Abstract
Objective
To assess the longitudinal effects of integrated spasticity management incorporating repeated cycles of botulinum toxin A type A (BoNT-A) over 2 years.
Methods
The Upper Limb International Spasticity study was a prospective, observational, cohort study following adult patients over 2 years of integrated upper-limb spasticity management including repeat botulinum toxin (BoNT-A) treatment (any commercially-available product).
Results
A total of 1,004 participants from 14 countries were enrolled, of which 953 underwent ≥ 1 BoNT-A injection cycle (median 4 cycles) and had ≥ 1 goal attainment scaling assessment. Most participants (55.9–64.6% across cycles 1–6) saw a therapist after BoNT-A treatment; the most frequent therapy intervention was passive stretch (70.1–79.8% across cycles 1–6). Patients achieved their goals as expected over repeated cycles; mean cumulated goal attainment scaling T-score at 2 years was 49.5 (49.1, 49.9). Mean goal attainment scaling change scores of ≥ 10 were maintained across up to 7 cycles. Higher rates of goal achievement were seen for primary goals related to passive vs active function (86.6% vs 71.4% achievement). Standardized measures of spasticity, pain, involuntary movements, active and passive function improved significantly over the study.
CONCLUSION
This large, international study provides evidence for benefit of repeated cycles of BoNT-A, over 2 years captured through person-centred goal attainment and standardized measures.
LAY ABSTRACT
This paper presents the primary efficacy results from the Upper Limb International Spasticity (ULIS-III) study, a large international longitudinal study that explored real-life clinical practice in the integrated management of upper-limb spasticity, using botulinum toxin-A (BoNT-A) in conjunction with physical therapies. The study provides evidence for the sustained functional benefit of repeated cycles of BoNT-A. In particular, those patients who continued to receive repeated injections for up to 7 cycles in 2 years showed relevant goal attainment. Standard outcome measures generally supported the rates of goal achievement; ratings of pain, involuntary movements, active and passive function all improved significantly over each treatment cycle. The successful results from ULIS-III highlight the importance of accurate and clear goal-setting during BoNT-A treatment to help target clinical intervention and the use of focused outcome measurement.
Key words: botulinum toxin A, goal attainment scaling, physical therapies, post-stroke spasticity, stroke rehabilitation
Spasticity is a common feature of upper motor neurone syndrome, which typically follows damage to the central nervous system. Botulinum toxin type A (BoNT-A) is shown in controlled studies to be a safe and effective focal intervention for reduction of spasticity (1-3), which is now recommended for use in routine clinical practice by national and international guidelines (4–6). However, while changes at the level of impairment are readily seen, changes at the level of function or activity have been more difficult to demonstrate (7–9). This is partly due to the wide heterogeneity of patient presentation and the diversity of individual expectations and goals for treatment (10).
Goal attainment scaling (GAS) (11) is increasingly accepted as a person-centred outcome measure for evaluating the attainment of individual goals for treatment and is now used to assess the effectiveness of rehabilitation in the areas that matter most to the patients and their caregivers (9, 10, 12). GAS has been shown to be sensitive to changes that occur following treatment of focal spasticity using BoNT-A (13–15), but a criticism of GAS is the theoretical lack of comparability in the data produced (16). Moreover, spasticity is a long-term condition, often requiring repeated treatment, and most of the studies to date have evaluated outcomes from just 1 or 2 injection cycles, mainly following stroke.
The Upper Limb International Spasticity (ULIS) study programme is a series of international observational studies designed to describe real-life clinical practice in the use of BoNT-A to manage upper limb spasticity (17). The first 2 studies (ULIS-I (18) and ULIS-II (19)) documented current practice and confirmed the feasibility of a common international dataset to collect prospective data incorporating GAS to capture outcomes across a range of goal areas, which included passive and active function, as well as pain and mobility. The third study (ULIS-III) describes the effects of integrated spasticity management incorporating repeated cycles of BoNT-A over 2 years in patients with spasticity of any aetiology. It introduces novel methods to: (i) systematically capture integrated approaches to spasticity management, including multidisciplinary therapy inputs; and (ii) the Upper Limb Spasticity Index, which combines GAS with targeted standardized measures (selected according to the patient’s priority goals for treatment) to provide comparability between different populations and practices.
This article presents the first primary effectiveness findings from the ULIS-III longitudinal study, reporting the population level recorded at each cycle. In addition, our previously-reported analysis of baseline and first cycle data identified significantly different injection intervals between the various BoNT-A products (20), and a secondary aim was to explore in more detail the longevity of these observations.
METHODS
Study design and participants
Full details of the ULIS-III methodology have been described previously (20, 21). In brief, ULIS-III (registered at clinicaltrials.gov NCT02454803) was an international observational, prospective, longitudinal cohort study following patients with upper-limb spasticity over 2 years, treated through integrated programmes incorporating pharmacological intervention (primarily botulinum toxin injections) and physical management, as delivered in real-life clinical practice. It was conducted in 14 countries across 4 continents. Clinicians treated patients in their normal way, guided by their local Summary of Product Characteristics and local therapeutic guidelines. Treatment was goal-directed, and patients could change their primary and/or secondary goals at the initial visit of each cycle. Patients participated in concomitant therapies as per usual local practice, and all activities (number, duration and types of non-pharmacological intervention) were documented in the Upper Limb Spasticity Therapy Recording schedule (ULSTR) (21).
The study was conducted in compliance with Guidelines for Good Pharmacoepidemiology Practices (GPP). Marketing authorization for the use of BoNT-A in this context was ensured for each participating country prior to the start of the study. Ethics approval and written informed consent to the recording of anonymous data was obtained in countries where this was required.
Study population
Specialist centres recruited up to 30 consecutive adult patients (≥ 18 years old) with upper-limb spasticity presenting for treatment with BoNT-A in routine clinical practice. Patients could be new to BoNT-A treatment or previously treated with BoNT, provided there had been at least a 12-week interval between the last injection and study entry. During the study, patients could be treated with any BoNT-A formulation, but the decision to treat was taken prior to, and independently from, the decision to offer enrolment to the patient for participation in the study.
Outcome assessment
There were 4 visit types in the study: baseline visit, injection (follow-up) visits, end of study visit, and early discontinuation visit. The timing of follow-up was at the discretion of the investigator; no additional visits were required.
