Abstract
Introduction
Traumatic brain injury is the leading cause of trauma-related death in children. We hypothesized that children with isolated traumatic brain injury would experience differential outcomes when treated at pediatric versus adult or combined trauma centers.
Methods
After institutional review board approval, the 2015 National Trauma Data Bank was queried for children up to age 16 years with isolated traumatic brain injury. Demographics and clinical outcomes were collected. Univariable and multivariable analyses were conducted to assess for predictors of in-hospital mortality and complications. Kaplan–Meier survival analysis was conducted.
Results
A total of 3,766 children with isolated traumatic brain injury were identified; 1,060 (28%) were treated at pediatric trauma centers, 1,909 (51%) at adult trauma centers, and 797 (21%) at combined trauma centers. Subjects were 5 years old (median, interquartile range 1–12 years), 63% male, and 64% white. Higher blood pressure and lower injury severity score were associated with reduced mortality (P < .05). Increasing injury severity score was associated with higher mortality by multivariable logistic regression (odds ratio 1.57, P < .0001). There were no survival differences among hospital types (P = .88).
Conclusion
Outcomes for children with isolated traumatic brain injury appear equal across different types of designated trauma centers. These findings may have implications for prehospital transport and triage guidelines.
Highlights
-
•
Traumatic brain injury (TBI) is the leading cause of trauma-related death in children.
-
•
Children with TBI are treated at pediatric trauma centers (PTCs), adult TCs (ATCs), and combined TCs (CTCs).
-
•
We utilized the ACS NTDB to evaluate outcomes for children with TBI at these centers.
-
•
Ventilator use and intensive care unit utilization were lowest at PTCs.
-
•
The 30-day mortality rates for children with isolated TBI were equal at PTCs, CTCs, and ATCs.
INTRODUCTION
More than 500,000 children sustain a traumatic brain injury (TBI) in the United States each year, resulting in more than 2,000 deaths annually. As such, it is the leading cause of trauma-related death in the pediatric population [1,2]. Most TBIs are mild (Glasgow Coma Scale [GCS] 13–15) and have mild associated symptoms such as headaches and difficulty concentrating [3]. Moderate (GCS 9–12) and severe (GCS 3–8) TBIs tend to be associated with worse functional outcomes and higher mortality [4,5].
Determining risk factors for poor outcomes after trauma has been of interest for health care providers and public health researchers alike. Recent studies have sought to establish whether the type of trauma facility plays a role in outcomes such as mortality, need for transfusions, rates of procedural interventions, and length of stay (LOS). Hospitals may be pediatric trauma centers (PTCs), adult trauma centers (ATCs), or combined trauma centers (CTCs). Some studies have demonstrated better outcomes such as mortality and overall function at PTCs [[6], [7], [8]]. For example, in blunt abdominal solid organ injury, it has been shown that pediatric patients treated at ATCs undergo more operative interventions than those treated at PTCs [9]. Yet other studies have not supported any difference in outcomes based on facility type [[10], [11], [12]].
However, there are limited data demonstrating whether children with TBI have disparate outcomes depending on the type of trauma facility where they are treated. A recent study found no difference in mortality between children at ATCs and ATCs with added qualifications in pediatrics (akin to CTCs) [13]. Although this may be relevant to a large number of hospitals in the country, it does not provide information that may address the possible need for triage to PTCs. Therefore, our aim is to identify whether children with TBI have different outcomes based on type of treating facility (PTC, ATC, or CTC). Owing to the specialized pediatric healthcare provider expertise and pediatric-specific resources available at PTCs, we hypothesized that children with TBI secondary to blunt trauma treated at PTCs would have better outcomes than children treated at ATCs or CTCs.
METHODS
Following institutional review board approval, a retrospective cohort study using the 2015 American College of Surgeons National Trauma Data Bank (NTDB) was performed. The NTDB is a voluntary repository for trauma centers and/or regional trauma registries in the United States. The NTDB is the largest aggregation of US trauma registry data ever assembled, contains more than 6 million cases, and is one of the leading performance improvement tools of trauma care.
