Abstract
Irreversible electroporation (IRE) of locally advanced pancreatic cancer is an increasingly used method for unresectable pancreatic cancer that can be used in cytoreduction followed by surgical treatment and shows promising results in palliative care.
IRE is an ablative technique where electric pulses cause damage to the cell membrane leading to apoptosis without the destruction of stroma. The application of IRE increases the concentration of hydrophobic regimens like bleomycin within the tumor, what could improve the effectiveness of treatment. This fusion of those two treatments is called electrochemotherapy. In this review, the authors will discuss the radiological perspective of possible beneficial role of irreversible electroporation in relation with chemotherapy in pancreatic cancer treatment.
Keywords: irreversible electroporation, pancreatic cancer, tumor ablation
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the neoplasms with the most fatal clinical course, resulting in extremely low 5-year survival rates of about 7% [1].
In 2017 in Poland 3508 new cases and simultaneously 4864 cases of death due to the pancreatic cancer were registered.
Early detection is expected to be the most important factor in the treatment of PDAC, but it remains unsuccessful, and the majority of cases are not treated effectively. As for many other cancers, it is suggested that surgery is the only possibility of cure. However, only 10-15% tumors are eligible for resection, with up to 20% of 5-year survival rates. According to the NCCN guidelines v.2.2017 PDAC was divided into four classes: resectable (10%), borderline resectable (5%), locally advanced pancreatic cancers (LAPC) (25%) and metastatic PDAC (60%) [2]. Even if negative margin resection was found, the adjuvant chemotherapy is needed, and local recurrences are quite frequent.
Borderline and locally advanced cancers with the infiltration of local vasculature or other tubular structures do not present any distant spread. Borderline ones are resectable with the extended surgery. LAPC presenting deep invasion of local structures is technically not possible to be resected. Application of neoadjuvant chemotherapy can lead to downstage the tumor enabling higher percentage of R0 resection and longer median overall survival [3,4]. The optimal neoadjuvant treatment is still not known, but intensively explored and discussed in the scientific literature. The principal opinion about neoadjuvant chemotherapy indicates now FOLFIRINOX and gemcitabine/nab-paclitaxel as new standards in advanced cases [5]. Other options in LAPC treatment include radiotherapy and other local ablative therapies (radiofrequency ablation, microwave ablation, cryoablation, irreversible electroporation – IRE, or non-thermal IRE – NT-IRE). For all of these methods, the main idea is simple cytoreduction and downsize of the tumor to allow R0 resection.
Minimally invasive ablation methods
There is a growing trend in application of ablative techniques in inoperable pancreatic cancer. They offer a cytoreduction, local tumor control, and pain relief. Thermal local ablations lead to thermal denaturation of ablated tissues and do not respect the tubular and neural structures, thus are highly risky in pancreatic region. This short review aims to highlight the main differences between the ablative methods.
Thermal ablation methods
Radiofrequency ablation
Radiofrequency ablation (RFA) (Table 1) is the most widely used ablative method in solid organs, especially in primary and secondary liver lesions. It is also used in the pancreatic field. RFA uses high frequency current, which generates high temperatures that cause coagulative necrosis. The procedure is performed open or percutaneously by placing the electrode in the center of the lesion with temperature settings of 90°C for 5 minutes long. To reduce the risk of complications, the safe distance 10 mm to duodenum and 15 mm to porto-mesenteric vessels should be maintained [6,7]. The thermal effect in RFA causes complications like duodenum and vessels injuries. Other adverse events include pancreatic fistula, porto-mesentheric thrombosis, bleeding, acute pancreatitis [8]. The incidence of complications increases with temperature > 100°C, without significant advantages. RFA shows heat-sink effect, where the bloodstream in adjacent vessels absorbs the heat, which is lowering the effectiveness of the procedure [9].
Table 1.
Selected studies on radiofrequency ablation
| Study | Number of patients | Approach | Follow-up | Overall survival, median (months) | Progression free time (months) | Regression/stable disease rate (%) | Progression rate (%) | Recurrence rate (%) | Complications rate (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Giardino et al. (2015) [92] | 200 | Open | 1 year | 19 | 13 | NA | NA | NA | 24.5 (12.5 RFA-related) |
2.5 |
| Girelli et al. (2010) [8] | 50 | Open | Median: 8 months | NA | NA | NA | NA | NA | 24 | 2 |
| Fegrachi et al. (2019) [10] | 17 | Open | NA | 9 | NA | NA | NA | NA | 53% (36% RFA-related) |
6 |
| Frigerio et al. (2013) [12] | 57 | Open | 12 | 19 | 10 | 3.5 without radiological signs of disease, 12.2 stable disease |
12.3 | NA | 14 (3.5 procedure-related) |
0 |
| Giardino et al. (2012) [13] | 167 | Open | 18 months (n = 107) |
25.6 | NA | NA | NA | NA | 28 (17.7 RFA-related) |
1.8 |
The results of a recently published phase II study on intraoperative RFA for LAPC reported a major complication rate of 53% using the Clavien-Dindo classification and 6% mortality [10].
Endoscopic guided radiofrequency ablation (EUS-RFA) provides better visualization of pancreatic field and does not result in major procedure related complications [11].
There are studies that support the prolonged survival rate after RFA in combination with chemotherapy, compared to RFA alone [12,13]. It is reported that RFA amplifies anti-cancer immune effect [14].
Microwave ablation
Microwave ablation (MWA) (Table 2) causes mechanical agitation of water molecules induced by microwaves, which results in thermal effect and leads to coagulative necrosis [15]. It can be performed percutaneously or intraoperatively. The generator is emitting microwaves through the antenna, which is placed directly in a tumor. This method shows high energy transmission through the tissues. As result, heat can be produced in larger areas. In comparison with RFA, MWA can be used for bigger lesions, it is characterized by higher temperatures and shorter ablation time [15]. Because of the difficulties in controlling the ablation zone size and high risk of side effects in the pancreatic field, this technique is not widely used in pancreatic cancer therapy. Despite that, some available studies show only minor complications (20-40%) and report the improvement of quality of life [16-18].
Table 2.
