Abstract
Objectives
To determine screening outcomes in women who have no recorded risk factors for breast cancer.
Methods
A retrospective population-based cohort study included all 1,026,137 mammography screening episodes in 323,082 women attending the BreastScreen Western Australia (part of national biennial screening) program between July 2007 and June 2017. Cancer detection rates (CDR) and interval cancer rates (ICR) were calculated in screening episodes with no recorded risk factors for breast cancer versus at least one risk factor stratified by age. CDR was further stratified by timeliness of screening (<27 versus ≥27 months); ICR was stratified by breast density.
Results
Amongst 566,948 screens (55.3%) that had no recorded risk factors, 2347 (40.9%) screen-detected cancers were observed. In screens with no risk factors, CDR was 50 (95%CI 48–52) per 10,000 screens and ICR was 7.9 (95%CI 7.4–8.4) per 10,000 women-years, estimates that were lower than screens with at least one risk factor (CDR 83 (95%CI 80–86) per 10,000 screens, ICR 12.2 (95%CI 11.5–13.0) per 10,000 women-years). Compared to timely screens with risk factors, delayed screens with no risk factors had similar CDR across all age groups and a higher proportion of node positive cancers (26.1% vs 20.7%). ICR was lowest in screens that had no risk factors nor dense breasts in all age groups.
Conclusions
Majority of screens had no recorded breast cancer risk factors, hence a substantial proportion of screen-detected cancers occur in these screening episodes. Our findings may not justify less frequent screening in women with no risk factors.
Keywords: Breast cancer, Population screening, Risk-stratified screening, Epidemiology
Highlights
-
•
40.9% of screen-detected breast cancers occurred in women with no risk factors.
-
•
Cancer detection rate was 50/10,000 in screens with no risk factors.
-
•
Cancer size and nodal status were no more favourable in screens with no risk factors.
-
•
Interval cancer rate was lowest in screens with no risk factors nor dense breasts.
-
•
Our findings may not justify less frequent screening in women with no risk factors.
1. Introduction
Mammography screening has been shown to reduce breast cancer deaths in average-risk women [1]. In Australia, similarly to organised breast screening in Europe, the publicly-funded BreastScreen program has been providing mammography screening for around 30 years, currently targeting women aged 50–74 years [2].
Both globally and within Australia, there is interest in risk-stratified breast cancer screening to improve benefit and possibly reduce the harms of screening [3]. This includes considering increasing screening frequency or using more sensitive imaging for women at increased risk, and conversely reducing screening frequency (longer time between screens) for those deemed at lower risk. To determine the increase in breast cancer risk associated with individual risk factors, we have previously reported screen-detected and inter-screen interval cancer rates by risk factors in contemporary Australian women [4]. However, little is known about screening outcomes in women who do not have any specific risk factors for breast cancer and who are presumed to be at or lower than population risk.
In this study, we investigate this under-researched group of screening participants who may be at the lower end of the risk spectrum and determine screening outcomes in women who have none of the breast cancer-related risk factors that are recorded by the BreastScreen program.
2. Material and methods
2.1. Participants
A retrospective cohort study was conducted using routinely collected clinical and administrative data in the BreastScreen Western Australia (BSWA) program. All mammography screening episodes in women aged ≥40 years in the BSWA program from 1 July 2007 to 30 June 2017 were included in the analyses.
Women aged ≥40 years are eligible for free mammograms every 2 years in the program. The target age-group that is actively invited was 50–69 until 30 June 2013 and 50 to 74 from 1 July 2013. Women aged ≥40 but below or above the target age range are not actively invited but are still able to take part in the screening program if they wish. BSWA classifies women with the following characteristics as high-risk allowing annual screening: strong family history (≥2 affected first-degree relatives, or ≥1 first-degree relative diagnosed at <50 years or with bilateral disease), personal history of breast and/or ovarian cancer, or previous diagnosis of Atypical Ductal Hyperplasia or Lobular Carcinoma in-situ (‘benign’ conditions associated with increased risk).