The age cutoff was based on the median age cutoff used by centers who enter data into the NTDB (age less than or equal to 16). Children with isolated head injury whose injury occurred in 2014 were included. TBI was defined using the Centers for Disease Control criteria [14] and included the following ICD-9 codes: 800–801.9, 803–804.9, 850–854.1, 950.1–950.3, 995.55, and 959.01. To precisely analyze treatment effects based on treatment center type, any subjects who were transferred in from another facility or transferred out to a higher acuity facility were excluded. Subjects with penetrating mechanisms of injury were also excluded a priori because of differences in recommendations and guidelines for the management in regard to neuroimaging, intracranial pressure monitoring, and surgical management, along with increased lethality seen with penetrating injuries [15].
Hospital types were categorized using American College of Surgeons (ACS) trauma center designation. Any hospital with a designated pediatric-level trauma center but without an adult-level trauma center was categorized as a PTC. Conversely, a hospital with only adult-level ACS designation was categorized as an ATC. A hospital with any combination of pediatric and adult designation was categorized as a CTC. State-level designations were not used because of the variability in criteria for these types of designations.
Demographics included age, sex, race, and physiologic parameters such as systolic blood pressure (SBP), heart rate (HR), injury severity score (ISS), and total Glasgow Coma Scale (GCS). The primary outcome was in-hospital mortality. Secondary outcomes included LOS, ventilator use, and intensive care unit (ICU) use.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Tennessee Health Science Center Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study, in accordance with the United States Code of Federal Regulations Title 45 (part 46, subpart D), was granted waiver of informed consent by the University of Tennessee Health Science Center Institutional Review Board.
Statistics
Descriptive statistics, univariate analyses for the primary and secondary outcomes, and multivariable modeling through the logistic regression approach were performed using SAS 9.3 (Cary, NC). In multivariable modeling, a P value of <.2 was used for entry into the logistic regression model. Nonparametric tests (Wilcoxon rank sum test or Kruskal–Wallis test) were used when appropriate. Missing or unknown values were excluded from statistical analysis. Kaplan–Meier survival analysis was used to compare survival among hospital types.
RESULTS
Demographics
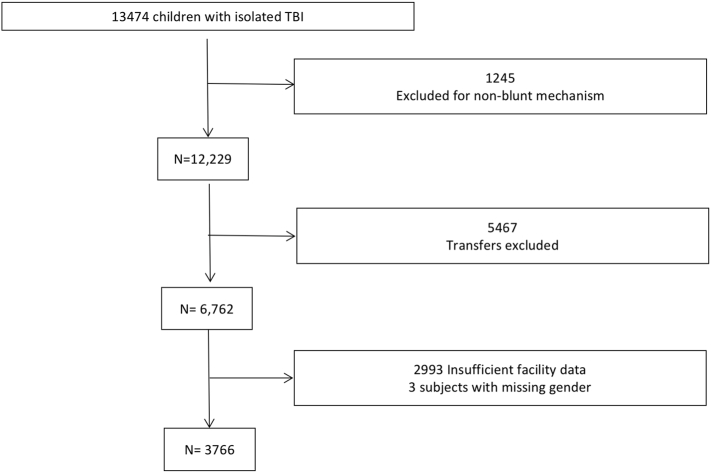
A total of 13,474 children with isolated TBI were identified from the NTDB. After exclusion of 1,245 with non-blunt mechanism of injury, 5,467 transfers, 2,993 with insufficient facility data, and 3 subjects with missing sex, 3,766 subjects remained for analysis (Fig 1). Of these, 1,060 (28%) were treated at PTCs, 1,909 (51%) at ATCs, and 797 (21%) at CTCs. The median age was 5 years (interquartile range [IQR] 1–12); subjects were 63% male, 64% white, and 93% non-Latino. Physiologic parameters included a median SBP of 120 (IQR 109–132) and median HR of 102 (IQR 88–120). The median ISS and GCS of the cohort were 4 (IQR 4–9) and 15 (IQR 12–15), respectively.
Fig 1.
Study population. A total of 13,474 children with isolated TBI were identified from the NTDB. After exclusion of 1,245 with non-blunt mechanism of injury, 5,467 transfers, 2,993 with insufficient facility data, and 3 subjects with missing sex, 3,766 subjects remained for analysis.
