Abstract
Objective
To investigate the effectiveness of modified rehabilitation programmes in comparison with standard rehabilitation programmes after total knee arthroplasty through randomized controlled trials.
Data sources
A search was conducted in PubMed, PubMed Central (PMC) and Cochrane Library databases in December 2020.
Study selection
Randomized controlled trials were reviewed if they compared a physiotherapy exercise intervention with usual or standard physiotherapy care, or if they compared 2 types of exercise physiotherapy interventions meeting the review criteria, after total knee arthroplasty for osteoarthritis. A total of 18 randomized controlled trials were included at the end of the screening process.
Data extraction
Two authors independently screened the literature, extracted data, and assessed the quality of included studies. The outcomes were knee extension, knee flexion, pain visual analogue scale, overall Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), 6-minute walking test, and Timed Up and Go test.
Data synthesis
There was no clear pattern regarding which combination of starting time-point and duration of the rehabilitation programme after total knee arthroplasty significantly improves the clinical outcome when comparing modified rehabilitation programmes with standard programmes. Moreover, no particular modification to the modified programmes could be solely attributed to the improved clinical outcome in the 2 studies that showed significant improvement.
Conclusion
Modified rehabilitation programmes do not result in systematic improvement in clinical outcome over one-size-fits-all-approaches after total knee arthroplasty.
LAY ABSTRACT
The aim of this study was to compare modified rehabilitation programmes with standard rehabilitation programmes after total knee replacement. A total of 18 randomized controlled trials were included at the end of the screening process. Six clinical outcomes were used for comparison. To our knowledge, this is the first study to compare modified and standard rehabilitation programmes based on the starting point and the duration of each programme. The results of the comparison showed that there is no clear pattern in the combination of starting time-point and duration of rehabilitation that significantly improves clinical outcomes. Moreover, improved clinical outcomes could not be attributed solely to any particular modification to the programmes. Accordingly, a one-size-fits-all approach to modified rehabilitation programmes does not result in systematic improvement in clinical outcome.
Key words: rehabilitation, exercise, physical therapy, total knee replacement
Total knee arthroplasty (TKA) is an established standard procedure to alleviate problems caused by advanced knee osteoarthritis (OA) (1). The annual worldwide incidence of TKA has increased steadily over the past 2 decades (2). The incidence of TKA in industrialized countries is 150–200/100,000 inhabitants (3).
After TKA, the patient’s movement is limited and restricted due to decreased muscle strength. For instance, muscle function has been found to be reduced by 20–25% at one month post-TKA (4), while, after one year, it remains lower than in healthy adults, with reports of 18% slower walking speed and 51% slower stairclimbing speed (5). Furthermore, range of motion (ROM) of the knee joint is reduced due to post-operation pain (6), haematoma and swelling (7). These limits complete functional recovery to only 67% of patients (8). In addition, the strength of the quadriceps muscle is reduced by 30.7% immediately after TKA, and by 50–60% after one month, despite the initiation of rehabilitation within 48 h after surgery (9).
Postoperative rehabilitation programmes are therefore of the highest importance, because they can improve function, outcome, and mobility in patients after TKA (10). These programmes consist of fitness components comprised of exercises for joints and muscles that include ROM, strength, walking, function, endurance, and balance. Regaining full ROM is essential to restore the natural capacity of movement that assists in regaining muscle strength by allowing full muscular contraction. Walking and functional exercises improve blood circulation and enable the ability to perform activities of daily living, such as standing, sitting, and stair climbing, to be regained (11). Endurance and balance exercises support the previous fitness components by increasing the duration of exercises and stability. An increase in endurance will enable subjects more time to exercise and to derive more benefit from exercises. Furthermore, improved balance will help patients to perform the exercises safely and smoothly, especially with exercises that require them to switch between legs. Regaining all these components is essential to reaching full recovery following a TKA.
In the clinical setting, post-operative rehabilitation programmes differ concerning the starting time-point and duration. Different arguments have been brought forward to support the diverse approaches; however, to date, the benefits of these approaches have not been directly investigated in a clinical setting. The lack of a universal definition of starting time-points and duration spans adds further complexity. Therefore, it is important to analyse whether a particular combination of starting time-point, and duration is more beneficial. Furthermore, since additional methods are being incorporated into modern rehabilitation programmes, it is worthwhile analysing whether these modifications improve the clinical outcome and prognosis and, if it is the case, which methods or exercises yield the best clinical improvements.
Therefore, the aim of this systematic review and meta-analysis of randomized controlled studies was to evaluate the effectiveness of different modified rehabilitation programmes vs standard care after TKA, and the effects of starting time-point and duration.
METHODS
Data sources
A search of Medline (PubMed), PubMed Central (PMC) and Cochrane Library databases was conducted. Exploded MeSH terms and keywords were used to generate sets for the following themes: total knee arthroplasty, total knee replacement, osteoarthritis, and rehabilitation approach. The Boolean terms “AND”, “OR” were used to find their intersection. Limitations were used, including English language publications between 2000 and 2020 and randomized control trial. In addition, the reference lists of all included studies were reviewed (see Appendix SI for complete search strategy and results). Furthermore, the outcomes were categorized and analysed for ROM (flexionextension), pain visual analogue scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire, 6-minute walking test (6MWT), and Timed Up and Go test (TUG). Comparisons were made between outcomes in the final assessment for each group.
The review was conducted using standard methodology outlined in the Cochrane Handbook (12) and the findings were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (12).
Selection criteria
Before beginning the systematic review and meta-analysis, a protocol was written outlining the search strategy, inclusion and exclusion criteria and outcomes of interest. Eligible studies were chosen based on the following criteria:
Examination of post-operative effect of the rehabilitation programme using physical rehabilitation methods (studies using music, medication, and supporting devices, such as knee-braces, were excluded).
Patients underwent unilateral or bilateral knee arthroplasty, since both surgeries have similar rehabilitation programmes.
Studies applied only to a post-operative rehabilitation programme.
Studies included a randomized design comparing a standard-based rehabilitation programme with a patient-modified programme.
Standard rehabilitation programmes must have contained at least 3 of the following 4 fitness components: strength, ROM, function and walking.
Studies calculated the mean and standard deviation (SD) of the parameters the studies measured.
Studies had to be published in English language.
Quality assessment
Two authors (WA, BR) independently assessed the risk of bias of the articles selected for detailed review. Methodological domains of the assessment, namely randomization sequence, allocation concealment, blinding, and conflicts of interest were graded according to the PEDro scale checklist (13).
