Abstract
Objective
COVID-19 can result in a broad spectrum of dysfunctions, some of which may persist for long periods, requiring long-term rehabilitation. A comprehensive screening tool is therefore necessary to identify these needs. To date, no data exist on satisfaction with medical and therapeutic interventions for COVID-19 in terms of quality and quantity. The aim of this study is to develop a survey for use with COVID-19 patients during and after the end of the acute phase of the disease.
Methods
Following the definition of dimensions by a group of experts, and a literature search, proven survey instruments were searched for suitable items. In addition, specific questions were developed based on symptoms, and answer options were created with regard to to the complexity of the questions.
Results
The COVID-19 Rehabilitation Needs Survey (C19-RehabNeS) consists of the established 36- item Short Form Survey (SF-36) together with the newly developed COVID-19-Rehabilitation Needs Questionnaire (C19-RehabNeQ) (11 further dimensions, respectively 57 items).
Conclusion
C19-RehabNeS is a comprehensive survey to assess functional limitations and rehabilitation needs during and after infection with SARS-CoV-2 (COVID-19). The strength of this survey is that it combines the assessment of important rehabilitation needs with assessment of satisfaction with the health services, treatment and therapy during the pandemic (C19-RehabNeQ) and assessment of patients’ quality of life (SF-36). The C19-RehabNeS survey also enables collection of systematic information on patients with Post-COVID-19 syndrome (Long-COVID-19).
LAY ABSTRACT
COVID-19 can cause a wide range of problems that affect several organ systems, resulting in long-term rehabilitation needs. A comprehensive screening instrument, that can identify these needs, is therefore necessary. The aim of this study is to develop a survey questionnaire for COVID-19 patients. A literature search was performed to identify current assessments concerning previously defined dimensions. A group of experts decided on the useful composition of possible questions. The resulting questionnaire (COVID-19 Rehabilitation Needs Survey; C19-RehabNeS) combines the 36-item Short Form (SF-36) with the newly developed COVID-19 Rehabilitation Needs Quesionnaire (C19-RehabNeQ) (with 11 dimensions and 57 items). The C19-RehabNeS is a comprehensive questionnaire for assessment of functional limitations during and after infection with SARS-CoV-2. The strength of the survey lies in the combination of assessment of 2 important issues: (i) rehabilitation needs and satisfaction with health services; and (ii) treatment and therapy during the pandemic. The C19-RehabNeS also enables collection of systematic information regarding rehabilitation and other treatments.
Key words: survey method, health service administration, physical and rehabilitation medicine, questionnaire design, Covid-19, SARS-CoV2
The outbreak of COVID-19 in Wuhan, China, in December 2019 quickly developed into a global pandemic. There is increasing evidence that infection with the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 can result in a wide range of dysfunctions in several organ systems (1). In addition to primary pulmonary restrictions, which are divided into different stages and may lead to pulmonary fibrosis (2–4), many studies show that there can also be damage to the cardiovascular (5, 6), gastrointestinal (7, 8), nervous (9, 10) and musculoskeletal systems (11). Effects on mental health, such as increased fatigue and mood-related disorders (12), as well as dermatological (13) and ophthalmological symptoms (14), should not be underestimated. In addition to the wide and variable range of symptoms, the possible long-term health consequences of SARS-CoV-2 infection are not fully understood at this stage. However, current knowledge indicates the possible development of Post-COVID-19 syndrome (Long-COVID-19), with restrictions lasting for months (15). COVID-19 infection reduces performance, quality of life and participation, in both vocational and personal life (16).
The wide range of possible symptoms leads to a necessity for multi-professional rehabilitation interventions in order to treat these functional deficits starting in the acute phase (17, 18). In addition to acute care rehabilitation, many patients will also require longterm and multidimensional treatment (19, 20). This is a core task of physical and rehabilitative medicine (21).
