Abstract
Basidiobolus ranarum is a known cause of subcutaneous zygomycosis. Recently, its etiologic role in gastrointestinal infections has been increasingly recognized. While the clinical presentation of the subcutaneous disease is quite characteristic and the disease is easy to diagnose, gastrointestinal basidiobolomycosis poses diagnostic difficulties; its clinical presentation is nonspecific, there are no identifiable risk factors, and all age groups are susceptible. The case of gastrointestinal basidiobolomycosis described in the present report occurred in a 41-year-old Indian male who had a history of repair of a left inguinal hernia 2 years earlier and who is native to the southern part of India, where the subcutaneous form of the disease is indigenous. Diagnosis is based on the isolation of B. ranarum from cultures of urine and demonstration of broad, sparsely septate hyphal elements in histopathologic sections of the colon, with characteristic eosinophilic infiltration and the Splendore-Hoeppli phenomenon. The titers of both immunoglobulin G (IgG) and IgM antibodies to locally produced antigen of the fungus were elevated. The patient failed to respond to 8 weeks of amphotericin B therapy, and the isolate was later found to be resistant to amphotericin B, itraconazole, fluconazole, and flucytosine but susceptible to ketoconazole and miconazole. One other noteworthy feature of the fungus was that the patient's serum showed raised levels of Th2-type cytokines (interleukins 4 and 10) and tumor necrosis factor alpha. The present report underscores the need to consider gastrointestinal basidiobolomycosis in the differential diagnosis of inflammatory bowel diseases and suggests that, perhaps, more time should be invested in developing standardized serologic reagents that can be used as part of a less invasive means of diagnosis of the disease.
CASE REPORT
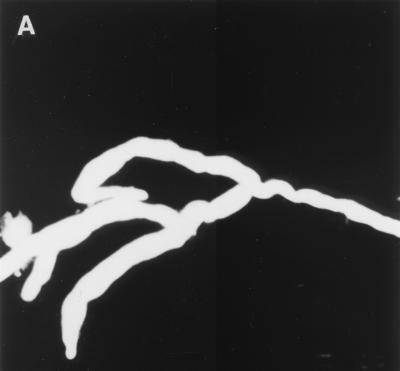
A 41-year-old, Indian male was admitted to Mubarak Al-Kabeer Hospital, Kuwait, in December 1999 with a history of abdominal pain of 20 days' duration. On examination, he was febrile (38.8°C) and his abdomen was tender and distended, particularly at the right hypochondrium and the right iliac fossa, where a palpable, nodular mass (9 by 5 cm) was located. The patient's history was not remarkable, except that he had a history of repair of a left inguinal hernia 2 years earlier. He was nondiabetic and nonneutropenic but had persistently low hemoglobin levels (7.7 to 8.7 g/dl). Ultrasound of the abdomen and pelvis confirmed the presence of a thick mass in the right iliac fossa and showed a marked thickening of the ascending colon and cecum and an increased echogenicity of the renal cortex, suggesting bilateral compromised function. Moreover, his prostate was markedly enlarged. There was fecal impaction in the colon. His blood urea nitrogen, serum creatinine, and uric acid levels were within the normal ranges. Examination of his urine showed many leukocytes and red blood cells, besides elevated levels of protein and bilirubin. On 11 January 2000, a right hemicolectomy was performed. The cecum, ascending colon, and hepatic flexure appeared to be involved, and portions of the cecum and the colon were also found to be adherent to the retroperitoneum and mesenteric lymph nodes. The resected material was sent for histopathological investigation. A presumptive diagnosis of intestinal tuberculosis was made, and a colon biopsy specimen was sent for confirmation of the presence of mycobacteria by culture, which later turned out to be negative. Since a colon biopsy specimen was not available for culture for fungi and we had seen a similar histopathological picture in our previous case of gastrointestinal basidiobolomycosis (9), we attempted to serodiagnose Basidiobolus ranarum infection with locally developed reagents. In the meantime, the patient developed difficulty in passing urine, and we examined and cultured his urine twice for the presence of fungi. On both occasions, several colonies of B. ranarum were isolated on modified Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.), and microscopic examination of the urine sediment with a 10% potassium hydroxide–calcofluor white (0.1%) mount revealed broad, sparsely septate hyphal elements (Fig. 1A).
FIG. 1.