Demographics for each facility type are listed in Table 1. A significant difference in age paralleled the types of trauma facilities. There were no differences in the sex ratio of patients; however, more white patients were treated at ATCs (71.7%) versus PTCs (52.5%) and CTCs (62.7%) (P < .0001). Insurance type also differed by facility type; patients treated at PTCs were less likely to have private insurance (45% vs 49%) and more likely to have Medicaid (40% vs 38% and 35%) (P = .003). There were no differences in median SBP (P = .33), HR (P = .6), or ISS (P = .84) among facility types. GCS was found to be different among center types, with a median of 15 at all center types (P = .007). On further inspection of subjects that had available GCS data, subjects with mild TBIs (GCS ≥ 13) comprised 74.2% overall, 81.1% of PTCs, 72% of CTCs, and 73% of ATCs. PTCs had fewer moderate TBIs (GCS 9–12) at 9.0% compared to 11.9% at CTCs and 13.7% at ATCs, and more severe TBIs (GCS 308) at 16.2% compared to 9.8% at PTCs and 13.3% at ATCs (P = .043).
Table 1.
Demographic characteristics of children with isolated TBI
|
Overall (n =3766) |
PTC (n = 1060) |
CTC (n = 797) |
ATC (n = 1909) |
P value | |
|---|---|---|---|---|---|
| Age (median, IQR) | 5 (1–12) | 4 (0–10) | 5 (1–13) | 6 (1–13) | <.0001 |
| Sex (n, %) | |||||
| Female | 1,407 (37) | 392 (37) | 310 (39) | 705 (37) | .6 |
| Male | 2,359 (63) | 668 (63) | 487 (61) | 1,204 (63) | |
| Race (n, %) | |||||
| White | 2,425 (64.4) | 556 (52.5) | 500 (62.7) | 1,369 (71.7) | <.0001 |
| Black | 595 (15.8) | 226 (21.3) | 151 (19) | 218 (11.4) | |
| Other | 746 (19.8) | 278 (26.2) | 146 (62.3) | 322 (16.9) | |
| Ethnicity (n, %) | |||||
| Hispanic/Latino | 151 (4) | 38 (3.6) | 23 (2.9) | 90 (4.7) | .01 |
| Non-Hispanic/Latino | 3,491 (92.7) | 987 (93.1) | 736 (92.4) | 1,768 (92.6) | |
| Unknown | 124 (3.3) | 35 (3.3) | 38 (4.7) | 51 (2.7) | |
| Insurance (n, %) | |||||
| Private/commercial | 1,807 (48%) | 476 (45%) | 390 (49%) | 941 (49%) | .003 |
| Medicaid | 1,398 (37%) | 427 (40%) | 301 (38%) | 670 (35%) | |
| Medicare | 7 (0.2%) | 1 (0.1%) | 0 (0%) | 6 (0.3%) | |
| Self-pay | 172 (4.6%) | 35 (3.3%) | 46 (5.8%) | 91 (4.8%) | |
| Other gov’t insurance | 114 (3.0%) | 49 (4.6%) | 9 (1.1%) | 56 (2.9%) | |
| Other | 99 (2.6%) | 20 (1.9%) | 13 (1.6%) | 66 (3.5%) | |
| Unknown | 169 (4.5%) | 52 (4.9%) | 38 (4.8%) | 79 (4.1%) | |
| SBP (median, IQR), n = 1,269 | 120 (109–132) | 118 (108–130) | 120 (110–133) | 119 (109–132) | .33 |
| HR (median, IQR), n = 1,513 | 102 (88–120) | 104 (89–123) | 102 (88–124) | 102 (88–120) | .6 |
| ISS (median, IQR), n = 3,704 | 4 (4–9) | 4 (1–9) | 4 (2–9) | 4 (4–9) | .84 |
