Abstract
Objective
The results of previous research into exercise interventions for children with cerebral palsy are inconsistent. The aim of this study is to assess the effectiveness of such exercise interventions.
Design
Systematic review and meta-analysis.
Methods
Systematic searches of the PubMed, Embase and Cochrane Library databases for randomized controlled trials involving exercise interventions for children with cerebral palsy, from inception to January 2020, were performed. Pooled weighted mean differences (WMDs) with 95% confidence intervals (95% CI) for gross motor function, gait speed, and muscle strength were calculated using random-effects models.
Results
A final total of 27 trials, including 834 children with cerebral palsy, were selected for quantitative analysis. Exercise interventions had no significant effect on the level of gross motor function (WMD 1.19; 95% CI −1.07 to 3.46; p = 0.302). However, exercise interventions were associated with higher levels of gait speed (WMD 0.05; 95% CI 0.00–0.10; p = 0.032) and muscle strength (WMD 0.92; 95% CI 0.19–1.64; p = 0.013).
Conclusion
These results suggest that exercise interventions may have beneficial effects on gait speed and muscle strength, but no significant effect on gross motor function in children with cerebral palsy.
LAY ABSTRACT
Cerebral palsy is the most common cause of physical impairment in children. This study evaluated the effectiveness of exercise interventions for children with cerebral palsy. Exercise interventions were significantly associated with increased gait speed and muscle strength, while gross motor function was not affected. Exercise interventions should therefore be used for children with cerebral palsy.
Key words: cerebral palsy, child, exercise, meta-analysis, systematic review
Cerebral palsy is the most common cause of physical impairment in children and is characterized by gait abnormalities (1–3). The characteristics of cerebral palsy are associated with damage to the immature brain, which causes subsequent primary impairments, including decreased muscle tone, loss of selective motor control, and impaired balance. Secondary impairments include muscle shortening or weakness and decreased range of motion (4, 5). The prevalence of cerebral palsy is approximately 2.1 in every 1,000 births, and children account for 74% of cases worldwide (6, 7). Children with cerebral palsy are significantly affected by epilepsy and by disorders in motor function, sensation, perception, communication, and behaviour, which significantly affect quality of life and result in huge economic and psychological burdens (8–11).
Currently, the primary therapeutic goals for cerebral palsy are aimed at improving mobility and upper limb function (12). Exercise interventions may also play an important role in improving muscle strength, endurance, and cardiorespiratory fitness. Several systematic reviews and meta-analyses have illustrated the potential role of exercise interventions for children with cerebral palsy; however, results regarding gross motor function, gait speed, and muscle strength are inconsistent (13–15). Exercise programmes usually include resistance and/ or aerobic training. Children with cerebral palsy have reduced muscle strength, and resistance exercise can maintain or increase muscle performance (16, 17), while aerobic training can improve cardiorespiratory fitness. Studies have found that muscle stretching can increase range of motion (18, 19). It is important to clarify the effectiveness of exercise interventions for treatment of cerebral palsy in children, and to determine the role of the type of training for children with cerebral palsy. A meta-analysis of randomized controlled trials (RCTs) of exercise interventions for children with cerebral palsy was therefore performed in order to assess the effectiveness of this treatment.
MATERIALS AND METHODS
Data sources, search strategy, and selection criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was applied to guide this metaanalysis (see checklist, Table SI1) (20). The study was designed as a meta-analysis of RCTs, with the aim of determining the effectiveness of exercise interventions for children with cerebral palsy. No restrictions were applied regarding published language and status of RCTs. The electronic databases of PubMed, EmBase, and Cochrane Library databases were systematically searched from their inception to January 2020. The core search terms were “cerebral palsy” AND “exercise” AND “randomized controlled trial”. Details of the search strategy for each database are shown in Appendix S11. The reference lists of relevant reviews or original articles were also searched manually to select any new eligible studies.
The literature search and study selection was conducted following a standardized flow, comprising 3 steps: (i) an initial literature screening, through reviewing title and abstracts, was conducted separately by 2 of the authors of this paper (ZT and GY); (ii) inconsistencies between author findings were checked and discussed; (iii) the full text of retrieved studies were independently reviewed by 2 authors (XL and JC), and inconsistency between authors was discussed to reach a consensus. The inclusion criteria for this metaanalysis was based on PICOS criteria: (i) Patients: children (< 18.0 years of age) with cerebral palsy, and diagnosed criteria of cerebral palsy was based on individual trial; (ii) Intervention: exercise intervention with no restrictions placed on exercise programme; (iii) Control: usual care, including background treatment and exercise strategies, which was also given in the intervention group; (iv) Outcomes: gross motor function, gait speed, and muscle strength; and (v) Study design: RCTs only. Studies designed as observational studies were excluded owing to various confounding factors that could overestimate the treatment effectiveness.
Data collection and quality assessment
Two authors (XL and JW) independently extracted the data from the included studies, and any disagreement was settled by group discussion. The extracted information included first authors’ surname, publication year, country, sample size, mean age of patients, percentage of male patients, disease status, measurement tool, intervention, control, follow-up duration, and reported outcomes. The Eastern countries was defined as East and Central Asia, and the Western countries including Europe, Australia, America, and South Africa. Study quality was assessed with the Jadad scale, which is based on randomization, concealment of the treatment allocation, blinding, completeness of follow-up, and use of intention-to-treat analysis (21). The Jadad scale ranges from 0 to 5, and studies scoring 4 or 5 were regarded as high quality.
Statistical analysis
The investigated outcomes were assigned as continuous data, and the weighted mean differences (WMDs) with 95% confidence intervals (95% CIs) was calculated based on mean, standard deviation (SD), and sample size for each individual trial. Then, the pooled WMDs and 95% CIs for gross motor function, gait speed, and muscle strength were calculated using the randomeffects model (22, 23). I2 and p-value for Q statistics were applied to assess the heterogeneity across included trials, and I2 > 50.0% or p < 0.10 was considered as significant heterogeneity (24, 25). Sensitivity analyses for gross motor function, gait speed, and muscle strength were conducted by excluding trials one by one, and then performing a pooled analysis of the remaining studies using the random-effects model (26). Subgroup analyses for gross motor function, gait speed, and muscle strength were conducted on the basis of country, mean age, proportion of male subjects, exercise type, follow-up, and study quality. The difference between subgroups was then assessed by interaction p-test (26). Publication biases were assessed by both qualitative (funnel plot) and quantitative (Egger and Begg tests) methods (27, 28). The inspection level for pooled outcomes are 2-sided, and p < 0.05 was regarded as statistically significant. STATA software (version 10.0; Stata Corporation, College Station, TX, USA) was used to conduct all statistical analyses.
