Abstract
Objective
To evaluate and assess the effectiveness of muscle strengthening and cardiovascular interventions in improving outcomes in poliomyelitis (polio) survivors.
Data sources
A systematic literature search was conducted in Medline, PubMed, CINAHL, PsychINFO, Web of Science, and Google Scholar for experimental and observational studies.
Study selection and extraction
Screening, data-extraction, risk of bias and quality assessment were carried out independently by the authors. The quality appraisal and risk of bias were assessed using the Downs and Black Checklist. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed to increase clarity of reporting.
Data synthesis
A total of 21 studies that met all the inclusion criteria were subjected to statistical analyses according to intervention (muscle strengthening or cardiovascular fitness). A random-effects metaanalysis showed a statistically significant effect for the exercise interventions favouring improvement in outcomes according to the International Classification of Functioning, Disability and Health (ICF).
Conclusion
This review provides further insight into the effects associated with muscle strengthening and cardiovascular interventions among polio survivors, and helps to further identify the current state of research in this area. Future research is needed, focusing on individualized approaches to exercise with polio survivors and specific exercise prescription recommendations, based on established frameworks, such as the ICF.
LAY ABSTRACT
Polio survivors are an ageing population and prone to functional decline. Multiple age-related diseases affect this population, in addition to Late Effects of Polio (LEoP). Exercise plays an important role in improving strength and overall cardiovascular fitness in these individuals, and clinicians face challenges when advising polio survivors on the optimal level of exercise to avoid producing pain and/or fatigue. Improvements in strength and cardiovascular fitness have the potential to translate into activities of daily living within this cohort. To our knowledge, this is the first systematic review and meta-analysis using a broad approach (i.e. including both experimental and observational studies) to capture and summarize the research to date regarding the role of muscle strengthening and aerobic conditioning exercise in polio survivors. This review provides valuable information for clinicians, which will help enable the development of specific exercise prescription appropriate to this population.
Key words: post-polio syndrome, International Classification of Functioning, Disability and Health framework, exercisebased intervention, rehabilitation
Poliomyelitis (polio) is a highly infectious, viral disease that affects the nervous system and can cause total paralysis (1). Based on published records, the World Health Organization (WHO) estimates there are 20 million polio survivors worldwide (1). Although outbreaks of polio have reduced significantly as a result of vaccination, 15–80% of all polio survivors develop post-polio conditions (2). Post-polio syndrome (PPS) is a clinical diagnosis in which symptoms may become apparent 15–30 years after exposure to polio (3, 4). PPS is characterized by progressive or new muscle weakness, generalized fatigue, muscle atrophy and pain (4, 5). Internationally, the cluster of signs and symptoms that include PPS features and additional biomechanical symptoms is referred to as Late Effects of Polio (LEoP) (6).
PPS can lead to significant disability, including inability to work, loss of mobility and loss of independence (4). Many individuals with PPS report being inactive, due to weakness and fatigue; symptoms that are perceived to worsen with activity (4). Reduced physical activity associated with muscle atrophy and deconditioning can then potentiate further fatigue and weakness and be linked with reduced muscle capacity and cardiovascular fitness, probably contributing to higher comorbidity rates and potential hospitalization (7). Aerobic fitness intervention modalities, such as walking, cycling, arm ergometry and water-based exercise, have been shown to be effective in attenuating decline in function in patients with PPS (8, 9). Furthermore, muscle strengthening training can also increase functional capacities in individuals with PPS (5), with previous research reporting improvements in isometric and isokinetic strength in individuals with PPS (4).
A recent systematic review initially raised concerns that polio survivors might overload weak muscles during exercise, causing an increase in symptoms, such as pain, fatigue and weakness (5). Despite this common concern, based on the methods used in this review, limited evidence was found to substantiate this (5). Earlier, a 2008 meta-analysis, limited by the number and quality of included studies, showed some cardiovascular outcomes could be improved in polio survivors, but apparent positive effects of strengthening were not significant (10). Such conflicting recommendations may cause clinicians and polio survivors to experience concerns about further risk of adverse outcomes when considering whether to pursue exercise programmes (8). The management of PPS symptoms is essential for maintaining quality of life and independence (11, 12). The benefits of exercise for those with non-communicable diseases comparable to PPS include improvements in productivity and wellbeing, and reduction in health system expenditure, further identified in a recent study focused around the role of the clinical exercise physiologist (13).
Clinicians can experience uncertainty when advising polio survivors on exercise, as divergent results and recommendations exist (10, 14). Limited modes of exercise have been studied in the literature with regard to this population, but aspects of exercise have been sufficiently tested to prompt this review. This systematic review and meta-analysis aims to summarize current knowledge of the effectiveness of muscle strengthening and cardiovascular interventions (and/or mixed interventions including both) in improving outcomes in polio survivors. The main hypothesis of this study is that these exercise interventions will improve outcomes in polio survivors above and beyond usual practice.
METHODS
Search strategy
The following computerized databases were searched for articles published from their respective inception dates to 20 February 2020, inclusive: Medline, PubMed, CINAHL, PsycINFO, Web of Science, and Google Scholar. Search terms were mapped to MeSH terms, or subject headings and synonyms were grouped together using Boolean operators. A range of search terms were used to identify the population, exercise interventions, and outcomes (see Table SI1). Both experimental (e.g. randomized controlled trials; RCTs) and observational (e.g. cohort studies) study designs were included in the search strategy and inclusion criteria. Results of the database searches were downloaded into Endnote X8 (Clarivate, Philadelphia, US) and duplicate papers were excluded. One author (AR) screened citation titles and abstracts for potentially relevant titles and abstracts. Article fulltext versions were then screened (AR and TL). Disagreements were resolved by consensus with the 2 remaining authors. Reference lists of all studies assessed against the eligibility criteria were also screened for additional literature.
Inclusion and exclusion criteria
Articles were included if: (i) the study targeted a sample of participants experiencing LEoP or PPS following a period of stable neurological function after 15 years; (ii) the focus was on original research; (iii) an exercise intervention was used; and (iv) publication was after 1980. Studies were excluded if they were: (i) conference or poster presentations; (ii) not original research; or (iii) not in English. Studies were not restricted by study design.
Quality assessment and risk of bias
Two independent reviewers (AR and TL) assessed the quality of the included studies using Downs and Black checklist (15) (Table SII1). The checklist consists of 27 questions addressing study reporting, external validity, internal validity (bias, confounding) and power. The quality index of the checklist has high criterion validity (r = 0.90), high internal consistency (KR- 20 = 0.89), test-retest (r = 0.88) and inter-rater (r = 0.75) reliability. For dimension reduction purposes, these items were reduced to 17 questions, with each question coded as either “yes”, “no”, or “undetermined”. The sum of each “yes”’ response contributed to the overall quality score, where higher scores indicate greater methodological quality. High-quality studies were categorised as 85–100%, moderate quality studies as 60–84%, and low quality as less than 59% (15). Two authors (AR and TL) rated each article independently. All disagreements (n = 15; 4% of all questions) were discussed at a consensus meeting and appropriate ratings decided on by the remaining authors.
Level of evidence
According to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework (16), the quality of evidence in this review can be utilised to help make recommendations to clinicians and polio survivors. Hence, based on risk of bias, inconsistency, indirectness, imprecision and other considerations, a summary of findings table was produced (Table I), highlighting overall certainty as well as the clinical importance of each key domain outcome.
Table I.
Summary of findings based on outcome domains and study design
| Certainty assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nr studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Impact | Certainty | |
| Activity and Participation (follow up: range 3 weeks to 15 months) | |||||||||
| 2/6a | Randomised trials and observational study designs | not serious | not serious | not serious | not serious | all plausible residual confounding would suggest spurious effect, while no effect was observedi | RCTs: Koopman et al. (3) and Murray et al. (4): Consistency in methods, interventions and outcomes (e.g. 6MWT, 2MWT, TUG), CIs considered not wide, homogenous. Observational: Bertelsen et al. (28), Brogardh et al. (46), Da Silva et al. (37), Davidson et al. (29), Sharma et al. (32), Skough et al. (38), Willen et al. (8): Some consistency in methods and activity outcomes. Interventions varied: strengthening, CV fitness, mixed, CIs considered not wide, homogenous. |
RCT only: ⊗⊗⊗⊗ HIGH All study designs: ⊗◯◯◯ VERY LOW |
|
| Body function mental and sensory (follow up: range 4 weeks to 10 months) | |||||||||
| 3/5 | randomised trials and observational study designs | seriousc
very seriousb,c |
not serious seriousd |
not serious seriousg |
not serious serioush |
all plausible residual confounding would suggest spurious effect, while no effect was observedi | RCTs: Koopman et al. (3), Oncu et al. (48) and Murray et al. (4) some consistency in methods, interventions and outcomes (e.g. all fatigue), CIs considered not wide Observational: Bertelsen et al. (28), Da Silva et al. (37), Davidson et al. (29), Dean et al. (25), Sharma et al. (32): Varied effect, CIs not wide, apparent improvement, interventions similar but outcomes varied. |
RCT only: ⊗⊗⊗◯ MODERATE All study designs: ⊗◯◯◯ VERY LOW |
|
| Body function lower (follow up: range 16 weeks to 10 months) | |||||||||
| 2/9 | randomised trials and observational study designs | very seriousb,c |
seriousd | not serious | serioush | publication bias strongly suspected, all plausible residual confounding would suggest spurious effect, while no effect was observedi | Jones et al. (47), Koopman et al. (3): Varied effect, wide CIs, apparent improvement, similar study characteristics (methods, interventions and outcomes). | All study designs: ⊗◯◯◯ VERY LOW |
|
| Body function cardiovascular (follow up: range 8 weeks to 10 months) | |||||||||
| 4/2 | randomised trials and observational study designs | very seriousb,c | seriousd | seriousf,g | serioush | all plausible residual confounding would suggest spurious effect, while no effect was observedi | Jones et al. (47), Kriz et al. (26), Koopman et al. (3), Oncu et al. (48): Varied effect, wide CIs, apparent improvement. | All study designs: ⊗◯◯◯ VERY LOW |
|
| Body function non-lower (follow up: range 4 weeks to 16 weeks) | |||||||||
| 3/2 | randomised trials and observational study design | very seriousb,c,d | seriouse | seriousf,g | serioush | all plausible residual confounding would suggest spurious effect, while no effect was observedi | Murray et al. (4), Chan et al. (33), Kriz et al. (26): Varied effect, wide CIs, apparent improvement. |
All study designs: ⊗◯◯◯ VERY LOW |
|
indicates ratio of RCT to non-RCT studies. CI: Confidence interval, CV: Cardiovascular fitness, RCT: randomised controlled trials, TUG: Timed up and go test, 6MWT: Six min walk test, 2MWT: Two min walk test Explanations:
no measure of random variability
limited adjustment of confounding
limited loss to followup, no intention to treat analysis
substantial heterogeneity
Differences in diagnostic criteria
Differences in outcome measures
Wide Confidence Intervals
as identified via funnel plot
Data extraction
The following data were extracted from included studies: author, study population, diagnosis criteria used, study design, followup time, type of exercise intervention, outcome measures, statistical analysis, and effects of the intervention. The International Classification of Functioning, Disability and Health (ICF) codes provide a framework for understanding the interactions of environment, conditions and personal factors on influencing body function and structure, activities and participation (17). Table SIII1 outlines second-level domain coding of the ICF for study outcome measures. Based on a proposed ICF Core Set for PPS (17), outcomes were grouped into the following ICF domains: (3) mental and sensory; and as a dual component domain (4) activity and participation.
