Abstract
Introduction
A systematic analysis of clinical trials was performed in order to assess the effectiveness and risks of bilateral renal denervation (RDN) in patients with chronic heart failure with reduced ejection fraction (HFrEF).
Methods
A systematic review was conducted of all clinical trials exploring the effectiveness of RDN in patients with HF who had reduced (<50%) EF. Primary outcomes were NYHA class, 6-min walk test, N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, left ventricular ejection fraction (LVEF) and other cardiac parameters including left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and left atrium diameter (LAD). Secondary outcomes were systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), glomerular filtration rate (GFR), and creatinine.
Results
Seven studies were included in this analysis. From baseline to 6 months after RDN, the pooled mean NYHA class was decreased (mean difference [MD], -0.9; 95% confidence interval [CI], -1.6 to -0.2; P = 0.018), the mean 6-min walk test was increased (MD, 79.5 m; 95% CI, 26.9 to 132.1; P = 0.003), and the average NT-proBNP level was decreased (MD, -520.6 pg/mL; 95% CI, -1128.4 to 87.2; P = 0.093). Bilateral RDN increased the LVEF (MD, 5.7%; 95% CI, 1.6 to 9.6; P = 0.004), decreased the LVESD (MD, -0.4 cm; 95% CI, -0.5 to -0.2; P < 0.001), decreased the LVEDD (MD, -0.5 cm; 95% CI, -0.6 to -0.3; P < 0.001), and decreased the LAD (MD, -0.4 cm; 95% CI, -0.8 to 0; P = 0.045). In addition, RDN significantly decreased systolic BP (MD, -9.4 mmHg; 95% CI, -16.3 to -2.4; P = 0.008) and diastolic BP (MD, -4.9 mmHg; 95% CI, -9.5 to -0.4; P = 0.033), and decreased HR (MD, -4.5 bpm; 95% CI, -8.2to -0.9; P = 0.015). RDN did not significantly change GFR (MD, 7.9; 95% CI, -5.0 to 20.8; P = 0.230), or serum creatinine levels (MD, -7.2; 95% CI, -23.7 to 9.4; P = 0.397).
Conclusion
Bilateral RDN appears safe and well-tolerated in patients with HF. RDN improved the signs and symptoms of HF and slightly decreased systolic and diastolic BP without affecting renal function in the clinical trials performed to date.
Keywords: Chronic heart failure, Renal denervation, Sympatho-excitation
Chronic heart failure, Renal denervation, Sympatho-excitation.
1. Introduction
Heart failure (HF) is a prevalent, widespread, and costly disease. Each year in the United States, heart failure affects over 800,000 new patients, contributes to more than 300,000 deaths, and costs 30 billion dollars [1]. The progressive pathophysiology of heart failure is characterized by maladaptive sympatho-excitation which adversely affects the cardiovascular and renal systems. Endovascular renal denervation (RDN) is a minimally invasive procedure that has been shown to effectively decrease blood pressure (BP) in patients with refractory hypertension [2]. In addition, RDN has provided improvements in alleviating the symptoms of HF by reducing sympathetic nerve activity and attenuating adverse cardiac remodeling in both HF animal models and in patients [3, 4, 5, 6], suggesting that RDN is a promising approach for the management of HF. Several clinical trials have been done to compare the effectiveness of RDN in the treatment of chronic HF, suggesting that RDN decreased N-terminal pro-B-type natriuretic peptide (NT-proBNP), increased in left ventricular ejection fraction (LVEF), and improved exercise tolerance in patients with chronic HF in RDN group over 6-month follow-up [4, 7]. Potential complications of RDN including renal artery stenosis or dissection, pseudoaneurysm at the femoral access site, and bradycardia are not common but require a concern. Given the lack of large clinical trials in HF patients, the relative benefits and safety of RDN in this patient population remain uncertain. We therefore carried out a systematic analysis of clinical trials in order to assess the efficacy and risks of bilateral RDN in patients with chronic HF with reduced ejection fraction (HFrEF).
2. Material and methods
2.1. Search strategy
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [8] and the reporting Meta-Analyses of Observational Studies in Epidemiology (MOOSE) [9].