Participants in BSWA program provide written consent for their data to be used for research and quality assurance each time they screen. Ethics approval for this study was obtained by the Governance, Evidence, Knowledge & Outcomes Ethics Committee, Quality Improvement Women's Health, Genetics & Mental Health, Women and Newborn Health Service (reference number 34263). An author responsible for data analysis (NN) had full access to all de-identified data in the study.
2.2. Measurement
The BSWA program routinely collects information on demographic characteristics, risk factors and breast symptoms using a self-administered registration form [2]. In addition, details and results of all screening mammograms and further assessments for recalled women are routinely recorded in the Mammographic Screening Registry. Data on interval cancers are collected according to BreastScreen national accreditation standards.
2.3. Exposures
Collected variables included age, screening round, and time since last screen for re-screens (repeat screens) as well as the following risk factors: personal history of breast and ovarian cancer, first-degree family history of breast cancer, hormone replacement therapy (HRT) in the past 6 months, a history of excision/biopsy of benign lump, and self-reported breast symptoms. Screens were classified as having none of these risk factors versus at least one risk factor. Age was categorised into four groups of 40–49, 50–59, 60–69, and ≥70 years.
BSWA recorded breast density only for women who had no abnormality on the mammogram and were not recalled for additional testing. Women were considered to have dense breasts if at least one of the two radiologists who double-read the mammogram visually classified it as showing heterogeneously or extremely dense breasts [5]. Secondary analyses for CDR included dense breasts from the previous screen within 27 months as a risk factor in the subset of women who had this information. Analyses for ICR included breast density from the current screen as a stratification variable.
2.4. Outcomes
Primary outcomes were cancer detection rates at screening (CDR) per 10,000 screens, and interval cancer rates (ICR) per 10,000 women-years.
Screen-detected cancers were defined as cancers detected following abnormal mammograms and recall for further testing. Pathological tumour size category and axillary nodal status were recorded for screen-detected cancers.
Interval cancers were defined as cancers in women whose screen results were negative, and in whom breast cancer diagnosis occurred before the next scheduled screen (two years for most women, or one year for women scheduled for annual screening). If women presented before 730 days in biennial screeners (365 days in annual screeners) with a symptom and then are diagnosed with a cancer in the same breast, they were considered to have interval cancers.
2.5. Statistical analysis
Characteristics of the cohort were summarised descriptively using means for age and percentages for categorical variables. 95% confidence intervals (95% CIs) were computed using the binomial distribution.
PROC GENMOD in SAS 9.4 (SAS Institute, Cary, North Carolina, US) was used to calculate CDR per 10,000 screens in re-screens and ICR per 10,000 women-years in all screens, and to compare screens that had no recorded risk factors and those that had at least one risk factor stratified by age groups. CDR was further stratified by timeliness of the screens in a biennial screening program (<27 months or ≥27 months since last screen) [6]. First (prevalent) screens were excluded when calculating CDR because CDR is known to be high in first screens regardless of risk factors [4]. CDR analysis was repeated including breast density information as determined at the previous screen within 27 months because breast density was only reported for women who had no abnormality on the current mammogram. ICR was further stratified by breast density since increased density has been shown to increase the risk of an interval cancer [7]. ICR included data for all screens since our earlier work showed no significant differences in ICR for first or repeat screens [4].
Distributions of pathological tumour size (pT) category and axillary nodal status of screen-detected cancers were stratified by risk factors and timeliness of the screens and the four strata were compared with the Chi-squared test.
All tests of statistical significance were two-sided. The level chosen for statistical significance was p < 0.05; p < 0.10 was considered weak evidence of association.
3. Results
Between 1 July 2007 and 31 June 2017, a total of 1,026,137 screens were performed in 323,082 women aged ≥40 years who attended BSWA. As reported in our earlier work, mean age at the time of screening was 58.5 (SD ±8.6) years (range 40–98) and the majority of screens were re-screens between 15th and 26th months (Supplementary Table 1) [4]. 459,121 screens (44.7%) had at least one risk factor (not including breast density information) (mean age 59.4 (SD ±8.5) years) and 566,948 screens (55.3%) had none of the recorded risk factors (mean age 57.8 (SD ±8.5) years).