As outlined in Table SIII1, the muscular function domain contains outcome measures such as isometric and dynamic strength, the cardiovascular domain contains outcome measures such as peak oxygen uptake, heart rate, blood pressure, and aerobic capacity (VO2) and mental and sensory domain contains pain and fatigue. The activity and participation domain contains outcome measures (from activities) such as 6-min walk test, 6-min arm test, Timed Up and Go test, 10-metre walk test, 2-min walk test, and (from participation) such as daily physical activity level scale for people with disabilities, Short-Form 36 (SF-36), physical component summary, mental component summary.
Data analysis
Following data extraction, effect sizes and their corresponding 95% confidence intervals (95% CI) were derived for each individual outcome. In the event multiple outcomes denoted a given ICF domain or component, a representative effect size was calculated by pooling the effect size of each outcome using Comprehensive Meta-analysis v3 (BioStat, Englewood, NJ USA). Data were sub-grouped into the respective interventions used (aerobic fitness, mixed, and muscle strengthening) and meta-analyses were completed on the combined body function domain, and each of its underlying components (lower limb, non-lower limb, cardiovascular, and mental and sensory), and a collated activity and participation domain. For all analyses, a generic inverse variance, random effects model was used. This model was adopted due to the anticipated differences among studies (study duration, outcome measures, and/or post-polio condition severity). Effect sizes were reported as Hedges g, with the magnitude of the effect defined using standardized conventions, where small, moderate, and large are represented by values of 0.20, 0.50, and 0.80, respectively (18). Significance was investigated through the use of p-values, where the alpha was set at ≤ 0.05. Heterogeneity was evaluated using Cochran’s Q, where the alpha was set at ≤ 0.10. In the event significance was reported, the I2 statistic was then explored to define the magnitude of heterogeneity about the result, where 0–40, 30–60, 50–90, and 75+ were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively (19). Leave-oneout sensitivity analyses were conducted when statistical heterogeneity was reported. In order to investigate the effects of intervention duration on the domains and/or components of the ICF, meta-regression was performed at the study level using a random-effects model. Publication bias was also investigated statistically through the Begg and Mazumdar’s rank correlation test and Eggers linear regression model, which were applied to each component and the overall analyses. If publication bias was detected, Duval and Tweedie’s trim and fill correction was applied and the resultant Hedges g and associated 95% CI were explored. Given the software used for these analyses the L0 estimator was used to formulate the correction. GRADEPro GDT (20) (McMaster University, Hamilton, Ontario, Canada) was used to develop a summary of findings table consistent with the GRADE handbook (16).
RESULTS
Systematic search and study quality
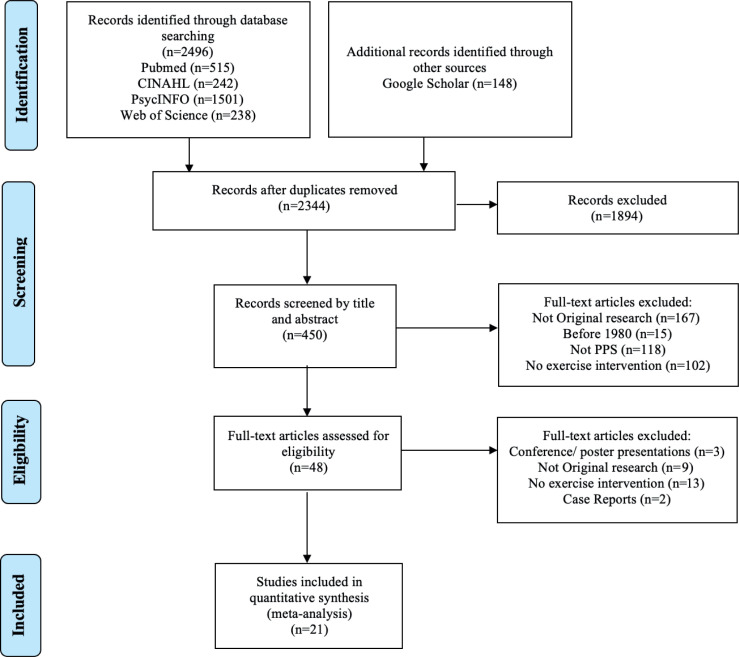
Fig. 1 shows the flow diagram of the systematic search, consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (21). The search strategy produced 2,644 citations from 6 databases. Following the removal of duplicates, 2,327 studies remained. After title and abstract screening, 48 studies were then assessed against the eligibility criteria, where 21 citations were then included and assessed for study quality, with the quality assessment scores ranging from 53% to 88%. The mean (standard deviation (SD)) score of study quality was found to be 72.5 (10.5) %. Individual scores for each study are also shown in Table SIII1. Of those studies satisfying study quality, there were 2 instances where more than one article was based on the same sample (3, 9, 22, 23), with the latter also examining both iso-metric and isokinetic exercise. Hence, the latter study in each circumstance of overlapping samples was removed from the analysis. Furthermore, one study (24) was divided into 2 cohorts to reflect 2 different intervention contexts (e.g. hospital-based compared with home-based).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (21) flow diagram outlining the identification and inclusion process for the quantitative review. PPS: post-polio syndrome.
Study descriptions
Of the studies included in this systematic review, 7 were RCTs, 4 were controlled trials, 7 were longitudinal studies, with the remaining 3 either cross-sectional, case-crossover or case-control design. Study duration ranged from 5 to 32 weeks, with sample size ranging from 5 to 68 participants with post-polio conditions. Of the included studies, 5 focused on aerobic fitness interventions (4, 24–27), 7 on mixed interventions (aerobic fitness and muscular strengthening/functional activities) (3, 9, 28–32) and 9 on muscular strengthening interventions (22, 23, 33–39). Table II summarizes the study population, sample size, study design, follow-up time, type of exercise intervention, outcome measures and statistical analysis and associations.
Table II.