Data extraction
Two authors (WA, BR) independently extracted the data from the included articles in forms; previously pilot-tested for feasibility and comprehensiveness. Data were extracted from each trial regarding participants (age, sex, group size), the content of the intervention (number of sessions, tools, and tests they used, standard care vs modified care or modified vs modified), setting and timing (starting point, duration), type of surgery and outcome. When a trial employed 2 variations in rehabilitation interventions, only one group was included (14–16).
For outcomes reported as continuous variables, mean and standard deviation were extracted. Outcomes with mean and confidence interval, or medians, and interquartile ranges were excluded.
Statistical analysis
For statistical analysis, Review Manager Software 5.3 (The Nordic Cochrane Collaboration, Copenhagen) was used. For continuous data, the inverse variance with arithmetic mean was considered. For dichotomous data, the Mantel-Haenszel method with odds ratio (OR) was considered. To evaluate heterogeneity, both χ2 and I-square (I2) statistical tests were performed. Value of χ2 > 0.5 along with I2 > 50% indicated heterogeneity. A fixed-effects model was used. If heterogeneity significantly affects the comparison, a random-effects model was used. The confidence interval (CI) was set at 95%. Values of p < 0.05 were considered statistically significant.
Defining the groups
The study defined the standard programme as control group based on the description of the rehabilitation programme in the RCTs; each study at least had to include 3 out of 4 fitness components (ROM, strength, walking or function) in the standard programme during the rehabilitation period. The experimental group was defined as the group that used the additional method plus the above-mentioned fitness components. The studies were categorized based on 2 factors commonly known to affect the outcome of the rehabilitation programmes: the starting time-point and duration of the rehabilitation programme. Accordingly, the studies were subdivided into 4 rehabilitation intervals: (i) early and short, (ii) early and long, (iii) late and short, and (iv) late and long. The interval subdivision of the studies between early and late rehabilitation was based on the wound-healing phases. After TKA the wound will be in the inflammatory phase between 4 and 6 days (17), which is a natural response to surgery. The signs elevate in the surrounding skin in exudate levels, erythema, heat, oedema, pain and functional disturbance (18). Some healthcare practitioners wait until the inflammation disappears before starting the rehabilitation programme, while others prefer to start within that phase, arguing that rehabilitation exercises boost the anti-inflammatory response and, thus, accelerate recovery. Therefore, in this review, the starting time-point factor was determined “early” when the rehabilitation programme started within the first week, while programmes that started after that time-point were considered as “late” starting programmes. There were 13 studies that started early (14–16, 18–27), while 5 studies started late (28–32). As for the duration factor, there is a lack of uniform criteria to define the length of the programme. In Germany the in-house rehabilitation programme has a maximum duration of 3 weeks. Accordingly, and for the sake of having a uniform anchor point, programmes that ran for less than 3 weeks were considered as “short” and programmes that ran more than 3 weeks as “long”. Nine studies employed short programmes (14–16, 18–23), while 9 studies employed long programmes (28–32).
When cross-checking the 2 factors, 8 studies started early and had a short duration, 4 studies started early and had a long duration, no studies started late and had a short duration, and 5 studies started late and had a long duration (Table I).
Table I.
Analysis of studies that used post-total knee arthroplasty rehabilitation programmes
| Combination | Number of studies | Studies |
|---|---|---|
| Early + Short | 9 | (Beaupe et al. 2001 (14); Bruun-Olsen et al. 2009 (18); Denis et al. 2006 (15); Labraca et al. 2011 (19); Lenssen et al. 2006 (20); Lenssen et al. 2008 (21); Mau-moeller et al. 2014 (22); Rahmaan et al. 2009 (16), Hardt et al. 2018 (23)) |
| Early + Long | 4 | (Ebert et al. 2013 (24); Stevens-Lapsley et al. 2012 (25); Bade et al. 2017 (26); Demircioglu et al. 2015 (27)) |
| Late + Short | 0 | |
| Late + Long | 5 | (Jakobsen et al. 2014 (28); Lastayo et al. 2009 (29); Liao et al. 2013 (30); Piva et al. 2017 (37), Schache et al. 2019 (32)) |
RESULTS
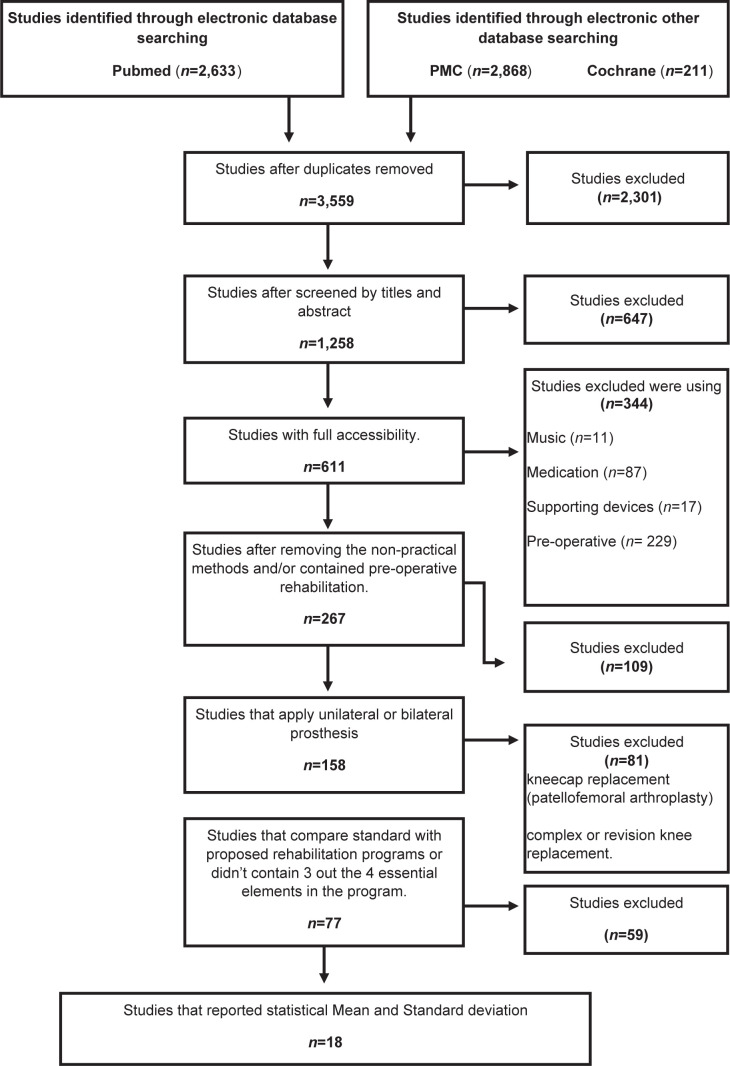
The initial search identified 267 potentially relevant studies. After reviewing titles and abstracts and applying the inclusion and exclusion criteria, only 18 articles (Bade et al. 2017 (26), Beaupre et al. 2001 (14), Bruun-Olsen et al. 2009 (18), Demircioglu et al. 2015 (27), Denis et al. 2006 (15), Ebert et al. 2013 (24), Hardt et al. 2018 (23), Jakobsen et al. 2014 (28), Labraca et al. 2011 (19), Lastayo et al. 2009 (29), Lenssen et al. 2006 (20), Lenssen et al. 2008 (21), Liao et al. 2013 (30), Mau-Moeller et al. 2014 (22), Piva et al. 2017 (37), Rahmann et al. 2009 (16), Schache et al. 2019 (32), Steven-Lapsley et al. 2012 (25) fulfilled the inclusion and exclusion criteria for the systematic review and meta-analysis (Fig. 1 and Table II).
