Abstract
The South American continent presents a great diversity of biomes, whose ecosystems are constantly threatened by the expansion of human activity. The emergence and re-emergence of viral populations with impact on the human population and ecosystem have shown increases in the last decades. In deference to the growing accumulation of genomic data, we explore the potential of South American-related public databases to detect signals that contribute to virosphere research. Therefore, our study aims to investigate public databases with emphasis on the surveillance of viruses with medical and ecological relevance. Herein, we profiled 120 “sequence read archives” metagenomes from 19 independent projects from the last decade. In a coarse view, our analyses identified only 0.38% of the total number of sequences from viruses, showing a higher proportion of RNA viruses. The metagenomes with the most important viral sequences in the analyzed environmental models were 1) aquatic samples from the Amazon River, 2) sewage from Brasilia, and 3) soil from the state of São Paulo, while the models of animal transmission were detected in mosquitoes from Rio Janeiro and Bats from Amazonia. Also, the classification of viral signals into operational taxonomic units (OTUs) (family) allowed us to infer from metadata a probable host range in the virome detected in each sample analyzed. Further, several motifs and viral sequences are related to specific viruses with emergence potential from Togaviridae, Arenaviridae, and Flaviviridae families. In this context, the exploration of public databases allowed us to evaluate the scope and informative capacity of sequences from third-party public databases and to detect signals related to viruses of clinical or environmental importance, which allowed us to infer traits associated with probable transmission routes or signals of ecological disequilibrium. The evaluation of our results showed that in most cases the size and type of the reference database, the percentage of guanine–cytosine (GC), and the length of the query sequences greatly influence the taxonomic classification of the sequences. In sum, our findings describe how the exploration of public genomic data can be exploited as an approach for epidemiological surveillance and the understanding of the virosphere.
Keywords: viral diversity, molecular evolution, emerging virus, metagenomic analysis, viral surveillance
Introduction
Viral emergencies showed a progressive increase in the last decades (Jones et al., 2008; Gould and Higgs, 2009; Ewald, 2011; Coffey et al., 2014; Nobre et al., 2016). Many factors linked to the expansion of human activity (agriculture, migration, urbanization, etc.) are indicated as agents that threaten the balance of viral communities in nature (Gould and Higgs, 2009; Woolhouse et al., 2012; Mahy, 2014). Currently, accumulated evidence reveals an overwhelming number of new viruses and routes of interaction that were not considered a few decades ago (Wong et al., 2007; Sobel Leonard et al., 2017; Nouri et al., 2018; Schoeman and Fielding, 2019). This impressive volume of new viral sequences allowed us to investigate the still hidden viral diversity that has great magnitude and actively participates in ecological processes (Koonin and Dolja, 2013; Posada-Cespedes and Seifert, 2017). Thus, the sequencing technologies permitted the observation with better resolution of the viral world, changing the old paradigm of parasitic entities to a category containing the largest and most variable genetic information, the virosphere (Shi et al., 2018). Nevertheless, the viral proportions observed to date are only a small fraction of the virosphere volume (Zhang et al., 2018).
Otherwise, the description of viral diversity presents great challenges. These entities have attributes that confer them a rapid response to the environment. Characteristics such as high mutational rate, high reproductive number, recombination, and reorganization, even in very divergent families, contribute to the wide diversity of viral groups (Skewes-Cox et al., 2014; Shi et al., 2016a; Gilbert et al., 2016). Thus, in contrast to microorganism classification methods where it is possible to classify operational taxonomic units (OTUs) from a few markers with high homology (e.g., RNA16S) (Hackl et al., 2004), virus detection is limited by the wide genetic mosaicism detected in the virosphere (Dolja and Koonin, 2018). Therefore, virus or viral trace detection considers alternative strategies to infer the presence of viral activity (Herath et al., 2017). Thus, the diversity discovered in the virosphere, largely explained by horizontal gene transfer (Cai et al., 2006; Gilbert et al., 2016; Wolf et al., 2018), reveals intricate genetic relationships that began to be described with greater resolution in the age of genomics (Xu, 2006).
The increasing addition of new viruses identified in the reference databases, advances in taxonomic classification (Krishnamurthy and Wang, 2017; Simmonds et al., 2017), and the implementation of new statistical and computational techniques make it possible to increase the informative capacity of viral sequences. The combined detection of multiple and diverse patterns in viral genes, such as protein domains or gene sequences of conserved genes, helps to detect OTUs of viral origin despite divergence between taxonomic groups. These innovations make it possible to detect molecular clues of viral origin that may indicate ecological processes, as well as biological phenomena in hosts (Hurst, 2011; Fermin, 2018; Sadeghi et al., 2021). In this sense, the profile of virus species and the abundance of each viral family detected in an organism can reveal key elements in the interaction with its environment. In addition, viral traces can be used as indicators of anomalous bacterial activity, environmental pollutants, or the presence of serious modifications in the ecosystem (Hurst, 2013; Fermin, 2018). This type of evidence allows the detection of large-scale phenomena in the environment and in non-culturable viruses (Roux et al., 2017; Wolf et al., 2020), thus contributing relevant sources of information for ecological monitoring (Fermin, 2018).
The exploration of the world of viral RNA reached a better resolution through the new tools, which permitted the accumulation of enough data to reveal key events in the evolution of this viral group (Duffy et al., 2008). The abundance and characteristics of replication of RNA viruses are favorable in the rapid expansion of its reproductive substrate, which can result in eventual emergencies (Holmes, 2009; Wang and Crameri, 2014). Conjunctions of factors such as diversity disturbances, increased density, and exposure of organisms to sources of infection (Bordería et al., 2011; Geoghegan and Holmes, 2017) are frequently associated with host jumps. However, several viral emergence events resulted from unknown viruses that had reduced sylvatic cycles, due to alterations in the environment, forcing them to occupy new niches and impacting humans (Weaver and Reisen, 2010; Castro et al., 2019). This is the case of several arboviruses and common viruses that infect vertebrates such as coronaviruses, bunyaviruses, and retroviruses (De Wit et al., 2016; Chouin-Carneiro, 2017; Lu et al., 2020). As a surveillance alternative, different research approaches indicate that viral migration pathways can be mapped by observing molecular evidence from viral lineages in a time–space context (Rosario and Breitbart, 2011; Geoghegan and Holmes, 2017).
Moreover, ecological and epidemiological aspects often overlap in the environment. It was indicated that most of the gastrointestinal diseases transmitted by recreational contact on water surfaces have their origin in human waste (Morace et al., 2002; Aw and Gin, 2010; Hjelmsø et al., 2019). Based on this, many countries and research groups have opted for molecular detection methods in aquatic environments as a solution to this problem. It is an important area of surveillance since fecal–oral transmission viruses show a high prevalence in watercourses. As a key feature to consider, fecal–oral transmission viruses show infective capacity at low frequencies (Lin and Ganesh, 2013). Thus, viruses such as reovirus, adenovirus, hepatitis, rotaviruses (RV), and human polyomaviruses are viral particles of waterborne transmission, which have high stability against environmental perturbations such as high UV degrees and long periods in the environment.
Therefore, the reanalysis of public databases to describe and explore various aspects of the virosphere is an accessible and powerful tool, which is at an early stage. As a first step, this work aims to investigate the large volume of publicly available sequencing data to detect viral sequences. Once viral evidence has been identified, we aim to label, categorize, and contextualize the data, according to the aspect to be analyzed through the collection of suitable metadata. For this purpose, we kept our focus on South America, mainly the Brazilian territory, whose tropical location is associated with the greatest biological diversity. In addition, this area presents a large number of biomes, intense agricultural activity, highly populated cities, and other socioeconomic factors related to the emergence and/or imbalance of viral communities. Depending on the geographical setting and the metagenomes available, our work also evaluates the ability to detect viral signals using the reference viral genomes available to date. In order to do this, we investigated if the knowledge of viral diversity related to a specific context can be used to drive responsible management of human-induced factors with a high impact on the biosphere.
Materials and Methods
To analyze the presence of viral patterns in public databases, we established a workflow that adapts the heterogeneity of the obtained samples to recover viral sequences using long-established and frequently used tools in sequence similarity (Blast-like) and comparison methods (HMMer-like) with an updated viral database.
Metagenomic Sequences
We choose metagenomic sequences of samples from Brazil and other countries in South America, using the keywords “Brazil,” “host,” “mosquitoes,” “South America,” and “virus” in the search engine of Sequence Read Archives (“SRA”) database. Also, we filtered the results to obtain sequences from “metagenomic,” “transcriptomic,” “soil,” “Environmental,” “Water,” “Freshwater,” “Host,” and others related to epidemiologically relevant samples.
Sequence Pre-Processing
The Fastq-dump and Prefetch programs from the SRAtoolkit package v2.3.2 were used to download and manage the files. The integrity of samples was analyzed using FASTQC v0.11.9, and data curation was performed with Fastp (Chen et al., 2018).
Processing and Analysis of Sequencing Reads
Mapping of viral sequences was performed with Bowtie 2 (Borozan et al., 2013) and the clustered-RVDB-v21 database (Goodacre et al., 2018), which allowed us to index more than 28,000 viruses as the subject for the mapping against the metagenome samples. The results in SAM format were filtered according to PRHED 20 quality, which considers a confidence percentage of 9% chance of error (Suzuki et al., 2017). We also filtered the mapped sequences in which the guanine–cytosine (GC) content ranged from 35% to 75%, using Python and R to perform these analyses. To determine the abundance of viral sequences, we calculated the percentage of reads that mapped with RVDB. Later, we checked the quality of these sequences with Aliview (Larsson, 2014). Once we detected the sequences with the first method (Bowtie 2), the sequences of putative viral origin were translated into the six reading frames (ORF) by EMBOSS (Rice et al., 2000) and subsequently mapped against clustered-RVDB protein (C-RVDB-v21, contains 3,899,699 protein sequences) against Blastp (BLAST+ v2.11.0) using a script based on the MetablastR package.
The results were associated with metadata and taxonomical classification and then filtered, and short reads with low-complexity regions were excluded. Was used clustered-RVDB (C-RVDB-v21) as a database, reporting only viral sequences that aligned with e-value >0.001 and coverage >75% with the level of taxonomic assignment (minimum inclusion criteria = family). Subsequently, we proceeded with a third method, where viral sequences were mapped using hidden Markov model viral profiles with the HMMer tool (Skewes-Cox et al., 2014; Goodacre et al., 2018), which used RVDB-prot (3,899,699) as a reference database to detect viral profiles (vFAMs—13,201 clusters) (Bigot et al., 2019).
Contig Assembly and Virus Mapping
We performed de novo assemblies of the filtered reads with SPADES (Bankevich et al., 2012) and CAP3 (Huang and Madan, 1999). In the same way that we proceeded with the reads, the assembled viral sequences with BLAST+ were inspected, using C-RVDB-v21 database as the first method. Then, the assemblies were filtered, using the same criteria of the reads that were based on GC content, e-value, and coverage. As a second method, each assembled sequence was also translated into the six ORFs by EMBOSS (Rice et al., 2000), analyzed with BLASTP against clustered-RVDB (C-RVDB-v21), and filtered by e-value, coverage, and low level of taxonomic assignment. Finally, contigs were mapped with HMMer using RVDB vFAMs, excluding e-value >0.001.
Analyses of Results
The results were analyzed using the R language where scripts were created using the packages Gtsummary, phyloR, Gmaps, Strings, SequinR, Biostrings, Rentrez, MetablastR, Pheatmap, and the n Python 3.8.0 language with the Biopython (https://biopython.org/) and pandas (https://pandas.pydata.org/) modules. Correlation analyses of diversity distribution detected by sample were performed according to the type of mapping method. The R packages used in correlation analysis were R, Hmisc, PerformanceAnalytics, and Corrplot packages.