Experimental study designs according to study design and intervention type (cardiovascular fitness, mixed intervention methods and muscle strengthening)
| Author | Study population | Diagnosis criteria used | Inclusion criteria | Study design | Intervention type and duration | Type of exercise intervention | Outcome measures | Statistical analysis | Effects of intervention |
|---|---|---|---|---|---|---|---|---|---|
| Agre et al. 1996 (44) | 12 participants (7 F and 5 M), 35-60 years | Halstead and Rossi (1985) | Excluded those with <3+/5 on manual strength testing | Longitudinal | Muscle strengthening activities (12 weeks) | Muscle strength: 4 days a week, cuffed ankle weights (~ 1 to 1.5kg = 13-14 RPE). Leg extension, hold 5 seconds. 1 rep every 30 secs, 6 reps at first then up to RPE 17/20 or 10 reps | Exercise compliance. Neuromuscular: Ankle weight lifted (kg), Isometric quads: peak torque, endurance holding time (sec), MVC (Nm) Tension time index (Nms) Isokinetic quads: quads peak torque (Nm), quads total work (Nm), hamstrings peak torque (Nm), hamstrings total work (Nm). EMG: Blocking (%), Jitter (usec), Macro EMG amplitude (mV). Serum CK | Wilcoxon matched pairs test. Friedman repeated measures ANOVA. Results in mean (SD), p < 0.05 | Positive association between strength training and ankle weight lifted |
| Agre et al. 1997 (45) | 7 participants (gender not reported), 35–65 years | Halstead and Rossi (1985) | Excluded those with <3+/5 on manual strength testing; allowed recent strength loss in 6 of 7 participants | Longitudinal | Muscle strengthening activities (12 weeks) | Muscle strength: 4 days a week ankle weights (~ 1 to 1.5kg = 13-14 RPE). Isokinetic (Tues/ Fri): Leg extension, hold 5 seconds. 3 x 12 reps rest 1 min. Isometric (Mon/ Thurs): 3 x 4 reps max. contractions 5 secs, rest 10 secs, 1 min between sets. Knee at 60 degrees from full extension | Exercise compliance. Neuromuscular: Ankle weight lifted (kg), Isometric quads: peak torque, endurance holding time (sec), tension time index (Nms), Isokinetic quads: quads peak torque (Nm), quads total work (Nm), hamstrings peak torque (Nm), hamstrings total work (Nm). EMG: Fiber density, Blocking (%), Jitter (usec), Macro EMG amplitude (mV). Serum CK | Wilcoxon matched pairs test. Friedman repeated measures ANOVA, with Holm’s post hoc comparisons. Results in mean (SD), p < 0.05 | Positive association between strength training and quad isometric (p < 0.05) and isokinetic (p < 0.05) |
| Bertelsen et al. 2009 (28) | 50 participants (30 Fand 20 M), age range 24-82 years, 4 dropped out due to illness within the follow up period. | Halstead and Rossi (1985) Informal PPS criteria; had acute polio and now have new problems | Allowed new problems related to PPS; 74% had reported recent strength decrease | Longitudinal (prospective uncontrolled intervention study) | Mixed: Aerobic fitness, muscle strengthening and functional exercises. (3 and 15 months) | Individualised physiotherapy-based approach. Physiotherapy and subsequent exercise programme including both muscle strength and aerobic fitness interventions. Consisting of a combination of exercise (included in 80% of programmes), massage (78%), stretching (72%), home training (72%), walking (26%), and/or balance training (24%) | 6MWT and timed-stands test | SF-36 and MF0-20 were converted into scales of 0 to 100. Non-parametric matchedpairs significance tests (Wilcoxon matched pair test) | Significantly improved 6MWT performance (BL: 378 m (SD 131), 3 months: 418 m (SD 122), 15 months: 419 m (SD 138); both p <0.001 to BL) and timed-stands test performance (BL: 31 sec (SD 7) , 3 months: 27 sec (SD 7), 15 months: 28 sec (SD 8); both p <0.001 to BL) |
| Brogardh et al. 2010 (46) | 5 participants (3 M and 2 F), aged 64 years (SD 6.7) , age range 55–71 years with late stages of polio | Halstead and Rossi (1985) | Excluded clinically unstable symptoms; Subjects had either PostPolio Class III clinically stable or Class IV clinically unstable polio | Case-controlled pilot study | Muscle strengthening activities (5 weeks) | Muscle strength: 2 x 30 min weekly sessions of WBV – standing knees flexed at 40–55°. Repetition duration and number was 40 sec and 4 reps (start of intervention) and increased to 60 sec and 10 reps | Isometric and isokinetic knee extensor and flexor strength - MVC (Nm) in less and more affected limbs. Gait performance – TUG, Comfortable and fast gait speeds tests, and the 6MWT | Mean relative difference = (diff pre to post/pretreatment x 100). Paired t-tests, p< 0.05 | Strength: Isokinetic KEXT (less affected limb: 125 (SD 43) to 123 Nm (SD 46), more affected limb: 54 (SD 35) to 56 Nm (SD 39), isokinetic KFLX (less affected limb: 64 (SD 32) to 66 Nm (37), more affected limb: 26 (SD 21) to 24 Nm (SD 20), Gait performance: TUG (11.0 (SD 2.0) to 10.9 sec (SD 1.9), comfortable gait speed (10.2 (SD 2.6) to 9.4 sec (SD 2.1), fast gait speed (7.2 (SD 1.9) to 7.1 sec (SD 1.7 ), and 6MWT (422 (SD 105) to 417 m (SD 92) |
| Chan et al. 2003 (33) | 10 post-polio patients (9 F and 1 M): 5 in training group (4 F and 1 M), 5 in control group (5F) | Post-polio diagnosis, affecting one or both upper limbs, moderate motor neuronal loss in median-innervated thenar muscles with MUNE 10-90 | Excluded those with MUNE <10, as increase in strength unlikely | RCT | Muscular strengthening activities (12 weeks) | 3 x 8 upper limb isometric contractions (50-70% MVC), 5 min rest between sets. 3 x weekly for 12 weeks | Thenar MVC, voluntary activation, estimated motor unit number, and surface detected motor unit action potential | One-way ANOVA. Post hoc analysis using Scheffe test. Training changes analysed using paired t-tests, between groups compared with independent t-tests | Improved thenar MVC force production and level of voluntary activation in contrast to control p < 0.05), while the estimated number of motor units and surface detected motor unit action potential remained similar (p>0.05). Compared to control MVC force increased 41%, voluntary activation improved 13%, estimated motor unit number was lower in the training group (30%), but these were greater in control at baseline (training: 45 (SD 16); control: 69 (SD 22) a.u.). Surface detected motor unit action potential increased in both groups (training: 389 (SD 53) to 370 (SD 56) and control: 215 (SD 29 to 238 (SD 31) |
| Da Silva et al. 2019 (37) | 21 with or without PPS (age: 40-85; Body weight less than 227 kgs) | National Institute of Health, 2015. PPS diagnosis not required but most participants had it; various criteria may have been used | Excluded those unable to tolerate weight bearing for 20 min | Random order, Crossover Exploratory Experimental Intervention | Muscular strengthening activities: WBV (4 week block of 8 sessions -crossover) | Two intervention groups with one group participating in low intensity WBV 4-week block of 8 sessions first (group Lo-Hi), and higher intensity WBV 4-week block of 8 sessions second | 10mWT, 2mWT, BPI Interference, Severity, PSQI, FSS | Descriptive statistics, Mann-Whitney U tests for between subject changes, Non-parametric Wilcoxon for within subject changes, Friedman's analysis of variance | Improvement in walking speed in Hi-Lo frequency group. Improvement in BPI pain severity after exposure to higher vibration. No significant change in 2mWT, PSQI or FSS |
| Davidson et al. 2009 (29) | 27 post-polio patients (17 M and 10 F), mean age of 56.4 years, age range 44-74 years | Informal PPS criteria; definite history of polio and new physical disability and symptoms typical of PPS | Allowed those with new physical disability and symptoms typical of PPS | Longitudinal | Mixed: both muscle strengthening and aerobic fitness activities (3 and 6 months) | An initial 3 per week for 3 weeks of a supervised exercise programme including a timed interval training circuit (based on CV fitness), stretching, hydrotherapy, relaxation techniques. Self-directed exercise until follow up | Muscle strength (sit to stand, grip strength of dominant hand), muscle endurance (10m shuttle walk test). Hospital anxiety and depression scale, Illness perception questionnaire | Non-parametric matched-pairs significance tests (Wilcoxon), Spearman rank correlations. Mann-Whitney test | Positive: Circuit training and shuttle test 29%), RPE, STS (20%) |
| Dean et al. 1991 (25) | 48 post-polio participants (38 F and 10 M, age ranging from 32 to 71 | Informal PPS criteria; confirmed history of poliomyelitis | Cross- sectional study | Aerobic fitness activities (6 weeks) | Two-min walking at 1.6 km/hr followed by an increase of 0.8 km/hr each min till a comfortable cadence was reached | Movement economy and cardio-respiratory conditioning based on movement economy index (MEI) and cardiorespiratory conditioning index (CRCI) based, maximum heart rate and VO2 | 2x2 ANOVA, Pearson product moment correlations and t-tests, p < 0.05 | MEIs were significantly different between the normal and reduced movement economy groups based on the manner in which the groups were categorised (p < 0.01). MEIs were not different for the conditioned and deconditioned groups (p > 0.05). CRCIs were significantly different between the normal and reduced conditioning groups based on the manner in which the groups were categorised (p <0.01). CRCIs were not significantly different for the groups with normal and reduced movement economy (p > 0.05) | |
| Einarsson 1991 (35) | 155 participants | Informal PPS criteria | Excluded those with <3+/5 on manual strength testing | Longitudinal study | Muscular strengthening activities (6 to 12 months post training) | 3 sessions/week of 12 sets of 8 isokinetic contractions, each at 180”/sec angular speed interposed with 12 sets of isolated 4-second isometric contractions at 30°, 60° | Isometric flexion and extension strength, Isokinetic flexion and extension strength, Fatigue Index and muscle biopsy | Non parametric Wilcoxon test, Spearman rank correlation test was used for analysis of correlation | Significant (p < 0.0l) increase (mean 29%) in isometric knee-extension muscle strength measured at 60° knee angle and in isokinetic knee-extension strength (mean 24%), measured as peak torques at angular velocities of 30°, 60°, 180° and 300° per second |
| Ernstoff et al. 1996 (30) | 12 (9 Fand 3 M). Aged 39 to 50 (mean 42 years), 5 lost to follow up. All but 4 had symptoms according to Halstead's criteria | Halstead (1987) | Excluded those unable to perform full knee extension or had severe weakness; those with <3/5 on quad manual strength testing | Longitudinal study | Mixed: both muscle strengthening and aerobic fitness activities (22 weeks) | 2 x week for 22 weeks. Group and home programmes. 60 min with 5 min warm up, low resistance, high rep ex for upper/lower/trunk. 5 mins cycling at 60-80% GXT | Muscle strength (highest peak torque from isokinetic concentric strength/isometric knee flexion dynamometer), Fatigue Index Evaluation, graded exercise test (GXT) bike ergo 30, 70, 100, 130 watts, muscle biopsy/CSA | Wilcoxon's signed rank test for statistical analysis. Spearman's rank correlation | Positive: 1) Less fatigue (reduction in peak torque) in weaker leg after training. 2) significant increases in strength of right elbow ext. Right wrist ext., hip abd laterally. 3) significant reduct in HR (133 vs 127 after), showing fitness |