3.1. Cancer detection rates and characteristics
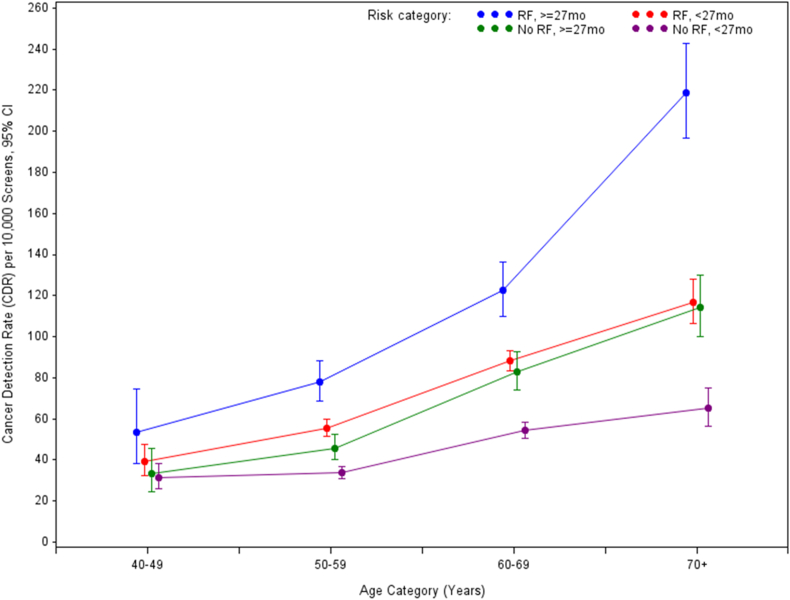
Of all screen-detected cancers, 40.9% were detected in screens with no risk factors (not including breast density information). In 408727 rescreens with at least one risk factor, 3395 cancers (2660 invasive, 734 in-situ, 1 unknown) were detected (CDR 83 (95%CI 80–86) per 10,000 screens), and in 468,850 rescreens with no risk factors, 2347 cancers (1824 invasive, 523 in-situ) were detected (CDR 50 (95%CI 48–52) per 10,000 screens). Timely screens (<27 months) with no risk factors had lower CDR compared to timely screens with at least one risk factor in women aged ≥50 (p < 0.001 in 50s, 60s and ≥70; p = 0.1166 in 40s) (Table 1, Fig. 1). However, delayed screens (≥27 months) with no risk factors had similar CDR to timely screens with at least one risk factor across all age groups (p = 0.3580 in 40s, p = 0.0890 in 50s, p = 0.3477 in 60s and p = 0.7917 in ≥70). The highest CDRs were observed in women with delayed screens and at least one risk factor.
Table 1.
Cancer detection rate in re-screens by age group, timeliness and risk factor grouping.
| Age | None of the breast cancer RFs |
At least one breast cancer RF |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timely screens (<27mo) |
Delayed screens (≥27mo) |
Timely screens (<27mo) |
Delayed screens (≥27mo) |
|||||||||||||
| Total | Cancer | CDR | 95% CI | Total | Cancer | CDR | 95% CI | Total | Cancer | CDR | 95% CI | Total | Cancer | CDR | 95% CI | |
| 40–49 | 34612 | 109 | 31† | 26–38 | 11781 | 39 | 33† | 24–45 | 27119 | 106 | 39 | 32–47 | 6364 | 34 | 53 | 38–75 |
| 50–59 | 145784 | 489 | 34∗∗ | 31–37 | 46617 | 213 | 46† | 40–52 | 127746 | 709 | 56 | 51–60 | 31593 | 246 | 78 | 68–88 |
| 60–69 | 145626 | 789 | 54∗∗ | 51–58 | 36294 | 301 | 83† | 74–93 | 137056 | 1206 | 88 | 83–93 | 26232 | 321 | 122 | 110–136 |
| 70 and over | 29027 | 189 | 65∗∗ | 56–75 | 19109 | 218 | 114† | 100–130 | 36967 | 431 | 117 | 106–128 | 15650 | 342 | 219 | 197–243 |
(calculated for each screening episode).