All primary and secondary goals and outcome assessment data were documented using the ULS Index (21) (Table SI1), which records:
Severity of spasticity presentation, level of impairment and confounding factors.
Goals for treatment captured using the Goal Attainment Scaling Evaluation of Outcome for Upper Limb Spasticity (GAS-eous) Tool (21), which uses a structured approach to goal-setting and evaluation of goal attainment, which is assimilated to yield a GAS T-score utilizing the GAS light method (22). At each baseline visit (i.e. each visit where new goals were set using GASeous), a goal parameter was recorded to define when and how a particular goal would be assessed. Baseline and target ratings were recorded, and baseline GAS scores were derived from the baseline goal rating. Goal attainment was evaluated at the next routine follow-up/re-injection visit.
-
Effectiveness as assessed by targeted standardized “rating scale” measures selected according to the chosen goal area. In addition to the Modified Ashworth Scale (MAS, which is assessed in all patients), the available targeted standardized measures include:
The Numbered-Graphic Pain Rating Scale (NPRS) (23) for pain goals;
Associated Reaction Rating Scale (ARRS) (24) for goals related to involuntary movements;
Upper Limb Spasticity adapted Neurological Impairment Scale (ULS-NIS) (25) for goals related to maintaining range of movement/avoiding contractures;
Arm Activity Measure (ArmA) (26) for goals related to passive and active function;
Functional Ambulation Category (FAC) (27) for goals related to mobility.
If goals are achieved as expected, the mean GAS T-score would be 50. The minimal clinically important change in GAS T-score from baseline is reported to be 10 (13). Training and proactive feedback on goals set from an early stage in the ULIS-III study helped to ensure a high-quality goal-setting process and the validity of GAS as a measure of achievement of intended goals for treatment (21).
Reporting of related adverse events followed the standard regulations related to spontaneous adverse event reporting for marketed products and was not collected as an outcome variable.
Statistical analysis
Assuming a type I error of 5% (2-sided), it was estimated that a sample size of at least 800 subjects would provide 90% power for comparisons between goal areas based on cumulated GAS T-score (assuming equal distribution of 115 patients in the 7 goal areas). This sample size would also allow at least 80% power for comparisons in the case of unbalanced distribution of sample sizes between goal areas (ratio up to 3:1). Assuming a dropout rate of 20% over 2 years, a total of at least 1,000 subjects was required for recruitment.
Primary analyses of effectiveness were based on the Effectiveness Population, which includes all patients who received at least one BoNT-A injection and had at least one post-baseline GAS assessment.
The primary outcome was the cumulated GAS T-score, defined as the mean of the individual GAS T-scores across all cycles per patient during the 2-year period. Mean and 95% confidence intervals (CI) GAS T-scores at baseline and after each injection cycle were also assessed for each individual goal area.
This study also reports descriptive statistics (n (%)) for categorical data and mean and 95% confidence intervals (95% CI) or median (inter-quartile range (IQR)) for scaled data. As fewer than 30 patients had more than 7 cycles, descriptive data are provided for up to 7 cycles only, in order to remain representative of the main population. Change from baseline for scaled data were analysed using paired t-tests. Spearman’s rank correlations between the 5-point primary goal achievement score (ranging from –2 to +2) for each goal area and change in the relevant standardized outcome measure were assessed for all primary goal observations (i.e. a patient could be counted more than once if he/she had multiple primary goals within the same goal area) over the follow-up period.
To examine differences in injection intervals between the different BoNT-A preparations while controlling for potential confounding factors, a multivariate additive linear regression model was developed to determine factors influencing the mean time to retreatment in patients who remained on the same BoNT-A formulation throughout the study. In the first step, univariate analyses were used to identify candidate covariates potentially associated with interval duration, including baseline and treatment characteristics. All variables with a p-value < 0.20 in the univariate analyses were entered into the multivariate model selection process along with age, sex, BoNT-A type and previous BoNT-A treatment, which were to be included in the multivariate model, irrespective of p-value in the univariate analysis step. Level 1 interactions of interest were also assessed. Other potential variates tested in the univariate model were concomitant therapy (type of therapists seen and number of therapy occasions), use of injection guidance technique, indexed BoNT-A dose, use of concomitant systemic anti-spasticity medications and concomitant treatment of lower-limb spasticity. To understand how repeat treatment affects treatment intervals, this study also performed a cycle-by-cycle analysis for the first 4 cycles, which included sufficient patient numbers per BoNT-A formulation for analysis.
All statistical evaluations were performed using the Statistical Analysis System (SAS V.9.4; SAS Institute, Inc, Cary, NC, USA). As this was a non-interventional study reflecting real-life clinical practice, missing data were expected, and no imputations were made. All statistical tests were 2-sided and performed at the 5% level of significance. In this observational study, no formal treatment comparisons were made. The p-values presented were interpreted only in the exploratory sense.
RESULTS
Patient disposition and baseline characteristics
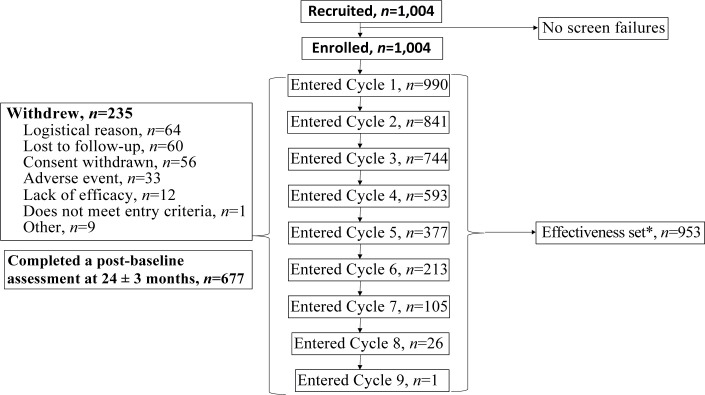
Between 14 January 2015 and 7 April 2017, a total of 1,004 participants were enrolled across the 58 active centres, of which 990 entered Cycle 1, and 953 underwent ≥ 1 BoNT-A injection cycle and had ≥ 1 GAS assessment and were included in the effectiveness population (Fig. 1).
Fig. 1.