| Fillyaw et al. 1991 (36) | 17 (6 lost to follow-up excluded from analysis) Halstead and Rossi criteria for post-polio, MMT fair +, both quads Age 51.3 (SD 12.3) | Halstead and Rossi (1985) | Excluded those with less than fair quads; <3/5 on manual strength testing | Controlled trial, randomised by muscle group (quads vs biceps) | Muscular strengthening activities (2 years) | 14 exercised quads muscle, 3 biceps. 10RM through knee ext. or elbow flex. without pain/fatigue. HEP based off 10RM 3 x 10 reps every other day. Set 1: 50% 10RM, 2: 75% 10RM, 3: 100% 10 RM. 5 mins. rest between | Maximum isometric torque (MIT), endurance integral (EI). 10 RM every 2 weeks | Analysis of variance between exercise and control group for MIT and EI using SAS General Linear Model | Positive: 1) Exercise and strength (10RM, mean increase 78%, p < 0,001). 2) Exercise and MIT: 8.4%, p = 0.04. 3) NS change in exercise and EI |
| Jones et al. 1989 (47) | 45 patients (37 completed the study) (age between 30 and 60 years) | Informal PPS criteria; hospital records | Adequate strength in at least one lower extremity to pedal an ‘exercycle’ and ’arm cycle ergometer’ | RCT | Aerobic fitness activities (16 weeks) | The training group trained at 70–75% of the heart rate plus resting heart rate on ergometer. 15–20 min exercise/session | Resting heart rate, beats per min, maximal heart rate, beats per min, Resting systolic blood pressure, mm Hg, Resting diastolic blood pressure, mm Hg, Maximum systolic blood pressure, mm Hg, Maximum diastolic blood pressure, mm Hg, Watts, Exercise times, Maximum expired volume, l/min, Maximum oxygen consumption, ml/min, Maximum carbon dioxide consumption, ml/min, Respiratory exchange ratio | Mean (SD) scores for pre and post treatment differences, multivariate analysis of variance was used to compare changes, Hotelling’s T2 for statistical test | Improvement in watts attained during testing, duration of testing, and VO2 max. Positive impa2ct of cardiorespiratory training on exercise group |
| Koopman et al. 2016 (3) | 68 participants (age between 18 and 75) | March of Dimes (2000) | Allowed those with walking ability, at least indoors, with or without a walking aid with or without a walking aid; and ability to cycle on a ergometer against a load of at least 25 W | Stratified multicentre single blinded RCT | Mixed: aerobic fitness, muscle strengthening and functional exercises. (>6 months) | Exercise Therapy: 3 sessions/week aerobic exercise on a cycle ergometer. Intensity increased from 60% to 70% heart rate reserve. Duration increased from 28 to 38 min | Submaximal heart rate during exercise, muscle strength (maximal isokinetic voluntary torque of quadriceps muscles), functional capacity (Timed-Up-and- Go test and 2-Min Walk test), and actual daily physical activity level | Primary analysis for efficacy: linear mixed models, with group and pre-treatment score of the outcome as covariates (primary analyses) | No beneficial effect of ET on fatigue, activities, or HRQoL compared with UC in patients with PPS |
| Kriz et al. 1992 (26) | 29 subjects at baseline, 20 at follow up | Informal PPS criteria | Physician screening; participants had to have adequate trunk and upper extremity strength for ergometry | RCT | Aerobic fitness activities (16 weeks) | Upper extremity aerobic exercise programme. 3 x per week for 20 min. Intensity at 70-75% HRR plus RHR | HRrest, HRmax, BP at rest, BP immediately post exercise, VO2max, RER, VEmax, RR | Change scores were compared using MANOVA. Univariate F-test was used to determine p < 0.05 | Positive: Exercise programme and fitness (VEMax - 17%, V VCO2 - 20%, VO2Max - 19%, 12% - Powe2 r, exercise time - 10%) |
| Murray et al. 2017 (4) | 55 subjects | Informal PPS criteria | Excluded those with severe weakness; those with unstable muscle groups per ACSM; severe fatigue or recent onset of weakness | Prospective, single blinded - RCT | Aerobic fitness activities (8 weeks) | Home-based arm ergometry at an intensity of 50%-70% maximum heart rate, compared with usual physiotherapy care | The 6-MAT, Fatigue Severity Scale, Physical Activity Scale for Individuals with Physical Disabilities SF-36 | Sample t-test for intergroup comparison and paired t-test for within group comparison. Linear regression modelling or Poisson regression. A significance level of p < 0.05 was set | No significant association between exercise and 6-MAT or 6MWT |
| Oncu et al. 2009 (48) | n = 15 hospitalbased programme and n = 13 in home-based exercise programme | Halstead (1991) | Allowed those with new lower limb weakness. Allowed those with ambulatory ability of 30 m in 60 sec | RCT | Aerobic fitness and stretching activities (8 weeks) | 3 session/week of 1.5 hours. Flexibility training, aerobic fitness on treadmill involving walking for 30 min with 3 rest periods at an intensity of 50–70% of pVO2 and at a level of 13–15 on the Borg Scale. Patients in group 2 performed flexibility and aerobic exercises. A walking programme was undertaken by the patients in group 2 as an aerobic exercise at 50–70% of pVO2 | FSS, FIS, Quality of life, heart rate, rhythm, Max oxygen consumption (pVO2) and carbon dioxide production (VCO2) | Mann–Whitney U test for numeric data, Fisher’s exact or chi-square tests for nominal data, nonparametric Wilcoxon test, Mann–Whitney U test for pre- vs postexercise differences | Improvement was observed in the parameters of fatigue and quality of life in both the hospital exercise group and the home exercise group. An increase in functional capacity was also found in the hospital exercise group |
| Sharma et al. 2014 (32) | 21 participants (13 F and 8 M) age between 18 and 65 | Halstead (1985) | Allowed body position change to reduce/ eliminate gravity for weaker muscle groups | Controlled trial | Aerobic fitness activities (4 weeks) | Group A: Performed exercise and lifestyle modification. Exercises were divided into 4 phases. Phase 1: warm up, gentle AROM; Phase 2: strengthening exercises, 8 muscle groups; Phase 3: aerobic exercise, 10 mins static cycling, moderate intensity (i.e. RPE of 13-15 on modified Borg's scale) Phase 4: Cool down, gentle PROM (5 reps) | FSS, 2MWD, Patient Reported Outcome Measurement Information System (PROMIS), Patient Health Questionnaire (PHQ-9) | Wilcoxon signed-rank test for within-group differences in FSS score, Kruskal-Wallis test for between groups differences, Mean difference in 2MWD within each group using paired t-test | Significant diference in FSS within group A and group B. For 2MWD, there was a statistically significant difference within group A and group B but no difference in group C. Physical function as measured by PROMIS, showed a statistically significant difference in group A and no difference in group B and group C. Statistically significant difference between groups in FSS score and PRoMiS score |
| Skough et al. 2008 (38) | 14 subjects at baseline and follow up (8 F and 6 M) | March of Dimes (2000) | Allowed those who were able to walk with or without a walking aid for 6 min | Randomized, placebocontrolled pilot study | Muscular strengthening activities (12 weeks) | Resistance training at 10-11 on the Borg Rate of Perceived Exertion scale for 30 min/session. the initial work-load was 50-60% of 1 repetition maximum (1RM) and was successively increased to an intensity of 70-80% of 1RM | Sit stand sit, timed up & go, 6-min walk, muscle strength measurement by means of dynamic dynamometer and short-form (SF)-36 questionnaire | Wilcox on signed-rank test was used to analyse differences within groups and Mann-Whitney U test for differences between groups. A p < 0.05 was taken as statistically significant | Positive: Significant associations between exercise programme and STS, 6MWT and muscle strength |
| Spector et al. 1996 (39) | 6 subjects at baseline and follow up | Informal PPS criteria | Excluded with study <3+/5 on manual strength testing; allowed limbs described ranging from asymptomatic to flaccid | Controlled those | Muscle strengthening, 10 weeks 4 to 6 weeks post training 5 months | Progressive resistance exercise of knee and elbow extensors representing both symptomatic and asymptomatic muscles | Fatigue Severity Scale, isometric and dynamic strength, MRI. Biopsies | t-tests | Positive: PRT and dynamic strength (3RM), 10 week and 5 months |
| Voorn et al. 2016 (49) | 44 participants (24 F and 20 M) | March of Dimes (2000) | Allowed those able to walk at least around their house | RCT | Mixed: aerobic fitness, muscle strengthening and functional exercises (3 x week, 4 months) | Home-based aerobic training programme on a bicycle ergometer 3 x weekly and a supervised group training 1xweek (muscle strengthening functional exercise) | Muscle endurance, Muscle strength (MVT). Resting HR, oxygen consumption at the AT, VO2 submax, RER submax, and RPE submax | Wilcoxon signed- rank test and Mann-Whitney U test were used. Linear regression model SPSS statistical software package | Training programme did not significantly improve muscle function nor CV fitness |
| Willen et al. 2001 (8) | 30 participants at baseline, 28 at follow up | Informal PPS criteria; late effects of polio | Excluded National Rehabilitation Post-Polio Limb Classification of I (no history of remote or recent weakness) | Controlled trial: Before-after tests | Mixed (Aerobic fitness, muscle strengthening and functional exercises) average of 5 months | 40 min of general fitness training session in warm water twice weekly | Peak load, Peak oxygen uptake, Peak HR, Berg balance scale, Visual analogue scale, Pain scale, Physical activity scale for the Elderly, and NHP. | Wilcoxon's signed-rank test and the Mann-Whitney U test A significance level of p = 0.05 was used throughout the study | Positive: exercise and function (lower HR and self-reported improvement in physical fitness) |
AROM: Active Range of Motion; BL: baseline; BPI: Brief Pain Inventory; CBT: Cognitive behavioural therapy; CK- Creatine Kinase; CRCI: Cardiorespiratory conditioning index; CSA : Cross-sectional area; CV: Cardiovascular; EI: Endurance integral; EMG: Electromyography; ET: Exercise therapy; Ext: Extension; F: female; FIS: Fatigue Impact Scale; Flex: Flexion; FSS: Fatigue severity scale; GLM: General linear model; GXT: Graded Exercise Test; HEP: Home exercise programme; HR: Heart rate; HRQoL: Health related quality of life; KEXT : knee extensor, KFLX : knee flexor; Kg: Kilogram; L: Litre; M: male; MAT- Min arm test; Max: Maximum; MEI: Movement economy index; MFI: Multidimensional fatigue inventory; MIT: Maximum isometric torque; mL: Millilitre; MRI: Magnetic Resonance Imaging; MUNE: Motor Unit Number Estimate; MVC: Maximal voluntary contraction; mV: millivolt; MVT: Muscle strength; MWT: Min Walk Test; Nm: Newton metre; NS: Not stated; NHP: Nottingham health profile; pVO2: Maximum oxygen consumption; PHQ: Patient health questionnaire; PPS: Post-polio syndrome; PROM: Passive Range of Motion; PROMIS: Patient reported outcome measurement information system; PSQI: Pittsburgh sleep quality index; RCT; Randomised controlled trial; RER: respiratory exchange ratio; RHR: Resting heart rate; RM: Repetition max; RPE: Rate of perceived exertion; SD: Standard deviation; Sec: Seconds; SF: short-form; SIP-68: Sickness Impact Profile; STS: Sit-to-stand; TUG: Timed up and go; usec: microsecond; UC: Usual care; VO2: Oxygen consumption; WBV: Whole body vibration.