RF, risk factor; CDR, cancer detection rate at screen per 10,000 screens, CI, confidence interval. ∗RF comprised any of the following: first-degree family history of breast cancer, HRT use, history of risk-relevant benign breast conditions, and self-reported symptoms (breast density information not included). ∗∗Difference to the ‘at least one RF, timely screens’ group in the same age group is statistically significant. †Difference to the ‘at least one RF, timely screens’ group in the same age group is not statistically significant.
Fig. 1.
Cancer detection rate in re-screens by age group, timeliness and risk factor grouping (calculated for each screening episode). “RF,≥27mo”, has at least one risk factor (not including breast density information) and returned after 27 months or longer; “RF,<27mo”, has at least one risk factor and returned within 27 months; “No RF,≥27mo”, does not have any risk factors and returned after 27 months or longer; “NoRF,<27mo”, does not have any risk factors and returned within 27 months.
After including breast density information in the risk factor classification, CDR in screens with no risk factors nor dense breasts was not substantially lower compared to timely screens with no risk factors not including breast density information (Table 1), especially in women older than 50 (Supplementary Table 2). Of the 2963 screen-detected cancers, 996 (33.6%) occurred in women with no risk factors nor dense breasts.
There was statistically significant overall difference in distributions of pathological tumour size category (p < 0.0001) and axillary nodal status (p = 0.0025) between cancers detected in timely and delayed screens with and without risk factors (not including breast density information). Delayed screens with at least one risk factor had the highest proportion of pT2 and pT3 cancers and positive nodes. Compared to timely screens with at least one risk factor, delayed screens with no risk factors group had similar proportions of pT2 and pT3 cancers but 5.4% more with positive nodes(Table 2).
Table 2.
Characteristics of the screen-detected cancers by timeliness and risk factor grouping.
| Characteristics | None of the RFs∗ |
At least one RF∗ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| timely screens (<27mo) |
Delayed screens (≥27mo) |
timely screens (<27mo) |
Delayed screens (≥27mo) |
||||||
| N | % | N | % | N | % | N | % | ||
| Pathological tumour size category | |||||||||
| pTis (DCIS) | 364 | 23.2% | 159 | 20.6% | 551 | 22.5% | 183 | 19.6% | |
| pT1a (≤5 mm) | 117 | 7.5% | 44 | 5.7% | 202 | 8.3% | 54 | 5.8% | |
| pT1b (>5 mm, ≤10 mm) | 327 | 20.9% | 161 | 20.9% | 502 | 20.5% | 152 | 16.2% | |
| pT1c (>10 mm, ≤20 mm) | 526 | 33.5% | 275 | 35.7% | 787 | 32.2% | 306 | 32.7% | |
| pT2 (>20 mm, ≤50 mm) | 219 | 14.0% | 117 | 15.2% | 362 | 14.8% | 210 | 22.4% | |
| pT3 (>50 mm) | 15 | 1.0% | 14 | 1.8% | 40 | 1.6% | 31 | 3.3% | |
| Unknown | 8 | . | 1 | . | 8∗∗ | . | 7 | . | |
| Total | 1568 | 770 | 2444 | 936 | |||||
| Axillary nodal status (invasive cancer) | |||||||||
| Negative for metastasis | 856 | 77.3% | 408 | 73.9% | 1342 | 79.3% | 507 | 72.9% | |
| Positive for metastasis | 251 | 22.7% | 144 | 26.1% | 351 | 20.7% | 188 | 27.1% | |
| Unknown | 105 | . | 60 | . | 208 | . | 64 | . | |
| Total | 1107 | 552 | 1693 | 695 | |||||
RF, risk factor; DCIS, ductal carcinoma in situ. ∗first-degree family history of breast cancer, HRT use, history of benign breast conditions, and self-reporting symptoms. Breast density was not included. ∗∗1 was also unclear whether it was DCIS or invasive.