Studies that evaluated the effect of RDN in patients with HF published before December 21, 2019, were identified using PubMed and EMBASE databases, MEDLINE, and ClinicalTrials.gov. The following keywords were used: renal denervation, renal sympathetic denervation and heart failure. References of relevant articles were also reviewed for any additional studies. We searched for studies in any language in which adult patients with HF received bilateral RDN.
2.2. Study selection
Studies that met each of the following criteria were included:
-
(1)
the study assessed the effectiveness of RDN in patients with HF who had reduced (<50%) EF.
-
(2)
the outcomes included NYHA class, 6-min walk test, N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, LVEF, left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and left atrium diameter (LAD), heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP), glomerular filtration rate (GFR), and creatinine.
-
(3)
the duration of follow-up was at least 6 months.
2.3. Data extraction
Two reviewers (Zhiqiu Xia and Li Han) independently extracted data. Any disagreement in opinion was resolved through discussion with all investigators. The following data were extracted from the included studies: publication year, methodology, number of patients, patient population, intervention including drugs they received, and outcomes.
Primary outcomes were NYHA class, 6-min walk test, NT-proBNP levels, LVEF and other cardiac parameters including LVESD, LVEDD, and LAD. Secondary outcomes were HR, SBP, DBP, GFR, and creatinine.
NYHA class, 6-min walk test, NT-proBNP levels, LVEF, LVESD, LVEDD, LAD, HR, SBP, DBP, GFR, and creatinine were treated as continuous variables, of which mean differences (MDs) with 95% confidence intervals (CIs) were calculated.
Using the formula shown in Chapter 7 of the Cochrane Handbook for Systematic Reviews of interventions, we estimated the mean and variance of the trials in which only median, interquartile range (IQR), and size were reported [10].
2.4. Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included studies [11]. Assessment scores of 0–3, 4–6, and 7–9 were considered as poor, fair, and good, respectively. Disagreements were resolved by consensus.
2.5. Data analysis
To assess net changes in the same outcome, only the data of patients that received renal denervation was extracted and calculated using a random-effects model. All parameters and units were normalized for comparison. Heterogeneity between studies was evaluated using a chi-squared test, and a P value of <0.10 was indicative of significant heterogeneity. Data analyses were done with Comprehensive Meta-analysis version 2.2. (Biostat Inc., Englewood, NJ)., and a P value of <0.05 was considered as significant in the analysis.
3. Results
3.1. Eligible studies
The initial search retrieved 52 publications. After excluding duplicates, 42 distinct articles were identified for title and abstract screen (Figure 1). Of these, 30 articles were excluded, of which three were studies about RDN for HF with preserved or normal EF, 22 were studies about HTN, renal diseases or Parkinson's disease, four were studies not mentioning RDN, and one was an animal study. Twelve studies were full-text read for further evaluation. Five were excluded because of no defined endpoints reported or incomplete study details. Therefore, seven studies were included in this analysis (Table 1) [4, 7, 12, 13, 14, 15, 16].