Selected studies on microwave ablation
| Study | Number of patients | Approach | Follow up | Overal survival, median (months) | Progression free time (months) | Regression/ stable disease rate (%) | Progression rate (%) | Recurrence rate (%) | Complications rate (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Carafiello et al. (2013), [16] | 10 | Open | Up to 12 months, mean 9.2 | NA | NA | NA | NA | NA | 30 | 0 |
| Lygidakis et al. (2007) [17] | 15 | Open | Up to 22 months | NA | NA | NA | NA | NA | 40 | 0 |
| Ierardi et al. (2018) [18] | 5 | Percutaneous | 6-12 months | NA | NA | 100 partial response after 1 month procedure | NA | 20 after 1 year |
20 | 0 |
Cryoablation
Cryoablation (Table 3) causes intra- and extracellular freezing. The formation of ice crystals in extracellular space changes the osmolarity and results in cell dehydration, leading to cell death. Cryoablation can be performed percutaneously or intraoperatively. During the procedure, needle-like probes are placed in a tumor; the argongas-based unit generates cold and is cooling the tumor to around –150°C. The longevity of this process depends on tumor size and the number of probes. Afterward, the tumor is rewarmed, and the whole process is repeated. The 5 mm safety distance from major structures should be obtained. In analyzed studies, the local tumor control and the quality of life was improved, with no significant difference in overall survival rate [19,20].
Table 3.
Selected studies on cryoablation
| Study | Number of patients | Approach | Follow up | Overal survival, median (months) | Progression free time | Regression /stable disease rate (%) | Progression rate (%) | Recurrence rate (%) | Complications rate (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Li et al. (2011) [19] | 68 | Open | Mean: 30.4 months |
350 days | NA | 65 (in 36 of 55 who had CT follow-up) | NA | NA | 42.9 | 0 |
| Song et al. (2014) [20] | 42 | Open | NA | 5 | NA | NA | NA | NA | NA | 0 |
High-intensity focused ultrasound
High-intensity focused ultrasound (HIFU) (Table 4) is a completely non-invasive method, which does not require electrodes placement. The HIFU transducer is placed above the tumor area, it generates ultrasonography beam in the intensities range of 100-1000 W/cm2. The soft tissues absorb the acoustic energy which is transformed to thermal energy and causes coagulative necrosis by two known mechanisms – cavitation and heat [21]. HIFU is also feasible for patients with metastatic disease. The most common complications include skin burn, pancreatitis, jaundice. In the review of 1717 pancreatic cancers treated with HIFU, the complications rate was 8.7% [22]. In analyzed studies, this method shows satisfactory results in pain management in majority of patients [23,24].
Table 4.
Selected studies on high-intensity ultrasound
| Study | Number of patients | Approach | Follow up | Overal survival, median (months) | Progression free time (months) | Regression/ stable disease rate (%) | Progression rate (%) | Recurrence rate (%) | Complications rate (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Marinova et al. (2018) [93] | 50 | Percutaneous, US-guided | 3 years | 16.2 | 6.8 | 972 regression of tumor volume | NA | 44 (10 local, 12 distant) | 64 | 0 |
| Sung et al. (2011) [94] | 46 | Percutaneous, US-guided | NA | 12.4 | NA | NA | NA | NA | 67.3 | 0 |
| Xiong et al. (2009) [95] | 89 | Percutaneous, US-guided | 36 months | 8.6 (stage II-IV) |
NA | 14.5 partial response 57.3 stable (no change) |
28.1 | NA | 11.2 | 0 |
| Zhao et al. (2017) [96] | 38 | Percutaneous, US-guided | NA | 10.3 (low power cumulative HIFU) 6 (traditional HIFU) |
NA | NA | NA | NA | NA | 0 |
| Yi et al. (2018) [24] | 87 | Percutaneous, US-guided | 16 | 12.2 | NA | 8 complete response 28.9 partial response 41.3 stable disease |
21.8 | NA | 28.7 | 0 |
Photodynamic therapy
Photodynamic therapy (PDT) (Table 5) is less frequently used method that exposes tumor to light following an intravenous administration of photosensitizers (e.g., meso-tetrahydroxyphenyl chlorin, verteporfin, porfirmer sodium), which enables the tumor ablation. Light is delivered through small fibers placed percutaneously [25]. There are only few studies on PDT in pancreatic cancer, among relatively small groups of patients (up to 16 per study). In comparison with dominant ablative techniques, PDT shows a shorter survival rate [25-27].
Table 5.
Selected studies on photodynamic therapy
| Study | Number of patients | Approach | Follow up | Overal survival, median (months) | Progression free time (months) | Regression rate/stable disease(%) | Progression rate (%) | Recurrence rate (%) | Complications rate (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bown et al. (2002) [25] | 16 | Percutaneous | NA | 12.5 | NA | NA | NA | 100 during follow up: 87.5 local, 12.5 distant | 40 | 0 |
| Hugget et al. (2014) [27] | 15 | Percutaneous | NA | 15.5 | NA | 85 stable at 1 month, 46 stable at 3 months |
15.3 distant metastases at 1 month | 87.5 local, 12.5 distant | 40 | 0 |
| DeWitt et al. (2018) [26] | 12 | EUS | 315 days | 11.5 | 2.6 | 25 stable, 8.3 partial response | 50 | NA | 58.3 | 8.3 |
Stereotactic body radiation therapy
Stereotactic body radiation therapy (SBRT) (Table 6) uses a high dose of tumor-targeted radiation with minimal margin. In comparing with standard radiotherapy, it is characterized by decreased destruction of surrounding tissues. The total radiation dose varies between studies, in analyzed studies it ranges between 12,5-50,0 Gy [28,29,97,98]. SBRT is usually combined with chemotherapy [29]. As pancreas is a mobile organ, the targeted tumor dose may be received by surrounding organs. The reported complications include ulceration and strictures in GI tract, duodenal perforation [30]. The less common complications are seen in hypofractionated treatment in compared to single fraction SBRT [31].
Table 6.