| GCS (median, IQR), n = 1,492 | 15 (12–15) | 15 (13–15) | 15 (12–15) | 15 (12–15) | .007 |
| TBI severity, n = 1,492, (n, %) | |||||
| Mild (GCS 13–15) | 1,107 (74.2) | 305 (81.1) | 249 (72) | 553 (73) | .043 |
| Moderate (GCS 9–12) | 191 (12.8) | 34 (9.0) | 41 (11.9) | 104 (13.7) | |
| Severe (GCS 3–8) | 194 (13.0) | 37 (9.8) | 56 (16.2) | 101 (13.3) | |
| Vent use (n, %) | 209 (5.6) | 41 (3.9) | 65 (8.2) | 103 (5.4) | <.0001 |
| ICU use (n, %) | 1,036 (27.5) | 259 (24.4) | 279 (35.0) | 498 (26.1) | <.0001 |
| LOS (median, IQR) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 1(1–2) | <.0001 |
| Mortality (n, %) | 16 (0.4) | 4 (0.4) | 6 (0.8) | 6 (0.3) | .268 |
| Disposition (n, %) | |||||
| Mortality | 16 (0.4) | 4 (0.4) | 6 (0.8) | 6 (0.3) | <.001 |
| Home | 2,681 (71%) | 906 (86%) | 708 (89%) | 1,067 (56%) | |
| Rehab | 47 (1.3%) | 8 (0.8%) | 24 (3.0%) | 15 (0.8%) | |
| Long-term care facility | 8 (0.2%) | 0 (0%) | 0 (0%) | 8 (0.4%) | |
| Home health | 39 (1.0%) | 0 (0%) | 12 (1.5%) | 27 (1.4%) | |
| Hospice | 1 (0.03%) | 0 (0%) | 0 (0%) | 1 (0.05%) | |
| Other | 7 (0.2%) | 1 (0.1%) | 4 (0.5%) | 2 (0.1%) | |
| Unknown | 967 (26%) | 141 (13%) | 43 (5.4%) | 783 (41%) |
Overall ventilator use was 5.6%, whereas 27.5% of subjects were admitted to the ICU at some point during their hospitalization. Nevertheless, median LOS was 1 day. Ventilator use was significantly more common in CTCs (8.2%) compared to PTCs (3.9%) and ATCs (5.4%) (P < .0001). ICU use was also higher in CTCs (35%) as compared to PTCs (24.4%) and ATCs (26.1%) (P < .0001). The overall mortality rate was 0.4%. There was no difference in mortality rate by center type (P = .27). Most patients were discharged to home (71%). Patients were more likely to be discharged to a long-term care facility (0.4%) or with hospice (0.05%) in ATCs, whereas patients were less likely to go home with home health (0%) or be discharged to rehab (0.75%) in PTCs (P < .001).
TBI Severity
Outcomes were compared by center type and TBI severity (Table 2). For mild TBI, there was no difference in ventilator use, LOS, or mortality between center types. ICU use was higher in CTCs at 33% compared to PTCs (20.7%) and ATCs (25.3%) (P < .0001). For moderate TBI, there was again no difference between center types in ventilator use or mortality. ICU use was significantly higher in ATCs (58.7%) compared to PTCs (34.8%) and CTCs (39%) (P = .01), and LOS was longer at ATCs (P < .043). Lastly, in severe TBI, an opposite trend was seen. ICU use was significantly lower at ATCs (50.5%) compared to PTCs (78.4%) and CTCs (73.2%) (P = .002), whereas LOS was shorter (P = .0001).
Table 2.