Fig. 1.
Flow-chart of the literature search in Pubmed, PMC, and Cochrane. PMC: PubMed Central.
Table II.
Summary of the 18 randomized controlled trials included.
| Study/country | First day of intervention | Period | Follow-up | Groups n = (women%) | Withdraw | Description of exercise/therapy | Outcomes |
|---|---|---|---|---|---|---|---|
| Bade et al. 2017 USA (26) | Day 4 | 11 w | 12 M | EXP1 (HI) = 84 n = 45, p = 54% EXP2 (LI) = 87 n = 52, p = 66% |
EXP1 = 0 EXP2 = 0 | Both groups: Patients were seen 3/w for the first 6/w and 2/w over the next 5 w. (26 sessions). (45 min each session) Both had education on healing. Hi group: high-intensity, progression-based, rehabilitation programme-based. PRE targeting. Weight-bearing, functional, balance, agility, and activity exercise (2 sets- 8 rep). 30 min walking 5/w. swimming, cycling, elliptical machine, stair climber. LI group: time-based rehabilitation programme. 1) iso and ROM ex for the first 4/w. 2) slower transition to w-b ex. 3) less progression in difficulty of w-b ex 4) no resistance beyond body weight or elastic bands 5) restricted activity outside of ADLs for the first 4/w gradually building to 30 min by the end of therapy (restricted to walking and low-resistance cycling) |
Stair-climbing test, TUG, 6MWT, ROM, MCS, SF-12, muscle strength, WOMAC |
| Beaupre et al. 2001 Canada (14) | Day 3 | 7 d | 6 M | EXP=40 n = 20, p = 50% CON = 40 n = 13, p = 30% | EXP = 9 CON = 11 | EXP1: standard rehabilitation (ROM ex + strength ex + functional ex) + CPM CON: Standard rehabilitation + SB |
Walking, A/ROM Ex, isometric knee extension, stair-climbing. |
| Bruun-Olsen et al. 2009 Norway (18) | Day 1 after Op | 6 d | 3 M | EXP= 30 n = 22, p = 73% CON = 33 n = 22, p = 67% |
EXP = 5 CON = 2 | EXP: CPM + active Ex: flexion/ extension exercises, active isometric contraction of the quadriceps, walking, climbing stairs (crutches), passive movement. CON: same programme without CPM |
ROM, pain, function, balance, walking |
| Demircioglu et al. 2015 Turkey (27) |
Day 1 | 6 w | 3 M | EXP=30 n = 28, p = 93% CON = 30 n = 29, p = 96% | EXP = 0 CON = 0 | Both groups started 30 min (ROM)-(CPM)/w, ankle ROM ex, isometric quadriceps ex, stand up with a walker and fully extend their knees and active and assisted ROM ex, Active ROM and isometric quadriceps exercise, mobilization, active hip abduction and adduction ex. A home ex programme was recommended. Closed kinetic chain ex, 15 min cryotherapy. EXP: 1st/D after surgery 30-min NMES on VM 5/D week, for 4-6 weeks* | Knee extension, flexion, pain, stiffness, function, TUG, SF-36. |
| Denis et al. 2006 Canada (15) | Day 2 after Op | 7-8 d | 2 years | EXP=28 n = 14, p = 51.9% CON = 27 n = 13, p= 46.4% |
EXP = 1 CON = 0 | EXP: CPM group 1 (35 min) + conventional CON: Group 2 (2h): respiratory and circulatory Ex, strength extension EX and extension knee alignment, A/P knee flexion, abduction and add of the hip in the horizontal plane, and knee extensor muscle Ex, functional Ex |
ROM (flexion-extension), TUG, WOMAC, length of stay |
| Ebert et al. 2013 Australia (24) | Day 2 after Op | 6 w | 6 w | EXP= 24 n = 7, p= 29% CON = 26 n = 7, p= 27% |
EXP = 0 CON = 0 | EXP: Lymphatic drainage+ conventional therapy CON: conventional therapy active- assisted knee flexion + (active knee flexion + hip and knee flexion + functional Ex + CPM + Cryotherapy | Active knee flexion and extension range of motion, lower limb girths (ankle, mid-patella, thigh, and calf), and pain |
| Hardt et al. 2018 Germany (23) | Day 1 | 7 d | 7±1 d | EXP =22 n = 3, p = 12.5% CON = 25 n = 4, p = 15.6% | EXP = 11 CON = 2 | EXP: Genusport knee trainer extra. CON: active and passive knee mobilization, gait training, assisted walking with crutches, strength exercises, stair-climbing, manual lymphatic drainage, and cryotherapy 3 times daily with ice packs. | Active and passive range of motion (ROM), pain, knee extension strength, TUG, 10-m Walk Test, 30-s Chair Stand Test, (KOOS), (KSS), |
| Jakobsen et al. 2014 Denmark (28) | 1 w after Op | 6 w | 26 w | EXP =35 n = 21, p=60% CON = 37 n = 16, p=57% |
EXP = 5 CON = 2 | EXP: warming up + knee ROM Ex + knee extensor stretches and 1-legged balance Ex + strength training + functional training + balance training (the programme was applied earlier) CON: Same programme |
Walking, ROM (flexion, extension), pain, 6MWt, KOOS, Qof, activity of daily living, Oxford knee score. |
| Labraca et al. 2011 Spain (19) | Within the first 24 | 4 d | 4 d | EXP = 153 n = 101, p = 73.1% CON =153 n=110, p = 81.4% |
EXP =15 CON = 18 | EXP: P/A ROM + Strength Exflexion/extension + breathing + Functional EX (the programme was applied earlier) CON: same programme |
ROM, muscle strength, pain, autonomy, gait, and balance |
| Lastayo et al. 2009 USA (29) | 1 - 4 years after Op | 12 w | 3 M | EXP = 9 n= 7, p = 77.7% CON =8 n = 6 p = 75% | EXP =0 CON = 0 | EXP: Strength Ex + ROM+ NMES+ Walk+ setups + wall squat (Eccentric (ECC) resistance Exmachine/additional) CON: same programme without ECC training |
Quadriceps volume, extension strength, TUG, 6MWT, stairs (ascending, descending) |
| Lenssen et al. 2006 Netherlands (20) | Day 1 | 4 d | 3 M | EXP = 21 n = 15, p = 71.4% CON = 22 n = 17, p = 77.2% |