Selected studies on stereotactic body radiation therapy
| Study | Number of patients |
Fractions | Follow-up | Overal survival, median (months) | Progression free time (months) | Regression/stable disease rate (%) | Progression rate (%) | Recurrence rate (%) | Complications rate (n/%) | Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Algappan et al. (2016) [28] | 208 | Single fraction dose 12.5-25 Gy Multifraction dose 25-45 Gy |
7.5 | > 5 fractions: 11 months ≥ 5 fractions: 14 months |
NA | NA | NA | 27% local progression at 1 year | NA | NA |
| Gurka et al. (2017) [29] | 38 | 25 Gy in 5 fractions Single fraction: 30 Gy |
NA | 14.3 | 9.2 | 3% partial response at 1 month 94% stable disease At 6 months local control in 82% |
3% at 1 month |
NA | Acute GI Grade I-II: n = 11 Late GI grade: n = 1 |
0 |
| Rwigema et al. (2011) [97] | 71 | 18-24 Gy in single fraction (68 patients) 24 Gy in 2 fractions (2 patients) 20 Gy in 2 fractions (1 patient) |
6 months | 10.3 | 71.7 at 6 months, 48.5 at 12 months |
Local control: 77.3 for tumor volume < 15 ml, 57.5 for tumor volume > 15 ml |
NA | 71.7 at 6 months, 48.5 at 12 months |
Acute – 38.1, grade I: n = 17, grade II: n = 8, grade III: n = 3 Late – 4.2, | n = 3 |
0 |
| Song et al. (2015) [98] | 59 | 35-50 Gy in 3-8 fractions | 10.9 | 12.2 | 13.85 months | 13.6 total remission, 52.5 partial remission, 30.3 stable disease | 13.6 | NA | Grade I-II acute and late: 61 Grade III: 1.7 |
0 |
SBRT is reported to improve local tumor control [32]. The systematic review and pooled analysis of 19 trials in patients with LAPC treated with SBRT by Petrelli et al. showed a median overall survival of 17 months, acute toxicity rate in the range of 0-36%, and chronic toxicity in the range of 0-11%.
Non-thermal tissue ablation
Irreversible electroporation
Irreversible electroporation (Table 7, Figure 1) is the result of high voltage electrical short pulses transmitted through electrodes placed around the ablated zone. The high voltage pulses are changing the „electroorganization” of cells and lead to higher permeability of cell membrane (reversible electroporation), or irreversible effect ending with cells apoptosis (IRE). Ultrahigh voltage short pulses (MV in nanosec) also destroy intracellular membranes, but up to now, the clinical applications of this potentially curative methods are not known. As these techniques do not involve tissue heating, their safety profile is favorable.
Table 7.
Results of representative studies on irreversible electroporation in locally advanced pancreatic cancer
| Study | Number of patients | Approach | Follow-up | Overal survival, median (months) | Progression free time (months) | Regression rate (%) |
Progression rate (%) |
Recurrence rate (%) |
Complications rate (%) |
Mortality rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Martin et al. (2012) [75] | 27 | Percutaneous, open | 90 days | NA | NA | NA | NA | 0 | 33 | 3.7 |
| Martin et al. (2013) [78] | 54 | Percutaneous, open | Median: 15 months |
20.0 | Local 14, distant 15 | NA | NA | 50 (15 patients local, 12 distant) |
59 | 2 |
| Martin et al. (2015) [49] | 200 | Open | 90 days | 24.9 | Local 12,4, distant 16,8 |
NA | NA | 29 | 37 | 2 |
| Kluger et al. (2015) [79] | 50 | Open | Median: 8.69 | 12.0 | NA | NA | NA | 58 (47 distant, 11 local) |
19 | 11 |
| Dunki-Jacobs et al. (2014) [80] | 65 | Percutaneous/open | 23 months | NA | 5.5 months in patients with recurrence, 12.6 months in patients without recurrence | NA | NA | 26.2 | 57 | NA |
| Månsson et al. (2016) [81] | 25 | Percutaneous | NA | 13.3 | NA | NA | NA | 72 (7 local, 10 distant, 1 both) |
44 | NA |
| Månsson et al. (2019) [82] | 24 | Percutaneous | NA | 13.3 | NA | NA | NA | NA | 25 | 4.1 |
| Paiella et al. (2015) [51] | 10 | Open | NA | 7.5 | NA | Partial response: 40 | NA | NA | 10 | 10 |
| Vogel et al. (2017) [83] | 15 | Open | NA | 16 | NA | NA | NA | NA | 53 | 13.3 |
| Scheffer et al. (2017) [76] | 25 | Percutaneous | 12 | 17 | 12 | NA | NA | NA | 40 | 0 |
| Zhang et al. (2017) [84] | 21 | Percutaneous | 1 month | NA | NA | 73.3 | NA | NA | “Mild” no number | NA |
| Lambert et al. (2016) [85] | 21 | Percutaneous/open | NA | 10.2 | NA | 24 | NA | 38 | 24 | 0 |
| Narayanan et al. (2017) [52] | 50 | Percutaneous | NA | 27.0 | NA | NA | NA | NA | 20 | 0 |
| Yan et al. (2016) [86] | 25 | Open | NA | NA | NA | Partial response: 36 | NA | 36 | 16 | NA |
| Leen et al. (2018) [87] | 75 | Percutaneous | 11.7 | 27.0 | 15 | Partial response: 31 | 3 | 38 | 25 | 0 |
| Belfiore et al. (2017) [88] |
29 | Percutaneous | 29 | 14.0 | NA | partial response 40.3 | NA | 3 (after 6 months) | NA | NA |
| Ruarus et al. (2019) [47] | 50 | Percutaneous | NA | 17.0 | 10 | NA | NA | 46 | 58 | 1/2 (2-4) 1 euthanasia |
| Holland et al. (2018) [89] | 152 | Open | NA | 30.7 | 22.8 | NA | NA | 21 | 18 | 2 |
| Yang et al. (2020) [90] | 74 | 69 open, 5 laparoscopic | 46.9 | 1 year 97.2, 2 years 53, 5 years 31,2 | 1 year 69.1, 2 years 48.7, 5 years 28.8 |
NA | NA | 52.7 (local 12.2, 40.5 distant) | NA | NA |
| Kwon et al. (2020) [91] | 12 | Open | 19.7 | 24.5 | 19.2 | NA | NA | NA | 75 (minor) | 8.3 |
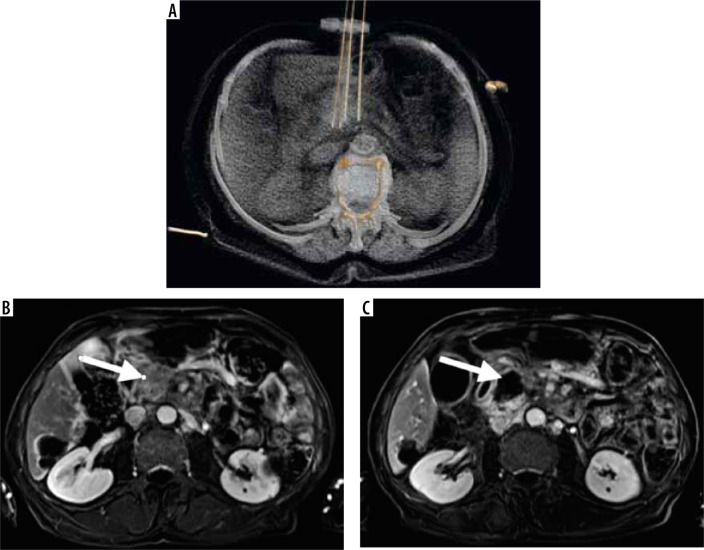
Figure 1.