RESULTS
Literature search
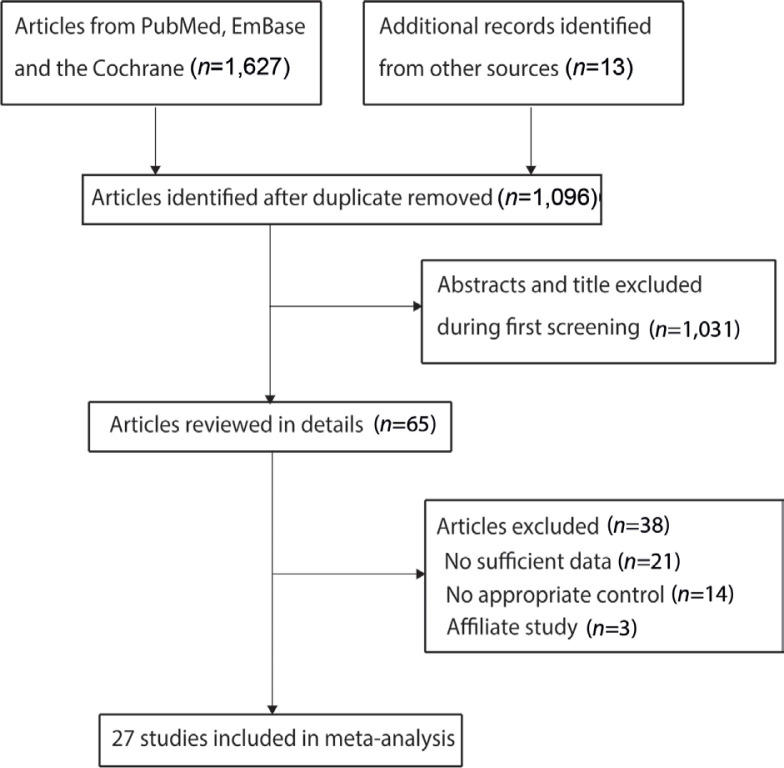
A total of 1,627 articles were identified from electronic searches, and 531 were excluded owing to duplicate topics. A total of 1,031 articles were excluded due to irrelevancy. A total of 65 studies were retrieved for further full-text evaluations, and 38 studies were excluded due to either insufficient data (n = 21), no appropriate control (n = 14), or affiliate study (n = 3). No new relevant reviews or original articles were found through manual searches of the reference lists. As a result, a final total of 27 RCTs met the inclusion criteria and were selected for the meta-analysis (29–55). Details of the literature search and study selection are shown in Fig. 1.
Fig. 1.
Flow diagram of study selection process.
Study characteristics
The baseline characteristics of the included studies are summarized in Table I. A total of 834 children with cerebral palsy were included from 27 separate trials. The included studies were all published between 2003 and 2019, and between 12 and 101 children were included in each individual trial. The mean age of included children ranged from 1.8 to 16.0 years, and the follow-up duration ranged from 1 to 12 months. Twenty-one studies were conducted in Western countries, while the remaining 6 studies were conducted in Eastern countries. Five trials scored 4 on the Jadad scale, 7 trials scored 3, 6 trials scored 2, and the remaining 9 trials scored 1 (Table II).
Table I.
Characteristics of studies included in the meta-analysis
| Study | Country | Sample size, n | Mean age, years | Males, % | Disease status | Measurement tool | Intervention | Control | Outcomes | Follow-up, months |
|---|---|---|---|---|---|---|---|---|---|---|
| Dodd et al. 2003 (29) | Australia | 21 | 13.0 | 47.6 | GMFCS levels I-III | ICF and GMFM | Resistance training | Normal activities | Gross motor function: 69.0 vs 75.3; gait speed: 0.8 m/s vs 0.84 m/s; muscle strength: 33.1 vs 25.5 | 6.0 |
| Engsberg et al. 2006 (30) | USA | 12 | 9.9 | 25.0 | GMFCS levels I-III | GMFM | Resistance training | No strength training | Gross motor function: 69.0 vs 71.4; gait speed: 0.91 m/s vs 0.79 m/s | 3.0 |
| Unger et al. 2006 (31) | South Africa | 37 | 16.0 | 61.3 | GMFCS levels I-III | Three-dimensional gait analysis | Resistance training | Normal school and therapy programme | Gait speed: 1.119 m/s vs 1.17 m/s | 2.0 |
| Liao et al. 2007 (32) | China | 20 | 7.4 | 60.0 | GMFCS levels I, II | GMFM | Resistance training | Regular physiotherapy programme | Gross motor function: 82.7 vs 80.6; gait speed: 1.012 m/s vs 0.98 m/s; muscle strength: 6.1 vs 6.2 | 1.5 |
| Seniorou et al. 2007 (33) | UK | 20 | 12.5 | 50.0 | GMFCS levels I-III | GMFM | Resistance training | Identical programme performed with no weights | Gross motor function: 55.6 vs 60.8; gait speed: 0.3 m/s vs 0.3 m/s; muscle strength: 1.3 vs 1.2 | 6.0 |
| Unnithan et al. 2007 (34) | Greece | 13 | 15.8 | 30.8 | GMFCS levels I-III | GMFM | Mixed training | Normal physical therapy | Gross motor function: 33.85 vs 30.76 | 3.0 |
| Verschuren et al. 2007 (35) | The Netherlands | 68 | 12.2 | 64.7 | GMFCS levels I, II | GMFM | Mixed training | Usual care | Gross motor function: 87.24 vs 90.11; muscle strength: 37.44 vs 38.48 | 12.0 |
| Lee et al. 2008 (36) | Korea | 17 | 6.3 | 58.8 | GMFCS levels II, III | GMFM | Resistance training | Conventional physiotherapy | Gross motor function: 62.7 vs 61.4; gait speed: 0.746 m/s vs 0.68 m/s; muscle strength: 13.2 vs 14.1 | 2.6 |
| Fowler et al. 2010 (37) | USA | 62 | 11.4 | 46.8 | GMFCS levels I-III | GMFM | Aerobic training | No cycling | Gross motor function: 70.8 vs 69.3; gait speed: 1.133 m/s vs 1.04 m/s; muscle strength: 0.89 kg vs 0.86 kg | 3.0 |
| Reid et al. 2010 (38) | Australia | 14 | 11.0 | 42.9 | GMFCS levels I-III | Biodex dynamometer | Resistance training | Normal activity | Muscle strength: 184.71 vs 211.81 | 1.5 |
| Scholtes et al. 2010 (39) | The Netherlands | 51 | 10.4 | 56.9 | GMFCS levels I-III | GMFM | Resistance training | Conventional physiotherapy programme | Gross motor function: 76.1 vs 73.1; gait speed: 1.03 m/s vs 1.07 m/s; muscle strength: 5.39 vs 4.48 | 4.0 |