A few studies (22, 23) have investigated healthrelated quality of life (using the 36-item Short Form survey; SF-36) of COVID-19 patients, and the results support the need for far-reaching rehabilitative therapies.
For the full assessment of patients, a comprehensive screening instrument is required to identify their rehabilitation needs, and to serve as a basis for the development of treatment concepts. However, there are currently only a few survey instruments that ask about the effects of a SARS-CoV-2 infection. A recently developed questionnaire can be conducted as a telephone interview (24). This tool comprises 19 questions on the biopsychosocial effects of COVID-19 and corresponding limitations in functioning (24). However, there are currently no other assessment tools known to the authors that specifically address the rehabilitation needs of COVID-19 patients. No study has yet been published that assesses these needs from a broader perspective.
The aim of this study was therefore to develop a multidimensional assessment tool for surveying the rehabilitation needs of patients during and after SARS-CoV-2 infection. It is important that the survey also records long-term symptoms that persist beyond the acute infection, referred to as Post-COVID-19-syndrome resp. Long-COVID-19 (15, 25–27).
METHODS
Development of the COVID-19 Rehabilitation Needs Survey (C19-RehabNeS) was carried out by a team of experts, comprising 5 specialists in physical and rehabilitative medicine, 1 physiotherapist, and 1 rehabilitation scientist with specific experience in the field of assessments, from Hannover Medical School, Department of Rehabilitation, Hanover, and Jena University Hospital, Institute for Physiotherapy, Jena, Germany. All clinicians were experienced in the management of patients with COVID-19 in the post-acute and long-term phase. In order to develop a comprehensive screening instrument, the team based the questionnaire on their clinical experience with COVID-19 patients, on screening tools already in use for patients with chronic conditions or impairments, and on standardized questionnaires measuring quality of life. The gained experience with the questionnaire used in the German cohort of the International Survey on Spinal Cord Injury (InSCI) (28) was used in developing the C19-RehabNeS.
The basis for the development of the survey and the assessment of need for rehabilitation resulted from the author’s (CL) daily work with COVID-19 patients. A large number of symptoms were observed (e.g. cough, fever, fatigue and musculoskeletal pain). Moreover, patients often reported helplessness, as they experienced stigmatization and rejection, not only from fellow citizens, but also from medical personnel, such as doctors, nurses and therapists. A literature search on existing impairments after a SARS-CoV-2 infection, was performed in June 2020. The aim was to record the wide range of symptoms known at that time and to include them in the survey. The results were compared and supplemented with the authors’ own experiences. A questionnaire, developed at the Department of Rehabilitation Medicine at Hannover Medical School, formed the basis from which to compile a specific international assessment for rehabilitation needs for COVID-19 patients. Additional standardized questionnaires were considered, based on the large-scale International Spinal Cord Injury Survey (InSCI) of the International Society of Physical and Rehabilitation Medicine and the International Society of Spinal Cord Injury, i.e. the German cohort coordinated by the researchers at Hannover Medical School. Although the aetiology and medical consequences of spinal cord injury are largely different from those of COVID-19, there are also overlaps in rehabilitation needs in terms of reduced quality of life and participation restrictions. The expert group therefore screened the survey for suitable items reflecting life satisfaction and functional deficits. The items found were traced back to their origin and the corresponding assessments were searched again for possible further items. The following questionnaires were screened:
Seekins Secondary Conditions Questionnaire (SCQ).The SCQ assesses the links between disability and other secondary diseases. The impact on many areas of life, such as mobility, mental health and medication-induced problems, is investigated. The SCQ contains 40 items and refers in its questions to the previous 3-month period (29).
SCQ Spinal Cord Injury Secondary Conditions Scale (SCISCS). The SCI-SCS is a 16-item questionnaire that records the secondary effects of spinal cord on health and physical function (29). It was used as part of the International Spinal Cord Injury Survey (InSCI) a multinational community survey based on the ICF Core Sets for SCI.