Patient flow. *Effectiveness set includes all patients who received at least one botulinum toxin A (BoNT-A) injection and had at least one post-baseline goal attainment scaling (GAS) assessment. 90 of the 235 withdrawals occurred after the first injection cycle. The number of treatment cycles given during the 2-year period depended on the patient’s condition, their treatment goals and local practice.
All BoNTA injections administered within the 2-year period were recorded, which proved to be up to 9 cycles. The study completed when the last patient completed the final study assessment visit on 3 June 2019. The main reasons for premature study withdrawal were logistics (e.g. ability to attend visits) and loss to follow-up. Overall 90 of the 235 withdrawals occurred after the first injection cycle.
Baseline characteristics are provided in Table I. Overall, 56% of patients were male and the mean (standard deviation; SD) age was 54 years (SD 15.39 years old. On average, patients were living with chronic spasticity, the mean time since onset of spasticity was 7.6 years (SD 9.4). For the large majority of subjects, ULS was secondary to a vascular event and spasticity was regionally distributed in the affected upper limb. At the time of study enrolment, two-thirds of the patients had previously received BoNT-A treatment for ULS, and one-third were naïve.
Table I.
Baseline demographics and severity of presentation
| Parameter | Effectiveness Set (n = 953)* |
|---|---|
| Age, years, mean (SD) | 54.0 (15.3) |
| Sex, n (%) Male | 537 (56) |
| Time since onset of the event leading to upper limb spasticity, years | |
| Mean (SD) | 7.6 (9.4) |
| Median (IQR) | 4.2 (6.5) |
| Diagnosis of condition leading to upper limb spasticity, n (%) | |
| Acquired brain injury (stroke/trauma/other) | 870 (91.3) |
| Spinal cord injury | 15 (1.6) |
| Progressive neurological condition | 20 (2.1) |
| Congenital | 44 (4.6) |
| Other | 4 (0.4) |
| Aetiology, n (%) | |
| Trauma | 71 (7.5) |
| Vascular (infarct or haemorrhage) | 786 (82.5) |
| Hypoxic | 25 (2.6) |
| Inflammatory/infective | 15 (1.6) |
| Tumour | 19 (2.0) |
| Degenerative | 12 (1.3) |
| Cerebral palsy | 10 (1.0) |
| Other | 15 (1.6) |
| Spasticity distribution, n (%) | |
| Focal (part of the limb) | 190 (19.9) |
| Regional | 763 (80.1) |
| Affected limb, n (%) | |
| Right arm | 409 (42.9) |
| Left arm | 488 (51.2) |
| Both arms | 56 (5.9) |
| From the Neurological Impairment Scale | |
| Motor impairment, n (%)** | n = 908* |
| Impaired muscle power | 783 (86.2) |
| Impaired control of voluntary movement | 540 (59.5) |
| Proximal motor impairment | 828 (91.2) |
| Distal motor impairment | 859 (94.6) |
| Confounding factors, n (%)** | n = 914* |
| Severe weakness | 416 (45.5) |
| Impaired mobility of joints | 631 (69.0) |
| Communication impairment | 397 (43.4) |
| Emotional/behaviour impairment | 309 (33.8) |
| Impaired cognitive function | 289 (31.6) |
| Sensation impairment (n = 913) | 484 (53.0) |
Not all patients had assessments of impairments; data are presented for patients with available data.
Defined as at least mild severity on the Upper Limb Spasticity adapted Neurological Impairment Scale (ULS-NIS), except for the presence of severe weakness, which was assessed as yes/no.
IQR: interquartile range; SD: standard deviation.
Spasticity management
The number of treatment cycles given during the follow-up period depended on the patient’s condition, their treatment goals and local practice. One of the key purposes of this study was to explore the number of injection cycles used and the circumstances governing this. Participants underwent a median (range) of 4 (1–9) BoNT-A injection cycles during the 2-year period. As expected, the number of patients requiring higher numbers of cycles progressively decreased and only one patient underwent 9 cycles of treatment. Only approximately one-third of patients had 5 or more cycles.
In Cycle 1, the most commonly injected BoNTA preparation was abobotulinumtoxinA (n = 602), followed by onabotulinumtoxinA (n = 241) and incobotulinumtoxinA (n = 104) (Table SII1). The large majority of patients were injected with the same BoNT-A preparation across the different cycles (see below), but there was a small overall trend towards increase in the mean injected dose of each preparation (i.e. those who required more frequent injection also tended to require higher doses).
Throughout the study, the most frequently injected segments of the subjects’ affected upper limb were the forearm (injected in 92.3% to 96.7% of patients across cycles 1–6) followed by the upper arm (injected in 70.0% to 72.9%), hand (39.6% to 47.4%) and shoulder (39.0% to 46.0%). The most frequently injected muscles included the flexor digitorum superficialis in the forearm (injected in 74.0% – 77.9% of patients across cycles 1–6) and the biceps brachii in the upper arm (42.7% to 52.3%). The pectoralis major was injected in 32.5% to 35.2% of patients. For most patients, an instrumental injection guidance technique was used for at least one muscle. The most frequently used guidance technique was electrical stimulation.
Between cycles 2 and 4, more than 60% of patients had a change in injection practice (e.g. change in dose, muscles injected, BoNT-A product, etc.), and in 90% of cases the reason was clinically related, and in more than half the cases, this was due to a change or modification of treatment goals (Table SIII1). In 99% of cases this involved a change in the muscles injected, in 90% of cases the BoNT-product remained the same.
At baseline and throughout the study, more than half of patients (55.9–64.6% across cycles 1–6) visited a therapist since their last spasticity clinic appointment, mainly physiotherapists.
Most patients were treated in individual sessions with a qualified therapist rather than in group sessions. Those patients who had more frequent injections also had more intensive therapy: the mean total amount of time that patients spent on therapy was 10.4 h (SD 20.4) for the 990 patients who entered Cycle 1 but increased to 12.5 h (SD 23.4) for the 213 patients entering Cycle 6. The most frequent physical therapy interventions set up by the therapist, but carried out by the patient/carer, were related to upper limb positioning, followed by splinting. The most frequent physical therapy interventions guided by a therapist (but also performed independently sometimes) were passive stretch, followed by strength training (Table SIV1).