| Gharib et al. 2011 (40) | Egypt | 30 | 11.6 | 53.3 | GMFCS level II | The Biodex Gait Trainer 2TM | Aerobic training | Identical programme performed with physical therapy exercise | Gait speed: 0.67 m/s vs 0.63 m/s | 3.0 |
| Johnston et al. 2011 (41) | USA | 34 | 9.5 | 53.8 | GMFCS levels II-IV | GMFM | Aerobic training | Strengthening exercise | Gross motor function: 63.3 vs 60.1; gait speed: 0.62 m/s vs 0.50 m/s; muscle strength: 3.58 vs 3.80 | 4.0 |
| Smania et al. 2011 (42) | Italy | 18 | 13.3 | 55.6 | GMFCS levels I-IV | WeeFIM | Aerobic training | Usual physiotherapy | Gait speed: 0.97 m/s vs 0.82 m/s | 1.5 |
| Olama et al. 2011 (43) | Egypt | 30 | 13.7 | 60.0 | NA | Bruininks- Oseretsity test | Aerobic training | Both groups received an exercise programme | Gross motor function: 44.09 vs 46.69; muscle strength: 29.50 vs 30.15 | 6.0 |
| Pandey et al. 2011 (44) | India | 18 | NA | 61.1 | NA | Lateral step up test | Resistance training | None were allowed to attend physiotherapy | Gait speed: 0.70 m/s vs 0.60 m/s; muscle strength: 6.3 vs 2.67 | 1.0 |
| Chrysagis et al. 2012 (45) | Greece | 22 | 16.0 | 59.1 | GMFCS levels I-III | GMFM | Aerobic training | Conventional physiotherapy | Gross motor function: 71.67 vs 65.13; gait speed: 0.997 m/s vs 0.78 m/s | 3.0 |
| Bryant et al. 2013 (46) | UK | 35 | 13.8 | 40.0 | GMFCS levels IV and V | GMFM | Aerobic exercise | Usual physiotherapy | Gross motor function: 1.87 vs 0.20 | 4.0 |
| Chen et al. 2013 (47) | China | 30 | 8.6 | 66.7 | GMFCS levels I-II | GMFM | Aerobic training | General physical activity at home | Gross motor function: 84.2 vs 81.0; muscle strength: 1.63 kg vs 1.35 kg | 3.0 |
| Mattern- Baxter et al. 2013 (48) | USA | 12 | 1.8 | 66.7 | GMFCS levels I-II | GMFM | Aerobic training | Weekly scheduled physiotherapy sessions | Gross motor function: 16.9 vs 13.89; gait speed: 0.699 m/s vs 2.40 m/s | 4.0 |
| Lee et al. 2015 (49) | Korea | 26 | 6.5 | 50.0 | GMFCS levels I-III | GMFM | Resistance training | General neurodevelopmental treatment | Gross motor function: 81.9 vs 81.3 | 1.5 |
| Mitchell et al. 2016 (50) | Australia | 101 | 11.8 | 51.5 | GMFCS levels I-II | 6MWT | Mixed training | Usual care | Muscle strength: 63.5 vs 46.8 | 5.0 |
| Cleary et al. 2017 (51) | Australia | 19 | 13.8 | 52.6 | GMFCS levels I-III | 6MWT | Aerobic training | Social/art activities | Muscle strength: 52.2 vs 24.7 | 5.0 |
| Peungsuwan et al. 2017 (52) | Thailand | 15 | 13.3 | 53.3 | GMFCS levels I-III | 6MWT | Resistance training | Usual care | Gait speed: 1.11 m/s vs 0.99 m/s; muscle strength: 11.13 vs 8.43 | 2.0 |
| Gibson et al. 2018 (53) | Australia | 42 | 12.5 | 64.3 | GMFCS levels I-III | GAS | Aerobic training | Usual care | Muscle strength: 25.6 vs 16.5 | 3.0 |
| Fosdahl et al. 2019 (54) | Norway | 37 | 10.2 | 56.8 | GMFCS levels I-II | 6MWT | Resistance training | Usual care | Gait speed: 1.04 m/s vs 1.03 m/s | 8.0 |
| Kara et al. 2019 (55) | Turkey | 30 | 11.5 | 46.7 | GMFCS levels I-III | GMFM | Resistance training | Usual care | Gross motor function: 97.22 vs 95.83; muscle strength: 4.94 vs 5.82 | 3.0 |
6MWT: Six-Minute Walk Test; GAS: Goal Attainment Scaling; GMFCS: Gross Motor Function Classification System; GMFM: Gross Motor Function Measure; ICF: International Classification of Functioning, Disability and Health; NA: not available; WeeFIM: Functional Independence Measure for Children.
Table II.
Quality assessment of included studies
| Study | Randomization | Blindness | Concealment of treatment allocation | Completeness of follow-up | ITT analysis | Total score |
|---|---|---|---|---|---|---|
| Dodd et al. 2003 (29) | 1 | 1 | 0 | 1 | 1 | 4 |
| Engsberg et al. 2006 (30) | 1 | 0 | 0 | 0 | 0 | 1 |
| Unger et al. 2006 (31) | 1 | 0 | 0 | 0 | 0 | 1 |
| Liao et al. 2007 (32) | 0 | 1 | 0 | 0 | 0 | 1 |
| Seniorou et al. 2007 (33) | 1 | 0 | 0 | 1 | 0 | 2 |
| Unnithan et al. 2007 (34) | 0 | 0 | 0 | 1 | 1 | 2 |
| Verschuren et al. 2007 (35) | 0 | 1 | 1 | 1 | 1 | 4 |
| Lee et al. 2008 (36) | 1 | 0 | 0 | 0 | 0 | 1 |
| Fowler et al. 2010 (37) | 1 | 0 | 0 | 1 | 1 | 3 |
| Reid et al. 2010 (38) | 0 | 0 | 0 | 1 | 1 | 2 |