3.2. Interval cancer rates
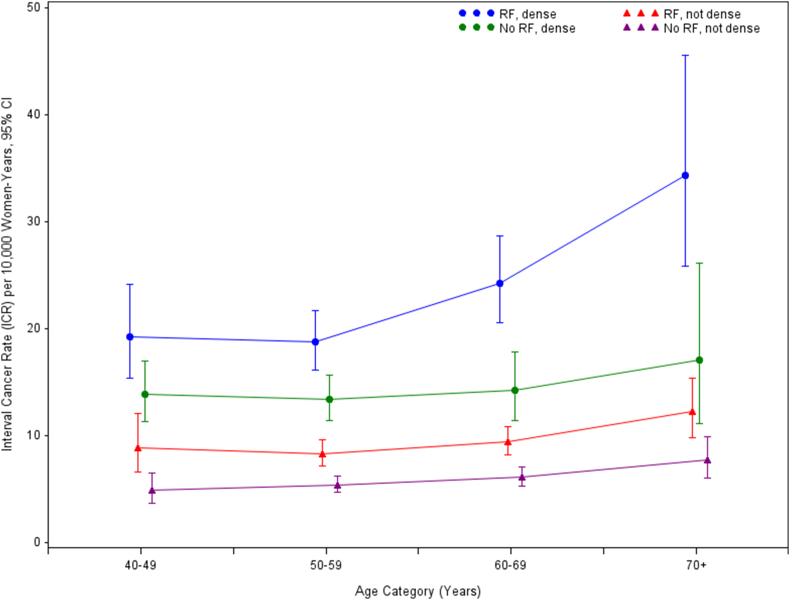
Approximately half (47.1%) of all interval cancers were detected in screens with no risk factors (not including breast density information). In 459121 screens with at least one risk factor, 987 interval cancers (950 invasive, 37 in-situ) were detected (ICR 12.2 (95%CI 11.5–13.0) per 10,000 screens), and in 566948 screens with no risk factors, 879 cancers (840 invasive, 39 in-situ) were detected (ICR 7.9 (95%CI 7.4–8.4) per 10,000 screens). Table 3 and Fig. 2 show ICR in all screens by age-group, breast density and presence or absence of all other risk factors. ICR was lowest in screens that had no risk factors nor dense breasts and the differences to the next lowest group, “at least one RF, not dense breasts”, were statistically significant in all age groups (p = 0.0050 in 40s, <0.0001 in 50s and 60s and p = 0.0070 in ≥70).
Table 3.
Interval cancer rate by age group, breast density and presence or absence of all other risk factors (calculated for each screening episode).
| Age | None of the RFs∗ |
At least one RF∗ |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not dense breasts |
Dense breasts |
Not dense breasts |
Dense breasts |
|||||||||||||
| Total | Cancer | ICR | 95% CI | Total | Cancer | ICR | 95% CI | Total | Cancer | ICR | 95% CI | Total | Cancer | ICR | 95% CI | |
| 40–49 | 49385 | 48 | 4.9 | 3.7–6.5 | 33226 | 91 | 13.8 | 11.3–17.0 | 26563 | 42 | 8.9∗∗ | 6.6–12.0 | 21701 | 75 | 19.2 | 15.3–24.1 |
| 50–59 | 171322 | 183 | 5.4 | 4.7–6.2 | 57133 | 151 | 13.4 | 11.4–15.7 | 114117 | 172 | 8.3∗∗ | 7.1–9.6 | 50254 | 173 | 18.7 | 16.1–21.7 |
| 60–69 | 154107 | 185 | 6.1 | 5.3–7.0 | 27683 | 78 | 14.3 | 11.4–17.8 | 112851 | 191 | 9.4∗∗ | 8.2–10.8 | 31528 | 139 | 24.3 | 20.5–28.7 |
| 70 and over | 41390 | 63 | 7.7 | 6.0–9.9 | 6247 | 21 | 17.0 | 11.1–26.1 | 33439 | 74 | 12.2∗∗ | 9.7–15.4 | 7683 | 48 | 34.3 | 25.9–45.5 |
RF, risk factor; ICR, interval cancer rate per 10,000 women-years; CI, confidence interval. ∗First-degree family history of breast cancer, HRT use, history of benign breast conditions, and self-reporting symptoms. ∗∗Difference to the ‘None of the RFs, No dense breasts’ group in the same age group is statistically significant.