A) 3D reconstruction of irreversible electroporation procedure performed under computed tomography guidance. Electrodes are placed on the tumor borders. Below, the T1 FATSAT image after contrast media administration with subtraction shows the pancreatic tumor before treatment (B) and the ablation zone after the procedure (C)
In the second half of the last century, the short (ms) high (300-500 V/cm) voltage electric pulses were applied to permeabilize the cell membrane, and to allow the chemical substances to enter the cells. This effect is reversible, so the method is called reversible electroporation (EP). When the hydrophobic chemotherapeutics are used, the intracellular concentration of them raised up to 1000 times (bleomycin), comparing to the same tissue concentrations without EP [33]. It indicates that many times lower chemotherapeutics doses could be used to reach effective intracellular concentrations of these toxic substances. For the first time this idea was exploited in practice by Eberhard Neumann and Kurt Rosenheck and described in “Journal of Membrane Biology” in 1972 [34] afterward it was described by Kulbacka et al. in 2017 [35]. The effect of temporary cell membrane electroporation has started multiple investigations in the delivery of many chemical substances, microorganisms, and nanoparticles into the cells. Electrochemotherapy is the modality when cytostatics are transported into the cells using electrical pulses.
The most commonly used is millisecond EP with relatively low voltage (up to 500 V/cm) to introduce genetic material, lipid nanoparticles, and some drugs into the cells [36,37]. The nanosecond pulsed electroporation field (nsPEF) results in permeabilization of cell membranes, including intracellular membranes with the loss of mitochondrial and nuclear membranes potential. It is the application of extremely high voltage (up to 300 kV/cm) in very short (10 ns) pulses [38].
The most extensively developed method is irreversible electroporation (IRE) using microsecond pulses of electric fields up to 2 kV/cm, via electrodes placed directly in the tumor. Until now, the efficacy of only a few drugs (bleomycin, cisplatin, gemcitabine) has been proven to be effective in clinical practice.
Discussion
Locally advanced pancreatic cancer is defined as a non-metastatic tumor that broadly adheres > 180° to major arteries in the pancreatic field, including aorta, celiac trunk, superior mesenteric artery or with unreconstructable involvement of superior mesenteric vein or portal vein. The presence of these features disqualify the tumor from primary radical resection. It applies to 25% of all pancreatic cancers [2]. The median overall survival rate in untreated LAPC is ranging from 5 to 11 months [39].
Chemotherapy remains the standard treatment of LAPC. In the past decade, the introduction of FOLFIRINOX (leucovorin, 5-fluorouracil, irinotecan, and oxaliplatin) and gemcitabine/nab-paclitaxel have prolonged the overall survival rate. Multiple trials are analyzing those two regimens. In the recently published study, Chan et al. have compared the overall survival time of 334 patients with LAPC in patients who received FOLFIRINOX vs. gemcitabine/nab-paclitaxel, showing 13.2 vs. 8.1 months adequately [40]. The goal of chemotherapy is to downsize the tumor and infiltration of surrounding vessels.
After completing neoadjuvant chemotherapy, restaging is performed according to Response Evaluation Criteria in Solid Tumors – RECIST 1.1 criteria based on CT imaging as standard.
However, there are research studies showing the superiority of Choi criteria (which additionally include tissue density analysis) and Positron Emission Tomography Response Criteria in Solid Tumors – PERCIST. In one of them, Granata et al. have compared results of restaging analyzed according to RECIST, Cho criteria, and PERCIST in 18 patients with LAPC. In RECIST evaluation based on CT images, 1 of 18 patients showed progression; in others stable disease was observed, whereas according to Choi criteria all patients showed partial response. PET-FDG was performed in 10 patients from this group, in evaluation based on PERCIST criteria patients showed partial response (6/10), stable disease (3/10) and progression disease (1/10). Moreover, the authors point out the potential role of MR in restaging, which enables the assessment of perfusion and diffusion [41]. Garcia-Figueiras et al. even suggest that MRI should become the modality of choice in the evaluation of solid tumors treatment effectiveness [42].
If regression is observed in restaging, the patient is suitable for surgery. In case of progression, further palliative options could be provided, including pain relief.
In the group of patients with partial response or non-progressive disease, ablative techniques can be offered. The application of these methods can lead to cytoreduction as well as local tumor control.
Irreversible electroporation is a relatively new, non-thermal ablative method that has been used in local treatment of focal lesions in kidneys, liver, prostate, lung, bones [43-45]. IRE induces electric pulses to cause damage to the cell membrane leading to apoptosis without the destruction of stroma. The preservation of stroma is the major advantage of IRE, as the pancreatic field is especially rich in structures like vessels, nerves, and bile ducts. As a non-thermal method, IRE does not show the heat-sink effect. These features distinguish IRE from thermal ablative treatments as radio frequency ablation, cryoablation, high intensity focused ultrasound, radiotherapy, and microwave ablation.
Nonetheless, researchers have noticed signs of thermal effect after IRE [46,47]. Fareja et al. have concluded that the thermal effect risk increases with the dose of energy used within the procedure; It is seen as coagulation rim close to the placement of the electrodes and on the borders of ablation zone [48].
IRE can be performed during open surgery, laparoscopic, or percutaneously under imaging guidance. Open surgery is preferred in the majority of published studies; it also enables the detection of peritoneal metastases. Another option is to perform the procedure during open surgery as adjuvant therapy to primary resection [49,50]. There is a growing trend in using percutaneous access, as it is less invasive and better tolerable by patients [51]. The most common complication after IRE is acute pancreatitis, resulting in abdominal pain in the first days after the procedure. More severe complications include the development of pancreatic fistula, duodenal perforation, bile leak, hemorrhage, and portal vein thrombosis [52]. Moris et al. have analyzed the Clavien-Dindo grade III or higher complications after IRE, comparing open, laparoscopic, and percutaneous procedure, where surprisingly, they were the most frequent in laparoscopic approach. The least procedure related morbidity was observed in the percutaneous approach [53].