German Spinal Cord Injury Survey (GerSCI). The GerSCI is part of the InSCI. The aim of this survey is to collect global and reliable data on people with spinal cord injury (SCI) in order to implement data on a national learning health system and to improve the living and care situations for people SCI in the long term. Some questions in the GerSCI were adapted to the specific country (30).
Model Disability Survey (MDS) project of the World Health Organization (WHO). The MDS is based on the ICF and was developed to collect data on life and related problems, especially in relation to the activity and impairment of people with and without disabilities (31).
World Health Organization Quality of Life (WHOQol5/BREF). The WHOQol5/BREF was developed to measure quality of life as a perception of the individual’s position in life in the context of culture, values, goals and expectations. The basic questionnaire (WHOQol100) consists of 100 items (32).
World Health Survey (WHS) 2001–2. The WHS was developed by the WHO to provide a valid, reliable and comparable tool for surveys worldwide. The content of the survey includes socio-demographic, health-specific and care aspects (33).
36-item Short-Form Survey (SF-36). The SF-36 comprises a total of 36 items on subjective mental, social and physical health, which are assigned to 8 dimensions. The score is calculated by adding the items marked with a cross on each scale. Prior to this, a conversion of the values is carried out on a scale ranging from 0 (poor quality of life) to 100 (best quality of life) (34). The SF-36 is suitable for both groupspecific and individual evaluations (35).
From the selected questionnaires, the useful elements were extracted and, if necessary, modified to meet the special requirements of the survey and the numerous facets of COVID-19. Finally, socio-economic and personal data, such as age, height and weight, were added. The Likert scale, yes/no options, multiple answers or free-text were allowed as possible answers, depending on the complexity and content of the question.
In a further elaboration, the team of experts identified thematic categories on which questions were formulated. These were developed to provide answers to the questions posed at the start, as well as additional socio-economic data on comparability and sub-grouping.
After compilation of all items, a joint discussion of each item took place according to importance and specificity. In order to develop a feasible questionnaire some items were deleted or reduced by consensus. The combination of infection time and symptoms still existing at the time of completing the survey appears to be useful for this purpose. A scientific link between these 2 pieces of information was established as an important basis. In addition to the newly developed COVID-19- Rehabilitation Needs Questionnaire (C19-RehabNeQ), it was decided to include an established and validated questionnaire on general quality of life in the survey (SF-36).
The final version of the C19-RehabNeS was developed after iterative discussion rounds and was approved by consensus.
RESULTS
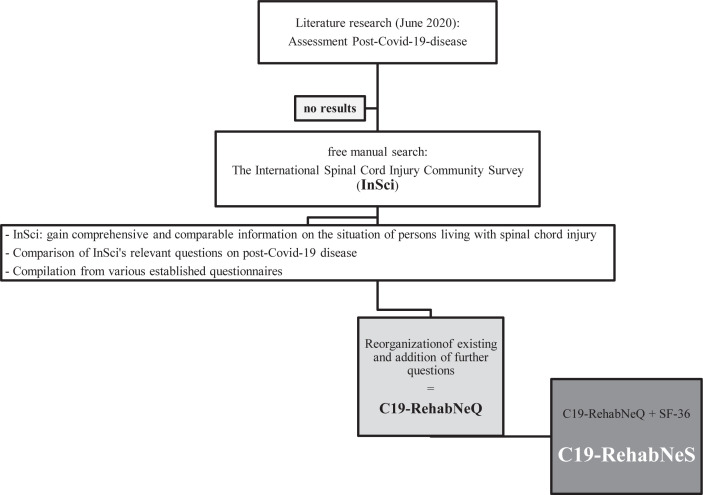
The C19-RehabNeS consists of 2 separate assessment tools: (i) the SF-36 on health-related quality of life; and (ii) the C19-RehabNeQ, as shown in Fig. 1. The C19-RehabNeS was first developed in German and the single dimensions were then translated into English. The items of the C19-RehabNeQ comprise individual questions from existing questionnaires and include newly developed questions to illustrate the particularities of a SARS-CoV-2 infection. Table I shows the number of questions used from the established questionnaires. A total of 57 items were assigned to 7 main categories, as follows:
Fig. 1.