Outcomes by TBI severity
| Severity | Variable | Overall | PTC | CTC | ATC | P value |
|---|---|---|---|---|---|---|
| Mild | Vent use (n, %) | 37 (3.3) | 11 (3.6) | 4 (1.6) | 22 (4.0) | .22 |
| ICU use (n, %) | 285 (25.8) | 63 (20.7) | 82 (32.9) | 140 (25.3) | <.0001 | |
| LOS (median, IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | .77 | |
| Mortality (n, %) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – | |
| Moderate | Vent use (n, %) | 26 (13.6) | 4 (8.7) | 6 (14.6) | 16 (15.4) | .53 |
| ICU use (n, %) | 93 (48.7) | 16 (34.8) | 16 (39.0) | 61 (58.7) | .010 | |
| LOS (median, IQR) | 2 (1–3) | 2 (1–2) | 2 (1–2) | 2 (1–4) | .043 | |
| Mortality (n, %) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – | |
| Severe | Vent use (n, %) | 77 (39.7) | 13 (35.1) | 25 (44.6) | 39 (38.6) | .63 |
| ICU use (n, %) | 121 (62.4) | 29 (78.4) | 41 (73.2) | 51 (50.5) | .0016 | |
| LOS (median, IQR) | 2 (1–5) | 3 (2–11) | 3 (2–10) | 1 (1–3) | .00013 | |
| Mortality (n, %) | 8 (4.1) | 2 (5.4) | 0 (0) | 6 (5.9) | .184 |
Outcomes: Univariable Analysis
Hospital type was not found to be associated with the primary outcome of mortality (P = .29). The odds of mortality appeared to be higher in CTCs and lower in ATCs when compared to PTCs, but these were not statistically significant (Table 3). Age, sex, and race were not associated with odds of mortality. A 1-unit increase in ISS was associated with 54% increased odds of mortality (P ≤ .0001). HR and GCS did not have a significant association with the likelihood of mortality, whereas SBP (odds ratio [OR] 1.06, P = .004) was associated with mortality on univariable analysis. However, because of the level of missingness, they could not be entered into the multivariable model.
Table 3.
Univariable analysis
| Variable | Univariable OR (95% CI) | P value |
|---|---|---|
| Hospital type | ||
| CTC versus PTC | 2 (0.56–7.1) | .13 |
| ATC versus PTC | 0.83 (0.23–2.9) | .31 |
| Age | 1.04 (0.96–1.1) | .32 |
| Sex | ||
| Male versus female | 0.77 (0.29–2.1) | .6 |
| Race⁎ | ||
| Black versus white | 1.02 (0.22–4.8) | .57 |
| Other versus white | 2.45 (0.85–7.1) | .12 |
| ISS | 1.54 (1.26–1.88) | <.0001 |
| Systolic BP⁎ | 1.06 (1.02–1.1) | .004 |
| HR⁎ | 0.98 (0.95–1.02) | .48 |
| GCS⁎ | 0.29 (0.08–1.03) | .055 |
Not entered into the multivariable model because of insignificant P value (race) or high level of missing data (SBP, HR, and GCS).
Outcomes: Multivariable Analysis
ISS and hospital type were included in a logistic regression model. The results of the model can be found in Table 4. The area under the curve for the model was 0.98 (Fig 2). Hospital type was not found to be a significant predictor in the final model. A 1-unit increase in ISS was associated with 57% increased odds of mortality (OR 1.57, 95% confidence interval [CI] 1.28–1.93, P ≤ .0001). No significant interactions were noted.
Table 4.
Multivariable logistic regression results
| Variable | Multivariable OR (95% CI) | P value |
|---|---|---|
| Hospital type | ||
| CTC versus PTC | 1.85 (0.47–7.3) | .091 |
| ATC versus PTC | 0.51 (0.13–1.9) | .072 |
| ISS | 1.57 (1.28–1.93) | <.0001 |
Fig 2.
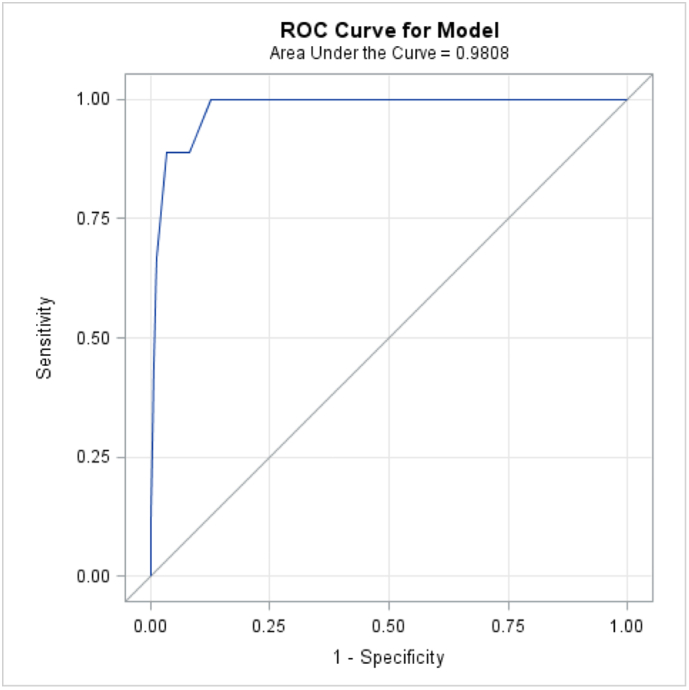
Area under the curve for logistic regression model. ISS and hospital type were included in a logistic regression model, and hospital type was not found to be a significant predictor in the final model (AUC = 0.98).