Analysis of Observed Diversity According to Environmental, Clinical, or Economic Impact
To analyze the host range of sample indicated by the contigs in each sample, metadata information (Fermin, 2018) that shows a taxonomic assignment of hosts in a comprehensive way was used with the following groups of classification: A (Animalia), Ar (Archaea), B (Bacteria), C (Chromista), F (Fungi), P (Plantae), or Pr (Protozoa). In order to identify groups of sequences according to their environmental, economic, veterinary, or clinical impact, our contig sequences were labeled with information from Viral Zone (Hulo et al., 2011). The labeling, taxonomy, and visual representation analyses were performed with the R language using the Rentrez and Sequinr packages. The workflow is shown graphically in Supplementary Figure S1.
In sum, we were able to analyze 19 bioprojects containing 120 SRA experiments, which represented virological diversity from several environments (Supplementary Table S1). In addition, we took into account evidence for, e.g., the presence of families with bioindicator potential, taxonomic groups with high emergence records, or relevant proteins.
Results
Viral Sequences
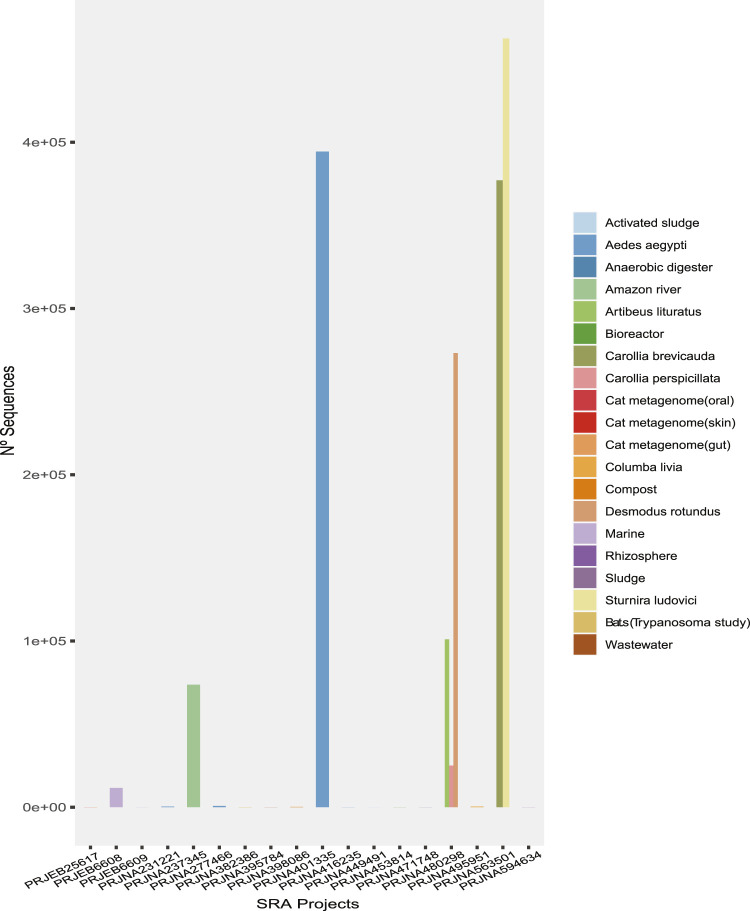
In the first phase of our analysis, 2,750,113 reads from viruses were detected from a total of 7.10E+08 reads in the metagenomes, corresponding to 0.38% of the reads in the 120 metagenomic samples from 19 projects, which represented the heterogeneous sampling origins (Figure 1). Moreover, clustering of the viral sequences by genome type (Table 1) revealed that the highest percentages of virus (Classified) recovered were dsRNA, ssRNA (+), dsDNA, ssRNA (−), and ssRNA-RT (Table 1), in this order.
FIGURE 1.
The number of sequences of probable viral origin identified in each metagenome analyzed.
TABLE 1.
Summary of rate of viral sequences by genome type.
| Genome | Amount (%) |
|---|---|
| dsDNA | 6.8947 |
| dsDNA-RT | 0.0001 |
| dsRNA | 43.4795 |
| ssDNA (+/−) | 0.0104 |
| ssDNA (−) | 0.0005 |
| ssRNA-RT | 12.1674 |
| ssRNA(+) | 17.4409 |
| ssRNA(+/−) | 18.6835 |
| ssRNA(−) | 1.3227 |
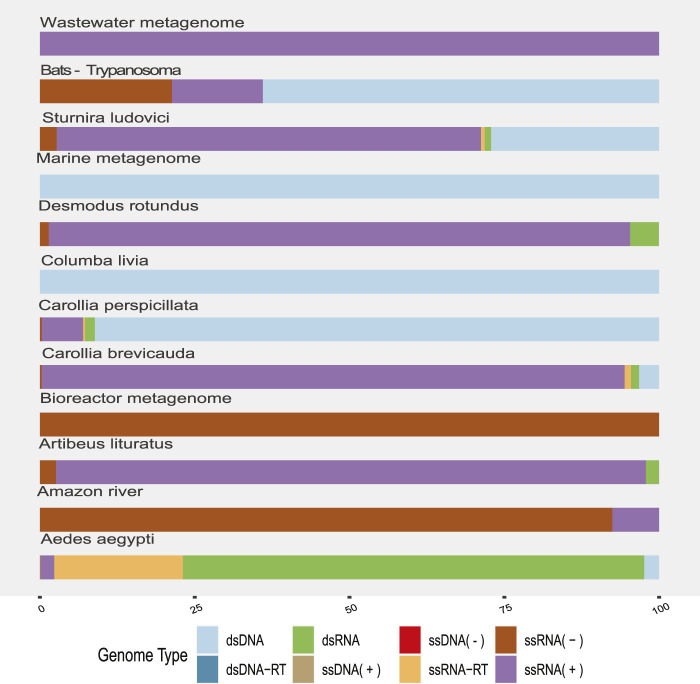
The highest proportion was observed in the dsRNA category from the Aedes aegypti samples (Figure 2) and corresponded to sequences identified as Reoviridae, Picobirnaviridae, Partitiviridae, Hypovirus, and Sedoreovirinae. This uneven distribution of sequences according to the genome in Aedes sp. samples could be biased by the methods to obtain and concentrate samples.
FIGURE 2.
Proportion of viral sequences according to the related genome type by sample origin. The colors allow to identify in the horizontal bars the proportion of sequences clustered according to their genome type (y-axis) by sample origin (x-axis).
RNA (+) viruses were found in more than 21% of the total samples, when they were added to the percentage of other RNA (+) viruses but classified within taxa of variable composition (RNA +/−), totaling more than 40% (Figure 2). In the remaining categories, viruses “ssRNA-RT,” which grouped the Orthoretrovirinae and Retroviridae families, stood out with 11% and a more homogeneous distribution in the samples. The viral sequences corresponding to ssDNA, dsRNA, and dsDNA-RT classes represented the lowest frequencies that we found. In agreement, we found the highest rate of RNA viruses over DNA in the environment (“Amazonia river,” “Marine,” “Activated sludge,” “Wastewater,” and “Bioreactor”) and bat metagenomes (Figure 2).
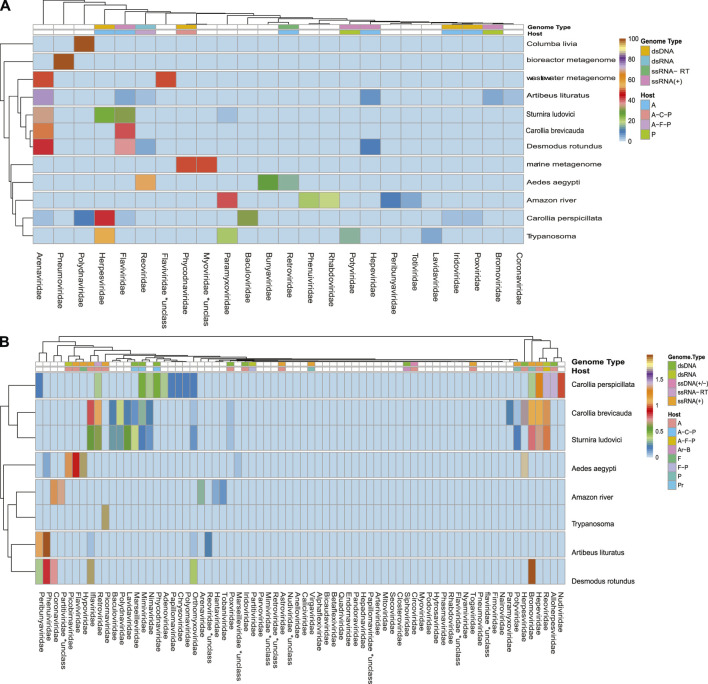
A broad observation of family rate distribution (Figure 3 and Supplementary Figure S4) indicated that most of the viral sequences were grouped in taxonomic categories, which have not yet been clarified. Otherwise, when grouping the sequences considering orders and families with epidemiological relevance (impact on human health or economy), we found a large number of sequences related to RNA (+) families (Arenaviridae, Hepaciviridae, Togaviridae, etc.) and RNA (−) (Phenuiviridae, Peribunyaviridae, and Hantaviridae). The sequences related to relevant dsDNA viruses were mostly classified in the families Alphaherpesviridae, Betaherpesviridae, Gamaherpesviridae, Picornaviridae, and Cytomegalovirus.
FIGURE 3.
Rate of viral sequences detected in each metagenome. (A) The proportions of families recovered with Blastn. (B) The proportions of families recovered using contigs translated with Blastp. The RVDB-v21 database reference was used in both methods.
When analyzing the data in a more restrictive way, allowing a minimum level of common taxonomic assignment in the family level, a percentage higher than 50% of all the sequences was indicated to be of viral origin; the methods were described by similarity search (Bowtie 2, and Blastp) and the presence of 78 families in the total of analyzed samples (Figure 3 and Supplementary Figure S4). Likewise, sequences reads and contigs translated into peptides (six ORF) were analyzed with HMMer (Supplementary Figure S9).
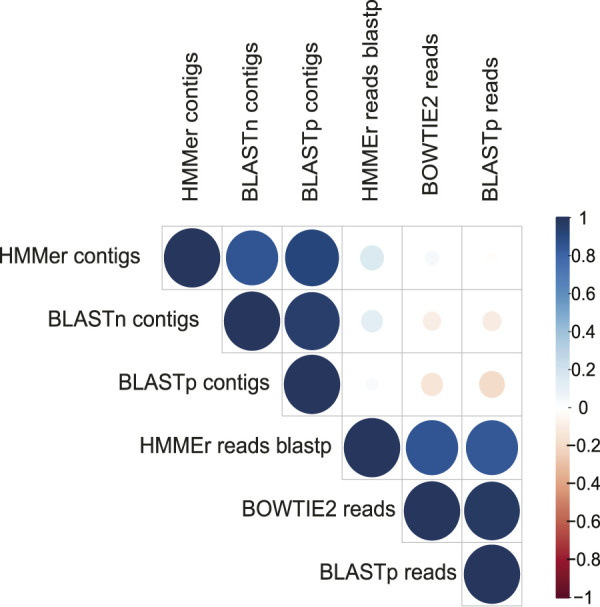
An overall evaluation of our results shows that more than 750,000 unique viral sequences were detected in reads (Bowtie 2), 170,000 unique sequences in translated reads (Blastp), 5,800 unique sequences in contigs, and 6,930 unique sequences in translated contigs.