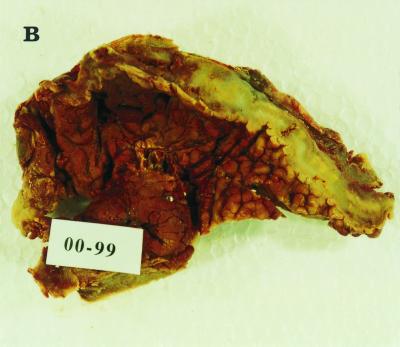
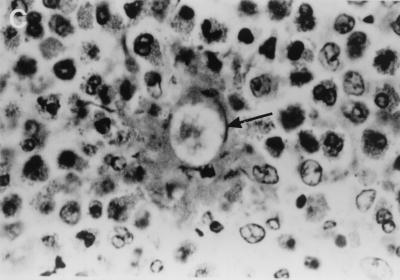
(A) KOH-calcofluor white mount showing broad, nonseptate, branched hyphae of B. ranarum in urine sediment. Magnification, ×200. (B) An open segment of large intestine showing a thick-walled mucosa with a cobblestone appearance mimicking Crohn's disease. (C) Hematoxylin-and-eosin-stained section of intestinal mucosa showing a cross section of a B. ranarum hypha (arrow) surrounded by the Splendore-Hoeppli phenomenon and many eosinophils. Magnification, ×1,000.
The patient was put on liposomal amphotericin B (AmBisome; neXstar Pharmaceuticals, San Dimas, Calif.) at 1 mg/kg of body weight for 4 weeks, but his condition did not improve. He continued to remain febrile and had repeated attacks of vomiting. He was put on total parenteral nutrition and was also managed symptomatically for fever and vomiting. The dose of liposomal amphotericin B was raised to 2 mg/kg, but despite 4 weeks of therapy, his condition deteriorated. The B. ranarum isolate was later found to be resistant to amphotericin B. By using the NCCLS reference method for broth dilution antifungal susceptibility testing of yeasts (11), the MICs for the isolate were determined to be as follows: amphotericin B, 4 μg/ml; flucytosine, >64 μg/ml; fluconazole, >64 μg/ml; itraconazole, 8 μg/ml; ketoconazole, 2 μg/ml; and miconazole, 0.5 μg/ml. Since the patient wanted to go back to India to be with his family, he was discharged and lost for follow-up.
Resected material received for histopathologic investigations consisted of a 39-cm piece of large intestine, which showed marked serosal congestion and a white exudate on the serosal surface. The mucosa was thickened and ulcerated and had a cobblestone appearance in some areas (Fig. 1B). Hematoxylin-and-eosin-stained sections showed a dense eosinophilic infiltrate with eosinophilic abscesses. Many noncaseating granulomas were present, and an area demonstrating the Splendore-Hoeppli phenomenon was observed (Fig. 1C), being composed of thick, eosinophilic, hyalanized material surrounding the fungal hyphae. Both cross and longitudinal sections of the broad, thin-walled hyphae stained by the Grocott staining method.
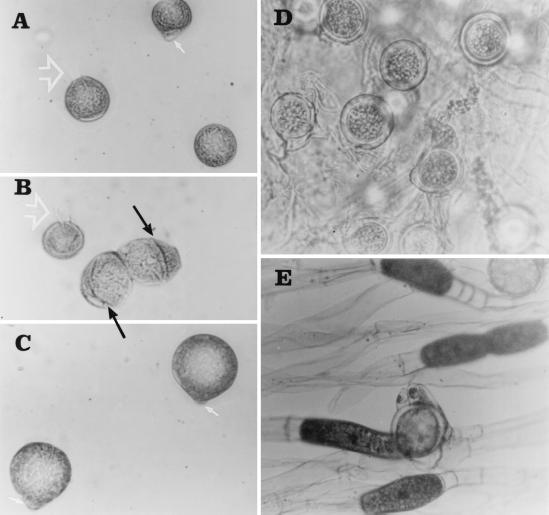
The B. ranarum isolate showed moderately fast growth at 30°C on Sabouraud dextrose agar, attaining a diameter of 37 mm in 4 days. The colony was rugose and smooth, appearing yellowish gray on the surface with a pale reverse, and had a waxy texture. As the growth progressed, formation of many satellite colonies was observed due to forced ejection of sporangioles (ballistospores). Microscopically, the hyphae were broad (diameter, 8 to 15 μm). The isolate also produced a large number of asexual spores, some of which were characterized by the presence of a hyphal tag and some of which were characterized by cleavage formation to produce meristospores (Fig. 2A to C). The isolate also produced a large number of smooth-walled zygospores (18 to 46 μm), some showing conjugation beaks, a characteristic feature of B. ranarum (Fig. 2D and E).