Goal attainment
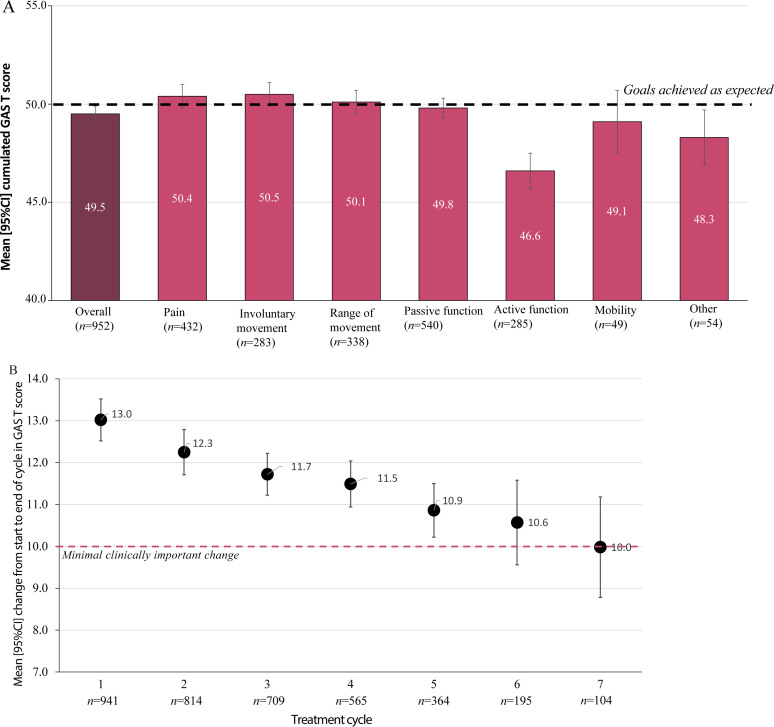
During the study period, the most commonly-chosen goals related to passive function (n = 541, 56.8%), followed by goals related to pain (n = 435, 45.6%), range of movements (n = 338, 35.5%), active function (n = 285, 29.9%) and involuntary movements (n = 283, 29.7%). Only a few patients chose goals related to mobility (n = 49, 5.1%) or other domains (n = 55, 5.8%). Overall, individual patients achieved their goals as expected over repeated cycles; mean (95% CI) GAS T-scores at baseline were 36.7 (36.5, 36.9) and the mean cumulated GAS T-score at 2 years was 49.5 (49.1, 49.9). Across the 6 goal domains, cumulated GAS T-scores were highest for involuntary movements (mean 50.5 (49.9, 51.2)) and pain relief (mean 50.4, (49.8, 50.9)) and lowest for active function (mean 46.6 (45.8, 47.5)) (Fig. 2A, Table SV1).
Fig. 2.
Goal attainment. (A) Cumulative goal attainment scaling (GAS) T-scores. (B) Cycle by cycle analysis of change in GAS T-scores over the first 7 cycles. 95% CI: 95% confidence interval.
As shown in Fig. 2B, the mean change in GAS T-score between the start and end of each evaluation cycle was highest at the first cycle (13.0 (SD 8.3)) and subsequently decreased with each cycle. Mean GAS change scores were maintained at or above the minimal clinical difference of ≥ 10 in the patients requiring up to 7 cycles.
Achievement within goal areas and evolution across the cycles
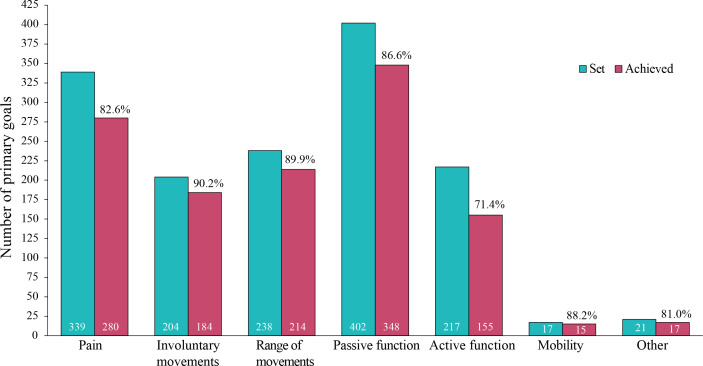
Overall, approximately 75% of all primary goals established in the study were achieved. Higher rates of goal achievement were seen for primary goals related to passive vs active function (86.6% vs 71.4% achievement) (Fig. 3).
Fig. 3.
Overall primary goal achievement by domain. Bars represent the number of goals set and achieved by domain. Percentages indicate the proportion of patients who achieved their primary goal. Error bars represent 95% confidence intervals (95% CIs).
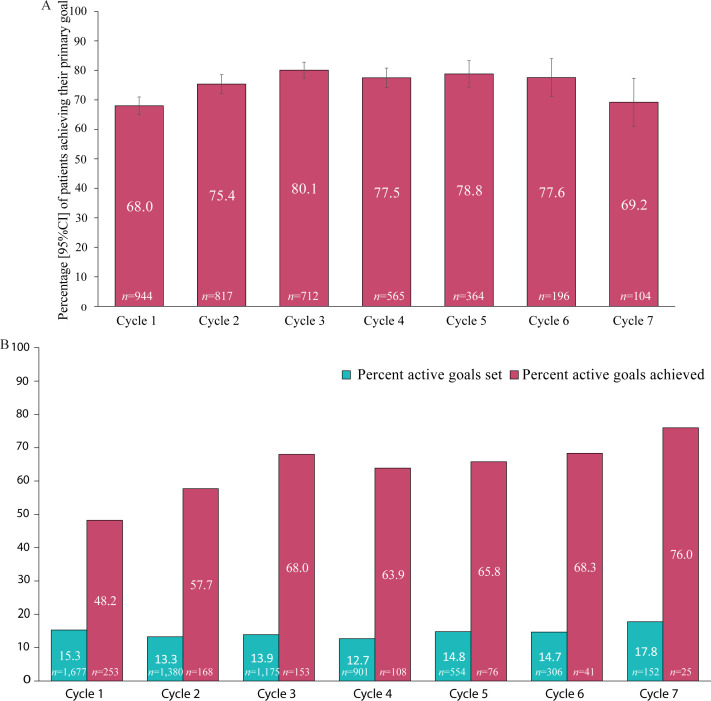
At the population level, rates of primary goal achievement increased in the first 3 cycles (from 68% to 80%) and then remained steady until cycle 5, but rates of achievement decreased successively from 78% to 69% for the subgroup of patients who had more than 5 cycles (Fig. 4A). A breakdown of trends in goal setting and rates of achievement across the 6 main goal domains is given in Table SV1 and Fig. S11. The proportion of goals set in each of the 6 domains remained more or less constant, but between cycles 4 and 7 there was an increase in the proportion of active function goals set (from 13% to 18%) and also a progressive increase in the rates of achievement (from 64% to 76%) (Fig. 4B). High rates of goal achievement were also sustained for goals related to involuntary movements, range of movement and passive function in the latter cycles.