Figure 1.
Flowchart of the literature search.
Table 1.
Main characteristics of included studies.
| No | Study | Country | Design | No. of Patients | Pt condition | Age, Mean (SD), y |
Males, % |
NYHA class | EF | Catheter type | Follow-up duration and drop-off | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RDN | Control | RDN | Control | |||||||||||
| 1 | Gao et al (2019) | China | Single-center, RCT Randomization method: random envelope | 60 | Chronic systolic HF (65.0% hypertension, 58.3% coronal heart disease, 11.7% atrial fibrillation, and 25% type 2 diabetes) | 59.0 (12.1) | 61.3 (11.1) | 83.3 | 73.0 | II-III | <40% | Stockert EP Shuttle radiofrequency generator (Johnson & Johnson Medical) | 6 months No drop-off | 9 |
| 2 | Drożdż et al (2019) | Poland | Open-label, RCT Randomization method: coin toss | 20 | Adult HF patients despite optimal medical treatment and resynchronization therapy (60% ischemic cardiomyopathy, and 40% dilated cardiomyopathy of unknown etiology) | 75.0 (65.0–81.0) | 71.0 (70.0–76.0) | 80.0 | 70.0 | II-III | <35% | Symplicity catheter (Medtronic) | 24 months No drop-off | 9 |
| 3 | Chen et al (2017) | China | Single center, open label, RCT Randomization method: not mentioned | 60 | CHF (25% hypertension, 57% cardiomyopathy, 18% ischemic cardiomyopathy). | 48.5 (8.4) | 50.5 (7.7) | 73.30 | 80.0 | ll-lV | ≤40% | Thermocool catheter (Biosense Webster) | 6 months No drop-off | 9 |
| 4 | Gao et al (2017) | China | Open label, single-arm study | 14 | CHF (29% hypertension, 14% dilated cardiomyopathy, 57% coronary artery disease). | 69.6 (5.7) | N/A | 85.7 | N/A | III-IV | <45% | Stockert EP Shuttle radiofrequency generator (Johnson & Johnson Medical) | 6 months No drop-off | 6 |
| 5 | Hopper et al (2017) | 8 study sites in Europe and Australia | Multi-center, open-label, single-arm study | 39 | Chronic systolic HF and renal impairment (62% ischemic heart failure) | 65 (11) | N/A | 87.0 | N/A | II-III | <40% | Symplicity Flex single-electrode catheter system (Medtronic) | 12 months One death by 12 months | 6 |
| 6 | Dai et al (2015) | China | Single center, open label, CT | 20 | CHF (40% dilated cardiomyopathy, 40% ischemic cardiomyopathy, 20% hypertensive cardiopathy). | 63 (10) | 64 (5) | 80.0 | 70.0 | III-IV | <40% | NR | 6 months No drop-off | 9 |
| 7 | Davies et al (2013) | UK | Open-label, single-arm study | 7 | CHF (71% ischemic HF, 29% unknown etiology) | 69 (7) | N/A | 71.4 | N/A | III-IV | <40% | Symplicity catheter (Medtronic) | 6 months No drop-off | 6 |
3.2. Characteristics of studies included in the meta-analysis
The main characteristics of the included studies are summarized in Table 1. All studies were published between 2013 to 2019. Patient follow-up duration ranged from 6 to 24 months. There were three randomized, control trials [4, 7, 13], one nonrandomized clinical trial [16], and three single-arm trials [12, 14, 15].
3.3. Primary outcomes
3.3.1. NYHA class
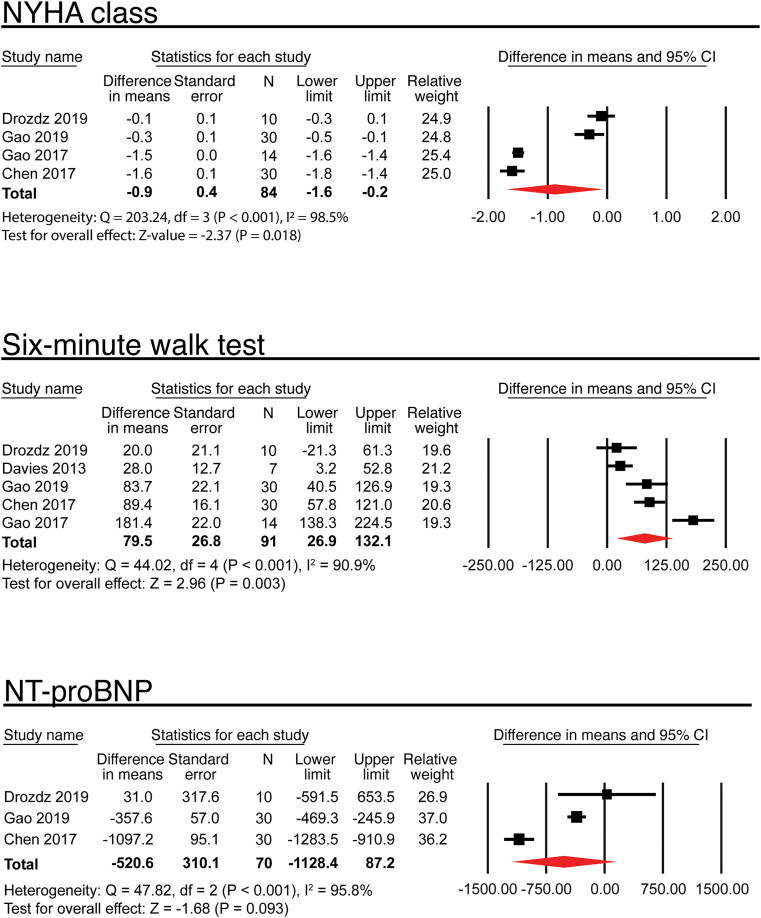
Four trials evaluated the effect of RDN on NYHA class (Figure 2) [4, 7, 13, 15]. From baseline to 6 months after RDN, the pooled mean NYHA class decreased by 0.9, consistent with an amelioration of heart failure symptoms (MD, -0.9; 95% CI, -1.6 to -0.2; P = 0.018).