Outcomes: Survival
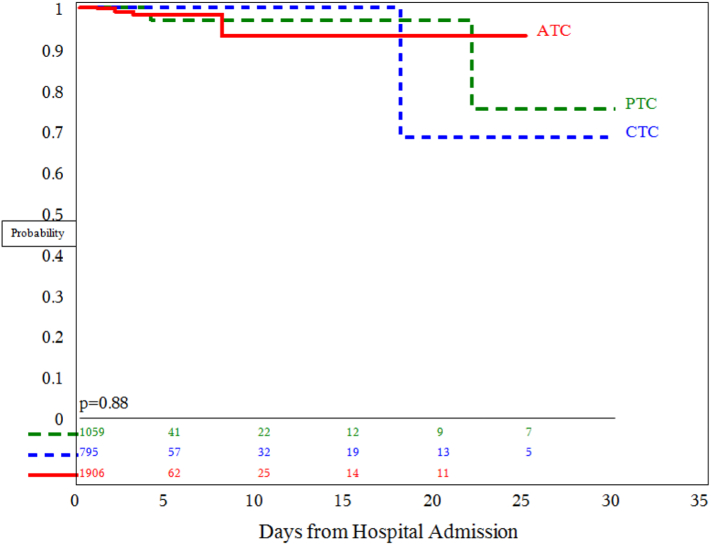
A Kaplan–Meier survival curve was calculated to compare survival probabilities by hospital type. There was no difference in survival among the 3 center types (P = .88, Fig 3).
Fig 3.
Kaplan–Meier survival plot. There was no difference in survival among the 3 center types for children with isolated TBI (P = .88).
DISCUSSION
In this study, one of the largest studies of children with isolated TBI in the United States, we demonstrate that treating hospital type (PTC, CTC, or ATC) is not a predictor of mortality for pediatric TBI patients. We found that children who suffer these injuries tend to be school-aged and male, and they are most commonly treated at ATCs. The racial distribution of children treated at PTCs may reflect the fact that free-standing children's hospitals are largely located in urban areas with heterogeneous populations. Physiologic characteristics were similar across hospital types, suggesting that the populations were similar. ICU use is high across center types, but LOS remained low, although slight differences were seen when stratified by TBI severity. This may be a reflection of initial triage protocols related to injury category rather than a requirement for the management of severe injuries. Ventilator use and ICU days were lowest at PTCs. In the multivariable model, only ISS was identified as a significant predictor of mortality.
Ovalle et al conducted a study of 7,057 pediatric patients with severe TBI (with and without additional injuries) treated at ATCs and ATCs with added qualifications in pediatrics [13]. Their population was limited to those with Abbreviated Injury Scale ≥ 3, which correlates with severe TBI, but, in contrast to our study, did include facilities with state trauma designations. They found that younger age was associated with increased mortality in this population; however, no gender differences were found. Similar to our results, they found no difference in terms of mortality when adjusting for hospital type in a multivariable model. When looking at severe TBIs, no mortality difference was seen between center types.
In contrast, Potoka et al studied 13,351 children from a statewide trauma registry who were treated at PTCs, ATCs with added qualifications (ATCs-AQ), or ATCs alone [16]. Children with severe TBI treated at PTCs had the lowest mortality rate of 6.6% (vs 8.8% in ATCs, P = .044). After controlling for ISS, they demonstrated continued improved survival in PTCs for children with head injuries as well as children with liver and spleen injuries. This was in contrast to our study, in which children had no survival difference across different trauma center types, either overall or in severe TBI.