Fig. 4.
Trends in goal achievement across the 7 cycles. (A) Primary goal achievement. (B) In active functioning.
Standardized outcome measures
All patients had muscle tone assessments using the MAS. Mean (SD) (95%CI) composite MAS scores decreased (improved) from 9.8 (SD 3.2) (9.6, 10.0) at baseline to 8.2 (SD 3.4) (7.9, 8.5) at 24 months (change of –1.8 (SD 2.8) (–2.0, –1.5)) indicating that patients achieved a sustained reduction in spasticity through repeated cycles of injection. As shown in Table II, standardized measures generally supported the rates of goal achievement and GAS T-scores. Ratings of pain, involuntary movements, active and passive function all improved significantly over each treatment cycle. In the few patients with mobility listed as one of their goals, FAC scores mostly remained unaltered throughout the study.
Table II.
Standardized measures of treatment effectiveness for areas relevant to goal domains
| Measure | Baseline Mean (95% CI) | Change Mean (95% CI) | p-value vs baseline |
|---|---|---|---|
| Pain score (range 0-10) | n = 346 | n =345 | <0.001 |
| 6.7 (6.5, 6.9) | -2.4 (-2.6, -2.2) | ||
| Associated Reaction Rating Scale (ARRS) (range 0-12) | n = 207 | n =201 | <0.001 |
| 7.4 (7.0, 7.7) | -1.4 (-1.6, -1.1) | ||
| Arm Activity (ArmA) scores; Passive function (range 0-28) | n = 435 | n = 429 | <0.001 |
| 14.1 (13.6, 14.6) | -2.9 (-3.3, -2.6) | ||
| Arm Activity (ArmA) scores; Active function (range 0-52) | n = 216 | n =207 | <0.001 |
| 39.8 (38.4, 41.2) | -3.2 (-4.3, -2.2) | ||
| Functional Ambulation Category (FAC) scores | n = 32 | n =30 | 0.071 |
| 3.7 (3.24; 4.13) | 0.3 (-0.1, 0.7) | ||
| ULS-NIS impaired mobility of joints* | n = 335 | n =335 | |
| Proximal (shoulder and elbow) | 1.8 (1.7, 2.0) | -0.2 (-0.3, -0.1) | <0.0001 |
| Distal (wrist and hand) | 2.3 (2.1, 2.5) | -0.4 (-0. 5, -0.2) | <0.0001 |
Paired t-test performed post-hoc. Scores are presented as the arithmetic mean of post-baseline scores across all evaluation cycles. Depending on in which areas goals were set at different visits, not all patients had both baseline and post-baseline assessments.
ULS-NIS: Upper Limb Spasticity adapted Neurological Impairment Scale.
Spearman’s rank correlations of the primary GAS goal achievement within each area and the relevant standardized outcome measures were more or less as anticipated. Strong correlations were not expected, but moderate correlations were seen between goal achievement for pain (rho –0.55, p < 0.0001, n = 902 observations); active function (rho –0.43, p < 0.0001, n = 274 observations) and passive function (rho –0.30, p < 0.0001, n = 627 observations). A weak but significant correlation was found for involuntary movement (rho –0.23, p < 0.0001, n = 300 observations), and the correlation between mobility goals and the FAC scores was rho 0.34, but non-significant (p = 0.13), possibly reflecting the very small number of observations (n = 21).
Time to re-injection
Injection intervals assessed for the 828/953 (86.9%) patients who remained on the same BoNT-A product during the study. The mean number of injection cycles was 3.8 (SD 1.7) for abobotulinumtoxinA (n = 555), 4.3 (SD 2.3) for onabotulinumtoxinA (n = 196), and 4.7 (SD 2.0) for incobotulinumtoxinA (n = 77).
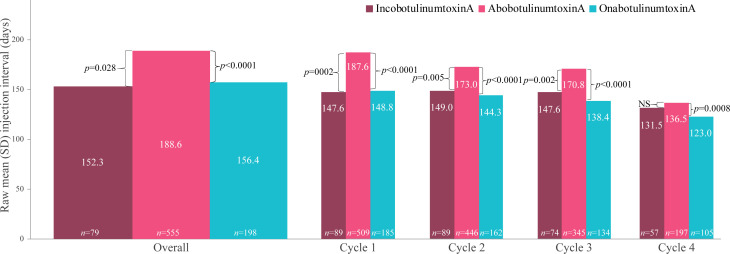
Across the study (cycles 1-9), the mean (SD) [range] injection interval (all BoNT-A products) was 177.6 (SD 81.9) [57–644] days. By product, the mean raw injection interval was 188.6 (SD 83.0) [57–644] for abobotulinumtoxinA, 156.4 (SD 79.9) [81–476] for onabotulinumtoxinA, and 152.3 (SD 61.5) [81–397] days for incobotulinumtoxinA. After conducting multivariable linear regression analyses using age, sex, concomitant physical therapies and other treatment factors identified in the univariate analyses (BoNT-A dose, previous treatment with a BoNT-A, duration of prior BoNT-A treatment, use of systemic anti-spasticity medications, use of physiotherapy, number of therapy visits, use of injection guidance and presence of lowerlimb spasticity had p < 0.2) as covariates, statistically significant differences in the overall injection intervals between abobotulinumtoxinA vs incobotulinumtoxinA and abobotulinumtoxinA vs onabotulinumtoxinA remained (Fig. 5).
Fig. 5.