The standard imaging method for follow-up after IRE is CT. However, as mentioned above, MRI could provide more information on processes occurring in the ablation zone, effects of treatment, and show possible progression signs.
The median overall survival rate after IRE procedure is estimated between 10 and 27 months among studies [54].
The heterogeneity of LAPC may impact the evaluation of IRE effectivness. Hypothetically, patients qualified for IRE may have a longer survival rate because of fewer risk factors. He et al. have compared the survival rate in patients after IRE with induction chemotherapy or chemotherapy alone. To avoid the differences between these groups that might impact the results, the Propensity Score Matching analysis was performed; 2 year overall survival was 57.9% in the first group vs. 18.1% in second group. Analogically, 2 year progression free survival was 31.4% vs. 7.1% [55].
In prospective PANFIRE II study, authors have compared the overall survival differences in patients who underwent IRE with or without systemic therapy. There were no survival benefits between the participants who have received FOLFIRINOX, gemcitabine, or no chemotherapy at all, suggesting that IRE was the most important factor in improved survival [56].
As IRE is causing the cell membranes permeability, it is possible to provide very high doses of chemotherapeutics parallel among the procedure. It will increase the drug concentration within the tumor without general toxicity and may improve the outcomes of systemic treatment. This is the background of electrochemotherapy [57,58].
Irreversible electroporation is proven to stimulate the immune system.
In the study by He et al., immune parameters were evaluated in patients after IRE. The results showed a transitory decrease, followed by a steady increase for CD4+ T cell, CD8+ T cell, NK cell, IL-2, C3, C4, and IgG. The opposite trend was seen for Treg cell, IL-6, and IL-10. It proves the immunomodulatory effect of IRE, which is caused by mild pancreatic inflammation after the procedure and probably the lysis of damaged cells. It was also conducted that CD8+ T cells may be a potentially prognostic parameter for overall survival and progression-free survival [59]. The consistent results were observed by Scheffer et al. with the conclusion that IRE may enable the application of immunotherapy, resulting in „in vivo vaccination” [60]. The study based on murine orthotopic pancreatic ductal adenocarcinoma provided encouraging data on the combination of IRE and anti-programmed cell death protein 1, which leads to selective tumor infiltration by CD8+ T cells and significantly prolongs survival [61].
Pain palliation
The majority of patients with pancreatic cancer experience pain as a prominent symptom, which impacts their quality of life. The pain in pancreatic cancer is predominantly localized in epigastrium, right upper quadrant, or in the back. Etiology is complex, including pancreatic duct blockage, increased parenchymal pressure, superimposed pancreatic inflammation in the tumor bed, and the most important factor – neuropathic pain associated with the infiltration of nerves [62]. Vascularization of the tumor and the growth of new nerve fibers with tumor progression intensifies the pain [63]. The psychological complex is also significant [64].
As the pain sensation is subjective, it can be evaluated and standardized by several tests and questionnaires. The most popular is Visual Analogue Scale (VAS), for pain assessment it uses a straight line with two endpoints defining „no pain” (= 0) and „the most unbearable pain” (= 100); the score is determined by choosing the point representing the current pain intensity [65]. Another widely used quality of life questionnaire is the European Organization for Research and Treatment Center Quality of Life Questionnaire C30 (EORTC QLQ-C30), with 30 assessable items divided into 15 modules of different aspects of quality of life including syndrome severity, global health status and functional assessment [66].
The conservative treatment of pain in pancreatic cancer is based on analgesic ladder rules, starting with non-opioid drugs like paracetamol, ibuprofen through weak opioids like tramadol and codeine to opiates as morphine, oxycodone, buprenorphine [67]. Vargas-Schaffer has discussed the limitations of the WHO analgesic ladder, including the fact that it could be less effective in neuropathic pain, which is dominant in pancreatic cancer [68]. The pharmacological management for chronic neuropathic pain was published by the International Association for the Study of Pain in 2007, with application of selective serotonin-norepinephrine reuptake inhibitors (SSNRI) and gabapentinoids [69]. Analgesics, in some cases, do not provide the satisfactory pain reduction, whereas they cause numerous side effects like dizziness, constipation, nausea, dyspepsia, anorexia, and more [70]. These symptoms may diminish the patient’s quality of life. Sharifi et al. reviewed the literature focusing on the potential use of cannabinoids in the treatment of pancreatic cancer and concluded that they might regulate the perception of pain as well as reduce tumor growth and invasion, inhibit angiogenesis, and lead to tumor cell death [71].
The invasive remedies include neurolytic coeliac plexus block (NCPB), thoracoscopic splanchnicectomy (TS), and rhizotomy. These methods were reviewed by Dobosz et al. and showed satisfactory results. However, the authors remarked that these procedures are used mostly among with the higher level of analgesic ladder drugs, resulting in the insufficient effects and higher complications rate [72]. Chemotherapy also has a potential to reduce pain in pancreatic cancer. The systematic review by Kristensen et al. concluded that chemotherapy with gemcitabine might lead to significant pain reduction in the majority of analyzed papers [73].
Ablative therapies that are feasible for the pancreatic field can cause local tumor control and pain relief. In comparison with other ablation techniques, IRE is characterized by shorter ablation time, which can diminish the rate of complications and postprocedural pain [74]. There are not many reports in literature focusing on pain relief after IRE procedure. The work by Martin et al. shows promising results that support IRE in pain management. In 90 days follow-up after IRE in 27 patients, the median fentanyl intake decreased from 75 µg to 25 µg [75].
The study of Scheffer et al. showed that up to 6 months after the procedure, the pain was moderately reduced, but after that time, it increased significantly, what was linked with the tumor regrowth [76]. Field et al. reported the strongest pain sensation among patients in 3 months post IRE, which decreased 6 months post IRE. There was no significant difference in pain perception between 1 month and 6 months after the procedure. However, the authors underline the relativity of patients reflects on symptom severity and subjectivity of used questionnaires [51].
Marsanic et al. has observed improved pain control after IRE in LAPC patients [77].