EXP =0 CON = 0 | EXP: A/P mobilization of the knee joint + active strengthening (quadriceps) + ADL functions treatment session (30 min), mean total of treatment sessions EXP- CPM more than the CON group CON: same programme | Passive flexion ROM, active ROM and passive extension ROM, functional status, length of stay, pain, satisfaction with treatment |
| Lenssen et al. 2008 Netherlands (21) | Day 1 after Op | 17 d | 3 M | EXP= 30 n = 18, p = 60% CON = 30 n = 21, p = 70% |
EXP = 0 CON = 0 | EXP: active and passive mobilization of the knee + strengthening of the quadriceps muscle + functional exercises + transfers from a supine position to sitting and from sitting to standing + walking and stair climbing CON: same programme |
Functional status, ROM, perceived effect, postoperative medication, satisfaction with treatment, quantity, duration, and nature of PT intervention |
| Liao et al. 2013 Taiwan (30) | At least 2 months after Op | 8 w | 8 w | EXP= 58 n = 46, p = 79.3% CON = 55 n = 37, p = 67.2% |
EXP = CON = | EXP: Exercises for strength + walking + endurance + 30 min function + 60 min balance CON: same programme without balance EX | Walking, balance, functional walking, pain, stiffness, function |
| Mau-Moeller et al. 2014 Germany (22) | Day 1 after Op | 3 w | 3 M | EXP1 (Sling) = 19 n = 7, p = 36.8% EXP2 CPM = 19 n=9, p = 47.3% |
EXP1 = 7 EXP2 = 10 | EXP1: Standard care + sling training (ST) EXP2: Standard care = A/P ROM Ex + Strength (quadriceps) + ADL Ex + walking + climbing stairs. Ex for pain and tolerance |
ROM, pain, physical activity, static posture control, function, QoL |
| Piva et al. 2017 USA (31) | After discharge | 3 d | 6 M | EXP=22 n = 18, p= 82% CON = 22 n = 13, p= 59% |
EXP = 0 CON = 0 | EXP: Warm up-5 min. Endurance- 20 min treadmill walking 50-75% intensity. Resistance ex (knee extensor, flex, hip extension, abduction) 60-80%. ((2 steps - 8 rep). Skilled ex 15 min. Education sessions. CON: Warm up - 15 min (bike). Endurance - 20 min (treadmill walking). Resistance ex - 40-50%. Both had home Ex |
Pain, function, stair- climbing, chair-standing, single-leg stance, 6MWT, gait speed, daily activity |
| Rahmann et al. 2009 Australia (16) | Day 4 | 14 d | 12 M | EXP= 18 n = 8 p= 44.4% CON = 17 n = 12, p= 70.5% |
EXP = 10 CON = 3 | EXP: Water programme: Hip adduction/abduction, squats, heel raises walk, lunges, stability Ex, hip extension, knee: walking, lunges, ROM CON: same programme without aquatic Ex |
Hip abductor strength, walking speed, self-reported disability (WOMAC), ROM, quadriceps + hamstring strength, function |
| Schache et al. 2019 Australia (32) | 2 w after Op | 6 w | 26 w | EXP =54 n = 39, p = 72% CON = 51 n = 30, p = 58% | EXP = 6 CON = 3 | EXP: Extra exercises targeting the strengthening of the hip abductor muscles CON: All participants received exercises to improve quadriceps, hamstring, and calf strength, increasing knee range of movement and improving walking and stair- climbing ability. These exercises have been described in detail previously.17 Manual therapy, including joint mobilization and massage, |
Pain, knee extension- flexion, hip strength, quadriceps strength, chair- stand test, stair-climbing test, 40 m fast-paced walk, TUG, step taps, 6MWT. |
| Steven-Lapsley et al. 2012 USA (25) |
Day 2 after Op | 6 w | 52 w | EXP =35 n = 20, p=57.1% CON = 31 n = 16, p=51.6% |
EXP = 5 CON = 6 | EXP: Exercises + NMES+ P/A ROM Ex + Functional Ex + ROM Ex + strengthening W/B non-W/B + walking CON: Passive (ROM) + cycling + flexibility + walking + functional training + strength |
Iso-quadriceps and hamstring torque and activation testing, NMES dose assessment, function, pain, ROM, health status questionnaires. |
D-day, W-week, M-month, EXP-experimental, CON-control, ROM-range of motion, A/P ROM-active/passive range of motion, TUG-time up and go test, 6MWT-6 minutes walking test, MCS-Mental Component Score, SF-12-Short Form Survey, ADL-activity of daily living, CPM-Continuous Passive Motion, SB-Slide Board, EX-exercise, NMES-Neuromuscular Electrical Stimulation, VM-Vastus medialis, KOOS-Knee Injury and Osteoarthritis Outcome Score, KSS-Knee Society Score, Qof-Quality of life, ECC-Eccentric, PT-Physiotherapy, OP-Operation, ST-Sling training, non-W/B non-weight bearing.
Characteristics of included studies
All studies were randomized controlled trial (RCTs) with a follow-up of between 6 weeks and 24 months, except for one study with only a 4-day follow-up. The total number of patients was 1,417 (Table II). Studies reported that there are no differences between groups on baseline measurement. Overall mean age was approximately 67.1 years (SD 2.5) and the percentage of females was 64.2%.
Interventions varied widely across studies. The specific rehabilitation programmes, which were compared with standard programmes, included water exercise (16), balance exercise (30), manual lymphatic drainage (24), neuromuscular electrical stimulation (NMES) (25, 27), comprehensive behavioural and exercise intervention (31), continuous passive motion (CPM) (15, 18, 21), sliding board (14), early high-intensity vs low intensity (26), strength exercise (23, 28, 29, 32), sling exercise (22), and fast track (19, 20). Six studies (21, 25, 26, 28, 29, 31) provided home exercises during the rehabilitation period.
The studies were subdivided into inpatient and out-patient groups (Table III)
Table III.