A more restrictive classification, allowing a minimum assignment criterion to include family, showed that the pairwise alignment and HMM comparison methods indicated similar diversity rates in the samples (Figure 3 and Supplementary Figure S4). Based on this, a correlatogram (Pearson’s correlation) was performed, which included the distribution of viral diversity detected in reads (Supplementary Table S8) and contigs (Supplementary Table S8) with each of the methods (Bowtie, Blastn, Blastp, and HMMer) in each type of sample. The correlogram (Figure 4) revealed a high level of correlation with significance between the distribution of diversity detected among contigs with Blastn, Blastp, and HMMer (pearson cor. >0.98). In the read category, it was possible to detect positive correlation >0.97 between the three methods (Bowtie, Blastp, and HMMer), demonstrating convergence between the diversity detected by pairwise alignment (diversity described by family) and comparison by hidden Markov models (diversity described by vFAMs detected) (Figure 4, Supplementary Figure S9). As expected, the contigs reveal a narrower diversity of families due to the exclusion of sequences in the assembly step, which is reflected in the correlation of their results. However, the lengthening of the sequences by assembly indicated a great diversity of proteins that served to indicate the presence of relevant viral species (Figures 5, 6).
FIGURE 4.
Correlatogram between the assigned family frequency distributions in the metagenomes obtained with all the methods used.
FIGURE 5.
Heatmaps of the proportions of sequencing reads classified into viral families by sample origin. Graphs (A and B) correspond to the highest proportion (families with >2% of total viral reads by sample) and the lowest proportion of reads (families with <2% of total viral reads by sample). The top columns represent annotations of the genome and host type of each identified family. Uncolored boxes correspond to unclassified families. Genome types are coded as dsDNA, dsRNA, ssDNA(+), ssRNA-RT, and ssRNA(+). Their hosts are coded here as A (Animalia), Ar (Archaea), B (Bacteria), C (Chromista), F (Fungi), and P (Plantae).
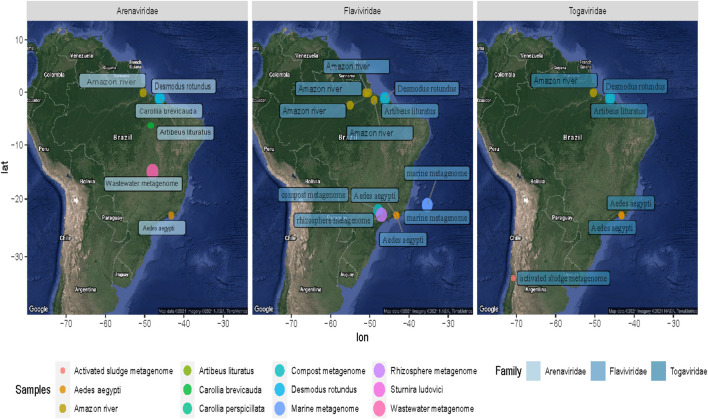
FIGURE 6.
Geographical distribution of the samples containing Arenavirus, Togavirus, and Flavivirus. The colored dots on the map symbolize the coordinates of each sampling group analyzed. The difference in size is proportional to the number of sequences of the family shown.
Some methods detected unique families; likewise, contig assembly in some samples allowed to describe and classify taxa not included by reads. Environmental samples such as rhizosphere, compost, and wastewater metagenome revealed viral sequences only after assembly (Figure 3). A total of 27 common families among reads and translated reads were detected: Baculoviridae, Togaviridae, Arenaviridae, Phycodnaviridae, Reoviridae, Mononegavirales, Protostomia, Retroviridae, Herpesviridae, Picobirnaviridae, Flaviviridae, Partitiviridae, Mitoviridae, Mimiviridae, Closteroviridae, Picornaviridae, Iflaviridae, Poxviridae, Marseilleviridae, Hepeviridae, Potyviridae, Iridoviridae, Astroviridae, Caliciviridae, Betaflexiviridae, and Alphaflexiviridae (Figure 3 and Supplementary Figure S4). In addition, the mapping of untranslated reads identified 76 families, indicating a significant number of sequences belonging to more than 40 viral families not detected in translated reads (Supplementary Figure S4). The distribution of families detected with contigs by Blastn and Blastp showed no significant differences (Figure 3). Moreover, the assemblers differed in the recovery of some viral species, since the efficiency of CAP3 or SPADEs is probably subject to the sequencing characteristics (transcriptomic, shotgun, pair-end, single-end, etc.) (Rosario and Breitbart, 2011; White et al., 2017). The two assembly approaches recovered in similar proportions of protein classes such as hypothetical (25%), photosystem (18%), nonstructural (8%), and structural (8%). Although no viral sequences larger than 4,000 nt were assembled with the techniques used, it was possible to detect important evidence of viruses such as RDRP, glycoproteins, nucleoproteins, and capsids (Figures 7, 8).
FIGURE 7.
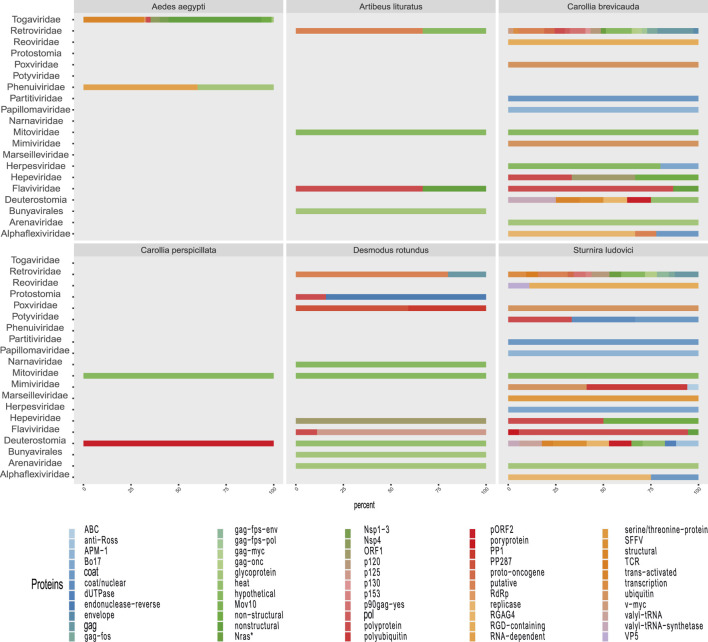
Proportion of contigs obtained related to viral proteins in each metagenome of organisms. The colors allow to identify in the horizontal bars the proportion of sequences related to viral proteins (x-axis) according to the viral family (y-axis) in each animal model.
FIGURE 8.
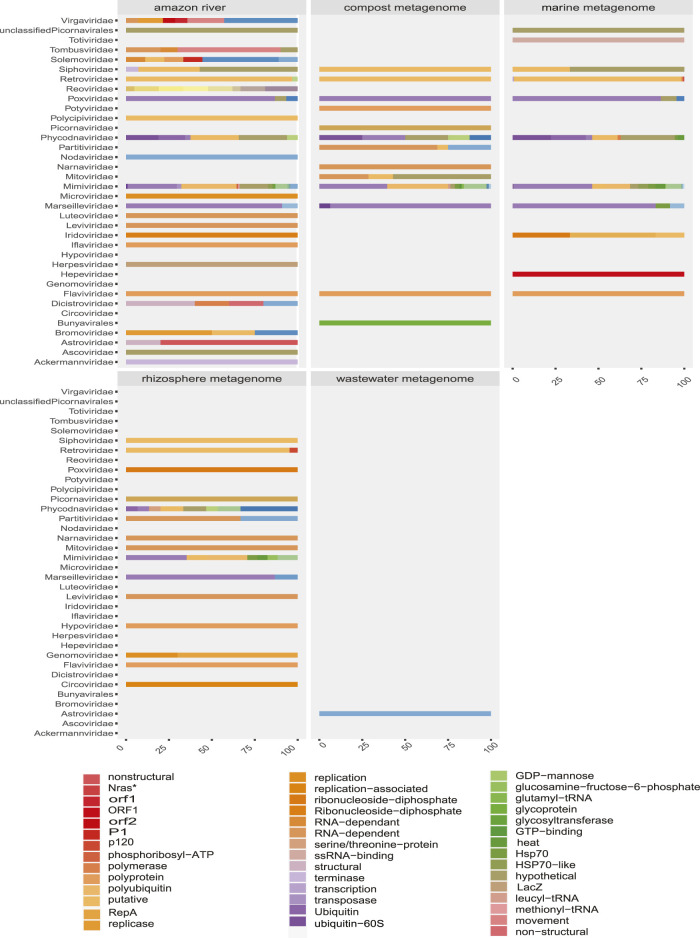
Proportion of contigs obtained related to viral proteins in each environmental metagenome. The colors allow to identify in the horizontal bars the rate of sequences related to viral proteins (x-axis) according to the viral family (y-axis) in each environmental model.
Due to the heterogeneity of the experiments, each sample type detected different levels of viral diversity, which were largely OTUs consistent with the expected viruses according to the type of sample (Figures 3, 5 and Supplementary Figures S4, S5). In addition, many samples analyzed showed a diversity of sequences that allowed classification at the species level.
Amazon River
In the metagenomes from the Amazon River, a higher amount of sequences from viruses related to the families Flaviviridae, Togaviridae, and Paramyxoviridae were detected. Flaviviridae sequences were represented by sequences related to the hepatitis C virus (RNA+). In the Alphaviridae group, we detected sequences related to Semliki Forest virus (length, ∼200 nt; e-value, 1.10e−66; identity, ∼91%).
In the low proportion (<1% total viral sequences), we identified sequences from Guanarito mammarenavirus (length, 300 nt; e-value, 8e−116; identity, ∼96%) and Phenuiviridae (Joao virus) families (length, ∼300 nt; e-value, 8e−116; identity, ∼96%). In addition, in the lower frequency of reads (<0.1), we were able to observe viral evidence, corresponding to plants (Betapartitiviurs, Bromoviridae, Virgaviridae, Tobamovirus, Tymovirus, and Goraviurs), fungi (Narnavirus), and Enterovirus, Paramyxoviridae, Bunyaviridae, Pestivirus, Phenuiviridae, Rhaboviridae, and Sedoreovirinae. The assembly also revealed some proteins signals of interesting viral traces, such as movement protein [Elderberry aureusvirus 1], VP1 [Mangshi virus], movement protein [Rice virus A], polymerase [Reticuloendotheliosis virus], RNA-dependent RNA polymerase [Rhizopus microsporus 23S narnavirus], and nonstructural protein [Spodoptera exigua iflavirus 1]. Furthermore, we found sequences indicating a diversity of glycoprotein-specific motifs and structural and nonstructural proteins of Venezuela equine encephalitis virus (length, ∼300 nt; e-value, 8e−116; identity, ∼96%) and Mosso das pedras virus (length, ∼300 nt; e-value, 8e−116; identity, ∼96%).
Aedes aegypti Samples
The number of viral sequences recovered from A. aegypti samples was high as compared with most of the analyzed metagenomes (Supplementary Figure S2). Nonetheless, the highest proportion of viral sequences in A. aegypti samples clustered in few viral families. Thus, the largest number of reads was grouped in unclassified families, endogenous elements, and Retrovirus sequences. Less frequently, close to 1%, we detected Flaviviridae- and Betaherpesvirinae-related sequences (Heatmap, Figure 5). The Flaviviridae family showed a high occurrence of reads grouped in Hepacivirus C or unclassified Flaviviruses. Otherwise, the sequences in very low frequency (<0.5%) were grouped in more than 70 groups, which allowed us to point out fragments of epidemiological interest. Also, the highest proportion of RNA (−) viruses were related to viruses like Phasi Charoen virus, a specific insect virus of the Bunyaviridae family. In an epidemiological context, our observation points out reads related to Togaviridae and fragments that mapped with polyproteins of Mosso das pedras virus (length, ∼300; e-value, 6e−73; identity, 100.00%) and Mucambu virus (length, 300 nt; e-value, 4e−50; identity, 85%), which are below the Venezuelan equine Encephalitis virus complex. In addition, the assembly allowed to detect longer sequences of Togaviridae family, corresponding to glycoproteins, SNPs, and structural and nonstructural proteins related to Venezuelan equine Encephalitis virus, Mucambo virus, and Rio Negro virus (length, 400–1,350; identity, >90%). Phenuiviridae was represented by glycoprotein and RDRP of Phasi Charoen-like phasivirus (Ferreira et al., 1994; Auguste et al., 2009; Lopes et al., 2014; Espósito and da Fonseca, 2015; Esposito and Fonsecada, 2017). The samples from Aedes showed an important rate of RNA viruses; they also indicated a high percentage of DNA sequences, which in most cases correspond to endogenous viruses (Figures 2, 5 and Supplementary Figure S5).