Conclusions
Irreversible electroporation should be considered as non-radical local ablative treatment feasible for locally advanced pancreatic cancer management. Irreversible electroporation is causing a small thermal effect in high voltage settings. A combination of intraprocedural chemotherapy and electrotherapy may increase the effectiveness of those two regimens and may be a future perspective for this group of patients. Irreversible electroporation creates a window for immunotherapy, which could enhance the treatment outcomes. For staging and restaging Choi criteria and PERCIST should be considered. The application of MRI as a follow-up study could provide more information on the ablation zone, including early signs of recurrent disease.
Although the provided data are showing promising results, more prospective clinical trials on electrochemotherapy and immunotherapy are needed, especially in the experienced research centers.
Conflicts of interest
The authors report no conflict of interest.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66: 271-289. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 1028-1061. [DOI] [PubMed] [Google Scholar]
- 3.Tachezy M, Gebauer F, Petersen C, et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA–a randomized multicenter phase III study (NCT01900327, DRKS00003893, ISRCTN82191749). BMC Cancer 2014; 14: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012; 118: 5749-5756. [DOI] [PubMed] [Google Scholar]
- 5.Suker M, Beumer BR, Sadot Eet al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016; 17: 801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paiella S, Salvia R, Girelli R, et al. Role of local ablative techniques (radiofrequency ablation and Irreversible Electroporation) in the treatment of pancreatic cancer. Updates Surg 2016; 68: 307-311. [DOI] [PubMed] [Google Scholar]
- 7.Fegrachi S, Walma MS, de Vries JJJ, et al. Safety of radiofrequency ablation in patients with locally advanced, unresectable pancreatic cancer: a phase II study. Eur J Surg Oncol 2019; 45: 2166-2172. [DOI] [PubMed] [Google Scholar]
- 8.Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010; 97: 220-225. [DOI] [PubMed] [Google Scholar]
- 9.Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015; 94: e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fegrachi S, Walma MS, de Vries JJJ, et al. Safety of radiofrequency ablation in patients with locally advanced, unresectable pancreatic cancer: a phase II study. Eur J Surg Oncol 2019; 45: 2166-2172. [DOI] [PubMed] [Google Scholar]
- 11.Crinò SF, D’Onofrio M, Bernardoni L, et al. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode: feasibility, safety, and technical success. J Gastrointestin Liver Dis 2018; 27: 67-72. [DOI] [PubMed] [Google Scholar]
- 12.Frigerio I, Girelli R, Giardino A, et al. Short term chemotherapy followed by radiofrequency ablation in stage III pancreatic cancer: results from a single center. J Hepatobiliary Pancreat Sci 2013; 20: 574-577. [DOI] [PubMed] [Google Scholar]
- 13.Giardino A, Girelli R, Frigerio I, et al. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB (Oxford) 2013; 15: 623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napoletano C, Taurino F, Biffoni M, et al. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int J Oncol 2008; 32: 481-490. [PubMed] [Google Scholar]
- 15.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005; 25 Suppl 1: S69-S83. [DOI] [PubMed] [Google Scholar]
- 16.Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg 2008; 6 Suppl 1: S65-S69. [DOI] [PubMed] [Google Scholar]
- 17.Lygidakis NJ, Sharma SK, Papastratis P, et al. Microwave ablation in locally advanced pancreatic carcinoma–a new look. Hepatogastroenterology 2007; 54: 1305-1310. [PubMed] [Google Scholar]
- 18.Ierardi AM, Biondetti P, Coppola A, et al. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg 2018; 7: 59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Chen X, Yang H, et al. Tumour cryoablation combined with palliative bypass surgery in the treatment of unresectable pancreatic cancer: a retrospective study of 142 patients. Postgrad Med J 2011; 87: 89-95. [DOI] [PubMed] [Google Scholar]
- 20.Song ZG, Hao JH, Gao S, et al. The outcome of cryoablation in treating advanced pancreatic cancer: a comparison with palliative bypass surgery alone. J Dig Dis 2014; 15: 561-569. [DOI] [PubMed] [Google Scholar]
- 21.Dubinsky TJ, Cuevas C, Dighe MK, et al. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol 2008; 190: 191-199. [DOI] [PubMed] [Google Scholar]
- 22.Yu T, Luo J. Adverse events of extracorporeal ultrasound-guided high intensity focused ultrasound therapy. PLoS One 2011; 6: e26110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinova M, Wilhelm-Buchstab T, Strunk H. Advanced pancreatic cancer: high-intensity focused ultrasound (HIFU) and other local ablative therapies. Rofo 2019; 191: 216-227 [Article in English, German]. [DOI] [PubMed] [Google Scholar]
- 24.Ji Y, Zhang Y, Zhu J, et al. Response of patients with locally advanced pancreatic adenocarcinoma to high-intensity focused ultrasound treatment: a single-center, prospective, case series in China. Cancer Manag Res 2018; 10: 4439-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bown SG, Rogowska AZ, Whitelaw DE, et al. Photodynamic therapy for cancer of the pancreas. Gut 2002; 50: 549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeWitt JM, Sandrasegaran K, O’Neil B, et al. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest Endosc 2019; 89: 390-398. [DOI] [PubMed] [Google Scholar]
- 27.Huggett MT, Jermyn M, Gillams A, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer 2014; 110: 1698-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol 2018; 41: 242-247. [DOI] [PubMed] [Google Scholar]
- 29.Gurka MK, Kim C, He AR, et al. Stereotactic body radiation therapy (SBRT) combined with chemotherapy for unresected pancreatic adenocarcinoma. Am J Clin Oncol 2017; 40: 152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comito T, Cozzi L, Clerici E, et al. Can stereotactic body radiation therapy be a viable and efficient therapeutic option for unresectable locally advanced pancreatic adenocarcinoma? Results of a phase 2 study. Technol Cancer Res Treat 2017; 16: 295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blakaj A, Stein SM, Khan SA, Johung KL. Review and current state of radiation therapy for locally advanced pancreatic adenocarcinoma. J Gastrointest Oncol 2018; 9: 1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tozzi A, Comito T, Alongi F, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol 2013; 8: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Probst U, Fuhrmann I, Beyer L, Wiggermann P. Electrochemotherapy as a new modality in interventional oncology: a review. Technol Cancer Res Treat 2018; 17: 1533033818785329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol 1972; 10: 279-290. [DOI] [PubMed] [Google Scholar]