| Scholtes et al. 2010 (39) | 1 | 0 | 0 | 1 | 1 | 3 |
| Gharib et al. 2011 (40) | 0 | 1 | 0 | 1 | 1 | 3 |
| Johnston et al. 2011 (41) | 1 | 0 | 0 | 0 | 0 | 1 |
| Smania et al. 2011 (42) | 1 | 0 | 0 | 1 | 1 | 3 |
| Olama et al. 2011 (43) | 1 | 0 | 0 | 0 | 0 | 1 |
| Pandey et al. 2011 (44) | 1 | 0 | 0 | 0 | 0 | 1 |
| Chrysagis et al. 2012 (45) | 1 | 1 | 0 | 1 | 0 | 3 |
| Bryant et al. 2013 (46) | 0 | 0 | 0 | 1 | 1 | 2 |
| Chen et al. 2013 (47) | 1 | 0 | 0 | 0 | 0 | 1 |
| Mattern-Baxter et al. 2013 (48) 0 | 0 | 0 | 0 | 1 | 1 | |
| Lee et al. 2015 (49) | 0 | 0 | 0 | 1 | 1 | 2 |
| Mitchell et al. 2016 (50) | 1 | 0 | 1 | 1 | 1 | 4 |
| Cleary et al. 2017 (51) | 1 | 0 | 0 | 1 | 1 | 3 |
| Peungsuwan et al. 2017 (52) | 1 | 0 | 0 | 1 | 0 | 2 |
| Gibson et al. 2018 (53) | 1 | 1 | 0 | 1 | 1 | 4 |
| Fosdahl et al. 2019 (54) | 1 | 1 | 0 | 1 | 1 | 4 |
| Kara et al. 2019 (55) | 1 | 1 | 0 | 1 | 0 | 3 |
1: low risk; 0: high risk; ITT: intention-to-treat
Gross motor function
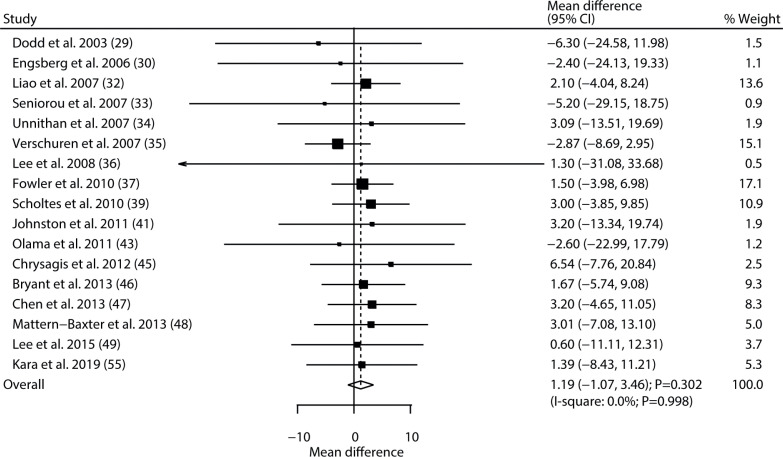
Data regarding the effect of exercise intervention on gross motor function were available in 17 of the selected trials. There was no significant difference between exercise and control for the level of gross motor function (WMD 1.19; 95% CI −1.07 to 3.46; p = 0.302; Fig. 2), and no evidence of heterogeneity was detected (I2= 0.0%; p = 0.998). The conclusion was robust and not altered by sequential exclusion of individual trials (Table III, Appendix S21). The results of subgroup analyses were consistent with the overall analysis in all subsets (Table IV). No significant publication bias for gross motor function was detected (p-value for Egger 0.738; p-value for Begg 0.174; Appendix S31).
Fig. 2.
Effect of exercise intervention on gross motor function in children with cerebral palsy. 95% CI: 95% confidence interval.
Table III.
Effect of exercise intervention on gross motor function, gait speed, and muscle strength when a study is omitted
| Study omitted | Gross motor function, WMD (95% CI) | Gait speed, WMD (95% CI) | Muscle strength, WMD (95% CI) |
|---|---|---|---|
| Dodd et al. 2003 (29) | 1.31 (–0.97 to 3.59) | 0.06 (0.01 to 0.11) | 0.89 (0.16 to 1.61) |
| Engsberg et al. 2006 (30) | 1.23 (–1.05 to 3.51) | 0.05 (0.00 to 0.10) | – |
| Unger et al. 2006 (31) | – | 0.06 (0.01 to 0.11) | – |
| Liao et al. 2007 (32) | 1.05 (–1.39 to 3.49) | 0.05 (0.00 to 0.11) | 1.04 (0.26 to 1.83) |
| Seniorou et al. 2007 (33) | 1.25 (–1.03 to 3.53) | 0.06 (0.01 to 0.12) | 1.07 (0.18 to 1.97) |
| Unnithan et al. 2007 (34) | 1.16 (–1.13 to 3.44) | – | – |
| Verschuren et al. 2007 (35) | 1.92 (–0.54 to 4.38) | – | 0.93 (0.20 to 1.66) |
| Lee et al. 2008 (36) | 1.19 (–1.08 to 3.46) | 0.05 (0.00 to 0.11) | 0.95 (0.21 to 1.68) |
| Fowler et al. 2010 (37) | 1.13 (–1.36 to 3.62) | 0.05 (–0.00 to 0.10) | 1.10 (0.16 to 2.05) |
| Reid et al. 2010 (38) | – | – | 0.92 (0.19 to 1.65) |
| Scholtes et al. 2010 (39) | 0.97 (–1.43 to 3.37) | 0.06 (0.01 to 0.11) | 0.93 (0.12 to 1.74) |
| Gharib et al. 2011 (40) | – | 0.06 (–0.00 to 0.12) | – |
| Johnston et al. 2011 (41) | 1.15 (–1.13 to 3.44) | 0.05 (0.00 to 0.10) | 0.97 (0.23 to 1.72) |
| Smania et al. 2011 (42) | – | 0.05 (–0.00 to 0.10) | – |
| Olama et al. 2011 (43) | 1.24 (–1.04 to 3.52) | – | 0.99 (0.25 to 1.74) |
| Pandey et al. 2011 (44) | – | 0.05 (–0.01 to 0.10) | 0.37 ( –0.06 to 0.80) |
| Chrysagis et al. 2012 (45) | 1.05 (–1.24 to 3.35) | 0.04 (–0.00 to 0.08) | – |
| Bryant et al. 2013 (46) | 1.14 (–1.24 to 3.52) | – | – |
| Chen et al. 2013 (47) | 1.01 (–1.36 to 3.38) | – | 1.04 (0.17 to 1.90) |
| Mattern-Baxter et al. 2013 (48) | 1.10 (–1.23 to 3.42) | 0.05 (0.02 to 0.09) | – |
| Lee et al. 2015 (49) | 1.22 (–1.09 to 3.52) | – | – |
| Mitchell et al. 2016 (50) | – | – | 0.82 (0.13 to 1.51) |
| Cleary et al. 2017 (51) | – | – | 0.91 (0.19 to 1.64) |
| Peungsuwan et al. 2017 (52) | – | 0.05 (–0.00 to 0.10) | 0.79 (0.06 to 1.53) |
| Gibson et al. 2018 (53) | – | – | 0.91 (0.19 to 1.64) |
| Fosdahl et al. 2019 (54) | – | 0.06 (0.00 to 0.11) | – |
| Kara et al. 2019 (55) | 1.18 (–1.15 to 3.51) | – | 0.89 (0.14 to 1.64) |
95% CI: 95% confidence interval; WMD: weighted mean difference.
Table IV.