Flowchart of development of the COVID-19 Rehabilitation Needs Survey (C19-RehabNeS).
Table I.
Items from existing questionnaires
| Questionnaire | Number of items used |
|---|---|
| Seekins Secondary Conditions Questionnaire (SCQ) | 8 |
| Spinal Cord Injury Secondary Conditions Scale (SCI-SCS) | 8 |
| German Spinal Cord Injury Survey (GerSCI) | 1 |
| Model Disability Survey (MDS) project of the World Health Organization (WHO) | 17 |
| World Health Organization Quality of Life (WHOQol5/BREF) | 6 |
| World Health Survey 2001–2 (WHS) | 1 |
Time of infection (1 item)
Health problems caused by SARS-CoV-2 (14 items)
Treatment (9 items)
Activity and participation (13 items)
Quality of life and general health (6 items)
Health service provisions (5 items)
Personal information (9 items)
The 11 dimensions developed and the corresponding items are shown in Table II.
Table II.
Structure of the COVID-19-Rehabilitation Needs Questionnaire (C19-RehabNeQ)
| Number | Dimension | Items | Origin | |
|---|---|---|---|---|
| 1 | Disease, symptoms | Positive COVID-19-testtest | 1 | new item |
| 2 | Health problems, symptoms | 2–15 | a 2–7, 10, 13 SCI-SCS like and SCQ 2, 13 | |
| a (problem severity) c (still problems) |
MDS 8, 9, 11, 12, 14, 15 own construction c 2–15 own construction |
|||
| 3 | Previous care provision | Hospital and/or outpatient treatments | 2–15 b (receiving therapy), 16-18 | b 2–15 GerSCI 16–18 new items |
| 4 | Rehabilitation interventions | 19–21 | New items | |
| 5 | Health services (out-patient) | 44 | MDS | |
| 6 | Barriers,expectations,satisfaction | 22–24, 45–48 | 22–24, 45, 47, 48 new items 46 World Health Survey 2001–2 |
|
| 7 | Activity and participation | ICF-list | 25–37 | MDS |
| 8 | Quality of life | Satisfaction | 38–43 | WHOQol |
| 9 | Personal data | Sex,age,height,bodyweight | 49–52 | New items |
| 10 | Marital status, vocational situation, living situation | 53–56 | 53 MDS | |
| 54 new item | ||||
| 55 GerSCI | ||||
| 56 new item | ||||
| 11 | Area of living | 57 | New item |
MDS: Model Disability Survey; WHOQol: World Health Organization Quality of Life; SCQ: Seekins Secondary Conditions Questionnaire; SCI-SCS: Spinal Cord Injury Secondary Conditions Scale.
DISCUSSION
Rehabilitation is an essential part of COVID-19 treatment, and should be applied equally during all phases of the disease course, starting with acute, through early post-acute and post-acute to long-term rehabilitation (36). For this purpose, suitable instruments are needed to assess the patient’s need for rehabilitation. Use of appropriate concepts, based on the ICF, is also needed to enable patients to return to activity and participation.
The C19-RehabNeS, described here, appears to be a practical and comprehensive survey for the assessment of functional limitations during and after infection with SARS-CoV-2. The strength of this survey lies in the combination of the important issues of rehabilitation needs and satisfaction with the availability of health services, treatment and therapy during the pandemic, and quality of life. The questions are based on expert consensus. Clinical experience was also an important factor in the development of the questionnaire. One author (CL) has treated many COVID-19 patients with mild course of the disease during the early months of the pandemic. This enabled a comprehensive view of the diversity of symptoms and existing functional deficits, which were considered in the selection of questions. In addition to discussion on the importance of individual questions among a panel of experts, special emphasis was placed on different answer options. The Likert scale was chosen to allow weighting, and free-text answers to reflect the dynamics of the disease.