- 35.Kulbacka J, Choromańska A, Rossowska J, et al. Cell membrane transport mechanisms: ion channels and electrical properties of cell membranes. Adv Anat Embryol Cell Biol 2017; 227: 39-58. [DOI] [PubMed] [Google Scholar]
- 36.Kulbacka J, Pucek A, Kotulska M, et al. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016; 110: 19-31. [DOI] [PubMed] [Google Scholar]
- 37.Kulbacka J, Pucek A, Wilk KA, et al. The Effect of millisecond pulsed electric fields (msPEF) on intracellular drug transport with negatively charged large nanocarriers made of solid lipid nanoparticles (SLN): in vitro study. J Membr Biol 2016; 249: 645-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulbacka J. Nanosecond pulsed electric fields (nsPEFs) impact and enhanced Photofrin II(®) delivery in photodynamic reaction in cancer and normal cells. Photodiagnosis Photodyn Ther 2015; 12: 621-629. [DOI] [PubMed] [Google Scholar]
- 39.Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Curr Probl Cancer 2002; 26: 176-275. [DOI] [PubMed] [Google Scholar]
- 40.Chan KKW, Guo H, Cheng S, et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: a population-based propensity score-weighted analysis. Cancer Med 2020; 9: 160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granata V, Fusco R, Setola SV, et al. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J Gastroenterol 2017; 23: 4767-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Figueiras R, Padhani AR, Baleato-González S. Therapy monitoring with functional and molecular MR imaging. Magn Reson Imaging Clin N Am 2016; 24: 261-288. [DOI] [PubMed] [Google Scholar]
- 43.Wendler JJ, Pech M, Köllermann J, et al. Upper-urinary-tract effects after irreversible electroporation (IRE) of human localised renal-cell carcinoma (RCC) in the IRENE pilot phase 2a ablate-and-resect study. Cardiovasc Intervent Radiol 2018; 41: 466-476. [DOI] [PubMed] [Google Scholar]
- 44.Wendler JJ, Klink F, Seifert S, et al. Irreversible electroporation of prostate cancer: patient-specific pretreatment simulation by electric field measurement in a 3D bioprinted textured prostate cancer model to achieve optimal electroporation parameters for image-guided focal ablation. Cardiovasc Intervent Radiol 2016; 39: 1668-1671. [DOI] [PubMed] [Google Scholar]
- 45.Vroomen LGPH, Petre EN, Cornelis FH, et al. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: what are the differences? Diagn Interv Imaging 2017; 98: 609-617. [DOI] [PubMed] [Google Scholar]
- 46.Vroomen LGPH, Scheffer HJ, Melenhorst MCAM, et al. MR and CT imaging characteristics and ablation zone volumetry of locally advanced pancreatic cancer treated with irreversible electroporation. Eur Radiol 2017; 27: 2521-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruarus AH, Vroomen LGPH, Puijk RS, et al. Conductivity rise during irreversible electroporation: true permeabilization or heat? Cardiovasc Intervent Radiol 2018; 41: 1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology 2013; 266: 462-470. [DOI] [PubMed] [Google Scholar]
- 49.Martin RC 2nd, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 2015; 262: 486-494. [DOI] [PubMed] [Google Scholar]
- 50.Simmerman E, Chung J, Lawson A, et al. Application of irreversible electroporation ablation as adjunctive treatment for margin enhancement: safety and efficacy. J Surg Res 2020; 246: 260-268. [DOI] [PubMed] [Google Scholar]
- 51.Paiella S, Butturini G, Frigerio I, et al. Safety and feasibility of irreversible electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg 2015; 32: 90-97. [DOI] [PubMed] [Google Scholar]
- 52.Field W, Rostas JW, Martin RCG. Quality of life assessment for patients undergoing irreversible electroporation (IRE) for treatment of locally advanced pancreatic cancer (LAPC). Am J Surg 2019; 218: 571-578. [DOI] [PubMed] [Google Scholar]
- 53.Narayanan G, Hosein PJ, Beulaygue IC, et al. Percutaneous image-guided irreversible electroporation for the treatment of unresectable, locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol 2017; 28: 342-348. [DOI] [PubMed] [Google Scholar]
- 54.Moris D, Machairas N, Tsilimigras DI, et al. Systematic review of surgical and percutaneous irreversible electroporation in the treatment of locally advanced pancreatic cancer. Ann Surg Oncol 2019; 26: 1657-1668. [DOI] [PubMed] [Google Scholar]
- 55.Lafranceschina S, Brunetti O, Delvecchio A, et al. Systematic review of irreversible electroporation role in management of locally advanced pancreatic cancer. Cancers (Basel) 2019; 11: 1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He C, Wang J, Zhang Y, et al. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: a propensity score matching analysis. Pancreatology 2020; 20: 477-484. [DOI] [PubMed] [Google Scholar]
- 57.Ruarus AH, Vroomen LGPH, Geboers B, et al. Percutaneous irreversible electroporation in locally advanced and recurrent pancreatic cancer (PANFIRE-2): a multicenter, prospective, single-arm, phase II study. Radiology 2020; 294: 212-220. [DOI] [PubMed] [Google Scholar]
- 58.Fernandes P, O’Donovan TR, McKenna SL, et al. Electrochemotherapy causes caspase-independent necrotic-like death in pancreatic cancer cells. Cancers (Basel) 2019; 11: 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He C, Wang J, Sun S, et al. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer. J Oncol 2019; 2019: 9346017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheffer HJ, Stam AGM, Geboers B, et al. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology 2019; 8: 1652532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Wen X, Tian L, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun 2019; 10: 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barreto SG, Saccone GT. Pancreatic nociception–revisiting the physiology and pathophysiology. Pancreatology 2012; 12: 104-112. [DOI] [PubMed] [Google Scholar]
- 63.Bapat AA, Hostetter G, Von Hoff DD, et al. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 2011; 11: 695-707. [DOI] [PubMed] [Google Scholar]
- 64.Védie AL, Neuzillet C. Pancreatic cancer: best supportive care. Presse Med 2019; 48 (3 Pt 2): e175-e185. [DOI] [PubMed] [Google Scholar]
- 65.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 2003; 4: 407-414. [DOI] [PubMed] [Google Scholar]
- 66.Shih CL, Chen CH, Sheu CF, et al. Validating and improving the reliability of the EORTC qlq-c30 using a multidimensional Rasch model. Value Health 2013; 16: 848-854. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization . (1996). Cancer pain relief: with a guide to opioid availability, 2nd ed. World Health Organization. Available at: https://apps.who.int/iris/handle/10665/37896 (Accessed: 29.08.2019).