Fig. 2.
Interval cancer rate in all screens by age group, breast density and risk factor grouping (calculated for each screening episode). “RF, dense”, has at least one risk factor (not including breast density information) and has dense breasts; “RF, not dense”, has at least one risk factor and does not have dense breasts; “No RF, dense”, does not have risk factors and has dense breasts; “No RF, not dense”, does not have risk factors and does not have dense breasts.
4. Discussion
This is the first population-based study to investigate screening outcomes in women with no recorded risk factors for breast cancer in a large cohort of participants in a mammography screening program (BSWA, Australia). In population screening, women are considered at ‘average’ (or population) risk, yet it is known that some will have risk factors for breast cancer and others will not, therefore screening participants will have a mixed risk profile. In the era of targeted therapies and precision medicine, risk-stratified cancer screening that tailors screening according to individual risks aligns with the concept of individualised healthcare [8]. Most studies examining screening outcomes by risk factors have focused on population subgroups at increased risk [9,10]. Instead, our study has focused on quantifying outcomes in those who have none of the risk factors that are recorded in the population screening program to inform ongoing discussions about potentially providing less intensive screening to those women who are at relatively lower risk. The evidence we report in the present study will be relevant to breast screening programs contemplating evaluation of risk-based screening, as is being planned in several countries [11,12], or indeed in trials in progress [13].
In our study, 55.3% of screening participants had no recorded risk factors, so even at a lower cancer detection rate, just less than half of screen-detected cancers (40.9%) occurred in women who had no risk factors. Although we did not have data about dense breasts at the time of the current screen in women who had screen-detected cancers, our secondary analysis found that 33.6% of screen-detected cancers had no risk factors nor dense breasts based on information from the prior screen within 27 months. This is consistent with Neal et al.‘s study that found that 33% of screen-detected cancers occurred in women without dense breasts, personal or family history of breast cancer [14].
When screens were stratified by timeliness (27 months was chosen as a cut-off value given that most population screening programs provide biennial screening allowing 3 months for women to turn up to re-screen since it is due at/after 24 months) and risk factor grouping, CDR was lower in timely screens with no risk factors compared to timely screens with at least one risk factor in women aged ≥50. However, CDR in delayed screens with no risk factors was similar to timely screens with at least one risk factor in all age groups. We acknowledge that this comparison may seem counter-intuitive given these are different groups, however this highlights the need for caution, or at least thorough evaluation, if longer screening intervals are contemplated as part of risk-stratified screening. Even after including breast density information (classified from the previous screens within 27 months), there was no substantial decrease in CDR in screens with no risk factors nor dense breasts compared to timely screens with no risk factors not including breast density information. Furthermore, there was a higher proportion of node positive cancers in delayed screens with no risk factors compared to timely screens with risk factors. Our observational data suggest that if risk-based screening is to be implemented in the future, prolonging the inter-screen interval beyond 2 years for those without any known conventional risk factors for breast cancer (so called ‘low-risk’ women) might not be appropriate. If we are to pursue risk-stratified programmatic screening, we need to find better methods to reliably identify women at lower risk who can have less frequent screening, and to investigate this in prospective trials. Clearly, such trials would include more comprehensive risk assessment beyond conventional risk factors as highlighted in ongoing studies and proposed screening and prevention approaches [[13], [15]]. [[,15] [][13], [15][].