For the existing and remaining symptoms of COVID-19, influence on quality of life and participation was recorded. The joint use in the C19-RehabNeS survey of the C19-RehabNeQ and the internationally established SF-36 enables assessment of quality of life compared with other studies (34, 37, 38). A further interesting aspect is the possibility to compare the results with those for patients with other upper respiratory tract infections, as the SF-36 is regularly used to examine the course of therapy and disease.
The C19-RehabNeQ was developed in July 2020. Due to the rapidly increasing number of studies on the topic and the increasing number of recovering patients, this instrument was designed to be adjusted dynamically. In addition to supplementing the C19-RehabNeQ with other, by now known and important, symptoms and long-term consequences, such as hair loss and dizziness, it is possible to add modular extensions to the RehabNeQ (Appendix SI1). In development, the expert group decided to supplement the survey, the RehabNeS, with other existing questionnaires according to individual needs. The first modular additions were the Fatigue Assessment Scale (39) and the Work Ability Index (40). Here, further use and evaluation are required to evaluate the benefits of such extensions, as the total length of the survey should not exceed a tolerable time in order to achieve a realistic response rate.
During development of the survey it had to be considered that the course of symptoms is highly variable. Some of the multiple, and not only functional, limitations caused by COVID-19 are mild and improve after a few days, while others may persist for weeks, months, or perhaps a lifetime. Further research during the coming months and years will provide information on this and form the basis for further adjustments to the RehabNeQ.
Another important aspect of the RehabNeQ is the initial evaluation of patient’s satisfaction with health services provision and related political decisions. The rapidly increasing number of infected persons in Germany in March and April 2020 led to many short-term ad hoc decisions being taken that affected all areas of the healthcare system. To date, there are no studies that show how these decisions and the associated processes have influenced the situation of infected persons. Furthermore, there is no current data showing satisfaction with access to healthcare and rehabilitation during the pandemic. In particular, rehabilitation and the associated therapeutic options should be considered as an intervention measure and not purely as aftercare.
A limitation of the RehabNeQ is that not only validated assessments were used in its development. Comparability of results is therefore limited until the test instrument has been used for further investigations or a globally validated sheet has been created. The findings regarding residual functional deficits were not diagnostically verified by a physician. Another point is that the infection of the respondents with SARS-CoV-2 was not additionally confirmed by an antibody test. In Germany, such tests are not routinely performed; PCR testing is the routine here. Therefore, including this information in the questionnaire was not helpful, although a comparison of the remaining restrictions with time of infection and antibody status would have been a further interesting aspect.
In developing the RehabNeQ, no sex-specific distinctions were made, although it is now known that patient’s sex influences the severity and course of the infection (41). However, in the survey planned using the C19-RehabNeS, collected data will be evaluated and compared on a sex-specific basis in order to take this aspect into account when planning individual rehabilitation programmes.
The C19-RehabNeS has already been used in a follow-up study of 1,027 COVID-19 patients in Germany. After a positive ethics vote from the Friedrich-Schiller-University Jena (registration number 2020-1834-Bef), the survey was conducted in cooperation with the health authorities of 3 Bavarian districts. The employees sent the C19-RehabNeS to all patients over 18 years of age who had tested positive for the virus with a cut-off date 18 July 2020. The current response rate is approximately 40%. This indicates good acceptance of the survey and its easy handling (42). Evaluation of the returned questionnaires is currently in progress. Initial results are expected in early 2021. In addition, the C19RehabNeS is being used in specialized consultations for post-COVID-19 patients in the outpatient clinic of Hannover Medical School, in order to enable optimal therapy planning by precisely determining the patients’ symptoms and associated limitations. In clinical use, patients indicated that the survey was comprehensive, but quick and easy to complete.