Meta-analyses: the effect of exercise interventions
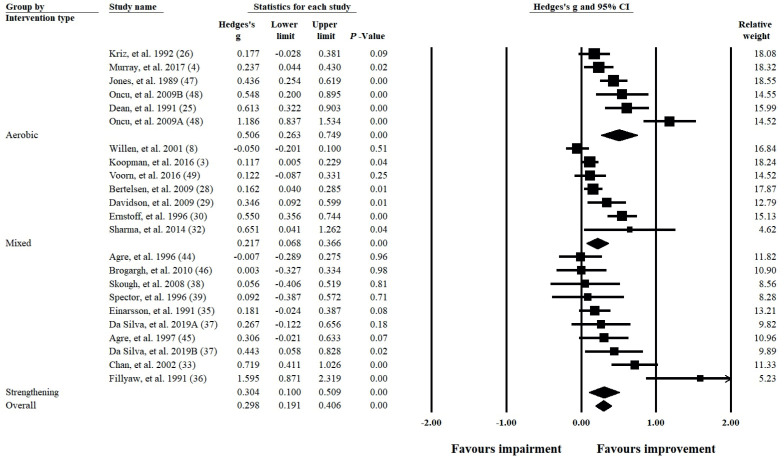
Fig. 2 shows the separate and combined effects of exercise interventions on the body function domain. When collated in this manner, aerobic, mixed and strengthening interventions were shown to have a small-to-moderate effect (g ranging from 0.217 to 0.506; all p < 0.01); however, heterogeneity ranged from substantial to considerable (all p < 0.01; I2 ranging from 70% to 83%). The overall effect on the body function domain indicated exercise interventions have a small positive effect (g = 0.298; 95% CI = 0.191– 0.406; p < 0.01); however, substantial to considerable heterogeneity was evident (Q(22) = 106.499; p < 0.01; I2 = 79%). To determine the robustness of these findings, a leave-one-out sensitivity analysis was completed and the output reported in Table SV(A–D)1. Table SVI1 (A shows a sensitivity analysis where both (9, 22) were removed from the analysis, as participants used in these studies may have been repeated. For the combined body function domain, the results of this analysis were comparable to the main analysis, where all interventions and the combined overall effect were significant (all p ≤ 0.01) and the same effect size ranges were maintained (g ranging from 0.237 to 0.506). All data were substantial to considerably heterogeneous (all p ≤ 0.01; I2 ranging from 73% to 83%).
Fig. 2.
Forest plot of the combined body function domains of the International Classification of Functioning, Disability and Health (ICF) disability framework. Studies are divided into their respective intervention types (aerobic fitness, mixed, and strengthening exercise).
Table III shows the meta-analysis summaries for each of the examined components and/or domains of the ICF. Within the body function domain, when all interventions were combined, small-to-moderate effects were identified in the lower limb, non-lower limb, and mental and sensory components (g ranging from 0.178 to 0.463; all p < 0.01). Only the cardiovascular component was not significant (g = 0.114; 95% CI = –0.034 to 0.262; p = 0.13). Significant heterogeneity was present for each of the components within the body function domain and ranged from moderate to considerable (all p ≤ 0.01; I2 ranging from 54% to 78%). Output from the leave-one-out sensitivity analysis is shown in Table SV(E, H, I and L)1.
Aerobic interventions within the body function domain were shown to produce significant small-tolarge effects in the non-lower limb, cardiovascular, and mental and sensory components (g ranging from 0.260 to 0.733; all p ≤ 0.01). Data were homogenous for the finding in the non-lower limb (Q(1) = 0.151; p = 0.70; I2 = 0%), while substantial heterogeneity was found within the cardiovascular and mental and sensory components (both p = 0.01; I2 ≥ 68%). The effect of aerobic interventions on the lower limb component was not explored, due to an insufficient number of studies (n = 1). Output from the leave-one-out sensitivity analysis is shown in Table SV(J and M)1 .
For mixed interventions within the body function domain, small improvements were found for the lower limb and mental and sensory components (g = 0.140 and 0.197 respectively; both p ≤ 0.05), while the cardiovascular component was not significant (g = 0.007; 95% CI = –0.169 to 0.183; p = 0.94). Data for mixed interventions were not interpreted for the non-lower limb component due to an insufficient number of studies (n = 1). For each of these findings, heterogeneity was not significant (all p ≥ 0.20; I2 ≤ 35%). Output from the leave-one-out sensitivity analysis is shown in Table SV(F and N)1. Removal of (9) resulted in significance being lost for mixed interventions in the lower limb component (g = 0.127; 95% CI = –0.028 to 0.281; p = 0.11), while there were insufficient study numbers to interpret the cardiovascular component (g = –0.092; 95% CI = –0.299 to 0.115; p = 0.38), shown in Table SVI1.
For strengthening interventions, a positive small-tomoderate effect was found for the lower limb and mental and sensory components (g = 0.232 and 0.356 respectively; both p ≤ 0.05). Although substantial heterogeneity was present for the lower limb (Q(6) = 18.614; p < 0.01; I2 = 68%), data were considered homogenous for the mental and sensory component (Q(1) = 0.398; p = 0.53; I2 = 0%). The main effect here appears to be between muscle strengthening exercise and fatigue, as fatigue measures accounted for 36% (13 of 36) compared with pain measures (19%) of the mental and sensory measures reported in the included studies. Within the non-lower limb component, muscle strengthening did not produce a significant effect (g = 0.337; 95% CI = –0.341 to 1.015; p = 0.33), but substantial to considerable heterogeneity was also present (Q(1) = 5.379; p = 0.02; I2 = 81%). Output from the leave-one-out sensitivity analysis is shown in Table SV(G and K)1. Removal of (22) resulted in a loss of significance for muscle strengthening in the lower limb component (g = 0.235; 95% CI = –0.049 to 0.519; p = 0.10; Table SVI (B)1), and data were substantially heterogeneous (Q(5) = 17.887; p < 0.01; I2 = 72%). This study did not contribute to the remaining components.
For the combined activity and participation domain, exercise interventions produced a small positive effect (g = 0.143; 95% CI = 0.088–0.198; p < 0.01). A small positive effect was also found for mixed interventions (g = 0.145; 95% CI = 0.086–0.204; p < 0.01). The aerobic and strengthening interventions were not significant (g = 0.173 and 0.071 respectively; both p≥0.09). All data sets in this domain were homogenous (all p≥0.73; I2 = 0%). Output from the leave-one-out sensitivity analysis is shown in Table SV(O–Q)1.