Although no mortality difference between PTCs and ATCs may be present, there may be other advantages that a PTC can provide. Using the same data source, Potoka and colleagues leveraged their robust state-level registry to evaluate functional outcomes in children with TBI [17]. They compared scores for feeding, locomotion, transfer mobility, social interaction, and expression among children aged 2–16 years. In children with TBI as the primary injury, functional outcomes in all 5 categories were significantly improved at PTCs compared to ATCs-AQ. When stratified by GCS, children with mild or moderate injuries showed no difference in functional outcomes by hospital type. However, children with severe head injury were found to have a significantly lower rate of dependency in the social interaction and expression categories when treated at PTCs versus ATCs-AQ. They suggest that this difference may be due to the specialized care provided by pediatric neurosurgeons. This study, however, was unable to look at functional outcomes because of limitations of the database.
We were able to look at overall patient disposition though and found that patients at ATCs were more likely to be discharged to a long-term care facility, whereas patients at PTCs more frequently were discharged home. Another study that also looked at the NTDB found that children with TBIs were more likely to be discharged home at a PTC [18]. This is similar to our findings. A study by Yanchar et al looked at isolated TBI outcomes by severity comparing PTCs and ATCs. They found that PTCs were far more likely to ensure neurocognitive follow-up at time of discharge for mild, moderate, and severe TBIs [19]. These differences may hint at the benefits a PTC may provide over an ATC when dealing with pediatric TBI.
Our study has several limitations. The NTDB is a convenience sample, with voluntary reporting, and thus, the quality of data collection may be variable among contributing centers. There are often missing data in database studies, and this affected our ability to use certain variables (eg, GCS, SBP) in the multivariable model. Missing data were substantial, and many patients had to be eliminated from the study altogether because of the extent of missingness, which limited our sample size. Rural or small centers may not submit to the NTDB, and hospitals may have different criteria for inclusion into the database; these conditions may lead to selection bias. Our decision to exclude transfer patients may skew our results; however, we found no difference in mortality outcomes in univariate analysis for transfer patients in our initial exploratory analyses. Furthermore, we excluded penetrating trauma in an effort to avoid confounding, and our results therefore are only applicable for blunt trauma. Finally, the NTDB does not contain data regarding long-term or functional outcomes. Disposition was examined as a surrogate for morbidity and long-term outcomes, but given the overall low mortality rate of children who sustain traumatic injuries, the analysis of long-term outcomes may provide better insight into clinically relevant outcome differences among trauma center types.
In conclusion, despite differences in resource utilization, survival outcomes for children with isolated traumatic brain injury appear equal across different types of trauma centers. This is reassuring, as most injured children in the United States are treated at non-PTCs. These data suggest that protocols to guide critical care of children with TBI should be developed and disseminated broadly to encompass all center types that care for injured children. Finally, long-term functional outcomes should be collected at the national level to develop an understanding of the true morbidity and mortality associated with childhood TBI and to help guide the appropriate triage and treatment of children with these injuries.
Author Contributions
Study conception and design: LVV, AG
Acquisition of data: LVV
Analysis and interpretation of data: LVV, RAL, MK, SN, AG
Drafting of manuscript: LVV, RAL, AG
All authors approved the final manuscript.
Conflicts of Interest
None
Funding Source
None
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Tennessee Health Science Center Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study, in accordance with the United States Code of Federal Regulations Title 45 (part 46, subpart D), was granted waiver of informed consent by the University of Tennessee Health Science Center Institutional Review Board.