Mean injection intervals across the study (cycles 1–9) and in the first 4 cycles. Statistical differences (p values) between brands were estimated using the multivariate model (brackets). Botulinum toxin (BoNT-A) doses remained relatively stable between cycles 1 and 4; mean doses were 814–859 U for abobotulinumtoxinA (Abo), 239–254 U for onabotulinumtoxinA (Ona) and 264–275 U for incobotulinumtoxinA (Inco). Factors explored in the regression model were: toxin, age, sex, previously treated for upper limb spasticity (ULS) with BoNT, time from event onset to Cycle 1, distribution of spasticity, dominance of affected limb, indication of lower limb spasticity, duration of BoNT-A prior to study, systemic antispastic medications (and ratio), injection guidance technique, mean indexed dose, any physiotherapy during the study and number of therapy visits. SD: standard deviation.
By cycle analysis of the first 4 cycles (Fig. 5) showed that patients treated with abobotulinumtoxinA had, on average, a significantly longer interval between injections in each of the first 3 cycles than patients treated with both onabotulinumtoxinA and incobotulinumtoxinA. Overall, the multivariate model gave comparative injection intervals of 18.6 days for abobotulinumtoxinA vs incobotulinumtoxinA (Cycle 1: 40.4 days, Cycle 2: 23.4 days, Cycle 3: 24.0 days, Cycle 4: 5.5 days) and 30.9 days for abobotulinumtoxinA vs onabotulinumtoxinA (Cycle 1: 36.8 days, Cycle 2: 27.8 days, Cycle 3: 32.1 days, Cycle 4: 15.3 days). No significant differences were detected in treatment duration between onabotulinumtoxinA and incobotulinumtoxinA, either overall or in the first 4 cycles.
DISCUSSION
To the best of our knowledge, ULIS-III is the largest, international observational cohort study to examine clinical practice and the effects of integrated treatment of upper limb spasticity in real-life clinical settings over a 2-year period. In addition, all 3 commercially-available BoNT-A products were used in the study. At the population level, the study provides evidence for the sustained functional benefit of repeated cycles of BoNT-A, captured through both person-centred goal attainment and standardized measures. Cumulated GAS T-scores demonstrated that the intended goals for treatment were achieved as expected. Moreover, when targeted in relation to the individual’s priority goals for treatment, the standardized measures showed statistically significant improvements from baseline in both active and passive function as well as reduction in pain and involuntary movements that were not only statistically significant, but of an order that was likely to be clinically important (24, 28, 29). Of note, the mean change in GAS T-scores between the start and end of each evaluation cycle was higher for the first few cycles than for subsequent cycles. There are several possible reasons for this. There may be greater potential for change earlier (vs later) in the treatment journey. Also, as this is a population-based analysis, patients requiring only a small number of treatment cycles in the 2 years may be expected to be the better responders than those requiring more frequent injection over the same period. Nevertheless, those who received repeated injections up to 7 cycles (n = 105) still met the criteria for a minimal clinically important change in the GAS T-score (10).
There are a number of points to highlight. Firstly, these results are analysed at population level, meaning that the mean figures presented for each cycle represent just those patients who had at least that number of cycles. Although nearly two-thirds (n = 590) had at least 4 cycles, the numbers drop off sharply thereafter, so that only approximately 10% had more than 6 cycles. This means that differences in goal attainment and other measures between the early and late cycles might simply reflect sampling bias, although it may also represent more appropriate selection of patients and interventions. A secondary analysis is underway to examine longitudinal change in terms of repeated measures for individual patients and to explore differences between the patients requiring more or less frequent injection (see below). Nevertheless, some useful inferences can be made from this population-level data. Most patients had 4 or fewer injections over 2 years, suggesting that, while 3-monthly injection cycles are permitted, they are not routine in clinical practice. It should be noted, however, that the majority of patients included in the study were receiving abobotulinumtoxinA, which was confirmed to have a longer injection interval than the other products, so its predominant use could therefore have skewed the overall number of injection cycles down (i.e. fewer injections) than might have been seen with more equal sample sizes for onabotulinumtoxinA and incobotulinumtoxinA. This concept is, to some extent, supported by the recent ASPIRE study, which found that the mean injection interval for onabotulinumtoxinA was 17.1 weeks (shorter than observed in ULIS-III) and the proportion of patients requiring ≥6 cycles within the 2-year follow-up (i.e. more frequent injections) was higher than observed in the current study (169/484 (35%) in ASPIRE vs 159/953 (20%) in ULIS-III) (30).
Secondly, changes in injection practice were very common, especially in the earlier cycles, and mainly reflected changes in the muscles injected due to a change or modification of treatment goals. These findings indicate that injectors, patients and carers were using goal-setting and GAS to reflect on the response and to tailor injections to changing needs over treatment. The slight overall trend towards increase in the mean injected doses throughout the cycles might also suggest that injectors were, understandably, more cautious with dosing in the early injection cycles and gained confidence in the benefit-risk of injections over time, especially up to 4 cycles. Increasing doses in the latter cycles may also reflect the fact that the small number of patients who required more frequent injection (6 or more cycles in 2 years) had more severe or resistant spasticity. Again, further analysis is required to understand these factors.
The most common goals for treatment were improvement in passive function and reduction in pain. This type of goal distribution remained broadly consistent with that previously reported in the first cycle of ULIS-III (20) and resonates with other observational studies (19, 30–32). While active function goals were less common, they were still relevant for approximately 1 in 5–6 patients. The increase in both the proportion of active function goals and their rates of achievement in the later cycles suggest that patients for whom active function is a priority aim of treatment continue to have sustained benefit, but may require more frequent injection than those with more passive or symptom/impairment-related goals. In the early cycles, goal achievement fell short of expectation for active function, suggesting that goal setting may have been over-ambitious. However, from cycle 4 onwards, the rates of achievement improved, possibly as both patients and their treating clinicians learned what outcomes can reasonably be expected together with the opportunity to continually fine-tune injection parameters to meet these active goals. These findings are important and resonate with clinical practice as they confirm that BoNT-A injection can produce improvements in active function for carefully selected patients who have underlying control of voluntary movements. Previous analyses of the ULIS-II study showed that patients with primary goals focused on active function had less motor impairment, contracture or soft-tissue shortening and a shorter time since stroke, whilst those with primary goals for passive function had the opposite (33). Future secondary analyses of this longitudinal dataset will allow us to further evaluate which goals best suit which patient subgroups, and, ultimately, to identify which patients are likely to be the best responders to treatment.