Chiroptera Samples
Our investigations for bat species in public databases revealed six bat metagenomes samples from three projects. The species Sturnira ludovici and Carollia brevicauda, corresponding to the SRA project PRJNA563501, which focuses on the analysis of vomer-nasal censoring; the project PRJNA480298 that analyzed endogenous viral elements in the species Desmodus rotundus, Carollia perspicillata, and Artibeus lituratus (brain tissue samples); and the project PRJNA382386, which studies the presence of Trypanosoma in bat blood samples. First, we observed that the species S. ludovici and C. brevicauda clustered together, presenting a similar profile in both high and low frequencies. These two species presented a high percentage of Flavivirus, Arenavirus, Herpesvirus, and Endogenous viral elements. The remaining species (D. rotundus, C. perspicillata, and A. lituratus), although sampled with the same methodology, showed a different viral profile but overlapping viral families. The diversity profile corresponding to the “Bats (blood)” study was not represented in the heatmap (Figure 5 and Supplementary Figure S5) because the low proportion of reads becomes incomparable when scaling the matrix of viral abundances. In addition, reads grouped in higher proportions showed lower family diversity (N < 4), which contrasted with a higher number of families in lower frequency (N > 8).
The identification of Arenavirus in D. rotundus, C. brevicauda, and S. ludovici was also verified by the presence of contigs from these viruses. These sequences corresponded to fragments between 200 and 700 nt of glycoprotein and L-segment regions of G. mammarenavirus (e-value, ∼2e−57; identity, 98.46%). These viral motifs were also detected with Blastp in reads and contigs.
Marine Metagenome
The detected viral sequences were from dsDNA families Cytomegalovirus, Myoviridae, and Lymphocryptovirus. A large number of viral reads from Prochlorococcus phage P-HM2 were detected (Myoviridae) (Supplementary Tables S2–S4).
Compost and Sugarcane Rhizosphere Metagenomes
Classification of reads pointed to a few viral groups (Beta Baculoviridae), Durnovirales, and Herpesvirales. Nevertheless, the assembly of the sequences revealed the presence of the families Flaviviridae, Mimiviridae, Narnaviridae, Partitiviridae, Poxviridae, and Retroviridae (Supplementary Tables S2, S3). The contigs from Flaviviridae (ssRNA+) were related to (length, 300–500 nt; e-value, 7e−39; identity, >90%) polyprotein of Bovine viral diarrhea virus 2. Mimiviridae (dsDNA) was represented by contigs related to CDS (methionyl-tRNA synthetase, leucyl-tRNA synthetase, glutamyl-tRNA synthetase, cyclophilin type peptidyl-prolyl cis-trans, Capsid, Hsp70) of Edafosvirus, Gaeavirus, Harvfovirus, Terrestrivirus, and Hokovirus HKV1 (length, 150–1,300 nt; e-value, 1e−07; identity, ∼90%). The contigs recovered in Mitoviridae corresponded to RDRP proteins, which at the species level were related to Colletotrichum fructicola (length, 150–580 nt; e-value, 5e−38; identity, ∼75%), R. microsporus (length, 190–450 nt; e-value, 6e−170; identity, ∼95%), and Pleurotus ostreatus virus (length, 200–850 nt; e-value, 6e−170; identity, ∼91%), whose hosts are mushrooms. The contigs grouped in Poxviridae indicated the presence of polyubiquitins related to Magpiepox virus, an avian Poxivirus (length, ∼400 nt; e-value, 2e−48; identity, ∼98%). The largest proportion of contigs was grouped in Retroviridae, related to the gag protein of Porcine endogenous retrovirus.
The second group of samples belongs to the project Metatranscriptome from sugarcane rhizosphere under drought stress, which was collected in February 2015 in São Carlos, Brazil. The analysis in the rhizome samples grouped a low number of sequences, which indicated the presence of Hypovirus, Fabavirus, and Retrovirus porcine viruses.
Wastewater
Although a low number of sequences could be recovered, we grouped sequences in the medical importance category related to G. mammarenavirus (length, 200 nt, 2e−40; identity, 97.06%, Guanarito Mammarenavirus isolate CVH-960201 segment L). Also, we detected sequences corresponding to Astrovirus, Norovirus, and Oxbow Orthohantavirus (length, ∼200 nt; identity, ∼86%). The assembly of reads allows recovery sequences of capsids related to human Astrovirus (length, ∼190 nt; identity, ∼89.19%).
Diversity Observed According to Host Range and Environmental and Clinical Impact
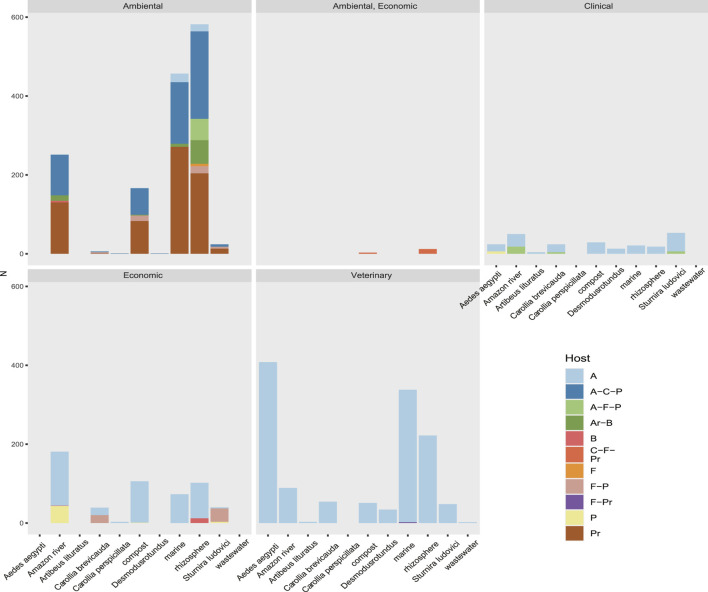
Using data on host range, ecosystem, and societal impact, we were able to classify the viral sequences and motifs detected in each sample, facilitating the broad observation of groups of interest (Figure 9 and Supplementary Figure S10). The observed clustering was consistent with the expected viral diversity in each sample type, showing in environmental models the highest host potential in “Aquatic metagenome,” “Compost metagenome,” “Marine metagenome,” and “Rizosphere metagenome.” Interestingly, we also detected a great diversity of viruses with environmental impact in samples corresponding to C. brevicauda (fruit-eating bat), S. ludovici (fruit-eating bat), and Desmodus rotundus (Hematophage) (Figure 9). The sequences related to viruses of clinical importance showed a proportional distribution in most of the samples, with a number of viral sequences being identified in “Wastewater metagenomic” and “C. brevicauda.” The highest proportion of sequences related to clinical significance was clustered in the Aquatic metagenome and S. ludovici samples. The observation of the number of viral sequences according to hosts (Supplementary Figure S10) was consistent with sample type, where the highest number of viral sequences having animals as hosts was detected in the animal model samples. In contrast, viral sequences related to infection in prokaryotes, fungi, bacteria, and plants were mostly detected in Aquatic, Compost, and Rizosphere metagenomes (Figure 9 and Supplementary Figure S10).
FIGURE 9.
Rate of sequences classified by their relevance clinical, environmental, economic, or veterinary relevance of the taxonomic group to which they belong. Also shown is the host range of the virome of each sample. Their hosts are coded here as A (Animalia), Ar (Archaea), B (Bacteria), C (Chromista), F (Fungi), and P (Plantae).
Analysis of Families Detected According to Length of Sequences
Depending on the informative capacity of the sequence, its size can directly influence the classification of viral signals in a metagenome. A histogram based on the length of the sequences identified in reads and contigs a heterogeneous distribution (alternate Supplementary Figures S6, S7) in the frequencies, which in reads is explained by the sequencing methods of the experiment. Reads of Illumina grouped in class intervals between 50 and 200 nt and Ion Torrent greater than 300 nt.
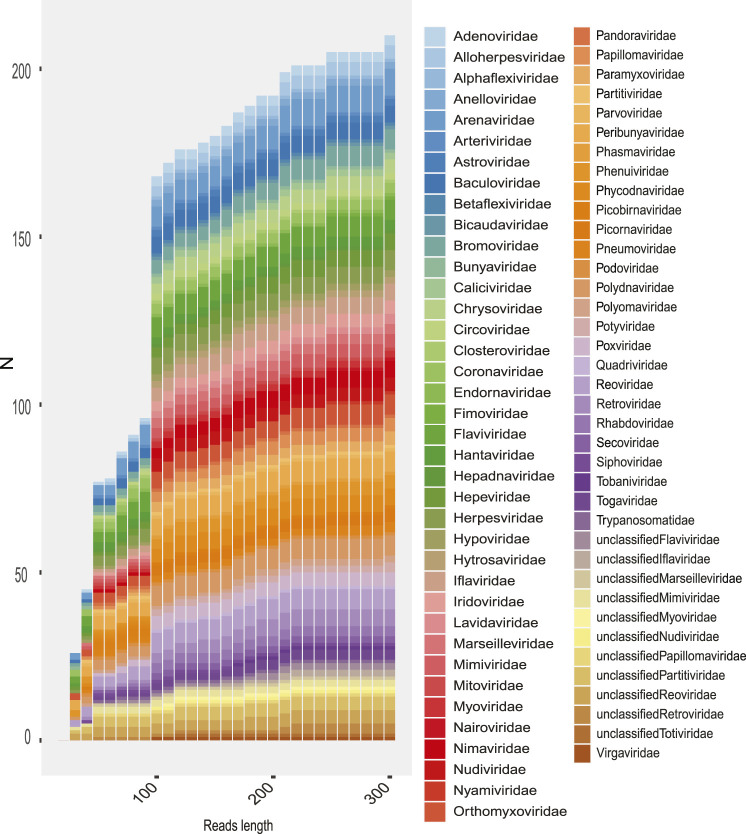
In order to evaluate how the size of the sequences influenced the taxonomic assignment (up to family level), we classified and compared the number of families detected as the length of the reads and contigs increased. We could observe, although to a lesser extent, that short sequences allow the identification of viral families, yet the highest informative capacity to classify is observed from 150 nt (Figure 10). This effect of read size on sequence identification is variable according to the type of experiment (Figure 10), but most indicated greater sensitivity with increasing read length.
FIGURE 10.
Rate of families recovered in the total metagenomes as the length of reads is increased. The number of families recovered is shown (y-axis) according to the length of the sequence (x-axis). The colored boxes represent the viral family.