Summary (inpatient-outpatient) of the patient rehabilitation situation
| Studies with inpatient rehabilitation (n = 9) | Studies with outpatient rehabilitation (n = 7) | Studies with both inpatient and outpatient (n = 1) |
|---|---|---|
| Beaupre et al. 2001 (14) Bruun-Oslen et al. 2009 (18) Denis et al. 2006 (15) Ebert et al. 2013 (24) Hardt et al. 2018 (23) Labraca et al. 2011 (19) Lessen et al. 2006 (20) Lessen et al. 2008 (21) Moeller et al. 2014 (22) Rahmann et al. 2008 (16) |
Bade et al. 2017 (26) Demircioglu et al. 2015 (27) Jakobsen et al. 2014 (28) Lastayo et al. 2009 (29) Liao et al. 2013 (30) Piva et al. 2017 (37) Schache et al. 2019 (32) |
Steven-Lapsley et al. 2011 (25) |
Quality assessment
In terms of quality, the mean PEDro score of the studies was 8.7 (Table IV). All studies reported eligibility criteria except for 3 studies (14, 18, 20). Two studies did not explain their randomization strategy clearly (20, 29). Blinding assessors occurred in 9 of 18 studies, respectively (19–21, 24, 26, 29–32).
Table IV.
PEDro scale included studies (n= 18)
| Study | Pedro Clinical Appraisal Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion, exclusion criteria | Randomization | Patients were casual extract | Groups presented similar income data | Blind patients | Blind therapists | Blind assessor | Measures obtained from more than 85% of initial subjects | All subjects received treatment or control | There was some comparative analysis between the groups | The analysis was satisfaction | Total/11 | |
| Bade et al. 2017 (26) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| Beaupre et al. 2001 (14) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 9 |
| Bruun-Olsen et al. 2009 (18) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 9 |
| Demircioglu et al 2015 (27) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 8 |
| Denis et al. 2006 (15) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 9 |
| Ebert et al. 2013 (24) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Hardt et al. 2018 (23) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 8 |
| Jakobsen et al. 2014 (28) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 8 | |
| Labraca et al. 2011 (19) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Lastayo et al. 2009 (29) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Lenssen et al. 2006 (20) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Lenssen et al. 2008 (21) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Liao et al. 2013 (30) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| Moeller et al. 2014 (22) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 8 |
| Piva et al. 2017 (37) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Rahmann et al. 2008 (16) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | 7 |
| Schache et al. 2019 (32) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Steven-Lapsley et al. 2012 (25) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | 7 |
Knee range of motion
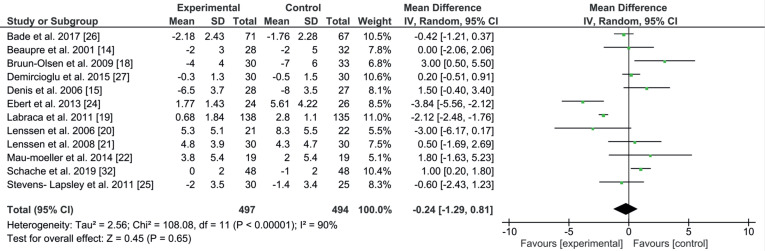
Knee ROM was measured with a standard goniometer, and covered active flexion and extension of the knee from different positions, e.g. during seated, supine, lying and prone positions. The results compared improvement and lack of it between experimental and control groups after the rehabilitation programme ended. The active extension ROM dataset was available for 12 studies (991 patients) (14, 15, 18–22, 24–27, 32), the mean and SD of the following-up period was 204.2 days (SD 220.1). The comparison for knee extension was numerically in favour of the experimental group (EE –0.24; 95% CI–1.29 to 0.81; p = 0.65, Fig. 2). In the active extension ROM, no significant differences were found in the 11 studies between the modified and the standard rehabilitation programmes after TKA. The studies shared the same starting point for the rehabilitation programme, which was within the first 36 h after surgery, the differences were in the duration and the method used, except for one study Schache et al. (32), which started 2 weeks after the operation. One study had an equal mean result between the 2 groups, so no preference was analysed for the methods used in either the experimental or control groups (14). Five studies out of the 12 reported improvements in the ROM mean in the experimental group (19, 20, 24–26). The other studies reported improvement in the ROM mean in the control group (15, 18, 21, 22, 27, 32). Three out of these 6 studies used CPM as the main method to regain the ROM, without considering the other methods or exercises, which could be a reason for these negative results (15, 18, 21). Heterogeneity was high, at 90%.
Fig. 2.
Forest plot of the knee extension comparison. SD: standard deviation; 95% CI: 95% confidence interval.
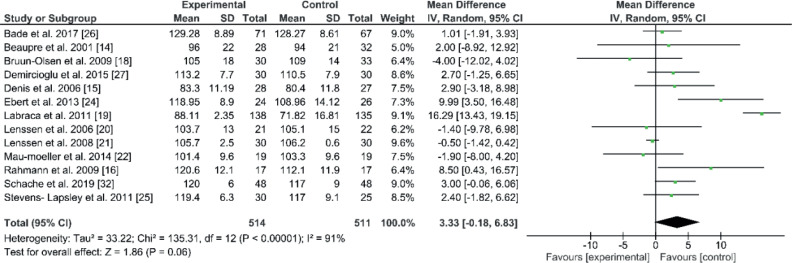
The active flexion ROM dataset was available for 13 studies (n=1.025 patients) (14–16, 18–22, 24–27, 32). The mean and SD for the following-up period was 218.8 days (SD 220.1), where the comparison for knee flexion was numerically in favour of the control group (EE 3.33; 95% CI –0.18 to 6.83; p=0.06, Fig. 3). In active flexion ROM, no significant differences were found in the 13 studies between modified and standard rehabilitation programmes after TKA. Eleven studies shared a starting time, which was within the first 4 days after surgery. Two studies started directly after discharge. Four studies found better mean improvement for the experimental group (18, 20–22). Two out of these 4 studies used CPM plus strength, function and walking exercises (21, 22). The third study used functional exercises (20), and the fourth used sling exercises (22). The other 9 studies showed better mean improvement for the control group. Seven studies used different methods (e.g. sliding board technique, active/ passive exercises and water exercises for ROM, NMES, extra strength exercise for hip), which were added to the individual programmes (14, 16, 19, 24, 25, 27, 32). The eighth study used early high-intensity progression (26). The ninth study used CPM parallel to strength and function exercises (15). Heterogeneity was also high, at 91%.
Fig. 3.
Forest plot of the knee flexion comparison. SD: standard deviation; 95% CI: 95% confidence interval.