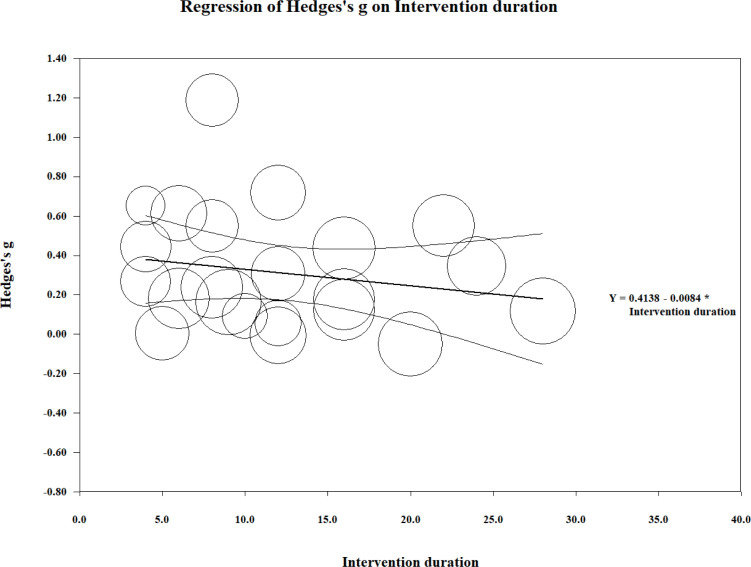
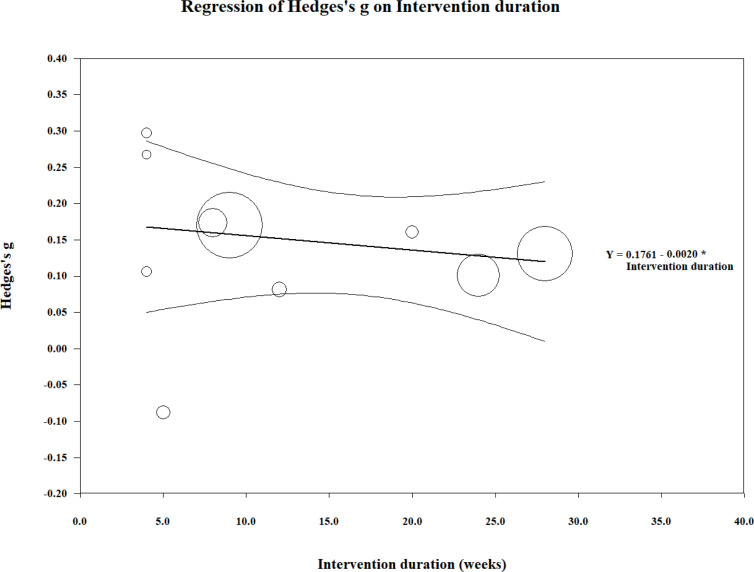
Meta-regression
Meta-regression was performed on the effect size estimates from the combined body function domain and the duration of the exercise intervention (Fig. 3). The overall test of the model was not significant (Q( 1 ) = 1.06; coefficient = –0.008; 95% CI –0.024 to 0.008; p = 0.30). In addition, the goodness of fit for this outcome was deemed significant (Q(20) = 87.810; p < 0.01), suggesting the dispersion of effects is outside the range expected from standard error alone. The analysis was conducted without (36), as the intervention duration by these authors far exceeded that of any other study and we believed it to be an outlier. Similar results were found for the activity and participation domain (Q(1) = 0.410; coefficient = –0.002; 95% CI = –0.008 to 0.004; p = 0.52; Fig. 4). However, goodness of fit was not significant (Q(8) = 2.310; p = 0.97).
Fig. 3.
Meta-regression analysis of intervention duration (weeks) and the effect size (g) for outcomes within the body function component of the International Classification of Functioning, Disability and Health (ICF) disability framework. Each study (n = 22) is depicted by a circle, with the circle size representing the relative weight attributed to each effect size. Note that (36) was removed from the analysis due to the long duration of the intervention.
Fig. 4.
Meta-regression analysis of intervention duration (weeks) and the effect size (g) for outcomes within the combined activity and participation components of the International Classification of Functioning, Disability and Health (ICF) disability framework. Each study (n = 10) is depicted by a circle, with the circle size representing the relative weight attributed to each effect size.
Publication bias
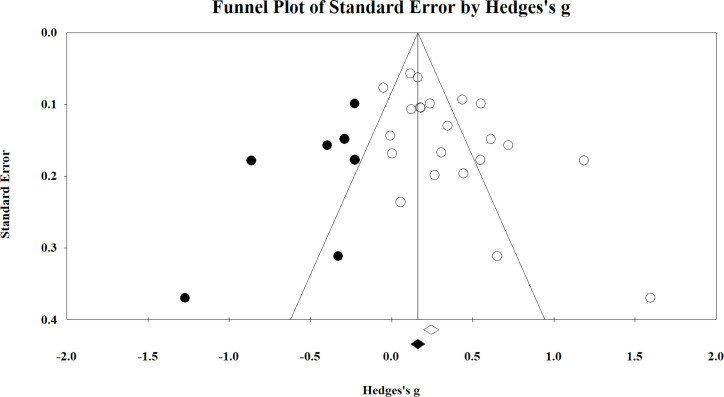
Possible publication bias was examined on the combined body function domain, each of the individual components, and the combined participation and activity domain. The funnel plot for the collated body function domain is shown in Fig. 5, where significant publication bias was identified using both the Begg and Mazumdar rank correlation test (Kendall’s τ = 0.299; p = 0.03 (1-tailed)) and Eggers linear regression method (intercept = 2.840; p = 0.01 (2-tailed)). Application of Duval and Tweedie’s trim and fill method indicated that 7 studies were missing to the left of the analysis (negative; implying that exercise interventions may impair outcomes within the body function component). These studies ranged from g = –0.233 to –1.276.
Fig. 5.
Funnel plot of the combined body function domain. The included and imputed studies are denoted by the white and black circles, respectively. Studies reported to the right of 0 represent exercise interventions having a positive effect on the respective domain and/or component.
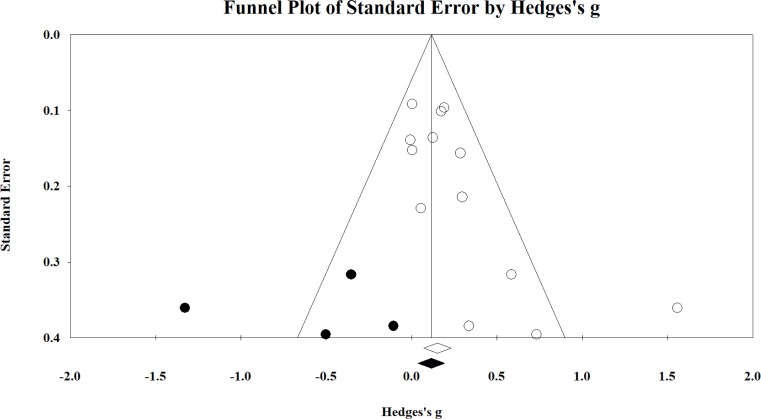
As outlined in Table III, there is limited evidence to suggest that exercise interventions are likely to have a debilitating effect on function (particularly up to the range shown statistically here). We consider it unlikely that such stark findings would have gone unpublished and, thus, have chosen to ignore this correction. However, even if the correction were accepted, a small beneficial effect on the body function domain still results (g = 0.175; 95% CI 0.046–0.303). Fig. 6 shows the funnel plot for the lower limb component of the body function domain, which also indicated significant publication bias (Kendall’s τ = 0.487; intercept = 2.100; both p ≤ 0.02). The analysis suggested that 4 studies were missing that imply exercise has a small-to-large negative effect on this component (g ranging from –0.113 to –1.333). Dissimilar to the commentary provided above on the combined body function domain, the correction results in the finding becoming nonsignificant (g = 0.120; 95% CI = –0.037–0.277). However, as stated above, we believe it is unlikely that studies with such prominent contrary findings to the present analysis would not have been published and thus, have chosen to ignore the corrected data. Publication bias was not evident for each of the remaining components of body function or in the combined activity and participation domain (all Kendall’s τ≤ 0.327; intercept≤ 2.839; all p≥0.08).
Table III.
Meta-analysis output for each of the components examined in the International Classification of Functioning, Disability and Health. Positive direction denotes the respective intervention mode having a beneficial effect on the respective domain and/ or component.
| ICF domain (component) | Intervention | Hedges g | 95% CI | p-value |
|---|---|---|---|---|
| Body function (lower limb) | Aerobic (n=1) | 0.585 | –0.036 to 1.205 | 0.06 |
| Mixed (n=5) | 0.140 | 0.002 to 0.277 | 0.05 | |
| Muscle strengthening (n=7) | 0.232 | –0.004 to 0.468 | 0.05* | |
| Overall (n=13) | 0.178 | 0.061 to 0.294 | <0.01* | |
| Body function (non-lower limb) | Aerobic (n=2) | 0.260 | 0.070 to 0.451 | 0.01 |
| Mixed (n=1) | 0.837 | 0.585 to 1.089 | <0.01 | |
| Muscle strengthening (n=2) | 0.337 | –0.341 to 1.015 | 0.33* | |
| Overall (n=5) | 0.463 | 0.315 to 0.611 | <0.01* | |
| Body function (cardiovascular) | Aerobic (n=5) | 0.373 | 0.100 to 0.646 | 0.01 |
| Mixed (n=3) | 0.007 | –0.169 to 0.183 | 0.94* | |
| Overall (n=8) | 0.114 | –0.034 to 0.262 | 0.13* | |
| Body function (mental and sensory) | Aerobic (n=4) | 0.733 | 0.146 to 1.175 | <0.01* |
| Mixed (n=4) | 0.197 | 0.080 to 0.314 | <0.01 | |
| Muscle strengthening (n=2) | 0.356 | 0.083 to 0.630 | 0.01 | |
| Overall (n=10) | 0.250 | 0.146 to 0.355 | <0.01* | |
| Activity and participation | Aerobic (n=1) | 0.173 | –0.024 to 0.371 | 0.09 |
| Mixed (n=5) | 0.145 | 0.086 to 0.204 | <0.01 | |
| Muscle strengthening (n=4) | 0.071 | –0.153 to 0.296 | 0.53 | |
| Overall (n=10) | 0.143 | 0.088 to 0.198 | <0.01 |
Denotes findings with significant and moderate to substantial heterogeneity. CI is confidence interval, ICF is International Classification of Functioning, Disability and Health, and n is the number of studies used in the respective analysis.
Fig. 6.
Funnel plot of the lower-limb component of the body function domain. The included and imputed studies are denoted by the white and black circles, respectively. Studies reported to the right of 0 represent exercise interventions having a positive effect on the respective domain and/or component.