In conclusion, C19-RehabNeS is the first comprehensive assessment tool to determine specific symptoms during and after COVID-19 infection, their impact on quality of life, and the resulting rehabilitation needs. It also provides information on whether patients have received appropriate treatment during and after COVID-19 and how satisfied they are with their treatment.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Lopez M, Bell K, Annaswamy T, Juengst S, Ifejika N. COVID-19 Guide for the Rehabilitation Clinician: A Review of Non-Pulmonary Manifestations and Complications. Am J Phys Med Rehabil 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salehi S, Reddy S, Gholamrezanezhad A. Long-term Pulmonary Consequences of Coronavirus Disease 2019 (COVID-19): What We Know and What to Expect. J Thorac Imaging 2020. [DOI] [PubMed] [Google Scholar]
- 4.Leo F, Wormanns D, Grohé C. COVID-19 aus Sicht der Pneumologie – Langzeitfolgen und Implikationen für die pneumologische Nachsorge. Dtsch Med Wochenschr 2020; 145: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 5.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 6.Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection; 2020. [Google Scholar]
- 8.Mao R, Qiu Y, He J-S, Tan J-Y, Li X-H, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic Features in Severe SARSCoV-2 Infection. N Engl J Med 2020; 382: 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand 2020; 142: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg Res 2020; 15: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey S, Biswas P, Ghosh R, Chatterjee S, Dubey MJ, Chatterjee S, et al. Psychosocial impact of COVID-19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020; 14: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abobaker A, Raba AA, Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loffredo L, Pacella F, Pacella E, Tiscione G, Oliva A, Violi F. Conjunctivitis and COVID-19: A meta-analysis. J Med Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamprecht B. Gibt es ein Post-COVID-Syndrom? Pneumologe (Berl) 2020: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falvey JR, Ferrante LE. Flattening the disability curve: Rehabilitation and recovery after COVID-19 infection. Heart Lung 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reißhauer A, Baack A, Liebl ME. Physiotherapie bei erwachsenen Patienten mit Verdacht oder Nachweis von COVID-19 an der Charité Universitätsmedizin Berlin. Phys Rehab Kur Med 2020; 30: 64–65. [Google Scholar]
- 18.Stierli S, Buss I, Redecker H, Baumberger M, Blättler E, Selb M, et al. Insights from an interprofessional post-COVID-19 rehabilitation unit: A speech and language therapy and respiratory medicine perspective. J Rehabil Med 2020; 52: jrm00100. [DOI] [PubMed] [Google Scholar]
- 19.Sheehy LM. Considerations for Postacute Rehabilitation for Survivors of COVID-19. JMIR Public Health Surveill 2020; 6: e19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebl ME, Gutenbrunner C, Glaesener JJ, Schwarzkopf S, Best N, Lichti G, et al. Frühe Rehabilitation bei COVID-19 – Best Practice Empfehlungen für die frühe Rehabilitation von Patient/innen mit COVID-19. Phys Rehab Kur Med 2020; 30: 129–134. [Google Scholar]
- 21.Gutenbrunner C, Pioch E. Die Rehabilitative Langzeitversorgung – eine Kernaufgabe der Physikalischen und Rehabilitativen Medizin. Phys Rehab Kur Med 2019; 29: 184–185. [Google Scholar]
- 22.Chen K-Y, Li T, Gong F-H, Zhang J-S, Li X-K. Predictors of Health-Related Quality of Life and Influencing Factors for COVID-19 Patients, a Follow-Up at One Month. Front Psychiatry 2020; 11: 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen HC, Nguyen MH, Do BN, Tran CQ, Nguyen TTP, Pham KM, et al. People with Suspected COVID-19 Symptoms Were More Likely Depressed and Had Lower Health-Related Quality of Life: The Potential Benefit of Health Literacy. J Clin Med 2020; 9: 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton IJ. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med 2020; 52: jrm00089. [DOI] [PubMed] [Google Scholar]