Description of Diversity
The starting point of our analysis focused on the results obtained in reads and contigs processed with Bowtie 2 and Blastn, where we obtained an interesting diversity classification, which was consistent with expected families in the samples. However, because of a large number of ambiguous short sequences that were related with virus, we resorted to analyzing diversity by alternative methods. In addition, a random sampling of sequences was performed to obtain an approximation of probable false positives due to pattern redundancy in nonviral reference proteins. We selected 10,000 random reads and 100 translated contigs (https://github.com/Fernando-Gmzr/Viral-signals-Public-metagenomes) and processed in Blastp to identify sequences related to nonviral origin with a high percentage of identity. The results of the random sampling of reads against reference proteins identified only sequences with a percentage of identity <60%, which belonged to sequences referenced by the RVDB as sequences of Guanarito virus (Arenaviridae) and Hepacivirus (Supplementary Table S6). These sequences were related to mammals and Actinobacteria. Only three contigs identified sequences with a high percentage of identity (proteomic reference of nonviral OTUs), which were related to the mammalian class (Supplementary Table S7).
Discussion
Viral Sequences
Virome screening of metagenome samples is limited, mainly by the experimental design of sample collection and sequencing strategies (Breitbart and Rohwer, 2005; Krishnamurthy and Wang, 2017; Obbard, 2018; Metsky et al., 2019). Even studies specifically designed to describe the virome of a sample face challenges that may bias the analyses. Although the viral database is growing, it is still at an early stage and represents only a small portion of the viral dark matter. Thus, sequencing reads are filtered based on homologous sequences, excluding those with the greatest divergence. In this way, the description of the virome in metagenomes is directly related to sequencing advances and the discovery of new viruses (Anthony et al., 2013; Zhang et al., 2019). For example, mimiviruses discovered within the last 2 decades represent a high rate of occurrence in environmental metagenomic samples, which would not be possible without the inclusion of Mimivirus genomes in databases.
On the other hand, the experimental design determines to a greater or lesser degree the presence of viral traces, which directly influence the actual composition of the observed virome (Shi et al., 2016b). The experimental design is influenced by important factors such as the exclusion of viral particles by filtering or the inclusion of foreign viruses by contamination (Posada-Cespedes and Seifert, 2017; Fitzpatrick et al., 2021). These limitations are common to all metagenomic analyses but have a greater effect on virome due to viral characteristics. However, metagenomic studies consider the sampling context and the nature of the sample to infer the composition of OTUs. In our study, the metagenomes analyzed whose characteristics were best suited to include sequences of viral origin showed that the most representative viral OTU rates were consistent with the expected composition. Thus, soil and compost samples showed an expected composition, which showed high representativeness of gigantoviruses and viruses related to farm feces. However, some of the metagenomes analyzed in this study, such as marine samples or Columba livia, revealed low rates of viral sequences, which are consistent with the type of experiment, sequencing, and nature of the sample. The marine samples revealed a high diversity of proteins, but most were associated with algal and protozoan infecting DNA viruses. In C. livia, the only sequences that showed homology with viruses were related to endoviruses, which in many cases are sequences included in the host genome.
A large proportion of metagenomic samples showed the occurrence of viral sequences, with RNA+ viruses as the most representative. Our results showed that of the total mapped reads (0.38%) of 730,253 sequences, 25% of the detected viral sequences corresponded to 64 viruses without full taxonomic classification (Supplementary Table S1). This finding suggested the presence of hidden viral diversity, whose exploration may be restricted by the sampling, methods, and low informative databases. Nevertheless, our results agreed with ecological estimates of viral diversity that reveal that the Baltimore RNA groups dominate the Virosphere eukaryotes, being the RNA+ type the most diverse in this category (Waldron et al., 2018; Wolf et al., 2018).
The results for each sample showed that the diversity detected was limited mainly by the quality and length of the sequences, which are highly dependent on the type of sequencing (Figure 10). In addition, the type of methodology and experimental design are directly related factors, since several samples describing interesting viral diversity were designed to capture transcriptome and virus. The composition of the viral diversity of each sample may show only viral families favored by the sampling methods and in some cases may also include viral OTUs due to contamination. Because of this, the description of viral diversity as an exploratory approach must consider a number of factors such as sample characteristics, metadata associated, families likely to be detected on the sample, method filtering, and the temporal–spatial location of the experiments.
A major limitation in our work was the redundant regions in the sequences, which can contribute to bias in the identification of sequences of viral origin (Marz et al., 2014; Herath et al., 2017; Krishnamurthy and Wang, 2017). Therefore, our approach focused on combining several methods to describe the diversity of each virome. Although each method differentially excluded a considerable proportion of sequences, inspection of reads and contigs with Bowtie 2, Blastn, and Blastp, validated with HMMer by hidden Markov models, confirmed more than 60% of the sequences as of viral origin. Moreover, the correlation between pairwise alignment methods (Bowtie 2 and Blast) and comparison methods (HMMer) revealed interesting results. In addition to the correlation between translated and untranslated reads and contigs, we also observed a correlation with the HMMer results. Unlike similarity methods, HMMer classifies sequences by evaluating their amino acid positional information against viral profiles, which may contain information related to more than one taxonomic family (Skewes-Cox et al., 2014).
The vFAMs are probabilistic models that due to their sensitivity are able to capture remote homologs, extending the informative capacity of the sequences to describe the viral diversity of a sample (Skewes-Cox et al., 2014). This method in combination with pairwise alignment methods proved to be an efficient tool to indicate the viral origin of the sequences. Also, this approach can be used to produce longer sequences or to capture signals from undiscovered viruses, which despite their divergence share patterns in their gene composition (Skewes-Cox et al., 2014; Bzhalava et al., 2018; Bigot et al., 2019).
The convergence in the classification of sequences by both paradigms proved to be efficient in revealing the first layer of viral diversity. The observed signal frequencies make it possible to coarse-view the composition of the virome of each sample (Figures 3, 5, 7, 8; Supplementary Figures S4, S5). In continuation, a more detailed observation shows viral traces of informative relevance in several samples.
Aedes aegypti
Aedes metagenomic samples were obtained from transcriptomics studies, whose focus was on RNAi and dsRNA profile; thus, we were able to detect reads related to dsRNA viruses such as Reoviridae, Picornaviridae, and Negeviruses. Our results corroborated those of other studies, indicating that the virome is constituted in a large proportion by specific insect viruses (Shi et al., 2020) and that the reads recovered in greater proportion corresponded to Phasi Charoen-like virus and Humaita-Tubiacanga virus, which were described as dominant species (Zakrzewski et al., 2018).
Otherwise, our findings point out the presence of short sequences from genes related to Venezuelan Equine Encephalitis virus, Mayaro virus complex, and Venezuelan equine encephalitis virus complex that belong to Alphavirus genus, which comprises viral species that showed emergence events in South America during the last decades (Auguste et al., 2009; Esposito and Fonsecada, 2017). These viruses are single-stranded positive-sense RNA viruses with spherical structures and envelopes that cause febrile diseases with possible encephalitis and arthritis. Although our findings do not represent any news about the circulation of alphaviruses in the region, the diversity of proteins found could suggest a probable focus of transmission. Based on this, the recovered sequences reinforce evidence on potentially emerging virus circulation in mosquitoes.
Amazon River
The detected viral sequences were obtained from quantitative metagenomic and metatranscriptomic analyses from two studies (June 2010 and July 2013) from the Amazon River. In general, the profile of the river samples showed viral evidence from representatives that infect hosts of various domains and kingdoms (Figures 7, 8). Thus, the revealed virome indicated circulation of a variety of infecting viruses of plant-, fungus, and bacteria-, protozoa-, and animal-infecting viruses. The authors have reported that the presence of high amounts of plant viruses in aquatic ecosystems may be associated with fecal contamination or contamination by agricultural activities. Our findings pointed out presented proteins related to species such as Tropical soda apple mosaic virus, Tomato necrotic streak virus, tomato Mosaic virus, or Rice virus A (Supplementary Tables S2, S3). Likewise, other sequences corresponding to gag proteins from porcine retrovirus, polyproteins from Bovine viral diarrhea virus 2, or proteins from avian Poxviridae could suggest transmission routes associated with farms. Although the region of the Amazonian rivers is surrounded by human settlements, our observations are based on low-frequency viral traces, requiring tests for the investigation of pollutants that quantify viral particles with greater precision.
Besides, the virome revealed several viral sequences associated with protozoa and soil bacteria. In this way, giant viruses (Mimivirus) were related to Edafovirus, hokovirus, and Indivirus groups. Also, we were able to observe sequences related to insect-specific viruses, such as proteins from Humaita-Tubiacanga virus or Phasi Charoen-like phasivirus. In the context of aquatic ecosystems, we found evidence of Phycodnaviridae, as well as sequences related to photosystem proteins. Infection in algae is a common phenomenon in marine ecosystems where viral activity functions as an important population regulator (Fuhrman, 1999; Fridman et al., 2017). Associated with animal viruses and with emerging potential, we point out with more emphasis evidence of viruses from Arenavirus and Togavirus families. In this way, these observations correspond to the north of Brazil collected in 2011 and 2013, indicating a broad profile whose traces could suggest probable contamination and a vast spectrum of waterborne viruses. Thus, the “SRA” obtained from these experiments allowed us to describe one of the most interesting results of viral diversity of the present study, which, despite its limitations, managed to reveal viral traces that could facilitate the investigation of ecological processes and transmission networks.
Marine Metagenome
The observed profiles of marine samples were collected in the Atlantic Ocean, Marine Ecoregion: Trindade and Martin Vaz Islands in 2010-10. The largest number of sequences clustered in photosystem genes (Supplementary Table S3). These genes are responsible for encoding key components of photosynthesis reactions (photosystem II (PSII)). Because of coevolution virus–algae, phage P-HM2 can express photosystems that help to protect its hosts from UV damage, while preventing photoinhibition and upregulating metabolic genes. The viral traces were related to Prochlorococcus sp. host, which is a cyanobacteria, considered as a major component of phytoplankton and the main contributor to primary productivity in tropical and subtropical oceans (Fuhrman, 1999; Waldron et al., 2018). The detection of viral evidence related to metabolism is used as an important indicator of viral activity, thereby allowing the detection of abnormal infection rates in important nodes of the ecosystem, such as algae. Due to the methodology used in the collection, the types of sequences expected were related to DNA-type viruses (Aro et al., 1993; Mann et al., 2003). In summary, these results, although they do not reveal the true diversity of viruses present due to the experimental method used, are sufficient evidence to indicate traces of viral activity in algae.
Soil Samples
This group of samples comes from two metatranscriptomic projects performed in the state of São Paulo, Brazil. The first one corresponds to the compost metatranscriptome, carried out in the city of São Carlos, whose samples were collected at locations 30 cm below the surface. The methodology of this project was designed to evaluate the composting microbial community growing with sugarcane bagasse. The virome profile of each sample was revealed to be consistent with samples associated with soil, plant debris, and fertilizers of animal origin. The largest amount of viral sequences was associated with the genus Klosneuvirus. This group, already described as soil giant viruses, possess a genome of ∼1.5 Mb, characterized by hosting aminoacyl transfer RNA synthetases for various amino acids and a great diversity of tRNA-modifying enzymes and translation factors (Schulz et al.). As in the Amazon River samples, the sequences indicated viral traces of Bovine viral diarrhea virus 2 or proteins associated with porcine Retroviruses (Supplementary Tables S2–S4). In this context, these sample results suggest probable exchange pathways that may explain the viral signals.
Wastewater
Wastewater samples were collected in Brasilia in May 2015 with the objective of monitoring pathogenic microorganisms in the urban environment. Although in a very low quantity, the viral traces identified in wastewater made it possible to reveal the presence of frequently infected Enteroviruses in human populations. Crucially, the sequences detected in this metagenome suggest, along with the other Arenavirus results, the presence of waterborne transmission.