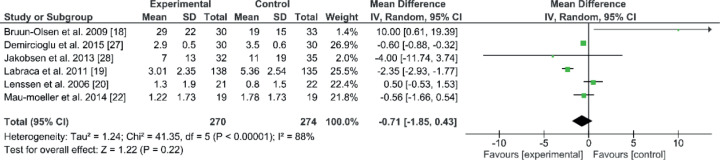
Pain
Pain was measured in 544 patients in 6 studies using a visual analogue scale (VAS). The results were taken after the rehabilitation programme ended. The mean and SD for the follow-up period was 91.6 days (SD 56.3). The comparison of VAS was numerically in favour of the experimental group (EE –0.71; 95% CI–1.85 to 0.43; p = 0.22, Fig. 4). No significant differences were found in the 6 studies between modified and standard rehabilitation programmes after TKA. The 6 studies used visual analogue scale (VAS) and calculated the mean and SD (18–20, 22, 27, 28). There was no significant difference in the combined result. All studies had an early starting rehabilitation point, within 48 h, except one study that started one week after surgery (28). Four studies out of 6 had better mean improvement in the experimental group than in the control group (19, 22, 27, 28). One of these 4 studies used progressive strength, in parallel with ROM exercises, strength, function and walking (28). The second study used sling exercise training (22). The third study used the same combination earlier than the control group, adding extra exercises, such as transfer and active daily living exercises (19). The fourth study used neuromuscular electrical stimulation (NMES) in parallel with strength, ROM, function, and walking exercises (27). The remaining 2 studies had better improvement in the control groups (18, 20). Heterogeneity was high, at 88%.
Fig. 4.
Forest plot of the visual analogue scale (VAS) comparison. SD: standard deviation; 95% CI: 95% confidence interval.
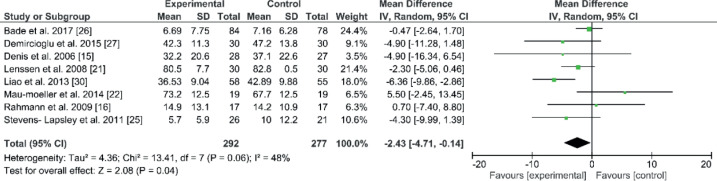
Western Ontario and McMaster Universities Osteoarthritis Index
The overall results of the WOMAC were chosen instead of 1 or 2 attributes. Eight studies with 569 patients used WOMAC to analyse pain, stiffness, and function. The mean and SD for the following-up period was 269.1 days (SD 233.1). The comparison of WOMAC resulted in a significant improvement in the experimental group (EE –2.43; 95% CI –4.71 to –0.14; p = 0.04, Fig. 5). Eight studies used a WOMAC questionnaire to collect information about pain, stiffness, and function in their patients (15, 16, 21, 22, 25–27, 30). Seven studies had an early starting point for rehabilitation, within 48 h, except one study, which started at least 2 months after surgery (30). The combined result was significant for the experimental groups. All of the studies shared the same programme components (strength, ROM, walking and function exercises). One study had significant improvement in the overall WOMAC at the end of the rehabilitation programme (30). Liao et al. (30) added balance and endurance exercises, which might explain this positive result for WOMAC in this study. Heterogeneity was moderate, at 48%.
Fig. 5.
Forest plot of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) comparison. SD: standard deviation; 95% CI: 95% confidence interval.
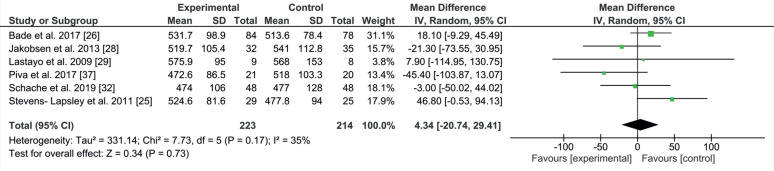
6-minute walking test
The 6MWT measures the maximal distance a subject is able to walk in 6 min, and is a moderately valid indicator of submaximal and maximal aerobic capacity (r = 0.53) in patients with knee OA (33). Six studies with 437 patients used 6MWT (25, 26, 28, 29, 31, 32). The mean and SD for the following-up period was 227.8 days (SD 111.9). All the studies had long rehabilitation programmes. The difference was in the starting point, where 4 studies started late and lasted long (28, 29, 31, 32) and the other 2 started early (25, 26). The comparison of 6MWT was numerically in favour of the control group (EE 4.34; 95% CI –20.74 to 29.41; p = 0.17, Fig. 6). The combined result showed no significant difference between groups. Heterogeneity was moderate, at 35%.
Fig. 6.
Forest plot of the 6-minute walking test (6MWT) comparison. SD: standard deviation; 95% CI: 95% confidence interval.
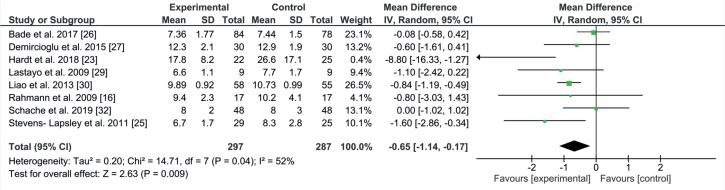
Timed Up and Go test
The TUG test measures the time to rise from an armchair, walk 3 m, turn around, and return to sitting in the same chair without physical assistance (34). Eight studies with 584 patients included TUG (16, 23, 25–27, 29, 30, 32). The mean and SD for the following-up period by days was 239.3 (SD 108.6). Comparison of the TUG test resulted in a significant improvement in the experimental group (EE –0.65; 95% CI –1.14 to –0.17; p = 0.009; Fig. 7). Eight studies used TUG in their measurements (16, 23, 25–27, 29, 30, 32). The combined result was significant for the experimental groups. All studies had almost the same programme components, consisting of strength, ROM and walking exercises. The difference was in the starting point; 3 studies started late (29, 30, 32) and the other 5 started early (16, 23, 25–27). All studies had long rehabilitation programmes, except for one study (25). Only one study showed a lack of difference between groups (16). Five studies had non-significant improvements in the experimental group (23, 25–27, 29). One study had an equal mean result (32). One study showed significant improvement in the experimental group (30). Heterogeneity was moderate, at 52%.
Fig. 7.
Forest plot of the Timed Up and Go (TUG) comparison. SD: standard deviation; 95% CI: 95% confidence interval.
DISCUSSION
The main finding of this study is that there is no specific rehabilitation method that can be recommended after TKA regardless of the time it will be applied after the operation.