- 25.Welte T. Herausforderung für die Innere Medizin: Komplexe COVID-19-Verläufe und die Rolle der Intensivmedizin bei ihrer Behandlung; 2020. [Google Scholar]
- 26.Lale Artun. Das Post-Covid-Syndrom : Genesen, aber nicht gesund. Frankfurter Allgemeine Zeitung 2020. Sep 28. [Google Scholar]
- 27.Sivan M. Remote assessment for identifying COVID-19 post-acute care needs. Aging clinical and experimental research 2020; 32: 2167–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross-Hemmi MH, Post MWM, Ehrmann C, Fekete C, Hasnan N, Middleton JW, et al. Study Protocol of the International Spinal Cord Injury (InSCI) Community Survey. Am J Phys Med Rehabil 2017; 96: S23-S34. [DOI] [PubMed] [Google Scholar]
- 29.Kalpakjian CZ, Scelza WM, Forchheimer MB, Toussaint LL. Preliminary reliability and validity of a Spinal Cord Injury Secondary Conditions Scale. J Spinal Cord Med 2007; 30: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bökel A, Blumenthal M, Egen C, Geng V, Gutenbrunner C. Querschnittlähmung in Deutschland Eine nationale Befragung (German Spinal Cord Injury Survey (GerSCI) Teil-projekt des Spinal Cord Injury Community Survey (InSCI)); 2019. [Google Scholar]
- 31.WHO & World Bank . Model Disability Survey: Providing evidence for accountability and decision-making; 2020. Available from: https://www.who.int/disabilities/data/mds.pdf.
- 32.Bonomi AE, Patrick DL, Bushnell DM, Martin M. Validation of the United States’ version of the World Health Organization Quality of Life (WHOQOL) instrument. Journal of Clinical Epidemiology 2000; 53: 1–12. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organisation (WHO) . WHO World Health survey; 2002. Available from: https://www.who.int/healthinfo/survey/whslongversionsurveymanual.pdf.
- 34.Morfeld M, Bullinger M. Der SF-36 Health Survey zur Erhebung und Dokumentation gesundheitsbezogener Lebensqualität. Phys Rehab Kur Med 2008; 18: 250–255. [Google Scholar]
- 35.Morfeld M, Bullinger M, Kirchberger. Fragebogen zum Gesundheitszustand: SF-36; deutsche Version des Short form-36 health survey: Hogrefe; 2011. [Google Scholar]
- 36.Gutenbrunner C, Stokes EK, Dreinhöfer K, Monsbakken J, Clarke S, Côté P, et al. Why Rehabilitation must have priority during and after the COVID-19-pandemic: A position statement of the Global Rehabilitation Alliance. J Rehabil Med 2020; 52: jrm00081. [DOI] [PubMed] [Google Scholar]
- 37.Ziebland S. The short form 36 health status questionnaire: clues from the Oxford region’s normative data about its usefulness in measuring health gain in population surveys. Journal of epidemiology and community health 1995; 49: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiklund I. Measuring quality of life in medicine. Scand J Prim Health Care Suppl 1990; 1: 11–14. [PubMed] [Google Scholar]
- 39.Michielsen HJ, Vries J de, van Heck GL. Psychometric qualities of a brief self-rated fatigue measure. J Psychosom Res 2003; 54: 345–352. [DOI] [PubMed] [Google Scholar]
- 40.Zwart BCH de, Frings-Dresen MHW, van Duivenbooden JC. Test-retest reliability of the Work Ability Index questionnaire. Occup Med (Lond) 2002; 52: 177–181. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal H, Das N, Nathani S, Saha S, Saini S, Kakar SS, et al. An Assessment on Impact of COVID-19 Infection in a Gender Specific Manner. Stem Cell Rev Rep 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iglesias C, Torgerson D. Does length of questionnaire matter? A randomised trial of response rates to a mailed questionnaire. J Health Serv Res Policy 2000; 5: 219–221. [DOI] [PubMed] [Google Scholar]