Figure 2.
Effects of renal denervation on NYHA class, 6-min walk test, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels.
3.3.2. Six-minute walk test
Five trials reported the effect of RDN on the 6-min walk test (Figure 2) [4, 7, 13, 14, 15]. Four reported mean (SD) [4, 7, 14, 15], and one reported median (IQR) [13]. The mean 6-min walk test was increased by 79.5 m (MD, 79.5 m; 95% CI, 26.9 to 132.1; P = 0.003).
3.3.3. NT-proBNP levels
Three trials reported the changes of NT-proBNP levels after RDN (Figure 2) [4, 7, 13], of which two reported mean (SD) [4, 7], and one reported median (IQR) [13]. The average NT-proBNP level was decreased by 520.6 pg/mL (MD, -520.6 pg/mL; 95% CI, -1128.4 to 87.2; P = 0.093). Two trials reported the changes of BNP levels. Dai et al observed that BNP level in the RDN group dropped from 629 ± 131 pg/mL to 460 ± 69 pg/mL at 24 h after denervation procedure [16]. Gao et al study demonstrated that BNP was decreased to 661.2 ± 368.2 pg/mL from 300.0 ± 249.3 pg/mL at 6 months after RDN [15].
3.3.4. Echocardiography
Six out of seven studies evaluated the effect of RDN on LVEF (Figure 3) [4, 7, 13, 14, 15, 16]. The combined data showed that RDN increased LVEF by 5.7% (MD, 5.7%; 95% CI, 1.6 to 9.6; P = 0.004). Three studies evaluated the effect of RDN on LVESD [7, 14, 15]. The combined data showed that RDN significantly decreased the LVESD by 0.4 cm (MD, -0.4 cm; 95% CI, -0.5 to -0.2; P < 0.001). Four studies evaluated the effect of RDN on LVEDD [4, 7, 14, 15]. The combined data showed that RDN significantly decreased the LVEDD by 0.5 cm (MD, -0.5 cm; 95% CI, -0.6 to -0.3; P < 0.001).
Figure 3.
Effects of renal denervation on left ventricular ejection fraction (LVEF), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and left atrium diameter (LAD).
Three studies evaluated the effect of RDN on LAD [4, 14, 15]. The combined data showed that the renal denervation significantly decreased the LAD by 0.4 cm (MD, -0.4 cm; 95% CI, -0.8 to 0; P = 0.045).
3.4. Secondary outcomes
3.4.1. Blood pressure (BP) and heart rate (HR)
All the seven included studies monitored BP changes. Hopper et al evaluated BP at 12 months after RDN showing that there was no significant BP change from baseline [12]. Dai et al monitored BP at 24 h after RDN demonstrating that there are no BP changes in the RDN group compared with baseline [16]. The changes of BP at 6 months following RDN were examined in five studies (Figure 4) [4, 7, 13, 14, 15]. The analysis demonstrated that RDN significantly decreased systolic BP by 9.4 mmHg (MD, -9.4 mmHg; 95% CI, -16.3 to -2.4; P = 0.008) and diastolic BP by 4.9 mmHg (MD, -4.9 mmHg; 95% CI, -9.5 to -0.4; P = 0.033). Four trials included changes of HR as an outcome of interest [4, 13, 14, 15], showing that RDN was associated with a reduction in HR (MD, -4.5 bpm; 95% CI, -8.2to -0.9; P = 0.015).
Figure 4.
Effects of renal denervation on systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR).