FIG. 2.
(A, B, and C) Sporangiospores of B. ranarum showing cleavage formation to produce meristospores (black arrow), knob-like adhesive tip (white arrow), and ballistospores with hyphal tag (white arrowhead). (D) Thick-walled zygospores. (E) A zygospore with a beak. Magnifications, ×400.
The patient's serum was tested for the presence of B. ranarum-specific antibodies by an immunodiffusion test (10) with a mycelial antigen prepared from the B. ranarum isolate from our previous patient (9). That patient's serum was used as a positive control reagent. The antigens were prepared by growing B. ranarum on Sabouraud dextrose broth at 37°C for 1 week, washing in phosphate-buffered saline (0.05 M; pH 7.4), and grinding with glass beads via a pestle and mortar. The resulting slurry was centrifuged at 15,000 × g, and the supernatant was filtered through a Nalgene filter unit (pore size, 0.45 μm), dialyzed against distilled water, and concentrated about 10 times by pervaporation. The protein content of the antigen was adjusted to 1 mg/ml. Two precipitin bands of identity were observed with the serum of our patient. It was further confirmed by demonstrating elevated levels of anti B. ranarum-specific immunoglobulin M (IgM) and IgG antibodies by a direct immunosorbent assay (enzyme-linked immunosorbent assay [ELISA]) described previously for the detection of antibodies to Mycobacterium tuberculosis (1). By using locally derived B. ranarum antigen in the ELISA, only IgG1 and IgG3 titers were selectively elevated in the patient's serum.
Assays for cytokines were carried out with the patient's serum by ELISA according to the instructions of the manufacturer and as described previously (13). The data were analyzed with Spectra Shell software for microplate-based assays (TECAN Austria GmbH, Salzburg, Austria). The minimum detectable concentrations in the assay were 5 pg/ml for tumor necrosis factor alpha (TNF-α), 5 pg/ml for gamma interferon, 5 pg/ml for interleukin-2 (IL-2), 5 pg/ml for IL-4, 1 pg/ml for IL-5, 3 pg/ml for IL-6, 5 pg/ml for IL-10, and 5 pg/ml for IL-12. Among the cytokines whose levels were measured, the levels of IL-4 (85 pg/ml), IL-10 (140 pg/ml), and TNF-α (256 pg/ml) were found to be significantly elevated (more than two times the control level).
Recent reports, mostly appearing from the Mayo Clinic in Scottsdale, Arizona (20), indicate that B. ranarum is also a potential etiologic agent of gastrointestinal and intra-abdominal infections. In a previous publication from Kuwait (9), we described a case of gastrointestinal basidiobolomycosis masquerading as Crohn's disease in an apparently healthy Bangladeshi male. This report presents yet another case of this disease in an Indian male involving the gastrointestinal tract with dissemination to the urinary tract.
Gastrointestinal infections due to B. ranarum have rarely been reported (2, 9). So far, there have been only 10 authentic cases of the disease in the English-language literature (5, 9, 14, 16, 17, 20, 21). They occurred in eight males and two females. Their ages ranged from 4 to 69 years. Eight of them were Americans, one was Brazilian, and one was Bangladeshi. No specific risk factors were identifiable, except that three of them had a history of diabetes mellitus. The condition may often simulate an inflammatory bowel disease (Crohn's disease or infective colitis) in clinical presentation and thus may be misdiagnosed. The mode of acquisition of the disease remains poorly understood. Ingestion of contaminated food or use of contaminated “toilet leaves” for cleaning of the skin after defecation has been considered the likely possibility (15). The patient described here was apparently healthy but had chronic anemia, and the only recognizable risk factor was the history of surgical correction of a left inguinal hernia 2 years earlier. The possibility that the fungus was implanted during the surgical procedure cannot be excluded. In this context it may be mentioned that our patient was from the southern part of India, where many cases of the subcutaneous form of the disease have been described (8). The only other patient in whom basidiobolomycosis developed following surgical intervention was an 8-year-old boy, who had had an appendectomy 5 months earlier (12). Our patient is similar to one described by Zavasky et al. (21), in that the infection disseminated to involve the urinary tract and the diagnosis was made by isolation of B. ranarum from urine samples. Moreover, the isolate in both the instances was resistant to amphotericin B.