Many meta-analyses have compared rehabilitation methods or compared the effect of rehabilitation programmes on the length of stay. Artz et al. (33) conducted a systematic review that found no differences in physical function and pain outcomes between physiotherapy with no supervision and home exercise with outpatient supervision in the short-term. The only difference in one study was that walking skills intervention was associated with long-term improvement in walking performance. A review by Sattler et al. (35) compared 4 different early supervised exercise therapy programmes, commencing within 48 h after TKA. The study reported that there are no significant differences between groups in the maximum knee flexion or knee society score after 6 weeks post-operation.
Regarding the movement in the knee joint, knee ROM is the main follow-up outcome after TKA, and it is believed to reflect patient progression, although it is a poor marker of implant success (13). For active knee extension and flexion ROM, comparison between standard and modified rehabilitation programmes was conducted. Active knee extension and flexion ROM were based on 12 studies with 991 patients, and 13 studies with 1,025 patients respectively. However, no significant differences were found between the standard and modified rehabilitation programmes after TKA. In addition, high heterogeneity was observed in the current meta-analyses of ROM, possible causes being the use of different measurement positions across studies and difficulties extracting data from studies, particularly in the case of active extension, where negative values can be misleading and the low reliability of the instruments employed (36). Furthermore, considering knee flexion, the programmes perhaps, missing the walking part of the programme gave this negative result, in contrast to other studies using continuous passive motion (CPM).
From these outcomes regarding both factors, knee extension and flexion, it seems that depending on CPM alone does not improve knee extension, but does improve knee flexion after TKA.
Pain is one of the most important outcomes after TKA. The persistence of pain after surgical procedures has become a major focus of interest, and its prevention now represents a challenge for caregivers as an index for the quality of healthcare (36). Pain was reported in 6 studies (18–20, 22, 27, 28). These studies used a combination of ROM, strength, function, and walking. Pain reduction post-TKA was reported in 4 different studies, each implementing a different additional method (19, 20, 27, 28). This suggests that no particular method has a unique advantage in reducing pain. This result corresponds with the result of a study by Chughtai et al. (37), that there were no clear guidelines to reduce pain while using different non-pharmacological therapy, such as NMES, transcutaneous electrical nerve stimulation (TENS) or cryotherapy.
Regarding WOMAC, the current study showed significant differences in the combined result. Six studies out of 8 reported improvements in the experimental group, 2 studies were early-short (15, 21), 3 were early-long (23, 25, 26) and one study was late-long (30). The 2 studies that reported improvement for the control group were early-short (16, 22). During the search, studies were identified that supported our findings and others contradicted it. A study by Hertog et al. (3) supported the early-long rehabilitation interval. On the other hand, in the same interval, a study by Mahomed et al. (38) demonstrated contradictory results from the study of Hertog et al. (3). Furthermore, a study by Piva et al. (39) found contradictory results from the latelong result. It seems that there is no specific method to improve WOMAC according to the time interval.
The 6MWT is one of the common tests used in studies, especially for interventions in the lower limbs (40). We found 6 studies that used 6MWT (25, 26, 28, 29, 31, 32). The studies shared the same programme components (strength, ROM, walking, and function exercises). Four out of 6 studies were late-long, and the other 2 were early-long. The result did not indicate that the starting point or the duration affect the 6MWT. This finding was supported by 2 studies, which applied different rehabilitation programmes with different starting points (41).
Finally, the TUG test is a timed test, in which participants start in a seated position in an armchair and then rise, go forward 3 m, turn around, and sit back down (29). Eight studies were found that used TUG in their trials (16, 23, 25–27, 29, 30, 32). The combined result was significant for the experimental group. As mentioned above, the studies had almost the same programme components. The difference was at the starting point. Referring to this result, we believe that the combined result is skewed, and, thus the current outcome must be considered with caution. Another interesting finding is that the starting point of the rehabilitation programmes does not seem to have any effect on the result, while the long period of rehabilitation suggests a clinical added value.
Study limitations
This meta-analysis has some limitations. There was a high level of heterogeneity between studies, and hence the data had to be interpreted with caution.With regard to the use of subgroup analysis to reduce heterogeneity, this was precluded by a lack of data
Conclusion
When comparing modified rehabilitation programmes with standard programmes post-TKA, there is no clear pattern in the combination of starting time-point and duration of rehabilitation that significantly improves clinical outcomes. Moreover, improved clinical out-comes could not be attributed solely to any particular modification to the programmes. Accordingly, a one-size-fits-all approach to modified rehabilitation programmes does not result in systematic improvement in clinical outcome.
ACKNOWLEDGEMENT
This work was supported by the German Academic Exchange Service (DAAD), Research Grant-Doctoral programmes in Germany, under grant 57129429.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge Aet al. Knee replacement. The Lancet 2012; 379: 1331–1340. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs, et al. The burden of musculoskeletal diseases in the United States. JAAOS 2008: 247. [Google Scholar]
- 3.Den Hertog, Kerstin Gliesche, Ju¨rgen Timm, Bernd Mu¨hlbauer, Sylvia Zebrowski. Pathway-controlled fast-track rehabilitation after total knee arthroplasty: a randomized prospective clinical study evaluating the recovery pattern, drug consumption, and length of stay. Arch Orthop Trauma Surg 2012; 132: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petterson SC, Mizner RL, Stevens JE, Raisis L, Bodenstab A, Newcomb Wet al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. J A & R. 2009; 61: 174–183. [DOI] [PubMed] [Google Scholar]
- 5.Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010; 40: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranawat CS, Ranawat AS, Mehta A. Total knee arthroplasty rehabilitation protocol:: what makes the difference?: What makes the difference? J Arthroplasty 2003; 18: 27–30. [DOI] [PubMed] [Google Scholar]
- 7.Cho W. Knee joint arthroplasty. Berlin: Springer; 2014. [Google Scholar]
- 8.Franklin PD, Li W, Ayers DC. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. Functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res 2008; 466: 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am 2005; 87: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gstoettner M, Raschner C, Dirnberger E, Leimser H, Krismer M. Preoperative proprioceptive training in patients with total knee arthroplasty. Knee 2011; 18: 265–270. [DOI] [PubMed] [Google Scholar]