References
- 1.Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., et al. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2011;60(5):1–32. [PubMed] [Google Scholar]
- 2.Keenan H.T., Bratton S.L. Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci. 2006;28(4–5):256–263. doi: 10.1159/000094152. [DOI] [PubMed] [Google Scholar]
- 3.Caskey R.C., Nance M.L. Management of pediatric mild traumatic brain injury. Adv Pediatr Infect Dis. 2014;61(1):271–286. doi: 10.1016/j.yapd.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Cicero M.X., Cross K.P. Predictive value of initial Glasgow Coma Scale score in pediatric trauma patients. Pediatr Emerg Care. 2013;29(1):43. doi: 10.1097/PEC.0b013e31827b52bf. [DOI] [PubMed] [Google Scholar]
- 5.Nesiama J.-A.O., Pirallo R.G., Lerner E.B., Hennes H. Does a prehospital Glasgow Coma Scale score predict pediatric outcomes? Pediatr Emerg Care. 2012;28(10):1027–1032. doi: 10.1097/PEC.0b013e31826cac31. [DOI] [PubMed] [Google Scholar]
- 6.Sathya C., Alali A.S., Wales P.W., Scales D.C., Karanicolas P.J., Burd R.S., et al. Mortality among injured children treated at different trauma center types. JAMA Surg. 2015;150(9):874–881. doi: 10.1001/jamasurg.2015.1121. [DOI] [PubMed] [Google Scholar]
- 7.Webman R.B., Carter E.A., Mittal S., Wang J., Sathya C., Nathens A.B., et al. Association between trauma center type and mortality among injured adolescent patients. JAMA Pediatr. 2016;170(8):780–786. doi: 10.1001/jamapediatrics.2016.0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrosyan M., Guner Y.S., Emami C.N., Ford H.R. Disparities in the delivery of pediatric trauma care. J Trauma. 2009;67(2 Suppl):S114–S119. doi: 10.1097/TA.0b013e3181ad3251. [DOI] [PubMed] [Google Scholar]
- 9.Safavi A., Skarsgard E.D., Rhee P., Zangbar B., Kulvatunyou N., Tang A., et al. Trauma center variation in the management of pediatric patients with blunt abdominal solid organ injury: a national trauma data bank analysis. J Pediatr Surg. 2016;51(3):499–502. doi: 10.1016/j.jpedsurg.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Osler T.M., Vane D.W., Tepas J.J., Rogers F.B., Shackford S.R., Badger G.J. Do pediatric trauma centers have better survival rates than adult trauma centers? An examination of the National Pediatric Trauma Registry. J Trauma. 2001;50(1):96–101. doi: 10.1097/00005373-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Miyata S., Cho J., Lebedevskiy O., Matsushima K., Bae E., Bliss D.W. Trauma experts versus pediatric experts: comparison of outcomes in pediatric penetrating injuries. J Surg Res. 2017;208:173–179. doi: 10.1016/j.jss.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 12.Rogers A.T., Gross B.W., Cook A.D., Rinehart C.D., Lynch C.A., Bradburn E.H., et al. Outcome differences in adolescent blunt severe polytrauma patients managed at pediatric versus adult trauma centers. J Trauma Acute Care Surg. 2017;83(6):1082–1087. doi: 10.1097/TA.0000000000001642. [DOI] [PubMed] [Google Scholar]
- 13.Ovalle F., Xu L., Pearson W.S., Spelke B., Sugerman D.E. Outcomes of pediatric severe traumatic brain injury patients treated in adult trauma centers with and without added qualifications in pediatrics—United States, 2009. Inj Epidemiol. 2014;1(1):15. doi: 10.1186/2197-1714-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faul M, Xu L, Wald M, Coronado VG. Traumatic brain injury in the United States. :74.
- 15.Mikhael M., Frost E., Cristancho M. Perioperative care for pediatric patients with penetrating brain injury: a review. J Neurosurg Anesthesiol. 2018;30(4):290–298. doi: 10.1097/ANA.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 16.Potoka D.A., Schall L.C., Gardner M.J., Stafford P.W., Peitzman A.B., Ford H.R. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49(2):237–245. doi: 10.1097/00005373-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Potoka D.A., Schall L.C., Ford H.R. Improved functional outcome for severely injured children treated at pediatric trauma centers. J Trauma. 2001;51(5):824–832. doi: 10.1097/00005373-200111000-00002. (discussion 832-834) [DOI] [PubMed] [Google Scholar]
- 18.Khalil M., Alawwa G., Pinto F., O’Neill P.A. Pediatric mortality at pediatric versus adult trauma centers. J Emerg Trauma Shock. 2021;14(3):128–135. doi: 10.4103/JETS.JETS_11_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanchar N.L., Lockyer L., Ball C.G., Assen S. Pediatric versus adult paradigms for management of adolescent injuries within a regional trauma system. J Pediatr Surg. 2021;56(3):512–519. doi: 10.1016/j.jpedsurg.2020.07.032. [DOI] [PubMed] [Google Scholar]