The current experience of treating patients with gastrointestinal basidiobolomycosis is limited to only a few cases. Surgical resection of the infected tissue and prolonged treatment with itraconazole appear to be the best available options. The patients who were treated with itraconazole showed evidence of complete resolution of the disease. Whether itraconazole alone is sufficient to eradicate the infection or simultaneous resection of the affected tissue is necessary to achieve a cure remains unclear. There is some reservation about the surgical debridement of the infected area because of the possibility of the spread of infection (3), a concern which is not shared by others (4, 14). Among the azoles, ketoconazole and fluconazole have also been found to be effective in a few reports of subcutaneous basidiobolomycosis (4, 6). Treatment of basidiobolomycosis with amphotericin B has given unsatisfactory results. Among the three patients in whom amphotericin B has been used, one died after 2 weeks of therapy and one had a marginal response (with ketoconazole); in the third patient, the isolate was resistant to amphotericin B, and therefore, itraconazole was subsequently used. Our patient failed to respond to 8 weeks of liposomal amphotericin B (AmBisome) therapy, and the isolate was later found to be resistant to this agent. However, it was susceptible to ketoconazole and miconazole. Yangco et al. (19) found that amphotericin B was active against only 50% of the isolates of Basidiobolus. Potassium iodide is another compound which has successfully been used for the treatment of subcutaneous basidiobolomycosis. Although KI has no known antifungal activity, its administration (30 mg/kg/day) to one patient with retroperitoneal basidiobolomycosis resulted in dramatic improvement (12). Within 6 weeks of therapy, the thoracolumbar mass and the suprarenal lesion resolved completely.
To the best of our knowledge, B. ranarum-specific immunoglobulin levels and cytokine responses in patients with basidiobolomycosis have not previously been reported. Our results, although based on a single patient, show significantly elevated levels of the Th2-type cytokines IL-4 and IL-10 and the macrophage cytokine TNF-α. The estimation of serum antibody levels showed elevated levels of B. ranarum mycelial antigen-specific IgG, IgG1, and IgG3. In other infections, all these antibodies have been shown to result from the effect of the Th2-type cytokines IL-4, IL-10, and TNF-α (7, 18). Although the results are quite preliminary, it is apparent that B. ranarum antibodies may be detected by both immunodiffusion and ELISA methods and that the elevated levels of the Th2-type cytokines in the present patient are at least consistent with the predominant eosinophilic infiltration seen at the site of infection (Fig. 1C).
The present report underscores the growing importance of B. ranarum in the etiology of gastrointestinal infections and the potential role of serology in its diagnosis. Since patients with gastrointestinal basidiobolomycosis are usually immunocompetent and the infection site is immunologically reactive, initiation of an excellent antibody response is expected. In five culture-proven cases of the disease (9, 14, 17, 20, 21), in which serology was used for the diagnosis, B. ranarum-specific precipitating antibodies were demonstrable for all of them. On the basis of current experience, serologic tests, if used with proper controls, appear to be promising for the presumptive diagnosis of gastrointestinal basidiobolomycosis.
Acknowledgments
We thank Fatima Hussain for carrying out the immunological studies.
The work was supported in part by Kuwait University Research grant MI 115.