Chiroptera
The investigated sequences were obtained from samples from the Brazilian Amazon region in Para state in 2014. The virus profile showed a great diversity of shared families among the analyzed bat species, while some species indicated the presence of unique families. According to a study carried out in 2020, which investigated “The Database of Bat-associated Viruses (DBatVir),” the viral percentages for the most representative families (>1%) were Coronaviridae (35%), Rhabdoviridae (26%), Paramyxoviridae (905%), Astroviridae (6.7%), Adenoviridae (3.4), Poluomaviridae (2.8), Reoviridae (2.3%), Circoviridae (2.1%), Herpesviridae (2.1%), Flaviviridae (2.0%), Picornaviridae (1.7%), Parvoviridae (1.5%), and Filoviridae (1.1%) (Letko et al., 2020). Otherwise, our analysis revealed a different viral profile. Although records of coronavirus in bats indicate very high frequency, our study detected very low proportions of coronavirus motifs in bats. Only the species D. rotundus and A. lituratus, which have different eating habits (hematophagous and fruit-eating), showed the presence of viral patterns related to “Infectious bronchitis virus,” an avian coronavirus. In contrast, families such as Herpesviridae and Flaviviridae (represented by hepatitis C in our analysis) showed a high frequency of occurrence (Figure 5 and Supplementary Figure S5).
All the ecological characteristics of Chiroptera evidence in this group are important vectors for interspecies transmission. Bats comprise a vast group (described as the second largest of mammals), where countless viral exchanges occur, connecting distant taxonomic categories. The wide range of habitats of Chiroptera results in the contact and transport of various groups of viruses (Brook and Dobson, 2015; Moratelli and Calisher, 2015). Thus, the coexistence with so many viral families facilitates routes for transmission of plant viruses, mycotic viruses, and vertebrate viruses (Brook and Dobson, 2015; Hayman, 2016). Arenaviruses, especially Mammarenaviruses in South America, deserve great attention since several countries such as Argentina, Brazil, and Venezuela are endemic regions of the virus that causes hemorrhagic fever (Parodi et al., 1966; Salas et al., 1991; Carroll et al., 2015; Gonzalez et al., 2018). In South America during the last 50 years, outbreaks related to these groups have occurred, resulting in major environmental disturbances (Safronetz et al., 2012; Carroll et al., 2015). Like Hantavirus, Mammarenaviruses frequently infect rodents and bats (Fernandes et al., 2019). In consequence, viral species that have small and closed cycles in rodents with specific habits, due to environmental alterations, can come into contact with vectors of large distributions, favoring the exposure to humans (Le et al., 2007; Warren and Sawyer, 2019). The occurrence of these factors increases in settlements of low economic stratum, where precarious houses with limited access to electricity and drinking water are favorable for the viral exchange (Jones et al., 2008; Mahy, 2014; Brook and Dobson, 2015; Afelt et al., 2018). This scenario, which frequently occurs as an interface between wild cycles and urban cycles, is a recurring risk factor in South America (Hahn et al., 2014; Nobre et al., 2016; Fernandes et al., 2019; Ellwanger et al., 2020). Due to rapid deforestation and advanced anthropization, the human impact on highly complex ecosystems such as the Amazon rainforest facilitates viral jumps (Afelt et al., 2018; de Mello Malta et al., 2020). In this context, our findings are in agreement with previous records of Arenavirus infecting several hosts (Coimbra et al., 1994; de Mello Malta et al., 2020). In sum, the analysis allowed detecting sequences and identifying corresponding viral profiles, where some viral species are common, and others could be specific viral traces of their ecotype, like plant and fungi viruses in frugivorous or Bovine viral diarrhea virus in hematophagous (Supplementary Tables S2, S3).
Biological Correlates of Our Findings
Based on this, the workflow applied in this study allowed us to take advantage of the sequences accumulated in public databases, to reveal in a coarse view the virome of organisms and specific environmental areas. The exploration of metagenomes of mosquitoes from Rio de Janeiro, bats from the Amazon region, or wastewater from Brasilia can serve as data that complement records or guide epidemiological investigations in relevant areas in the future. As a result, the analysis in such heterogeneous samples revealed traces of viral activity in different hosts and environments.
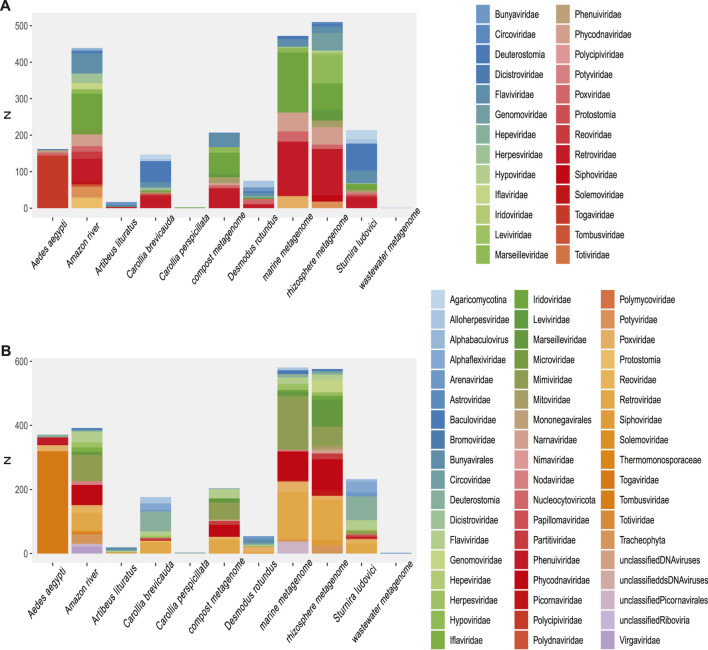
In this scenario, the retrieved tracks point to probable foci of Alphavirus, and Arenavirus transmission in the Amazon, Brasilia, and Rio de Janeiro regions, which are related to potentially emerging viral groups (Figure 4).
The analysis of third-party public databases as an exploratory approach to virus identification is subject to major challenges and limitations related to the nature of the viruses and to the database that is affected by experimental design and other factors that we cannot control, such as lab contamination. However, the ubiquity and resistance of viruses in the environment, the high reproduction in foci of infection, and the strategic choice of transmission sources for monitoring establish viral detection as an important tool. Our findings allowed us to figure out the virus detection and description capability, with reference tools and databases, which are still in their initial stages. Accordingly, it is expected that in the near future, the progressive discovery of new viruses, the optimization of analysis strategies (e.g., hidden Markov models and machine learning), and the exponential accumulation of data will enable new large-scale perspectives to study biodiversity. Consequently, the informative capacity of viral sequences, in addition to being useful epidemiologically, has the potential to contribute to the understanding of the virosphere (Schulz et al., 2018).
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://github.com/Fernando-GMzr/Viral-signals-Public-metagenomes.
Author Contributions
FM, LM, JM, and PG generated the data. FM and CF analyzed the data and wrote the manuscript.
Funding
We thank for the grant #2014/06090-4 and #2014/17766-9, FAPESP São Paulo Research Foundation (FAPESP). We also thank CNPq for the grant #421160/2016-0. These grants helped to acquire the computational resources that we used in this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.722857/full#supplementary-material
workflow used in the present work to capture viral signals using different methods.
Number spots and viral sequences by sra experiment. On the x-axis are distributed the analyzed experiments where viral sequences were identified. Red bar represents total reads by SRA, Blue bar reads corresponding to viral sequences.
Rate of viral sequences (reads) detected in each metagenome. A) It shows the proportions of families recovered with Bowtie2. B) It shows the proportions of families recovered using reads translated with blastp. In both methods the RVDB-v21 database reference was used.
Geographic distribution of the metagenomes samples. The different colors indicate the type of host or environment from which the sample was extracted.
Frequency histogram of the length of reads retrieved from all metagenomes.
Frequency histogram of the length of translated contigs retrived from all metagenomes.
Heatmaps of the proportions of contigs classified into viral families by sample origin. Graphs A and B correspond to the highest proportion (families with >2% of total viral reads by sample) and lowest proportion of reads (families with <2% of total viral reads by sample). The upper columns represent annotations of the genome and host type of each identified family. Uncolored boxes correspond to unclassified families. Genome types are coded as dsDNA, dsRNA, ssDNA(+), ssRNA-RT, ssRNA(+). Their hosts are coded here as A (Animalia), Ar (Archaea), B (Bacteria), C (Chromista), F (Fungi), P (Plantae).
Range of hosts detected by metagenome type.
Rate of vFAMs detected in reads (A) and contigs (B) using hiden markov models (HAMMer).
Rate of families recovered per metagenome sample according to the length of reads.
Proportion of sequences of viral origin detected in each SRA with their respective associated metadata information.
Rate of viral protein signals according to family and type of metagenome analyzed.Also, the table describes functional information of the related proteins.
Table of viral signals related to metadata and description of the related protein.
Results of contigs processed by blastn. It shows the sequences with their metadata and taxonomical information.
Metadata used to classify families by genome type and host.
Table of results of random sampling analysis of reads against Blastp using a non-viral database. it shows the sequences with a high percentage of identity (>70%).
Table of results of random sampling analysis of contigs against Blastp using a non-viral database. it shows the sequences with a high percentage of identity (>70%).
Tables of Distribution of the frequencies obtained by taxonomic assignment (Family) in each sample by pairwise alignment (Bowtie, Blast) and Hidden Markov comparison methods (HMMer).