- 11.Chehuen Marcel, Gabriel G Cucato, Celso Ricardo F Carvalho, Raphael M Ritti-Dias, Nelson Wolosker, Anthony S Leichtet al. Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: a randomized controlled trial. J Sci Med Sport 2017; 20: 886–892. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–9, W64. [DOI] [PubMed] [Google Scholar]
- 13.Florez-García M, Fernando García-Pérez, Rafael Curbelo, Irene Pérez-Porta, Betina Nishishinya, Maria Piedad Rosario Lozanoet al. Efficacy and safety of home-based exercises versus individualized supervised outpatient physical therapy programs after total knee arthroplasty: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2017; 25: 3340–3353. [DOI] [PubMed] [Google Scholar]
- 14.Beaupré LA, Davies DM, Jones CA, Cinats JG. Exercise combined with continuous passive motion or slider board therapy compared with exercise only: a randomized controlled trial of patients following total knee arthroplasty. Phys Ther 2001. [PubMed] [Google Scholar]
- 15.Denis M, Moffet H, Caron F, Ouellet D, Paquet J, Nolet L. Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Phys Ther 2006; 86: 174–185. [PubMed] [Google Scholar]
- 16.Rahmann AE, Brauer SG, Nitz JC. A specific inpatient aquatic physiotherapy program improves strength after total hip or knee replacement surgery: a randomized controlled trial. Arch Phys Med Rehabil 2009; 90: 745–755. [DOI] [PubMed] [Google Scholar]
- 17.Mayers B. Wound Management: principles and practices. Upper Saddle River, NJ: Pearson Education Inc.; 2008, p. 12–16. [Google Scholar]
- 18.Bruun-Olsen V, Heiberg KE, Mengshoel AM. Continuous passive motion as an adjunct to active exercises in early rehabilitation following total knee arthroplasty – a randomized controlled trial. Disabil Rehabil 2009; 31: 277–283. [DOI] [PubMed] [Google Scholar]
- 19.Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil 2011; 25: 557–566. [DOI] [PubMed] [Google Scholar]
- 20.Lenssen, Crijns YH, Waltjé EM, van Steyn MJ, Geesink RJ, van den Brandt PAet al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord 2006; 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenssen TA, van Steyn MJ, Crijns YH, Waltjé EM, Roox GM, Geesink RJ, van den Brandt PA, De Bie RA. Effectiveness of prolonged use of continuous passive motion (CPM), as an adjunct to physiotherapy, after total knee arthroplasty. BMC Musculoskelet Disord 2008; 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mau-Moeller A, Behrens M, Finze S, Bruhn S, Bader R, Mittelmeier W. The effect of continuous passive motion and sling exercise training on clinical and functional outcomes following total knee arthroplasty: a randomized activecontrolled clinical study. Health Qual Life Outcomes 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardt S, Schulz MRG, Pfitzner T, Wassilew G, Horstmann H, Liodakis E, et al. Improved early outcome after TKA through an app-based active muscle training programme-a randomized-controlled trial. Knee Surg Sports Traumatol Arthrosc 2018; 26: 3429–3437. [DOI] [PubMed] [Google Scholar]
- 24.Ebert JR, Joss B, Jardine B, Wood DJ. Randomized trial investigating the efficacy of manual lymphatic drainage to improve early outcome after total knee arthroplasty. Arch Phys Med Rehabil 2013; 94: 2103–2111. [DOI] [PubMed] [Google Scholar]
- 25.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther 2012; 92: 210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bade MJ, Struessel T, Dayton M, Foran J, Kim RH, Miner T, et al. Early high-intensity versus low-intensity rehabilitation after total knee arthroplasty: a randomized controlled trial. Arthritis Care Res (Hoboken) 2017; 69: 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demircioglu DT, Paker N, Erbil E, Bugdayci D, Emre TY. The effect of neuromuscular electrical stimulation on functional status and quality of life after knee arthroplasty: a randomized controlled study. J Phys Ther Sci 2015; 27: 2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res (Hoboken) 2014; 66: 1856–1866. [DOI] [PubMed] [Google Scholar]
- 29.LaStayo PC, Meier W, Marcus RL, Mizner R, Dibble L, Peters C. Reversing muscle and mobility deficits 1 to 4 years after TKA: a pilot study. Clin Orthop Relat Res 2009; 467: 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao CD, Liou TH, Huang YY, Huang YC. Effects of balance training on functional outcome after total knee replacement in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2013; 27: 697–709. [DOI] [PubMed] [Google Scholar]
- 31.Piva SR, Almeida GJ, Gil AB, DiGioia AM, Helsel DL, Sowa GA. Effect of comprehensive behavioral and exercise intervention on physical function and activity participation after total knee replacement: a pilot randomized study. Arthritis Care Res (Hoboken) 2017; 69: 1855–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schache MB, McClelland JA, Webster KE. Incorporating hip abductor strengthening exercises into a rehabilitation program did not improve outcomes in people following total knee arthroplasty: a randomised trial. J Physiother 2019; 65: 136–143. [DOI] [PubMed] [Google Scholar]
- 33.Artz N, Elvers KT, Lowe CM, Sackley C, Jepson P, Beswick AD. Effectiveness of physiotherapy exercise following total knee replacement: systematic review and meta-analysis. BMC Musculoskelet Disord 2015; 16: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podsiadlo D RS. The Timed “Up & Go” a test of basic functional mobility for frail elderly persons: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 35.Sattler LN, Hing WA, Vertullo CJ. What is the evidence to support early supervised exercise therapy after primary total knee replacement? A systematic review and meta-analysis. BMC Musculoskelet Disord 2019; 20: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cleffken B, van Breukelen G, Brink P, van Mameren H, Olde Damink S. Digital goniometric measurement of knee joint motion. Evaluation of usefulness for research settings and clinical practice. Knee 2007; 14: 385–389. [DOI] [PubMed] [Google Scholar]
- 37.Chughtai M, Elmallah RDK, Mistry JB, Bhave A, Cherian JJ, McGinn TL, et al. Nonpharmacologic pain management and muscle strengthening following total knee arthroplasty. J Knee Surg 2016; 29: 194–200. [DOI] [PubMed] [Google Scholar]
- 38.Mahomed NN, Davis AM, Hawker G, Badley E, Davey JR, Syed KA, et al. Inpatient compared with home-based rehabilitation following primary unilateral total hip or knee replacement: a randomized controlled trial. J Bone Joint Surg Am 2008; 90: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 39.Piva SR, Schneider MJ, Moore CG, Catelani MB, Gil AB, Klatt BA, et al. Effectiveness of later-stage exercise programs vs usual medical care on physical function and activity after total knee replacement: a randomized clinical trial. JAMA Netw Open 2019; 2: e190018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox PD, Frengopoulos CA, Hunter SW, Sealy CM, Deathe AB, Payne MWC. Impact of course configuration on 6-minute walk test performance of people with lower extremity amputations. Physiother Can 2017; 69: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruun-Olsen V, Heiberg KE, Wahl AK, Mengshoel AM. The immediate and long-term effects of a walking-skill program compared to usual physiotherapy care in patients who have undergone total knee arthroplasty (TKA): a randomized controlled trial. Disabil Rehabil 2013; 35: 2008–2015. [DOI] [PubMed] [Google Scholar]