Subgroup analyses for investigated outcomes
| Outcomes | Factors | Groups | WMD and 95% CI | p-value | Heterogeneity, % | p-value for heterogeneity | p-value between subgroups |
|---|---|---|---|---|---|---|---|
| Gross motor function | Country | Eastern | 2.22 (-2.21 to 6.65) | 0.326 | 0.0 | 0.987 | 0.596 |
| Western | 0.83 (-1.81 to 3.46) | 0.538 | 0.0 | 0.983 | |||
| Mean age, years | > 12.0 | -0.71 (-4.69 to 3.26) | 0.725 | 0.0 | 0.852 | 0.252 | |
| < 12.0 | 2.11 (-0.65 to 4.87) | 0.133 | 0.0 | 1.000 | |||
| Percentage male, % | > 50.0 | 1.21 (-1.63 to 4.05) | 0.403 | 0.0 | 0.963 | 0.984 | |
| < 50.0 | 1.16 (-2.60 to 4.93) | 0.545 | 0.0 | 0.975 | |||
| Exercise type | Resistance | 1.46 (-2.24 to 5.15) | 0.440 | 0.0 | 0.987 | 0.390 | |
| Aerobic | 2.25 (-1.11 to 5.61) | 0.189 | 0.0 | 0.993 | |||
| Mixed | -2.22 (-7.71 to 3.28) | 0.429 | 0.0 | 0.507 | |||
| Follow-up, months | > 6.0 | -3.24 (-8.47 to 1.98) | 0.224 | 0.0 | 0.985 | 0.065 | |
| < 6.0 | 2.22 (-0.29 to 4.73) | 0.084 | 0.0 | 1.000 | |||
| Study quality | High | -3.19 (-8.73 to 2.36) | 0.260 | 0.0 | 0.726 | 0.090 | |
| Low | 2.07 (-0.41 to 4.55) | 0.102 | 0.0 | 1.000 | |||
| Gait speed | Country | Eastern | 0.10 (0.02 to 0.17) | 0.016 | 0.0 | 0.966 | 0.209 |
| Western | 0.04 (-0.02 to 0.11) | 0.194 | 43.5 | 0.053 | |||
| Mean age, years | > 12.0 | 0.06 (-0.03 to 0.16) | 0.202 | 53.9 | 0.055 | 0.519 | |
| < 12.0 | 0.04 (-0.03 to 0.11) | 0.285 | 12.7 | 0.328 | |||
| Percentage male, % | > 50.0 | 0.05 (-0.00 to 0.11) | 0.068 | 41.8 | 0.056 | 0.826 | |
| < 50.0 | 0.07 (-0.07 to 0.21) | 0.352 | 0.0 | 0.727 | |||
| Exercise type | Resistance | 0.03 (-0.02 to 0.08) | 0.237 | 0.0 | 0.763 | 0.169 | |
| Aerobic | 0.10 (-0.02 to 0.22) | 0.112 | 63.4 | 0.018 | |||
| Follow-up, months | > 6.0 | -0.00 (-0.08 to 0.07) | 0.990 | 0.0 | 0.960 | 0.122 | |
| < 6.0 | 0.07 (0.01 to 0.13) | 0.024 | 36.3 | 0.092 | |||
| Study quality | High | -0.00 (-0.15 to 0.14) | 0.980 | 0.0 | 0.775 | 0.459 | |
| Low | 0.06 (0.01 to 0.12) | 0.032 | 37.1 | 0.079 | |||
| Muscle strength | Country | Eastern | 1.37 (-0.50 to 3.24) | 0.152 | 92.7 | < 0.001 | < 0.001 |
| Western | 0.38 (-0.20 to 0.96) | 0.205 | 53.0 | 0.015 | |||
| Mean age, years | > 12.0 | 0.77 (-0.73 to 2.28) | 0.312 | 32.9 | 0.177 | < 0.001 | |
| < 12.0 | 0.37 (-0.20 to 0.93) | 0.204 | 61.2 | 0.008 | |||
| Percentage male, % | > 50.0 | 1.04 (0.04 to 2.03) | 0.042 | 85.1 | < 0.001 | < 0.001 | |
| < 50.0 | 0.20 (-0.62 to 1.01) | 0.639 | 7.1 | 0.358 | |||
| Exercise type | Resistance | 1.34 (0.08 to 2.60) | 0.037 | 87.6 | < 0.001 | < 0.001 | |
| Aerobic | 0.06 (-0.13 to 0.25) | 0.526 | 0.0 | 0.781 | |||
| Mixed | 7.83 (-9.56 to 25.21) | 0.377 | 85.7 | 0.008 | |||
| Follow-up, months | > 6.0 | 0.09 (-0.34 to 0.53) | 0.682 | 0.0 | 0.560 | 0.356 | |
| < 6.0 | 1.17 (0.22 to 2.11) | 0.015 | 87.4 | < 0.001 | |||
| Study quality | High | 7.85 (-1.52 to 17.22) | 0.101 | 57.2 | 0.072 | 0.008 | |
| Low | 0.80 (0.11 to 1.50) | 0.024 | 85.7 | < 0.001 |
95% CI: 95% confidence interval; WMD: weighted mean difference
Gait speed
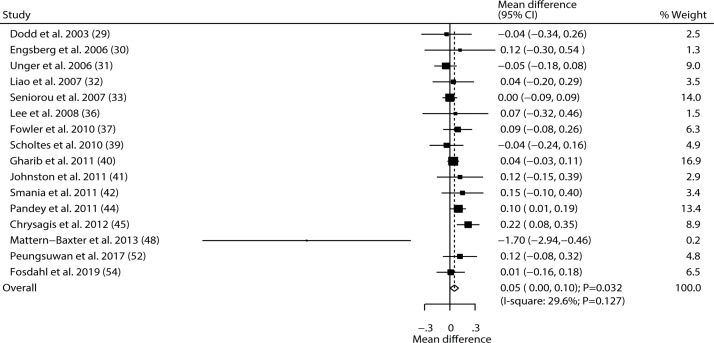
Data regarding the effect of exercise intervention on gait speed were available in 16 of the selected trials. Exercise intervention was associated with higher gait speed than those in control groups (WMD 0.05; 95% CI 0.00–0.10; p = 0.032; Fig. 3), and non-significant heterogeneity was detected across these trials (I2 = 29.6%; p = 0.127). This conclusion was altered when excluding the studies conducted by Fowler et al., 2010 (37), Gharib et al., 2011 (40), Smania et al., 2011 (42), Pandey et al., 2011 (44), Chrysagis., 2012 (45), or Peungsuwan et al., 2017 (52) (Table III, Appendix S21). Subgroup analysis revealed that a more significant effect of exercise intervention on gait speed was detected if the study was conducted in an Eastern country, if follow-up was< 6.0 months, and in studies with lower quality (Table IV). There was no significant publication bias for gait speed (p-value for Egger 0.541; p-value for Begg 0.893; Appendix S31).