DISCUSSION
This systematic review summarizes the role of exercise for muscular strength and cardiovascular fitness in polio survivors. The 21 studies were grouped into respective ICF domains and/or components based on the ICF codes (40) and administered to quantitative synthesis. The overall results for the body function component (motor function, cardiovascular, mental and sensory domains) show that interventions have an effect favouring improvement in the body function of polio survivors. Results indicating improvement were also found for measures of activity and participation related to exercise. These findings provide clarification for a 2008 meta-analysis, which questioned the inclusion of muscular strengthening interventions in research (10), and build on the conclusions of the 2010 and 2011 reviews that stated that rehabilitation interventions seemed effective (14, 41).
Study heterogeneity and quality
Heterogeneity varied among the findings of this study. Although some findings were homogeneous (the combined activity and participation components), most findings displayed moderate to substantial heterogeneity, which remained following sensitivity analyses. This may be explained by when this set of studies was published (all since 2001), in relation to the emergence of the ICF framework (2001), which oriented researchers to human functioning, resulting in more frequent inclusion of activity and participation measures. These studies were also of a higher quality: scoring 71+% (Table SIV1) except for one (31) (at 59%). In addition, we observed a difference in quality (based on our assessment using a reduced Downs and Black checklist (15), when comparing studies published before and after 2001. Studies in this review published since 2001 appeared to be of higher quality, when assessed using this quality appraisal checklist.
International Classification of Functioning, Disability and Health
The results were presented in ICF components and domains, to enable meaningful interpretations to be made in a familiar framework of disability. The body function component is split into domains of motor function, cardiovascular fitness, and mental and sensory, enabling the key topics of this review to be discerned. The activity and participation components’ domains are combined, as each is oriented to the performance of tasks. Combining these latter 2 domains can provide context for potential positive outcomes and prognosis for social independence (40). The Core Set for PPS proposed by Bocker et al. (2016) helped to narrow the ICF categories, providing clearer directions for assessment and documentation in clinical practice and research (17). This was an overall strength of this review outlining the effectiveness of muscular strengthening and aerobic fitness activities across a range of domains (e.g. body function – cardiovascular d450: walking), while still allowing specificity to individual outcome items (e.g. 6-min walk test as measure of fitness).
Intervention duration
Studies ranged in duration from 5 to 32 weeks. The meta-regression analyses of intervention durations vs effect (Figs 3 and 4) excluded the 2-year study (36), which was an outlier that skewed the regression line. This study (36) showed the strongest effect size amongst the studies, suggesting continued gains in the long term could be established in focal muscle groups when a non-fatiguing protocol is established in-clinic and continued as a home programme. Particularly able, motivated, and resourced polio survivors may have been recruited in this study, possibly lowering the attrition risk and biasing the outcomes. Current evidence seems to suggest progression of symptoms may not be as rapid as anticipated (8); however, without further long-term studies, it is difficult to confirm the rate of deterioration or the maintenance of key gains in the active population.
The body function domain analysis shows the dominant cluster within the remaining studies being between 4 and 16 weeks of intervention, this is reflective of interventional exercise studies, which look for measurable effects within several months. A similar analysis for activity and participation domains (Fig. 4) shows a weak overall regression line. Both sets of domains had an apparent negative (waning) effect slope in response to duration. A subtle gradual worsening of symptoms amongst participants may explain this effect slope, or the protocols may not have managed fatigue adequately. Polio survivors can be affected by fatigue within and between sessions, and serially. We recommend exercise protocols that acknowledge and limit fatigue, concurrent with education on fatigue management, with the aim of improving long-term motivation and adherence to exercise.
Effect sizes
Small-to-moderate improvement effects due to exercise interventions were seen in the motor function components and mental and sensory domain, while effects that appeared to be of clinical interest were identified in the cardiovascular component (not significant) and activity and participation domains, as outlined in Table III.
Although the effect sizes were modest, clinicians and polio survivors should derive confidence from further evidence of exercise favouring improvement across a range of contexts. What might be most reassuring, is that the strongest exercise effect was in outcomes within the mental and sensory domain (outcomes related to pain and fatigue) favouring improvement in 2 highly prevalent symptoms in post-polio conditions. This challenges the findings of an earlier systematic review and meta-analysis (10), identifying no associations between exercise and improved fatigue management, and may be due to the increased number of studies included in the current research.
Body function domains
Motor function component
A prominent component of polio sequelae is increasing weakness across muscle groups (4). Exercise can be perceived by polio survivors and clinicians as disadvantageous to maintaining function, due to discerning effects such as pain and fatigue. Long-term stress on polio-surviving motor units is widely accepted as precipitating muscle weakness through the degradation or loss of these motor units (10). Among the studies examined in the current analysis, strength outcomes in 5 studies (31, 33, 34, 38, 39) had limited effects on either improvement or impairment. This demonstrates the need for further high-quality mode-testing studies to discern intervention modes with potential adverse effects across LEoP and PPS populations.
In their 2016 article, Vroon et al (9) discuss nuances of strength and cardiovascular exercise prescription in this population: anaerobic threshold as a tolerance limit, musculature chronically utilised being adapted to higher loading, the limitations of non-whole body exercise, and individualization (14). We agree that these factors are significant contributors to the heterogeneity of results within studies on polio survivors, and need to be balanced against participation outcomes.
Exercise prescription criteria for polio survivors engaging in strengthening activities, such as those summarised by Gonzalez et al. in 2010 (14), should be utilised. Most studies in this analysis excluded candidates with severe weakness or excluded individuals’ muscle groups with a manual grade of less than 3/5. Further, studies highlight the importance of monitoring and responding to personspecific limits or adverse events during interventions with individualized modifications (5, 29). Without applying itemized criteria to set exercise participation limits, harmful or null overall exercise effects may arise in this population (5). It is essential that clinicians adhere to these tenants (prescription and exclusion) of exercise when treating polio survivors. The variety of measures and muscle groups strengthened effectively and safely across the included studies in this review indicates that strength exercise is suitable for polio survivors; a finding that is consistent with previous literature (10).
Cardiovascular component
The criteria of included studies allowed a broad range of assistive device use, limb bracing use, and fatigue profiles amongst participants. Exclusions usually reflected the physical requirements of the cardiovascular intervention mode and the severity of existing weakness. Barriers to polio survivors maintaining or improving cardiovascular fitness include: global and peripheral fatigue, muscle weakness profile, use of assistive devices, activity choices, and the risk of falls (29). Thus, an individual’s profile determines the feasibility of fitness exercise mode.
Clinically, exercise mode decisions should be similarly based on polio survivors’ ability and symptoms, accommodating any evident body limitations and assistive technology use (5). The studies incorporating cardiovascular domain interventions used combinations of limb use, body position and interface. This demonstrates polio survivors’ tolerance of a variety of cardiovascular exercise modes already available in clinical settings.
Mental and sensory component
The results of this review indicated links between muscle strengthening exercise and mental and sensory component, particularly fatigue (36% of the mental and sensory measures reported in the included studies). Fatigue is multidimensional and complex, and the measures used in the included studies (FSS, FIS, MFI-20, VAS) are non-specific to body system or condition (32). Fatigue is a pervasive symptom amongst polio survivors and is more prevalent than weakness (5). We recommend that consistent use of the fatigue measures should be used with polio survivors in research and clinical settings, and strict muscle pain and fatigue avoidance protocols should be adopted as demonstrated, consistent with previous protocols outlined in the literature (36, 42). In contrast, pain measures accounted for 19% of the mental and sensory measures reported, a representation much lower than expected, as it is important to monitor pain during exercise so that symptoms of pain or soreness in the polio survivors involved are not excessive (2). The presence of only one mental and sensory weakness measure among the included studies may be explained by the abundance of objective motor function testing measures performed. The inclusion of subjective weakness as a mental and sensory evaluation measure could capture the lived experience of functional strength during activity and may add scope to studies of non-motor oriented post-polio conditions (43).
Limitations
This systematic review and meta-analysis addressed limitations across the literature regarding polio survivors exercising and previous meta-analyses, such as (10); there were a number of points of improvement. A key assumption of the analysis carried out was that independent studies were unique cohorts. This was not the case in 2 examples: subjects in (22) were recruited from the cohort of 12 subjects originally studied in (23). Similarly (9) followed the same cohort as (3). Furthermore, our systematic search of the literature only included studies available in English; hence, we recommend that future reviews include languages other than English within searches, particularly given contemporary translation options outlined in the Cochrane Handbook (19). It is possible that such studies could influence the publication bias highlighted in this review. Further research into more individualized approaches to exercise prescription for polio survivors would greatly advance research in this area.
CONCLUSION
The findings of this review and analysis provide “very low level evidence” (according to the Grading of Recommendations Assessment, Development and Evaluate; GRADE) to polio survivors, clinicians and researchers. The main findings of this review relate specifically to changes in body function, and activity and participation, and include evidence of effect on improved functioning without furthering debility in polio survivors. This systematic review and meta-analysis provides additional insights into effects associated with exercise, across various types of interventions, in polio survivors, and advances the level of methodological quality of research in this area. Although there was evidence demonstrating effect across domains, due to inherent biases within the literature to date, further and high-quality primary exercisefocused research is required in order to strengthen the certainty of evidence regarding important research questions about the ongoing health of polio survivors.