Our work highlights that one population subgroup had a relatively low ICR - women with no risk factors nor dense breasts had lower ICR than those with risk factors and/or dense breasts. This also suggests that screening programs might be enhanced by routine collection and reporting of outcomes by breast density to better understand the burden of interval cancers. This is consistent with previous reports that dense tissue compromises cancer detection on mammography [16].
Although the focus of this study was on women who had no recorded risk factors, our findings also highlighted the high CDR and less favourable cancer characteristics in women with risk factors who had delayed screens. The effect of delaying re-screening may be larger because some of the women with risk factors may have had a supplementary examination between routine screening outside of the public screening program. This shows that it is important for screening programs to support timely re-screening for women with risk factors. A remarkably high ICR was also observed in women with risk factors and dense breasts. BSWA informs women and their primary care physicians of their breast density, with the anticipation that these women may receive follow-up discussions in primary care about the pros and cons of supplementary examinations.
Our study showed that the risks of both screen-detected and interval cancers are diverse between those with risk factors and those without, and the differences are more pronounced in older age groups. This supports the importance of individualised decision-making about breast cancer screening particularly in older women as highlighted in the EUSOMA/SIOG guidelines for management of breast cancer in older women [17]. The balance between benefits and harms of breast screening may vary greatly in older women due to more diverse breast cancer risk and individuals’ health and life expectancy.
The major limitation of this study is that the BSWA did not record some important risk factors such as second-degree family history, established high-risk gene mutations (e.g. BRCA1/2), ancestry associated with these mutations, or radiotherapy to the chest. However, very few women with these strong risk factor would have been included in this study since women with known cancer-predisposing genes are generally managed in specialised clinics because they require alternative screening or risk-reducing strategies. Furthermore, BSWA does not systematically collect information about in-situ cancers in the inter-screen intervals therefore in-situ cancers may be underestimated amongst interval cancers. However, the proportion of in-situ cancers in all interval cancers in this study (4.1%) was comparable to 4.6% reported in a Norwegian study on a population-based breast cancer screening program [18]. We are also cognisant that the study focused on cancer detection and interval cancer rates, and did not assess long-term health outcomes, however our work represents the first step in documenting outcomes in screening participants who do not have a risk factor for breast cancer.
5. Conclusions
A substantial proportion of screen-detected cancers occurred in screening participants who did not have any recorded risk factors for breast cancer – these are the majority participants in Australia's screening program. Although CDR and ICR were relatively low in screens with no risk factors, delayed screens (≥27 months) with no risk factors had similar CDR and a higher proportion of node positive cancers compared to timely screens (<27 months) with at least one risk factor. Our findings may not align with the proposed concept of de-intensify screening in those deemed at lower risk based on conventional risk factors, or at least do not justify less frequent screening in women with none of the risk factors routinely recorded in screening services. The findings from our study may inform ongoing discussion in the BreastScreen program and other breast screening programs world-wide about potentially providing less intensive screening to women who are at or lower than population risk based on conventional risk factors, and may assist planning of future research to generate evidence on effectiveness and cost-effectiveness of this approach.
Conflicts of interest
This work was partly funded by the National Breast Cancer Foundation (NBCF Australia) Chair in Breast Cancer Prevention (grant #EC-21-001), and a National Health and Medical Research Council Investigator (Leader) grant (#1194410), awarded to NH.