Fig. 3.
Effect of exercise intervention on gait speed in children with cerebral palsy. 95% CI: 95% confidence interval.
Muscle strength
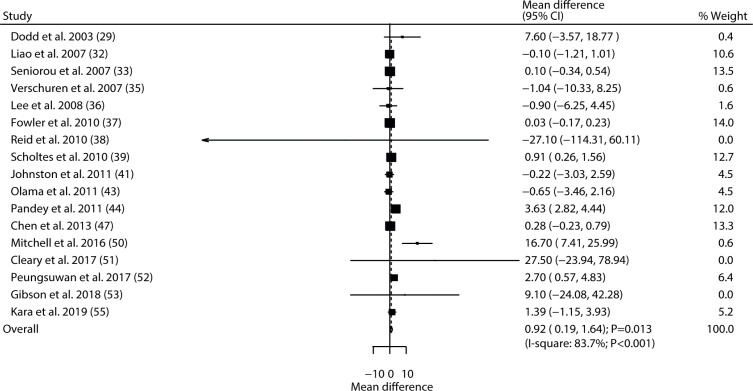
Data for the effect of exercise intervention on muscle strength were available in 17 trials. The pooled result found exercise intervention was associated with an improvement in muscle strength (WMD 0.92; 95% CI 0.19–1.64; p = 0.013; Fig. 4), and significant heterogeneity was seen among the included trials (I2 = 83.7%; p < 0.001). This conclusion was changed into non-significant difference after excluding the study conducted by Pandey et al., 2011 (44) (Table III, Appendix S21). Subgroup analyses revealed that the significant effect of exercise intervention on muscle strength was observed mainly when the proportion of males was ≥50%, when patients had received resistance training, and when follow-up was < 6.0 months, and in studies with lower quality (Table IV). No significant publication bias for muscle strength was detected (p-value for Egger 0.115; p-value for Begg 0.387; Appendix S31).
Fig. 4.
Effect of exercise intervention on muscle strength in children with cerebral palsy. 95% CI: 95% confidence interval.
DISCUSSION
This meta-analysis of RCTs of children with cerebral palsy assessed the effectiveness of exercise interventions on gross motor function, gait speed, and muscle strength in these patients. The quantitative analysis was based on 834 children with cerebral palsy from 27 RCTs, and the broad characteristics of patients were included. The meta-analysis revealed that exercise interventions are not associated with improved gross motor function in children with cerebral palsy, but were associated with increased gait speed and muscle strength. Meta-analysis also revealed that the effect of exercise intervention on muscle strength could be affected by country, mean age, proportion of male subjects, exercise type, and study quality.
Several systematic reviews and meta-analyses have been conducted previously to investigate the effectiveness of exercise interventions for patients with cerebral palsy. Bania et al. conducted a meta-analysis of 9 studies to investigate the effect of activity training in children with cerebral palsy (13), and reported that activity training did not result in significant effects on activity or participation. A meta-analysis by Booth et al., based on 11 RCTs, found that functional gait training conferred a significant increase in walking speed in children and young adults with cerebral palsy (14). A Cochrane review found that aerobic exercise could improve gross motor function, but did not affect gait speed, and that resistance training did not result in any beneficial effect on gait speed, gross motor function, participation, or quality of life in children with cerebral palsy (15). However, several outcomes were not addressed in Bania et al.’s study (13), and the other 2 studies included both children and adults (14, 15). Several additional studies have since been published, which should be taken into account when evaluating the effectiveness of exercise interventions for children with cerebral palsy.
Although the results of the current meta-analysis showed that exercise intervention has no significant effect on gross motor function, a trend of improvement was observed in the pooled conclusion and sensitivity analysis. All the studies included in the meta-analysis reported similar results, and no significant difference in the level of gross motor function between the exercise and control groups. Potential reasons for these results are that the effectiveness of exercise interventions on gross motor function could be affected by the type and intensity of the exercise programme, the amount of exercise could be affected by the age of the children, and the effectiveness of exercise interventions could be affected by compliance and by guardians. These factors could induce potential non-significant differences for children after long-term exercise interventions.
This meta-analysis revealed that exercise intervention could significantly increase gait speed in children with cerebral palsy. Most studies reported no significant effect of exercise intervention on gait speed, but 2 of the included trials reported a conclusion similar to the pooled conclusion. Pandey et al. found that task-specific strength training of the lower limbs was associated with a significant increase in gait speed after one month (44). The study conducted by Chrysagis et al. included 22 adolescents (age range 13–19 years) and found that a treadmill programme was associated with increased gait speed compared with conventional physiotherapy (45). The potential reason for this is that manual correction by the physical therapist could enhance walking ability, and the exercise programme involved repetitive movements in the lower limbs during training (56). Moreover, the change in weightbearing from the pelvis could improve hip extension, knee collapse, and foot clearance (56). Sensitivity analysis found that the pooled conclusion was not stable after sequentially excluding individual trials. The potential reason for this could be the lower or upper limit of 95% CI was close to zero and further RCTs are needed to verify this result.
The pooled results of this study reveal that exercise interventions are associated with increased muscle strength in children with cerebral palsy. Although most included trials reported that exercise interventions had no significant effect on muscle strength, 4 of the studies found that exercise intervention could significantly increase muscle strength. Scholtes et al. found that children with 12 weeks of functional progressive resistance exercise had increased muscle strength (39). Pandey et al. reported that task-specific strength training of the lower limbs could significantly increase muscle strength (44). Mitchell et al. found that webbased training for activity capacity and performance could significantly increase functional strength and walking endurance in children with unilateral cerebral palsy (57). Peungsuwan et al. reported that children with cerebral palsy had increased muscle strength after following a combined strength and endurance training programme (58). Subgroup analyses revealed that exercise intervention significantly enhanced muscle strength when the proportion of males was ≥50%, when patients received resistance training, when follow-up was < 6.0 months, and in studies with lower quality. These results could be explained by the amount of exercise, and the type of exercise programme is significantly related to the increased muscle strength. Moreover, the effect of exercise intervention was more evident after shorter follow-up. , the results of this study should be recommend cautiously because of the significant difference between groups was observed in the subgroup of studies with low quality.
Study limitations
This study has several limitations. First, the types of exercise intervention were different across included trials, making direct comparisons problematic. Secondly, the disease status ranged from I to V (Gross Motor Function Classification System; GMFCS), and there were differences in baseline gross motor function, gait speed, and muscle strength. Thirdly, the heterogeneity for muscle strength among the included trials was not fully explained by sensitivity and subgroup analyses. Fourthly, most of the included trials had low to moderate quality, and the results of these studies should be viewed with caution. Finally, meta-analyses based on pooled data have inherent limitations, including inevitable publication bias and restricted details.
