distribution Systems. Int J Environ Res Public Health. 2017;14(3):276. doi: 10.3390/ijerph14030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersson F., Baadsgaard M., Thygesen L.C. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39:95–98. doi: 10.1177/1403494811408483. [DOI] [PubMed] [Google Scholar]
- 23.Muller Clemma J, MacLehose Richard F. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–970. doi: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olshan A.F., Faustman E.M. Nitrosatable drug exposure during pregnancy and adverse pregnancy outcome. Int J Epidemiol. 1989;18:891–899. doi: 10.1093/ije/18.4.891. [DOI] [PubMed] [Google Scholar]
- 25.Park J.W., Piknova B., Jenkins A., Hellinga D., Parver L.M., Schechter A.N. Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye. Sci Rep. 2020;10:13166. doi: 10.1038/s41598-020-69272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavalli P. Prevention of Neural Tube Defects and proper folate periconceptional supplementation. J Prenat Med. 2008;2:40–41. [PMC free article] [PubMed] [Google Scholar]
- 27.Friberg A.K., Jørgensen F.S. Few Danish pregnant women follow guidelines on periconceptional use of folic acid. Dan Med J. 2015;62:A5019. [PubMed] [Google Scholar]
- 28.UNESDA (Union of European Soft Drinks Associations); 2018. Industry Volume Data: Denmark. https://www.unesda.eu/consumption/[accessed 6 August 2021].
- 29.Environmental Protection Agency . 2nd edition. Ministry of Environment of Denmark; 2021. Kvaliteten af det danske drikkevand – For perioden 2017-2019 [in Danish: The quality of the Danish drinking water – For the period 2017-2019] Grundvand og drikkevand nr. 3 ISBN: 978-87-7038-251-9. Downloaded on the web 23 July 2021. https://www2.mst.dk/Udgiv/publikationer/2020/12/978-87-7038-251-9.pdf. [Google Scholar]
- 30.Leung M., Kioumourtzoglou M.A., Raz R., Weisskopf M.G. Bias due to Selection on Live Births in Studies of Environmental Exposures during Pregnancy: A Simulation Study. Environ Health Perspect. 2021;129:47001. doi: 10.1289/EHP7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen A.M.L., Liew Z., Riis A.H., et al. Nitrosatable drug exposure during pregnancy and risk of stillbirth. Pharmacoepidemiol Drug Saf. 2019;28:1204–1210. doi: 10.1002/pds.4867. [DOI] [PubMed] [Google Scholar]
- 32.Lytzen R., Vejlstrup N., Bjerre J., Petersen O.B., Leenskjold S., Dodd J.K., Jørgensen F.S., Søndergaard L. Live-Born Major Congenital Heart Disease in Denmark: Incidence, Detection Rate, and Termination of Pregnancy Rate From 1996 to 2013. JAMA Cardiol. 2018 Sep 1;3(9):829–837. doi: 10.1001/jamacardio.2018.2009. PMID: 30027209; PMCID: PMC6233653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Barisic I., Loane M., Addor M.C., Bailey L.M., Gatt M., Klungsoyr K., Mokoroa O., Nelen V., Neville A.J., O'Mahony M., Pierini A., Rissmann A., Verellen-Dumoulin C., de Walle H.E.K., Wiesel A., Wisniewska K., de Jong-van den Berg L.T.W., Dolk H., Khoshnood B., Garne E. Congenital clubfoot in Europe: A population-based study. Am J Med Genet A. 2019;179(4):595–601. doi: 10.1002/ajmg.a.61067. Epub 2019 Feb 10. PMID: 30740879. [DOI] [PubMed] [Google Scholar]
- 34.Hansen B., Thorling L., Schullehner J., Termansen M., Dalgaard T. Groundwater nitrate response to sustainable nitrogen management. Sci Rep. 2017;7:8566. doi: 10.1038/s41598-017-07147-2. Online: http://www.nature.com/articles/s41598-017-07147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schullehner J., Hansen B. Nitrate exposure from drinking water in Denmark over the last 35 years. Environ Res Lett. 2014:9. [Google Scholar]
- 36.Hansen B., Voutchkova D.D., Sandersen P.B.E., Kallesøe A., Thorling L., Møller I., Madsen R.B., Jakobsen R., Aamand J., Maurya P., Kim H. Assessment of complex subsurface redox structures for sustainable development of agriculture and the environment. Environ Res Lett. 2021;16 doi: 10.1088/1748-9326/abda6d. [DOI] [Google Scholar]
- 37.Jacks G., Sharma V.P. Nitrogen circulation and nitrate in ground water in an agricultural catchment in southern India. Environmental Geology. 1983;5(2):61–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to privacy concerns, the data from this study are not available to outside researchers unless they have approval from the Danish Data Protection Agency.