Acknowledgement
We would like to thank Dr Sonia El-Zaemey and Ms Kim Kee Ooi with the BreastScreen WA for extracting and cleaning the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.01.015.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Myers E.R., Moorman P., Gierisch J.M., Havrilesky L.J., Grimm L.J., Ghate S., et al. Benefits and harms of breast cancer screening: a systematic review. JAMA. 2015;314(15):1615–1634. doi: 10.1001/jama.2015.13183. [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare . AIHW; Canberra: 2019. BreastScreen Australia data dictionary: version 1.2. [Google Scholar]
- 3.Pashayan N., Morris S., Gilbert F.J., Pharoah P.D.P. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a life-table model. JAMA Oncol. 2018;4(11):1504–1510. doi: 10.1001/jamaoncol.2018.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noguchi N., Marinovich M.L., Wylie E.J., Lund H.G., Houssami N. Screening outcomes by risk factor and age: evidence from BreastScreen WA for discussions of risk-stratified population screening. Med J Aust. 2021;215(8):359–365. doi: 10.5694/mja2.51216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Orsi C., Sickles E., Mendelson E., Morris E. American College of Radiology; Reston, VA: 2013. ACR BI-RADS® Atlas, breast imaging reporting and data system. [Google Scholar]
- 6.Spiegelman D., Hertzmark E. Easy SAS Calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 7.Houssami N., Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Canc. 2017;3(1):12–13. doi: 10.1038/s41523-017-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trimboli R.M., Rossi P.G., Battisti N.M.L., et al. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insight Imag. 2020;11:105. doi: 10.1186/s13244-020-00905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J.M., Ichikawa L.E., Wernli K.J., Bowles E., Specht J.M., Kerlikowske K., et al. Digital mammography and breast tomosynthesis performance in women with a personal history of breast cancer, 2007–2016. Radiology. 2021;300(2):290–300. doi: 10.1148/radiol.2021204581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadatmand S.M.D., Geuzinge H.A.M., Rutgers E.J.T.P., Mann R.M.M.D., de Roy van Zuidewijn D.B.W.M.D., Zonderland H.M.M.D., et al. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol. 2019;20(8):1136–1147. doi: 10.1016/S1470-2045(19)30275-X. [DOI] [PubMed] [Google Scholar]
- 11.Shieh Y., Eklund M., Madlensky L., Sawyer S.D., Thompson C.K., Stover Fiscalini A., et al. Breast cancer screening in the precision medicine era: risk-based screening in a population-based trial. JNCI : J Natl Cancer Inst. 2017;109(5):djw290. doi: 10.1093/jnci/djw290. [DOI] [PubMed] [Google Scholar]
- 12.Rainey L., van der Waal D., Jervaeus A., Wengström Y., Evans D.G., Donnelly L.S., et al. Are we ready for the challenge of implementing risk-based breast cancer screening and primary prevention? Breast. 2018;39:24–32. doi: 10.1016/j.breast.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Esserman L.J. The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Canc. 2017;3(1):34–37. doi: 10.1038/s41523-017-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neal C.H., Rahman W.T., Joe A.I., Noroozian M., Pinsky R.W., Helvie M.A. Harms of restrictive risk-based mammographic breast cancer screening. Am J Roentgenol. 2018;210(1):228–234. doi: 10.2214/AJR.17.18120. [DOI] [PubMed] [Google Scholar]
- 15.Pashayan N., Antoniou A.C., Ivanus U., et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. 2020;17:687–705. doi: 10.1038/s41571-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moshina N., Sebuødegård S., Lee C.I., Akslen L.A., Tsuruda K.M., Elmore J.G., et al. Automated volumetric analysis of mammographic density in a screening setting: worse outcomes for women with dense breasts. Radiology. 2018;288(2):343–352. doi: 10.1148/radiol.2018172972. [DOI] [PubMed] [Google Scholar]
- 17.Biganzoli L., Battisti N.M.L., Wildiers H., et al. Updated recommendations regarding the managemtn of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG) Lancet Oncol. 2021;22(7):e327–e340. doi: 10.1016/S1470-2045(20)30741-5. [DOI] [PubMed] [Google Scholar]
- 18.Hofvind S., Sagstad S., Sebuødegård S., Chen Y., Roman M., Lee C.I. Interval breast cancer rates and histopathologic tumor characteristics after false-positive findings at mammography in a population-based screening program. Radiology. 2017;287(1):162159–162167. doi: 10.1148/radiol.2017162159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.