This study found that exercise interventions in children with cerebral palsy were significantly associated with increased gait speed and muscle strength, but had no significant effect on gross motor function. Further large-scale RCTs are needed to verify the findings of this study.
ACKNOWLEDGEMENTS
This study was supported by Research on the application of integrated services for the disabled (four square step test; FSST), grant number JB2017-16-2.
The authors have no conflicts of interests to declare.
http://www.medicaljournals.se/jrm/content/?doi = 10.2340/16501977-2772
REFERENCES
- 1.Yu Y, Chen X, Cao S, Wu, Zhang X, Chen X. Gait synergetic neuromuscular control in children with cerebral palsy at different gross motor function classification system levels. J Neurophysiol 2019; 121: 1680–1691. [DOI] [PubMed] [Google Scholar]
- 2.Papageorgiou E, Nieuwenhuys A, Vandekerckhove I, Van Campenhout A, Ortibus E, Desloovere K. Systematic review on gait classifications in children with cerebral palsy: an update. Gait Posture 2019; 69: 209–223. [DOI] [PubMed] [Google Scholar]
- 3.Appleton RE, Gupta R. Cerebral palsy: not always what it seems. Arch Dis Child 2019; 104: 809–814. [DOI] [PubMed] [Google Scholar]
- 4.Cerebral Palsy Follow-up Program and Norwegian Cerebral Palsy Register, Annual Report. 2017. [Cited 2020 Jun 5]. Available from: https://oslo-universitetssykehus.no/avdelinger/barne-og-ungdomsklinikken/barneavdeling-for-nevrofag/cpop-cerebral-parese-oppfolgingsprogram#%C3%A5rsrapporter. [Google Scholar]
- 5.James RG, Michael HS, Steven EK, Tom FN. The identification and treatment of gait problems in cerebral palsy, 2nd edn. Cambridge, UK: Mac Keith Press; 2009. [Google Scholar]
- 6.McKinnon CT, Meehan EM, Harvey AR, Antolovich GC, Morgan PE. Prevalence and characteristics of pain in children and young adults with cerebral palsy: a systematic review. Dev Med Child Neurol 2019; 61: 305–314. [DOI] [PubMed] [Google Scholar]
- 7.Ostojic K, Paget SP, Morrow AM. Management of pain in children and adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol 2019; 61: 315–321. [DOI] [PubMed] [Google Scholar]
- 8.Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral palsy – trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr 2017; 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto TPS, Fonseca ST, Goncalves RV, Souza TR, Vaz DV, Silva PLP, et al. Mechanisms contributing to gait speed and metabolic cost in children with unilateral cerebral palsy. Braz J Phys Ther 2018; 22: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degelaen M, De Borre L, Buyl R, Kerckhofs E, De Meirleir L, Dan B. Effect of supporting 3D-garment on gait postural stability in children with bilateral spastic cerebral palsy. NeuroRehabilitation 2016; 39: 175–181. [DOI] [PubMed] [Google Scholar]
- 11.Qu D, Guan LJ. [Study on the clinical types and complications of 1323 cases of infantile cerebral palsy.] J Chin Pediatr Integr Tradit West Med 2017; 009: 451–454. (in Chinese). [Google Scholar]
- 12.Vargus-Adams JN, Martin LK. Domains of importance for parents, medical professionals and youth with cerebral palsy considering treatment outcomes. Child Care Health Dev 2011; 37: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bania T, Chiu HC, Billis E. Activity training on the ground in children with cerebral palsy: systematic review and meta-analysis. Physiother Theory Pract 2019; 35: 810–821. [DOI] [PubMed] [Google Scholar]
- 14.Booth ATC, Buizer AI, Meyns P, Oude Lansink ILB, Steenbrink F, van der Krogt MM. The efficacy of functional gait training in children and young adults with cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol 2018; 60: 866–883. [DOI] [PubMed] [Google Scholar]
- 15.Ryan JM, Cassidy EE, Noorduyn SG, O’Connell NE. Exercise interventions for cerebral palsy. The Cochrane database of systematic reviews 2017; 6: Cd011660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol 1998; 40: 100–107. [DOI] [PubMed] [Google Scholar]
- 17.Verschuren O, Ada L, Maltais DB, Gorter JW, Scianni A, Ketelaar M. Muscle strengthening in children and adolescents with spastic cerebral palsy: considerations for future resistance training protocols. Phys Ther 2011; 91: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Wiart L, Darrah J, Kembhavi G. Stretching with children with cerebral palsy: what do we know and where are we going? Pediatr Phys Ther 2008; 20: 173–178. [DOI] [PubMed] [Google Scholar]
- 19.Harvey LA, Katalinic OM, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contracture: an abridged republication of a Cochrane Systematic Review. J Physiother 2017; 63: 67–75. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Xiang C, Zhou YH, Jiang A, Qin YY, He J. Effect of statins on cardiovascular events in patients with mild to moderate chronic kidney disease: a systematic review and meta-analysis of randomized clinical trials. BMC Cardiovasc Disord 2014; 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 23.Ades AE, Lu G, Higgins JP. The interpretation of randomeffects meta-analysis in decision models. Medical Decision Making 2005; 25: 646–654. [DOI] [PubMed] [Google Scholar]
- 24.Deeks JJ, Higgins J, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Oxford: The Cochrane Collaboration; 2008, p. 243–296. [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed) 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey SG, Altman DG, editors. Systematic reviews in health care: metaanalysis in context, 2nd edn. London: BMJ Books; 2001, p. 312. [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res ed) 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 29.Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol 2003; 45: 652–657. [DOI] [PubMed] [Google Scholar]
- 30.Engsberg JR, Ross SA, Collins DR. Increasing ankle strength to improve gait and function in children with cerebral palsy: a pilot study. Pediatr Phys Ther 2006; 18: 266–275. [DOI] [PubMed] [Google Scholar]
- 31.Unger M, Faure M, Frieg A. Strength training in adolescent learners with cerebral palsy: a randomized controlled trial. Clin Rehabil 2006; 20: 469–477. [DOI] [PubMed] [Google Scholar]
- 32.Liao HF, Liu YC, Liu WY, Lin YT. Effectiveness of loaded sit-to-stand resistance exercise for children with mild spastic diplegia: a randomized clinical trial. Arch Phys Med Rehabil 2007; 88: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seniorou M, Thompson N, Harrington M, Theologis T. Recovery of muscle strength following multi-level orthopaedic surgery in diplegic cerebral palsy. Gait Posture 2007; 26: 475–481. [DOI] [PubMed] [Google Scholar]