The authors have no conflict of interest to declare.
http://www.medicaljournals.se/jrm/content/?doi = 10.2340/16501977-2832
REFERENCES
- 1.Jones KM, Balalla S, Theadom A, Jackman G, Feigin VL. A systematic review of the worldwide prevalence of survivors of poliomyelitis reported in 31 studies. BMJ Open 2017: e015470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.March of Dimes Steering Committee on Post-Polio Syndrome, editors . March of Dimes International Conference on Post-Polio Syndrome: Identifying best practices in diagnosis and care [monograph on the Internet.] March of Dimes; 2001. [Cited 2021 April 8]. Available from: https://www.polioaustralia.org.au/wp-content/uploads/2010/09/PPS-Identifying-Best-Practices-in-Diagnosis-Care.pdf. [Google Scholar]
- 3.Koopman FS, Voorn EL, Beelen A, Bleijenberg G, de Visser M, Brehm MA, et al. . No reduction of severe fatigue in patients with postpolio syndrome by exercise therapy or cognitive behavioral therapy: results of an RCT. Neurorehabil Neural Repair 2016; 30: 402–410. [DOI] [PubMed] [Google Scholar]
- 4.Murray D, Hardiman O, Campion A, Vance R, Horgan F, Meldrum D. The effects of a home-based arm ergometry exercise programme on physical fitness, fatigue and activity in polio survivors: a randomised controlled trial. Clin Rehabil 2017; 31: 913–925. [DOI] [PubMed] [Google Scholar]
- 5.Koopman FS, Beelen A, Gilhus NE, de Visser M, Nollet F. Treatment for postpolio syndrome. Cochrane Database Syst Rev 2015; (5): CD007818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post-Polio Syndrome Conference Consensus Group. 2nd European Polio Conference: Post-Polio Syndrome Conference. A condition without boundaries. J Rehab Med 2014; 46: 568–608. [Google Scholar]
- 7.Lo JK, Robinson LR. Post-polio syndrome and the late effects of poliomyelitis: Part 2. treatment, management, and prognosis. Muscle Nerve 2018; 58: 760–769. [DOI] [PubMed] [Google Scholar]
- 8.Willen C, Hou L, Sunnerhagen KS. A very long term longitudinal follow up of persons with late effects of polio. Eur J Phys Rehabil Med 2020; 56: 155–159. [DOI] [PubMed] [Google Scholar]
- 9.Voorn EL, Koopman FS, Brehm MA, Beelen A, de Haan A, Gerrits KHL, et al. . Aerobic exercise training in post-polio syndrome: process evaluation of a randomized controlled trial. Plos One 2016; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y-L, Lee C, Chiu T-Y, Lee S-J, Lee H-C, Wang M-WTTJ. Effects of exercise training, lifestyle modification and modality on fitness and post-polio syndrome in polio survivors: meta analysis. Formosan Journal of Physical Therapy – FJPT 2008; 33: 409–420. [Google Scholar]
- 11.Sulaiman SK, Aldersey HM, Fayed N, Kaka B, Okyere C. Quality of life assessment scales in polio survivors: a scoping review. Qual Life Res 2019; 28: 2341–2357. [DOI] [PubMed] [Google Scholar]
- 12.Shing SLH, Chipika RH, Finegan E, Murray D, Hardiman O, Bede P. Post-polio syndrome: more than just a lower motor neuron disease. Frontiers Neurol 2019; 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce A, Longhurst G. The role of the clinical exercise physiologist in reducing the burden of chronic disease in New Zealand. Int J Environ Res Pub Health 2021; 18: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. Lancet Neurol 2010; 9: 634–642. [DOI] [PubMed] [Google Scholar]
- 15.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epi Com Health 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schunemann H, Brozek J, Guyatt GH, Oxman AD. GRADE handbook for grading quality of evidence and strength of recommendations. The Grade Handbook, The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group; 2013. [Google Scholar]
- 17.Bocker B, Schueler C, Best N, Smolenski U. Evaluation of function, disability and participation of patients with motor neuron disease. J Disab Rehab 2016; 2: 164–171. [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins SE, Rothstein HR. Introduction to meta-analysis. Chichester (UK: ): John Wiley and Sons; 2009. [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. . Cochrane handbook of systematic reviews of interventions. Chichester (UK: ): John Wiley and Sons; 2020. [Google Scholar]
- 20.GRADEPro GDT. GRADEpro Guideline Development Tool [Software]. In: Evidence Prime I, editor.; 2020; McMaster University, Hamilton, Ontario, Canada; 2020. [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analysis: the PRISMA statement. PLoS One 2009; 6: e1000097. [PMC free article] [PubMed] [Google Scholar]
- 22.Agre JC, Rodriguez AA, Franke TM. Strength, endurance, and work capacity after muscle strengthening exercise in postpolio subjects. Arch Phys Med Rehabil 1997; 78: 681–686. [DOI] [PubMed] [Google Scholar]
- 23.Agre JC, Rodriquez AA, Franke TM, Swiggum ER, Harmon RL, Curt JT. Low-intensity, alternate-day exercise improves muscle performance without apparent adverse effect in postpolio patients. Am J Phys Med Rehabil 1996; 75: 50–58. [DOI] [PubMed] [Google Scholar]
- 24.Oncu J, Durmaz B, Karapolat H. Short-term effects of aerobic exercise on functional capacity, fatigue, and quality of life in patients with post-polio syndrome. Clin Rehabil 2009; 23: 155–163. [DOI] [PubMed] [Google Scholar]
- 25.Dean E, Ross J. Effect of modified aerobic training on movement energetics in polio survivors. Orthopedics 1991; 14: 1243–1246. [DOI] [PubMed] [Google Scholar]
- 26.Kriz JL, Jones DR, Speier JL, Canine JK, Owen RR, Serfass RC. Cardiorespiratory responses to upper extremity aerobic training by postpolio subjects. Arch Phys Med Rehabil 1992; 73: 49–54. [PubMed] [Google Scholar]
- 27.Jones DR, Speier J, Canine K, Owen R, Stull GA. Cardiorespiratory responses to aerobic training by patients with postpoliomyelitis sequelae. JAMA 1989; 261: 3255–3258. [PubMed] [Google Scholar]
- 28.Bertelsen M, Broberg S, Madsen E. Outcome of physiotherapy as part of a multidisciplinary rehabilitation in an unselected polio population with one-year follow-up: an uncontrolled study. J Rehabil Med 2009; 41: 85–87. [DOI] [PubMed] [Google Scholar]
- 29.Davidson AC, Auyeung V, Luff R, Holland M, Hodgkiss A, Weinman J. Prolonged benefit in post-polio syndrome from comprehensive rehabilitation: a pilot study. Disabil Rehabil 2009; 31: 309–317. [DOI] [PubMed] [Google Scholar]
- 30.Ernstoff B, Wetterqvist H, Kvist H, Grimby G. Endurance training effect on individuals with postpoliomyelitis. Arch Phys Med Rehabil 1996; 77: 843–848. [DOI] [PubMed] [Google Scholar]
- 31.Willen C, Sunnerhagen KS, Grimby G. Dynamic water exercise in individuals with late poliomyelitis. Arch Phys Med Rehabil 2001; 82: 66–72. [DOI] [PubMed] [Google Scholar]
- 32.Sharma SS, Sheth MS, Vyas NJ. Fatigue and functional capacity in persons with post-polio syndrome: short-term effects of exercise and lifestyle modification compared to lifestyle modification alone. Disabil CBR Inclus Dev 2014; 25: 78–91. [Google Scholar]
- 33.Chan KM, Amirjani N, Sumrain M, Clarke A, Strohschein FJ. Randomized controlled trial of strength training in post-polio patients. Muscle Nerve 2003; 27: 332–338. [DOI] [PubMed] [Google Scholar]
- 34.Brogårdh C, Flansbjer U, Lexell J. No effects of whole-body vibration training on muscle strength and gait performance in persons with late effects of polio: a pilot study. Arch Phys Med Rehabil 2010; 91: 1474–1477. [DOI] [PubMed] [Google Scholar]
- 35.Einarsson G. Muscle conditioning in late poliomyelitis. Arch Phys Med Rehabil 1991; 72: 11–14. [PubMed] [Google Scholar]
- 36.Fillyaw MJ, Badger GJ, Goodwin GD, Bradley WG, Fries TJ, Shukla A. The effects of long-term non-fatiguing resistance exercise in subjects with post-polio syndrome. Orthopedics 1991; 14: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 37.Da Silva CP, Szot CL, deSa N. Whole body vibration on people with sequelae of polio. Physiother Theory Pract 2019; 35: 554–564. [DOI] [PubMed] [Google Scholar]
- 38.Skough K, Krossen C, Heiwe S, Theorell H, Borg K. Effects of resistance training in combination with coenzyme Q10 supplementation in patients with post-polio: a pilot study. J Rehabil Med 2008; 40: 773–775. [DOI] [PubMed] [Google Scholar]
- 39.Spector SA, Gordon PL, Feuerstein IM, Sivakumar K, Hurley BF, Dalakas MC. Strength gains without muscle injury after strength training in patients with postpolio muscular atrophy. Muscle Nerve 1996; 19: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 40.Riis-Djernaes LM, Jensen CM, Madsen E, Maribo T. Should rehabilitation goals reflect all aspects of functioning in relation to a biopsychosocial ICF perspective? Disabil Rehabil 2019; p. 1–6. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.Albdulraheem IS, Saka MJ, Saka AO. Postpolio syndrome: epidemiology, pathogenesis and management. J Infect Dis Immun 2011; 3: 247–257. [Google Scholar]
- 42.Lexell J, Jonasson SB, Brogardh C. Psychometric properties of three fatigue rating scales in individuals with late effects of polio. Ann Rehabil Med 2018; 42: 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lygren H, Jones K, Grenstad T, Dreyer V, Farbu E, Rekand T. Perceived disability, fatigue, pain and measured isometric muscle strength in patients with post-polio symptoms. Physiother Res Int 2007; 12: 39–49. [DOI] [PubMed] [Google Scholar]