References
- Afelt A., Devaux C., Serra-Cobo J., Frutos R. (2018). “Bats, Bat-Borne Viruses, and Environmental Changes,” in Bats. London, UK: Intechopen. 10.5772/intechopen.74377 [DOI] [Google Scholar]
- Anthony S. J., Epstein J. H., Murray K. A., Navarrete-Macias I., Zambrana-Torrelio C. M., Solovyov A., et al. (2013). A Strategy to Estimate Unknown Viral Diversity in Mammals. mBio 4, e00598–13. 10.1128/mBio.00598-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E.-M., Virgin I., Andersson B. (1993). Photoinhibition of Photosystem II. Inactivation, Protein Damage and Turnover. Biochim. Biophys. Acta (Bba) - Bioenerg. 1143, 113–134. 10.1016/0005-2728(93)90134-2 [DOI] [PubMed] [Google Scholar]
- Auguste A. J., Volk S. M., Arrigo N. C., Martinez R., Ramkissoon V., Adams A. P., et al. (2009). Isolation and Phylogenetic Analysis of Mucambo Virus (Venezuelan Equine Encephalitis Complex Subtype IIIA) in Trinidad. Virology 392, 123–130. 10.1016/j.virol.2009.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw T. G., Gin K. Y. H. (2010). Environmental Surveillance and Molecular Characterization of Human Enteric Viruses in Tropical Urban Wastewaters. J. Appl. Microbiol. 109, 716–730. 10.1111/j.1365-2672.2010.04701.x [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: A New Genome Assembly Algorithm and its Applications to Single-Cell Sequencing. J. Comput. Biol. 19, 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot T., Temmam S., Pérot P., Eloit M. (2019). RVDB-prot, a Reference Viral Protein Database and its HMM Profiles [version 1; Peer Review: 2 Approved with Reservations]. F1000Research 8, 530. 10.12688/f1000research.18776.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordería A. V., Stapleford K. A., Vignuzzi M. (2011). RNA Virus Population Diversity: Implications for Inter-species Transmission. Curr. Opin. Virol. 1, 643–648. 10.1016/j.coviro.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Borozan I., Watt S. N., Ferretti V. (2013). Evaluation of Alignment Algorithms for Discovery and Identification of Pathogens Using RNA-Seq. PLoS One 8, e76935. 10.1371/journal.pone.0076935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart M., Rohwer F. (2005). Here a Virus, There a Virus, Everywhere the Same Virus. Trends Microbiol. 13, 278–284. 10.1016/j.tim.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Brook C. E., Dobson A. P. (2015). Bats as 'special' Reservoirs for Emerging Zoonotic Pathogens. Trends Microbiol. 23, 172–180. 10.1016/j.tim.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzhalava Z., Hultin E., Dillner J. (2018). Extension of the Viral Ecology in Humans Using Viral Profile Hidden Markov Models. PLoS ONE 13, e0190938. 10.1371/journal.pone.0190938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Wong Y. F., Zhou H., Xie Y., Liu Z. Q., Jiang Z. H., et al. (2006). The Comparative Study of Sprague-Dawley and Lewis Rats in Adjuvant-Induced Arthritis. Naunyn Schmied Arch. Pharmacol. 373, 140–147. 10.1007/s00210-006-0062-5 [DOI] [PubMed] [Google Scholar]
- Carroll M. W., Matthews D. A., Hiscox J. A., Elmore M. J., Pollakis G., Rambaut A., et al. (2015). Temporal and Spatial Analysis of the 2014-2015 Ebola Virus Outbreak in West Africa. Nature 524, 97–101. 10.1038/nature14594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M. C., Baeza A., Codeço C. T., Cucunubá Z. M., Dal’Asta A. P., De Leo G. A., et al. (2019). Development, Environmental Degradation, and Disease Spread in the Brazilian Amazon. Plos Biol. 17, e3000526. 10.1371/journal.pbio.3000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. (2018). Fastp: An Ultra-fast All-In-One FASTQ Preprocessor. Bioinformatics 34, i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouin-Carneiro T., dos Santos F. B. (2017). “Transmission of Major Arboviruses in Brazil: The Role of Aedes aegypti and Aedes albopictus Vectors,” in Biological Control of Pest and Vector Insects (InTech). 10.5772/66946 [DOI] [Google Scholar]
- Coffey L. L., Page B. L., Greninger A. L., Herring B. L., Russell R. C., Doggett S. L., et al. (2014). Enhanced Arbovirus Surveillance with Deep Sequencing: Identification of Novel Rhabdoviruses and Bunyaviruses in Australian Mosquitoes. Virology 448, 146–158. 10.1016/j.virol.2013.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra T. L. M., Nassar E. S., de Souza L. T. M., Ferreira I. B., Rocco I. M., Burattini M. N., et al. (1994). New Arenavirus Isolated in Brazil. The Lancet 343, 391–392. 10.1016/S0140-6736(94)91226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello Malta F., Amgarten D., Nastri A. C. d. S. S., Ho Y.-L., Boas Casadio L. V., Basqueira M., et al. (2020). Sabiá Virus-like Mammarenavirus in Patient with Fatal Hemorrhagic Fever, Brazil, 2020. Emerg. Infect. Dis. 26, 1332–1334. 10.3201/EID2606.200099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit E., Van Doremalen N., Falzarano D., Munster V. J. (2016). SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat. Rev. Microbiol. 14, 523–534. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja V. V., Koonin E. V. (2018). Metagenomics Reshapes the Concepts of RNA Virus Evolution by Revealing Extensive Horizontal Virus Transfer. Virus. Res. 244, 36–52. 10.1016/j.virusres.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S., Shackelton L. A., Holmes E. C. (2008). Rates of Evolutionary Change in Viruses: Patterns and Determinants. Nat. Rev. Genet. 9, 267–276. 10.1038/nrg2323 [DOI] [PubMed] [Google Scholar]
- Ellwanger J. H., Kulmann-Leal B., Kaminski V. L., Valverde-Villegas J. M., Veiga A. B. G. D., Spilki F. R., et al. (2020). Beyond Diversity Loss and Climate Change: Impacts of Amazon Deforestation on Infectious Diseases and Public Health. Acad. Bras. Ciênc. 92, e20191375. 10.1590/0001-3765202020191375 [DOI] [PubMed] [Google Scholar]
- Espósito D. L. A., da Fonseca B. A. L. (2015). Complete Genome Sequence of Mayaro Virus ( Togaviridae, Alphavirus ) Strain BeAr 20290 from Brazil. Genome Announc 3, e01372–15. 10.1128/genomeA.01372-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D. L. A., Fonseca B. A. L. d. (2017). Will Mayaro Virus Be Responsible for the Next Outbreak of an Arthropod-Borne Virus in Brazil? Braz. J. Infect. Dis. 21, 540–544. 10.1016/j.bjid.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald P. W. (2011). Evolution of Virulence, Environmental Change, and the Threat Posed by Emerging and Chronic Diseases. Ecol. Res. 26, 1017–1026. 10.1007/s11284-011-0874-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin G. (2018). “Host Range, Host-Virus Interactions, and Virus Transmission,” in Viruses: Molecular Biology, Host Interactions, and Applications to Biotechnology. (Elsevier: ), 101–134. 10.1016/B978-0-12-811257-1.00005-X [DOI] [Google Scholar]
- Fernandes J., Oliveira R. C. d., Coelho T. A., Martins R. M. B., Caetano K. A. A., Horta M. A. P., et al. (2019). Rodent-borne Viruses Survey in Rural Settlers from Central Brazil. Mem. Inst. Oswaldo Cruz 114, 180448. 10.1590/0074-02760180448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira I. B., Pereira L. E., Rocco I. M., Marti A. T., Souza L. T. M. d., Iversson L. B. (1994). Surveillance of Arbovirus Infections in the atlantic forest Region, State of São Paulo, Brazil: I. Detection of Hemagglutination-Inhibition Antibodies in Wild Birds between 1978 and 1990. Rev. Inst. Med. Trop. S. Paulo 36, 265–274. 10.1590/S0036-46651994000300011 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A. H., Rupnik A., O'Shea H., Crispie F., Keaveney S., Cotter P. (2021). High Throughput Sequencing for the Detection and Characterization of RNA Viruses. Front. Microbiol. 12, 621719. 10.3389/fmicb.2021.621719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman S., Flores-Uribe J., Larom S., Alalouf O., Liran O., Yacoby I., et al. (2017). A Myovirus Encoding Both Photosystem I and II Proteins Enhances Cyclic Electron Flow in Infected Prochlorococcus Cells. Nat. Microbiol. 2, 1350–1357. 10.1038/s41564-017-0002-9 [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A. (1999). Marine Viruses and Their Biogeochemical and Ecological Effects. Nature 399, 541–548. 10.1038/21119 [DOI] [PubMed] [Google Scholar]
- Geoghegan J. L., Holmes E. C. (2017). Predicting Virus Emergence amid Evolutionary Noise. Open Biol. 7, 170189. 10.1098/rsob.170189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Peccoud J., Chateigner A., Moumen B., Cordaux R., Herniou E. A. (2016). Continuous Influx of Genetic Material from Host to Virus Populations. PLOS Genet. 12, e1005838. 10.1371/journal.pgen.1005838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J., Souris M., Valdivia-granda W. (2018). Hemorrhagic Fever Viruses. Methods Mol. Biol. 1604, 3–31. 10.1007/978-1-4939-6981-4_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre N., Aljanahi A., Nandakumar S., Mikailov M., Khan A. S. (2018). A Reference Viral Database (RVDB) to Enhance Bioinformatics Analysis of High-Throughput Sequencing for Novel Virus Detection. mSphere 3, e00069–18. 10.1128/mSphereDirect.00069-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. A., Higgs S. (2009). Impact of Climate Change and Other Factors on Emerging Arbovirus Diseases. Trans. R. Soc. Trop. Med. Hyg. 103, 109–121. 10.1016/j.trstmh.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl E., Zechmeister-Boltenstern S., Bodrossy L., Sessitsch A. (2004). Comparison of Diversities and Compositions of Bacterial Populations Inhabiting Natural forest Soils. Appl. Environ. Microbiol. 70, 5057–5065. 10.1128/AEM.70.9.5057-5065.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. B., Gangnon R. E., Barcellos C., Asner G. P., Patz J. A. (2014). Influence of Deforestation, Logging, and Fire on Malaria in the Brazilian Amazon. PLoS ONE 9, e85725. 10.1371/journal.pone.0085725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman D. T. S. (2016). Bats as Viral Reservoirs. Annu. Rev. Virol. 3, 77–99. 10.1146/annurev-virology-110615-042203 [DOI] [PubMed] [Google Scholar]
- Herath D., Jayasundara D., Ackland D., Saeed I., Tang S.-L., Halgamuge S. (2017). Assessing Species Diversity Using Metavirome Data: Methods and Challenges. Comput. Struct. Biotechnol. J. 15, 447–455. 10.1016/j.csbj.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmsø M. H., Mollerup S., Jensen R. H., Pietroni C., Lukjancenko O., Schultz A. C., et al. (2019). Metagenomic Analysis of Viruses in Toilet Waste from Long Distance Flights-A New Procedure for Global Infectious Disease Surveillance. PLoS ONE 14, e0210368. 10.1371/journal.pone.0210368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C. E. (2009). The Evolution and Emergence of RNA Viruses. Oxford: OUP Oxford. [Google Scholar]
- Huang X., Madan A. (1999). CAP3: A DNA Sequence Assembly Program. Genome Res. 9, 868–877. 10.1101/gr.9.9.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulo C., de Castro E., Masson P., Bougueleret L., Bairoch A., Xenarios I., et al. (2011). ViralZone: a Knowledge Resource to Understand Virus Diversity. Nucleic Acids Res. 39, D576–D582. 10.1093/NAR/GKQ901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C. J. (2011). Studies in Viral Ecology. 10.1002/9781118025666 [DOI] [Google Scholar]
- Hurst C. J. (2013). Viral Ecology. [Google Scholar]
- Jones K. E., Patel N. G., Levy M. A., Storeygard A., Balk D., Gittleman J. L., et al. (2008). Global Trends in Emerging Infectious Diseases. Nature 451, 990–993. 10.1038/NATURE06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. (2013). A Virocentric Perspective on the Evolution of Life. Curr. Opin. Virol. 3, 546–557. 10.1016/j.coviro.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S. R., Wang D. (2017). Origins and Challenges of Viral Dark Matter. Virus. Res. 239, 136–142. 10.1016/j.virusres.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Larsson A. (2014). AliView: a Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 30, 3276–3278. 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T. M., Wong H. H., Tay F. P. L., Fang S., Keng C.-T., Tan Y. J., et al. (2007). Expression, post-translational Modification and Biochemical Characterization of Proteins Encoded by Subgenomic mRNA8 of the Severe Acute Respiratory Syndrome Coronavirus. FEBS J. 274, 4211–4222. 10.1111/j.1742-4658.2007.05947.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Seifert S. N., Olival K. J., Plowright R. K., Munster V. J. (2020). Bat-borne Virus Diversity, Spillover and Emergence. Nat. Rev. Microbiol. 18, 461–471. 10.1038/s41579-020-0394-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Ganesh A. (2013). Water Quality Indicators: Bacteria, Coliphages, Enteric Viruses. Int. J. Environ. Health Res. 23, 484–506. 10.1080/09603123.2013.769201 [DOI] [PubMed] [Google Scholar]