Strengths and limitations
The authors recognize a number of strengths and limitations of this study. Strengths include its large size, wide international representation and the “real-world” design, all of which help to ensure the generalisability of the findings, as do the inclusion of all aetiologies and all BoNT-A products. The use of standardized outcome measurements targeted on the goals for treatment, along with documentation of associated physical therapies, were key design features for the study and translated well to routine practice. Other international longitudinal observational studies such as the Early-BIRD (31) and ASPIRE (29) studies have examined the longitudinal effects of a single BoNT-A product, but ULIS-III is the first published longitudinal study to include spasticity of any aetiology and any BoNT-A product studied over a period of 2 years.
Limitations of this study include the lack of a control group and the level of missing data, which are inherent to all observational studies performed in routine clinical practice of this type. Although there was wide geographical spread, some countries had only a few active sites, while others had several, so the findings may not be truly representative. In this study, patients were predominantly treated with abobotulinumtoxinA, which may have introduced some selection bias, although several centres use all 3 products. While the study was not designed or powered to compare the different products, our results indicate that abobotulinumtoxinA has a longer injection interval than the other products, and so the predominant use of this formulation may have impacted on the limited number of treatment cycles needed within the study period compared with patients treated with the other products. Similarly, the smaller numbers of patients who used incobotulinumtoxinA and onabotulinumtoxinA limited the cycle-by-cycle analysis of the first 4 treatment cycles.
CONCLUSION AND FUTURE RESEARCH
The ULIS programme was originally initiated to understand who are the best responders to treatment, and progress towards achieving this aim has evolved with each successive study in the series (17). The successful results from ULIS-III highlight the importance of accurate and clear goal-setting during BoNT-A treatment to help target clinical intervention and the use of focused outcome measurement. The authors believe that this approach could form the basis of a common core dataset for prospective, systematic recording of longitudinal outcomes in the conduct of routine clinical practice to support the development of a large international database of sufficient size and breadth to promote future interrogation and subset analysis going forward.
The rich ULIS-III dataset not only provides detailed description of real-life clinical practice, but also supports examination of the various treatment components (including non-pharmacological interventions) in ways that were not previously possible. The “primary” findings presented here provide the necessary foundation for planned secondary analyses looking at the longitudinal evolution of patient-level treatment and goal attainment over 4–6 cycles. It will be possible to understand whether individual patients maintain the same treatment goals, or if these change over time, and how. The detailed collection of associated physical therapies will also allow exploration of which therapies are used to support which treatment goals, the evolution of best-practice therapies over time and, ultimately, which therapies are associated with best response. It will be possible to compare outcomes for new patients with those on an established treatment regimen. We also plan to evaluate the characteristics of the subgroup of patients who required 6 or more treatment cycles within the 2-year timeframe. It will be important to understand if these are the more severely affected patients, whether they have different goals requiring more repeat cycles, or if it is simply a country, region or site effect. Finally, for those countries with enough sites and patients, this dataset will provide useful insights into national practice.
ACKNOWLEDGEMENTS
The authors thank all the investigators and patients who participated in this trial. We also thank Anita Chadha-Patel, PhD, of ACP Clinical Communications Ltd (Hertfordshire, UK) for providing medical writing support, which was funded by Ipsen (Paris, France) in accordance with Good Publication Practice guidelines.
Footnotes
http://www.medicaljournals.se/jrm/content/?doi = 10.2340/16501977-2801
Funding
This work was supported by Ipsen Pharma.
Conflicts of interest
LTS, KF, JJ, AB and SA all received honoraria from Ipsen for undertaking this research. LTS and SA have a specific interest in outcomes evaluation and have published extensively on the use of GAS in this context, as well as a number of the other standardized measures. All of these tools are freely available, however, and they have no personal financial interest in any of the material mentioned in this article. KF has a specific interest in outcomes evaluation and the use of the International Classification of Functioning, Disability and Health in clinical settings. He has no personal financial interest in any of the material mentioned in this article. JJ has a particular interest in clinical and instrumental spasticity evaluation methods, goal-setting, treatment strategies/techniques and outcome measurement. He has no personal or financial interest in any of the material mentioned in this article. AB has a particular interest in inclusion and patient-centred care and has no personal or financial interest in any of the material mentioned in this article. PM and AL are employees of Ipsen.
Data sharing
Ipsen will share aggregated data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
REFERENCES
- 1.Gracies JM, Brashear A, Jech R, McAllister P, Banach M, Valkovic P, et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. Lancet Neurol 2015; 14: 992–1001. [DOI] [PubMed] [Google Scholar]
- 2.Elovic EP, Munin MC, Kanovsky P, Hanschmann A, Hiersemenzel R, Marciniak C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle Nerve 2016; 53: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson DM, Gracies JM, Yablon SA, Barbano R, Brashear A. Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. J Neurol Neurosurg Psychiatry 2009; 80: 380–385. [DOI] [PubMed] [Google Scholar]
- 4.Royal College Physicians (UK) . Spasticity in adults: management using botulinum toxin. National guidelines; 2018. [accessed January 2021]. Available from: https://www.rcplondon.ac.uk/guidelines-policy/spasticity-adults-management-using-botulinum-toxin. [Google Scholar]