REFERENCES
- 1.Amoudy H A, Al-Asmer A B H, Abul A T, Mustafa A S. Evaluation of complex and defined antigens of Mycobacterium tuberculosis in an IgG-specific ELISA for the diagnosis of tuberculosis. Med Princ Pract. 1997;6:103–109. [Google Scholar]
- 2.Bittencourt A L, Ayala M A R, Ramos E A G. A new form of abdominal zygomycosis different from mucormycosis: report of two cases and review of the literature. Am J Trop Med Hyg. 1979;28:564–569. [PubMed] [Google Scholar]
- 3.Cameroon H M. Entomophthoromycosis. In: Mahgoub E S, editor. Tropical mycoses. Beerse, Belgium: Janssen Research Council; 1990. pp. 186–198. [Google Scholar]
- 4.Davis S R, Ellis D H, Goldwater P, Dimitoiou S, Byard R. First human culture-proven Australian case of entomophthoromycosis caused by Basidiobolus ranarum. J Med Vet Mycol. 1994;32:225–230. doi: 10.1080/02681219480000291. [DOI] [PubMed] [Google Scholar]
- 5.de Aguair E, Moraes W C, Londero A T. Gastrointestinal entomophthoromycosis caused by Basidiobolus haptosporus. Mycopathologia. 1980;72:101–105. doi: 10.1007/BF00493818. [DOI] [PubMed] [Google Scholar]
- 6.Drouhet E, Dupont B. Laboratory and clinical assessment of ketoconazole in deep-seated mycosis. Am J Med. 1983;74:30–46. doi: 10.1016/0002-9343(83)90512-0. [DOI] [PubMed] [Google Scholar]
- 7.Hussain R, Kifayet A, Dojki M, Dockrell H M. Selective correlation on interferon-gamma, tumour necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor with immunoglobulin G1 and immunoglobulin G3 subclass antibody in leprosy. Immunology. 1999;98:238–243. doi: 10.1046/j.1365-2567.1999.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamalam A, Sentamilsilvi G, Thambiah A S. Entomophthoromycosis. Proc R Coll Phys Edinburgh. 1992;22:52–59. [Google Scholar]
- 9.Khan Z U, Parkash B, Kapoor M, Medda J P, Chandy R. Basidiobolomycosis of the rectum masquerading as Crohn's disease. Clin Infect Dis. 1998;26:521–523. doi: 10.1086/517107. [DOI] [PubMed] [Google Scholar]
- 10.Khan Z U, Sandhu R S, Randhawa H S, Menon M P S, Dusaj I S. Allergic bronchopulmonary aspergillosis: a study of 46 cases with special reference to laboratory aspects. Scand J Respir Dis. 1976;57:73–87. [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.Nazir Z, Hassan R, Pervaiz S, Alam M, Moazam F. Invasive retroperitoneal infection due to Basidiobolus ranarum and response to potassium iodide—case report and review of the literature. Ann Trop Pediar. 1997;17:161–164. doi: 10.1080/02724936.1997.11747880. [DOI] [PubMed] [Google Scholar]
- 13.Pacsa A S, Agarwal R, Elbishbishi E A, Chaturvedi U C, Nagar R, Mustafa A S. Role of interleukin-12 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2000;28:151–155. doi: 10.1111/j.1574-695X.2000.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 14.Pasha T M, Leighton J A, Smilack J D, Heppel J, Colby T V, Kaufman L. Basidiobolomycosis: an unusual fungal infection mimicking inflammatory bowel disease. Gastroenterology. 1997;112:250–254. doi: 10.1016/s0016-5085(97)70242-7. [DOI] [PubMed] [Google Scholar]
- 15.Ribes J A, Vanover-Sams C L, Baker D J. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schimidt J H, Howard R J, Chen J L, Pierson K K. First culture-proven case of gastrointestinal entomophthoromycosis in the United States: a case report and review of the literature. Mycopathologia. 1980;95:101–104. doi: 10.1007/BF00437168. [DOI] [PubMed] [Google Scholar]
- 17.Smilack J D. Gastrointestinal basidiobolomycosis. Clin Infect Dis. 1998;27:663–664. doi: 10.1086/517154. [DOI] [PubMed] [Google Scholar]
- 18.Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 19.Yangco B G, Okafor J I, Testrake D. In vitro susceptibilities of human and wild-type isolates of Basidiobolus and Conidiobolus species. Antimicrob Agents Chemother. 1984;25:413–416. doi: 10.1128/aac.25.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yousef O M, Smilack J D, Kerr D M, Ramsey R, Rosati L, Colby T V. Gastrointestinal basidiobolomycosis: morphological findings in the cluster of six cases. Am J Clin Pathol. 1999;112:610–616. doi: 10.1093/ajcp/112.5.610. [DOI] [PubMed] [Google Scholar]
- 21.Zavasky D-M, Samovitz W, Loftus T, Segal H, Carroll K. Gastrointestinal zygomycotic infection caused by Basidiobolus ranarum: case report and review. Clin Infect Dis. 1999;28:1244–1248. doi: 10.1086/514781. [DOI] [PubMed] [Google Scholar]