- Lopes N., Nozawa C., Linhares R. E. C., Linhares C. (2014). Características gerais e epidemiologia dos arbovírus emergentes no Brasil. Revista Pan-Amazônica de Saúde 5, 55–64. 10.5123/S2176-62232014000300007 [DOI] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. (2020). Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. The Lancet 395, 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W. J. (2014). Emerging and Reemerging Virus Diseases of Vertebrates☆. Elsevier. 10.1016/b978-0-12-801238-3.02564-2 [DOI] [Google Scholar]
- Mann N. H., Cook A., Millard A., Bailey S., Clokie M. (2003). Bacterial Photosynthesis Genes in a Virus. Nature 424, 741. 10.1038/424741a [DOI] [PubMed] [Google Scholar]
- Marz M., Beerenwinkel N., Drosten C., Fricke M., Frishman D., Hofacker I. L., et al. (2014). Challenges in RNA Virus Bioinformatics. Bioinformatics 30, 1793–1799. 10.1093/bioinformatics/btu105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsky H. C., Siddle K. J., Siddle K. J., Gladden-Young A., Qu J., Yang D. K., et al. (2019). Capturing Sequence Diversity in Metagenomes with Comprehensive and Scalable Probe Design. Nat. Biotechnol. 37, 160–168. 10.1038/s41587-018-0006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morace G., Aulicino F. A., Angelozzi C., Costanzo L., Donadio F., Rapicetta M. (2002). Microbial Quality of Wastewater: Detection of Hepatitis A Virus by Reverse Transcriptase-Polymerase Chain Reaction. J. Appl. Microbiol. 92, 828–836. 10.1046/j.1365-2672.2002.01587.x [DOI] [PubMed] [Google Scholar]
- Moratelli R., Calisher C. H. (2015). Bats and Zoonotic Viruses: Can We Confidently Link Bats with Emerging Deadly Viruses? Mem. Inst. Oswaldo Cruz 110, 1–22. 10.1590/0074-02760150048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre C. A., Sampaio G., Borma L. S., Castilla-Rubio J. C., Silva J. S., Cardoso M. (2016). Land-use and Climate Change Risks in the Amazon and the Need of a Novel Sustainable Development Paradigm. Proc. Natl. Acad. Sci. USA 113, 10759–10768. 10.1073/pnas.1605516113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri S., Matsumura E. E., Kuo Y.-W., Falk B. W. (2018). Insect-specific Viruses: from Discovery to Potential Translational Applications. Curr. Opin. Virol. 33, 33–41. 10.1016/j.coviro.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Obbard D. J. (2018). Expansion of the Metazoan Virosphere: Progress, Pitfalls, and Prospects. Curr. Opin. Virol. 31, 17–23. 10.1016/j.coviro.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Parodi A. S., Coto C. E., Boxaca M., Lajmanovich S. n., Gonzlez S. (1966). Characteristics of Junin Virus. Archiv f Virusforschung 19, 393–402. 10.1007/BF01250608 [DOI] [PubMed] [Google Scholar]
- Posada-Cespedes S., Seifert D., Beerenwinkel N. (2017). Recent Advances in Inferring Viral Diversity from High-Throughput Sequencing Data. Virus. Res. 239, 17–32. 10.1016/j.virusres.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. (2000). EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16, 276–277. 10.1016/S0168-9525(00)02024-2 [DOI] [PubMed] [Google Scholar]
- Rosario K., Breitbart M. (2011). Exploring the Viral World through Metagenomics. Curr. Opin. Virol. 1, 289–297. 10.1016/j.coviro.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Roux S., Emerson J. B., Eloe-Fadrosh E. A., Sullivan M. B. (2017). Benchmarking Viromics: an In Silico Evaluation of Metagenome-Enabled Estimates of Viral Community Composition and Diversity. PeerJ 5, e3817. 10.7717/peerj.3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi M., Tomaru Y., Ahola T. (2021). RNA Viruses in Aquatic Unicellular Eukaryotes. Viruses 13, 362. 10.3390/v13030362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronetz D., Feldmann H., Falzarano D. (2012). Arenaviruses and Filoviruses. Med. Microbiol., 546–558. 10.1016/B978-0-7020-4089-4.00068-8 [DOI] [Google Scholar]
- Salas R., Pacheco M. E., Ramos B., Taibo M. E., Jaimes E., Vasquez C., et al. (1991). Venezuelan Haemorrhagic Fever. The Lancet 338, 1033–1036. 10.1016/0140-6736(91)91899-6 [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B. C. (2019). Coronavirus Envelope Protein: Current Knowledge. Virol. J. 16, 69. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz F., Alteio L., Goudeau D., Ryan E. M., Yu F. B., Malmstrom R. R., et al. (2018). Hidden Diversity of Soil Giant Viruses. Nat. Commun. 9, 4881. 10.1038/s41467-018-07335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Lin X.-D., Tian J.-H., Chen L.-J., Chen X., Li C.-X., et al. (2016b). Redefining the Invertebrate RNA Virosphere. Nature 540, 539–543. 10.1038/nature20167 [DOI] [PubMed] [Google Scholar]
- Shi M., Lin X.-D., Vasilakis N., Tian J.-H., Li C.-X., Chen L.-J., et al. (2016a). Divergent Viruses Discovered in Arthropods and Vertebrates Revise the Evolutionary History of the Flaviviridae and Related Viruses. J. Virol. 90, 659–669. 10.1128/jvi.02036-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Zhang Y.-Z., Holmes E. C. (2018). Meta-transcriptomics and the Evolutionary Biology of RNA Viruses. Virus. Res. 243, 83–90. 10.1016/j.virusres.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Zhao L., Atoni E., Zeng W., Hu X., Matthijnssens J., et al. (2020). The Conservation of a Core Virome in Aedes Mosquitoes Across Different Developmental Stages and Continents. mSystems, 2020.04.23.058701. 10.1101/2020.04.23.058701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Adams M. J., Benkő M., Breitbart M., Brister J. R., Carstens E. B., et al. (2017). Virus Taxonomy in the Age of Metagenomics. Nat. Rev. Microbiol. 15, 161–168. 10.1038/nrmicro.2016.177 [DOI] [PubMed] [Google Scholar]
- Skewes-Cox P., Sharpton T. J., Pollard K. S., DeRisi J. L. (2014). Profile Hidden Markov Models for the Detection of Viruses within Metagenomic Sequence Data. PLoS One 9, e105067. 10.1371/journal.pone.0105067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Frangeul L., Dickson L. B., Blanc H., Verdier Y., Vinh J., et al. (2017). Uncovering the Repertoire of Endogenous Flaviviral Elements in Aedes Mosquito Genomes. J. Virol. 9, e00571–17. 10.1128/JVI.00571-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel Leonard A., Weissman D. B., Greenbaum B., Ghedin E., Koelle K. (2017). Transmission Bottleneck Size Estimation from Pathogen Deep-Sequencing Data, with an Application to Human Influenza A Virus. J. Virol. 91, e00171–17. 10.1128/JVI.00171-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron F. M., Stone G. N., Obbard D. J. (2018). Metagenomic Sequencing Suggests a Diversity of RNA Interference-like Responses to Viruses across Multicellular Eukaryotes. Plos Genet. 14, e1007533. 10.1371/journal.pgen.1007533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-F., Crameri G. (2014). Emerging Zoonotic Viral Diseases. Available at www.onehealthinitiative.com ([Accessed December 26, 2020]. [DOI] [PubMed] [Google Scholar]
- Warren C. J., Sawyer S. L. (2019). How Host Genetics Dictates Successful Viral Zoonosis. Plos Biol. 17, e3000217. 10.1371/journal.pbio.3000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Reisen W. K. (2010). Present and Future Arboviral Threats. Antiviral Res. 85, 328–345. 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. J., Wang J., Hall R. J. (2017). Assessing the Impact of Assemblers on Virus Detection in a De Novo Metagenomic Analysis Pipeline. J. Comput. Biol. 24, 874–881. 10.1089/cmb.2017.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y. I., Kazlauskas D., Iranzo J., Lucía-Sanz A., Kuhn J. H., Krupovic M., et al. (2018). Origins and Evolution of the Global RNA Virome. mBio 9, e02329–18. 10.1128/mBio.02329-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y. I., Silas S., Wang Y., Wu S., Bocek M., Kazlauskas D., et al. (2020). Doubling of the Known Set of RNA Viruses by Metagenomic Analysis of an Aquatic Virome. Nat. Microbiol. 5, 1262–1270. 10.1038/s41564-020-0755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S., Lau S., Woo P., Yuen K.-Y. (2007). Bats as a Continuing Source of Emerging Infections in Humans. Rev. Med. Virol. 17, 67–91. 10.1002/rmv.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M., Scott F., Hudson Z., Howey R., Chase-Topping M. (2012). Human Viruses: Discovery and Emergence. Phil. Trans. R. Soc. B 367, 2864–2871. 10.1098/rstb.2011.0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. (2006). Microbial Ecology in the Age of Genomics and Metagenomics: Concepts, Tools, and Recent Advances. Mol. Ecol. 15, 1713–1731. 10.1111/j.1365-294X.2006.02882.x [DOI] [PubMed] [Google Scholar]
- Zakrzewski M., Rašić G., Darbro J., Krause L., Poo Y. S., Filipović I., et al. (2018). Mapping the Virome in Wild-Caught Aedes aegypti from Cairns and Bangkok. Sci. Rep. 8, 4690. 10.1038/s41598-018-22945-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-Z., Chen Y.-M., Wang W., Qin X.-C., Holmes E. C. (2019). Expanding the RNA Virosphere by Unbiased Metagenomics. Annu. Rev. Virol. 6, 119–139. 10.1146/annurev-virology-092818-015851 [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Z., Wu W.-C., Shi M., Holmes E. C. (2018). The Diversity, Evolution and Origins of Vertebrate RNA Viruses. Curr. Opin. Virol. 31, 9–16. 10.1016/j.coviro.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
workflow used in the present work to capture viral signals using different methods.
Number spots and viral sequences by sra experiment. On the x-axis are distributed the analyzed experiments where viral sequences were identified. Red bar represents total reads by SRA, Blue bar reads corresponding to viral sequences.
Rate of viral sequences (reads) detected in each metagenome. A) It shows the proportions of families recovered with Bowtie2. B) It shows the proportions of families recovered using reads translated with blastp. In both methods the RVDB-v21 database reference was used.
Geographic distribution of the metagenomes samples. The different colors indicate the type of host or environment from which the sample was extracted.
Frequency histogram of the length of reads retrieved from all metagenomes.
Frequency histogram of the length of translated contigs retrived from all metagenomes.
Heatmaps of the proportions of contigs classified into viral families by sample origin. Graphs A and B correspond to the highest proportion (families with >2% of total viral reads by sample) and lowest proportion of reads (families with <2% of total viral reads by sample). The upper columns represent annotations of the genome and host type of each identified family. Uncolored boxes correspond to unclassified families. Genome types are coded as dsDNA, dsRNA, ssDNA(+), ssRNA-RT, ssRNA(+). Their hosts are coded here as A (Animalia), Ar (Archaea), B (Bacteria), C (Chromista), F (Fungi), P (Plantae).
Range of hosts detected by metagenome type.
Rate of vFAMs detected in reads (A) and contigs (B) using hiden markov models (HAMMer).
Rate of families recovered per metagenome sample according to the length of reads.
Proportion of sequences of viral origin detected in each SRA with their respective associated metadata information.
Rate of viral protein signals according to family and type of metagenome analyzed.Also, the table describes functional information of the related proteins.
Table of viral signals related to metadata and description of the related protein.
Results of contigs processed by blastn. It shows the sequences with their metadata and taxonomical information.
Metadata used to classify families by genome type and host.
Table of results of random sampling analysis of reads against Blastp using a non-viral database. it shows the sequences with a high percentage of identity (>70%).
Table of results of random sampling analysis of contigs against Blastp using a non-viral database. it shows the sequences with a high percentage of identity (>70%).
Tables of Distribution of the frequencies obtained by taxonomic assignment (Family) in each sample by pairwise alignment (Bowtie, Blast) and Hidden Markov comparison methods (HMMer).
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://github.com/Fernando-GMzr/Viral-signals-Public-metagenomes.