- 5.Sheean G, Lannin NA, Turner-Stokes L, Rawicki B, Snow BJ. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: international consensus statement. Eur J Neurol 2010; 17 (Suppl 2): 74–93. [DOI] [PubMed] [Google Scholar]
- 6.Simpson DM, Hallett M, Ashman EJ. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache. Neurology 2016; 86: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashford S, Slade M, Malaprade F, Turner-Stokes L. Evaluation of functional outcome measures for the hemiparetic upper limb: a systematic review. J Rehabil Med 2008; 40: 787–795. [DOI] [PubMed] [Google Scholar]
- 8.Ashford S, Turner-Stokes L. Systematic review of upper-limb function measurement methods in botulinum toxin intervention for focal spasticity. Physiother Res Int 2013; 18: 178–189. [DOI] [PubMed] [Google Scholar]
- 9.Sunnerhagen KS, Olver J, Francisco GE. Assessing and treating functional impairment in poststroke spasticity. Neurology 2013; 80 (Suppl 2): S35–44. [DOI] [PubMed] [Google Scholar]
- 10.Turner-Stokes L, Ashford S, Esquenazi A, Wissel J, Ward AB, Francisco G, et al. A comprehensive person-centered approach to adult spastic paresis: a consensus-based framework. Eur J Phys Rehabil Med 2018; 54: 605–617. [DOI] [PubMed] [Google Scholar]
- 11.Kiresuk T, Sherman R. Goal attainment scaling: a general method of evaluating comprehensive mental health programmes. Commun Mental Health J 1968; 4: 443–453. [DOI] [PubMed] [Google Scholar]
- 12.Krasny-Pacini A, Hiebel J, Pauly F, Godon S, Chevignard M. Goal attainment scaling in rehabilitation: a literature-based update. Ann Phys Rehabil Med 2013; 56: 212–230. [DOI] [PubMed] [Google Scholar]
- 13.Ashford S, Turner-Stokes L. Goal attainment for spasticity management using botulinum toxin. Physiother Res Int 2006; 11: 24–34. [DOI] [PubMed] [Google Scholar]
- 14.Ward AB, Wissel J, Borg J, Ertzgaard P, Herrmann C, Kulkarni J, et al. Functional goal achievement in post-stroke spasticity patients: the BOTOX Economic Spasticity Trial (BEST). J Rehabil Med 2014; 46: 504–513. [DOI] [PubMed] [Google Scholar]
- 15.Ashford S, Fheodoroff K, Jacinto J, Turner-Stokes L. Common goal areas in the treatment of upper limb spasticity: a multicentre analysis. Clin Rehabil 2016; 30: 617–622. [DOI] [PubMed] [Google Scholar]
- 16.Tennant A. Goal attainment scaling: current methodological challenges. Disabil Rehabil 2007; 29: 1583–1588. [DOI] [PubMed] [Google Scholar]
- 17.Turner-Stokes L, Fheodoroff K, Jacinto J, Maisonobe P, Ashford S. ULIS (Upper Limb International Spasticity), a 10-year odyssey: an international, multicentric, longitudinal cohort of person-centered spasticity management in real-life practice. The J Int Soc Phys Rehabil Med 2019; 2: 138–150. [Google Scholar]
- 18.Bakheit AM, Zakine B, Maisonobe P, Aymard C, Fhedoroff K, Hefter H, et al. The profile of patients and current practice of treatment of upper limb muscle spasticity with botulinum toxin type A: an international survey. Int J Rehabil Res 2010; 33: 199–204. [DOI] [PubMed] [Google Scholar]
- 19.Turner-Stokes L, Fhedoroff K, Jacinto J, Maisonobe P. Results from the Upper Limb International Spasticity Study-II (ULIS- II): a large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open 2013; 3: e002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner-Stokes L, Jacinto J, Fheodoroff K, Brashear A, Maisonobe P, Lysandropoulos A, et al. Assessing the effectiveness of upper-limb spasticity management using a structured approach to goal setting and outcome measurement: first cycle results from the ULIS (Upper Limb International Spasticity)-III study. J Rehabil Med 2021; 53: jrm00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner-Stokes L, Ashford SA, Jacinto J, Maisonobe P, Balcaitiene J, Fheodoroff K. Impact of integrated upper limb spasticity management including botulinum toxin A on patient-centred goal attainment: rationale and protocol for an international, prospective, longitudinal cohort study (ULIS-III). BMJ Open 2016; 6: e011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner-Stokes L. Goal Attainment Scaling (GAS) in Rehabilitation: a practical guide. Clinical Rehabilitation 2009; 23: 362–370. [DOI] [PubMed] [Google Scholar]
- 23.Turner-Stokes L, Disler R, Shaw A, Williams H. Screening for pain in patients with cognitive and communication difficulties: evaluation of the SPIN-screen. Clin Med 2008; 8: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macfarlane A, Turner-Stokes L, De Souza L. The associated reaction rating scale: a clinical tool to measure associated reactions in the hemiplegic upper limb. Clin Rehabil 2002; 16: 726–735. [DOI] [PubMed] [Google Scholar]
- 25.Turner-Stokes L, Thu A, Williams H, Casey R, Rose H, Siegert RJ. The Neurological Impairment Scale: reliability and validity as a predictor of functional outcome in neurorehabilitation. Disabil Rehabil 2014; 36: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashford S, Slade M, Nair A, Turner-Stokes L. Arm Activity measure (ArmA) application for recording functional gain following focal spasticity treatment. Int J Ther Rehabil 2014; 21: 10–17. [Google Scholar]
- 27.Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil 2007; 88: 1314–1319. [DOI] [PubMed] [Google Scholar]
- 28.Ashford S, Turner-Stokes L, Siegert R, Slade M. Initial psychometric evaluation of the Arm Activity Measure (ArmA): a measure of activity in the hemiparetic arm. Clin Rehabil 2013; 27: 728–740. [DOI] [PubMed] [Google Scholar]
- 29.Michener LA, Snyder AR, Leggin BG. Responsiveness of the numeric pain rating scale in patients with shoulder pain and the effect of surgical status. J Sport Rehabil 2011; 20: 115–128. [DOI] [PubMed] [Google Scholar]
- 30.Francisco GE, Jost WH, Bavikatte G, Bandari DS, Tang SFT, Munin MC, et al. Individualized onabotulinumtoxina treatment for upper limb spasticity resulted in high clinician- and patient-reported satisfaction: long-term observational results from the ASPIRE study. PM R 2020; 12: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esquenazi A, Mayer N, Lee S, Brashear A, Elovic E, Francisco GE, et al. Patient registry of outcomes in spasticity care. Am J Phys Med Rehabil 2012; 91: 729–746. [DOI] [PubMed] [Google Scholar]
- 32.Wissel J, Fheodoroff K, Hoonhorst M, Müngersdorf M, Gallien P, Meier N, et al. Effectiveness of abobotulinumtoxinA in post-stroke upper limb spasticity in relation to timing of treatment. Front Neurol 2020; 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fheodoroff K, Ashford S, Jacinto J, Maisonobe P, Balcaitiene J, Turner-Stokes L. Factors influencing goal attainment in patients with post-stroke upper limb spasticity following treatment with botulinum toxin A in real-life clinical practice: sub-analyses from the Upper Limb International Spasticity (ULIS)-II Study. Toxins 2015; 7: 1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]