- 68.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twentyfour years of experience. Can Fam Physician 2010; 56: 514-517, e202-205. [PMC free article] [PubMed] [Google Scholar]
- 69.Dworkin RH, O’Connor AB, Kent J, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013; 154: 2249-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wickham RJ. Cancer pain management: opioid analgesics, part 2. J Adv Pract Oncol 2017; 8: 588-607. [PMC free article] [PubMed] [Google Scholar]
- 71.Sharafi G, He H, Nikfarjam M. Potential use of cannabinoids for the treatment of pancreatic cancer. J Pancreat Cancer 2019; 5: 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dobosz Ł, Kaczor M, Stefaniak TJ. Pain in pancreatic cancer: review of medical and surgical remedies. ANZ J Surg 2016; 86: 756-761. [DOI] [PubMed] [Google Scholar]
- 73.Kristensen A, Vagnildhaug OM, Grønberg BH, et al. Does chemotherapy improve health-related quality of life in advanced pancreatic cancer? A systematic review. Crit Rev Oncol Hematol 2016; 99: 286-298. [DOI] [PubMed] [Google Scholar]
- 74.Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver 2010; 4 (Suppl 1): S99-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 2012; 215: 361-369. [DOI] [PubMed] [Google Scholar]
- 76.Scheffer HJ, Vroomen LG, de Jong MC, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology 2017; 282: 585-597. [DOI] [PubMed] [Google Scholar]
- 77.Marsanic P, Mellano A, Sottile Aet al. Irreversible electroporation as treatment of locally advanced and as margin accentuation in borderline resectable pancreatic adenocarcinoma. Med Biol Eng Comput 2017; 55: 1123-1127. [DOI] [PubMed] [Google Scholar]
- 78.Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013; 20 Suppl 3: S443-S449. [DOI] [PubMed] [Google Scholar]
- 79.Kluger MD, Epelboym I, Schrope BA, et al. Single-institution experience with irreversible electroporation for T4 pancreatic cancer: first 50 patients. Ann Surg Oncol 2016; 23: 1736-1743. [DOI] [PubMed] [Google Scholar]
- 80.Dunki-Jacobs EM, Philips P, Martin RC 2nd. Evaluation of resistance as a measure of successful tumor ablation during irreversible electroporation of the pancreas. J Am Coll Surg 2014; 218: 179-187. [DOI] [PubMed] [Google Scholar]
- 81.Månsson C, Brahmstaedt R, Nilsson A, et al. Percutaneous irreversible electroporation for treatment of locally advanced pancreatic cancer following chemotherapy or radiochemotherapy. Eur J Surg Oncol 2016; 42: 1401-1406. [DOI] [PubMed] [Google Scholar]
- 82.Månsson C, Brahmstaedt R, Nygren P, et al. Percutaneous irreversible electroporation as first-line treatment of locally advanced pancreatic cancer. Anticancer Res 2019; 39: 2509-2512. [DOI] [PubMed] [Google Scholar]
- 83.Vogel JA, Rombouts SJ, de Rooij T, et al. Induction chemotherapy followed by resection or irreversible electroporation in locally advanced pancreatic cancer (IMPALA): a prospective cohort study. Ann Surg Oncol 2017; 24: 2734-2743. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Shi J, Zeng J, et al. Percutaneous irreversible electroporation for ablation of locally advanced pancreatic cancer: experience from a Chinese institution. Pancreas 2017; 46: e12-e14. [DOI] [PubMed] [Google Scholar]
- 85.Lambert L, Horejs J, Krska Z, et al. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: general hospital cancer center experience. Neoplasma 2016; 63: 269-273. [DOI] [PubMed] [Google Scholar]
- 86.Yan L, Chen YL, Su M, et al. A Single-institution experience with open irreversible electroporation for locally advanced pancreatic carcinoma. Chin Med J (Engl) 2016; 129: 2920-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leen E, Picard J, Stebbing J, et al. Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma. J Gastrointest Oncol 2018; 9: 275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belfiore G, Belfiore MP, Reginelli A, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol 2017; 34: 38. [DOI] [PubMed] [Google Scholar]
- 89.Holland MM, Bhutiani N, Kruse EJ, et al. A prospective, multiinstitution assessment of irreversible electroporation for treatment of locally advanced pancreatic adenocarcinoma: initial outcomes from the AHPBA pancreatic registry. HPB (Oxford) 2019; 21: 1024-1031. [DOI] [PubMed] [Google Scholar]
- 90.Yang PC, Huang KW, Pua U, et al. Prognostic factor analysis of irreversible electroporation for locally advanced pancreatic cancer–a multi-institutional clinical study in Asia. Eur J Surg Oncol 2020; 46: 811-817. [DOI] [PubMed] [Google Scholar]
- 91.Kwon JH, Chung MJ, Park JY, et al. Initial experience of irreversible electroporation for locally advanced pancreatic cancer in a Korean population. Acta Radiol 2021; 62: 164-171. [DOI] [PubMed] [Google Scholar]
- 92.Giardino A, Girelli R, Frigerio I, et al. Two hundred consecutive patients treated with radiofrequency ablation for stage III pancreatic cancer: results from a single institution. Eur J Surg Oncol 2015; 41: S4-S5. [Google Scholar]
- 93.Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med 2019; 40: 625-637. [DOI] [PubMed] [Google Scholar]
- 94.Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011; 40: 1080-1086. [DOI] [PubMed] [Google Scholar]
- 95.Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009; 10: 123-129. [PubMed] [Google Scholar]
- 96.Zhao J, Zhao F, Shi Y, et al. The efficacy of a new high intensity focused ultrasound therapy for locally advanced pancreatic cancer. J Cancer Res Clin Oncol 2017; 143: 2105-2111. [DOI] [PubMed] [Google Scholar]
- 97.Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol 2011; 34: 63-69. [DOI] [PubMed] [Google Scholar]
- 98.Song Y, Yuan Z, Li F, et al. Analysis of clinical efficacy of CyberKnife((R)) treatment for locally advanced pancreatic cancer. Onco Targets Ther 2015; 8: 1427-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]