- 34.Unnithan VB, Katsimanis G, Evangelinou C, Kosmas C, Kandrali I, Kellis E. Effect of strength and aerobic training in children with cerebral palsy. Med Sci Sports Exerc 2007; 39: 1902–1909. [DOI] [PubMed] [Google Scholar]
- 35.Verschuren O, Ketelaar M, Gorter JW, Helders PJ, Uiterwaal CS, Takken T. Exercise training program in children and adolescents with cerebral palsy: a randomized controlled trial. Arch Pediatr Adolesc Med 2007; 161: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Sung IY, Yoo JY. Therapeutic effects of strengthening exercise on gait function of cerebral palsy. Disabil Rehabil 2008; 30: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 37.Fowler EG, Knutson LM, Demuth SK, Siebert KL, Simms VD, Sugi MH, et al. Pediatric endurance and limb strengthening (PEDALS) for children with cerebral palsy using stationary cycling: a randomized controlled trial. Phys Ther 2010; 90: 367–381. [DOI] [PubMed] [Google Scholar]
- 38.Reid S, Hamer P, Alderson J, Lloyd D. Neuromuscular adaptations to eccentric strength training in children and adolescents with cerebral palsy. Dev Med Child Neurol 2010; 52: 358–363. [DOI] [PubMed] [Google Scholar]
- 39.Scholtes VA, Becher JG, Comuth A, Dekkers H, Van Dijk L, Dallmeijer AJ. Effectiveness of functional progressive resistance exercise strength training on muscle strength and mobility in children with cerebral palsy: a randomized controlled trial. Dev Med Child Neurol 2010; 52: e107–e113. [DOI] [PubMed] [Google Scholar]
- 40.Gharib NM, El-Maksoud GM, Rezk-Allah SS. Efficacy of gait trainer as an adjunct to traditional physical therapy on walking performance in hemiparetic cerebral palsied children: a randomized controlled trial. Clin Rehabil 2011; 25: 924–934. [DOI] [PubMed] [Google Scholar]
- 41.Johnston TE, Watson KE, Ross SA, Gates PE, Gaughan JP, Lauer RT, et al. Effects of a supported speed treadmill training exercise program on impairment and function for children with cerebral palsy. Dev Med Child Neurol 2011; 53: 742–750. [DOI] [PubMed] [Google Scholar]
- 42.Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. Am J Phys Med Rehabil 2011; 90: 137–149. [DOI] [PubMed] [Google Scholar]
- 43.Olama KA. Endurance exercises versus treadmill training in improving muscle strength and functional activities in hemiparetic cerebral palsy. Egyptian J Med Hum Genet 2011; 12: 193–199. [Google Scholar]
- 44.Pandey DP, Tyagi V. Effect of functional strength training on functional motor performance in young children with cerebral palsy. Ind J Physiother Occupat Ther 2011; 5: 52–55. [Google Scholar]
- 45.Chrysagis N, Skordilis EK, Stavrou N, Grammatopoulou E, Koutsouki D. The effect of treadmill training on gross motor function and walking speed in ambulatory adolescents with cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil 2012; 91: 747–760. [DOI] [PubMed] [Google Scholar]
- 46.Bryant E, Pountney T, Williams H, Edelman N. Can a sixweek exercise intervention improve gross motor function for non-ambulant children with cerebral palsy? A pilot randomized controlled trial. Clin Rehabil 2013; 27: 150–159. [DOI] [PubMed] [Google Scholar]
- 47.Chen CL, Chen CY, Liaw MY, Chung CY, Wang CJ, Hong WH. Efficacy of home-based virtual cycling training on bone mineral density in ambulatory children with cerebral palsy. Osteoporos Int 2013; 24: 1399–1406. [DOI] [PubMed] [Google Scholar]
- 48.Mattern-Baxter K, McNeil S, Mansoor JK. Effects of home-based locomotor treadmill training on gross motor function in young children with cerebral palsy: a quasirandomized controlled trial. Arch Phys Med Rehabil 2013; 94: 2061–2067. [DOI] [PubMed] [Google Scholar]
- 49.Lee M, Ko Y, Shin MM, Lee W. The effects of progressive functional training on lower limb muscle architecture and motor function in children with spastic cerebral palsy. J Phys Ther Sci 2015; 27: 1581–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell LE, Ziviani J, Boyd RN. A randomized controlled trial of web-based training to increase activity in children with cerebral palsy. Dev Med Child Neurol 2016; 58: 767–773. [DOI] [PubMed] [Google Scholar]
- 51.Cleary SL, Taylor NF, Dodd KJ, Shields N. An aerobic exercise program for young people with cerebral palsy in specialist schools: A phase I randomized controlled trial. Dev Neurorehabil 2017; 20: 331–338. [DOI] [PubMed] [Google Scholar]
- 52.Peungsuwan P, Parasin P, Siritaratiwat W, Prasertnu J, Yamauchi J. Effects of Combined Exercise Training on Functional Performance in Children With Cerebral Palsy: A Randomized-Controlled Study. Pediatr Phys Ther 2017; 29: 39–46. [DOI] [PubMed] [Google Scholar]
- 53.Gibson N, Chappell A, Blackmore AM, Morris S, Williams G, Bear N, et al. The effect of a running intervention on running ability and participation in children with cerebral palsy: a randomized controlled trial. Disabil Rehabil 2018; 40: 3041–3049. [DOI] [PubMed] [Google Scholar]
- 54.Fosdahl MA, Jahnsen R, Kvalheim K, Holm I. Effect of a Combined Stretching and Strength Training Program on Gait Function in Children with Cerebral Palsy, GMFCS Level I & II: A Randomized Controlled Trial. Medicina (Kaunas) 2019; 55: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaya Kara O, Livanelioglu A, Yardımcı BN, Soylu AR. The Effects of Functional Progressive Strength and Power Training in Children With Unilateral Cerebral Palsy. Pediatr Phys Ther 2019; 31: 286–295. [DOI] [PubMed] [Google Scholar]
- 56.Day JA, Fox EJ, Lowe J, Swales HB, Behrman AL. Locomotor training with partial body weight support on a treadmill in a nonambulatory child with spastic tetraplegic cerebral palsy: a case report. Pediatr Phys Ther 2004; 16: 106–113. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell LE, Ziviani J, Boyd RN. A randomized controlled trial of web-based training to increase activity in children with cerebral palsy. Dev Med Child Neurol 2016; 58: 767–773. [DOI] [PubMed] [Google Scholar]
- 58.Peungsuwan P, Parasin P, Siritaratiwat W, Prasertnu J, Yamauchi J. Effects of Combined Exercise Training on Functional Performance in Children With Cerebral Palsy: A Randomized-Controlled Study. Pediatr Phys Ther 2017; 29: 39–46. [DOI] [PubMed] [Google Scholar]