3.4.2. GFR and creatinine
All seven studies reported GFR or creatinine and no study reported both parameters. Two trials evaluated the effect of RDN on GFR at 6 months following RDN (Figure 5) [4, 13]. The analysis did not demonstrate a statistically significant effect of RDN on GFR from baseline to 6-month post-RDN (MD, 7.9; 95% CI, -5.0 to 20.8; P = 0.230). Hopper et al demonstrated that there was no significant change in GFR at12 months following RDN [12]. Two trials reported the effect of RDN on serum creatinine levels [14, 15]. Similarly, mean creatinine levels did not change 6 months after RDN (MD, -7.2; 95% CI, -23.7 to 9.4; P = 0.397). Dai et al showed creatinine levels didn't change significantly at 24 h after RDN [16].
Figure 5.
Effects of renal denervation on glomerular filtration rate (GFR), and creatinine.
3.4.3. Medicine titration and readmission
No study reported severe adverse events during ablation procedure and follow-up periods. Three included studies reported down titration of loop diuretics in RDN group [4, 14, 15]. Chen et al reported that the use of loops in RDN group was decreased compared with control group at 6 months after RDN from 46.7% at baseline to 23.3% in RDN group at 6 months post RDN [4]. Gao et al observed that six patients reduced or discontinued use of loop diuretics, and the use of and angiotensin receptor blockers (ARBs) were also decreased [15]. Davies et al reported that four patients reduced or stopped loop diuretics in RND group due to improvement of peripheral edema [14].
Dai et al reported that two patients in RDN group were readmitted due to worsening heart failure with mean hospital stay of 5 ± 3 days, while eight patients were readmitted due to worsening heart failure with mean hospital stay of 9 ± 5 days in the control group [16]. In Drożdż study, three patients in RDN group and two in control group were readmitted for decompensation of heart failure during 12 months of follow-up, while whereas during 24 months of follow-up, four patients in RDN group and five patients in control group were readmitted during 24 months of follow-up [13]. Hopper et al reported 12 hospitalizations due to decompensated HF in eight patients during 12 months of follow-up [12].
4. Discussion
This meta-analysis showed that RDN significantly improved the symptoms of HFrEF as evidenced by decreases in NYHA class and increased 6-min walk distances. This meta-analysis also showed echocardiographic improvements in left ventricular function (LVEF, LVESD) and congestion (LAD, LVEDD) after RDN, suggesting that these echocardiographic improvements could drive symptomatic improvement. Moreover, renal denervation decreased systolic and diastolic BP as well as HR without affecting renal function.
Renal denervation was originally performed to treat patients with resistant hypertension. Initial clinical studies showed that RDN was efficient in decreasing BP in patients with hypertension [2, 17], which was however not supported by the observations from the Symplicity HTN-3 study [18]. Subsequently, several trials (DENERHTN, SPYRAL HTN-OFF MED, RADIANCE-HTN SOLO, and SPYRAL HTN-ON MED) demonstrated that RDN deceased BP more in comparison with sham regardless of pharmacotherapy use [19, 20, 21, 22]. A recent meta-analysis of RCTs showed that RDN provides significant but modest reduction in ambulatory and office blood pressure in patients with hypertension [23]. Chronic heart failure (CHF) has also been proposed as another indication of RDN. A major problem in the development of HF is excessive sympatho-excitation that promotes cardiac dysfunction and affects other important organs such as the kidneys. In the setting of CHF, renal spinal afferents are sensitized and provide increased input to the central nervous system causing excessive sympathetic outflow to the heart, the kidneys, and the peripheral vasculature. Activation of renal efferent nerves leads to increased renal vascular resistance and activation of the renin-angiotensin-aldosterone system contributing to sodium and water retention [24]. RDN, by reducing both afferent and efferent renal nerve activity, can affect global sympathetic tone and volume status [25]. Several animal experiments have shown that RDN improves cardiac function, decreases ventricular arrythmia and ameliorates renal damage, suggesting beneficial effects of RDN in chronic HF [26, 27]. However, whether RDN is effective in treating patients with chronic HF is still unclear. Our study shows that RDN significantly improved symptoms related to HF, suggesting an improvement in the quality of life. The possible mechanism is RDN suppresses sympathetic tone and the renin-angiotensin-aldosterone system, leading to the amelioration of cardiac fibrosis and improvement cardiac contractility.
Our study reviewed two potential safety endpoints: hemodynamics and renal function. Our study suggests that bilateral RDN slightly decreased systolic and diastolic BP as well as HR in chronic HF; therefore, hemodynamics should be taken into consideration prior to and after the RDN procedure as hypotension and bradycardia may further compromise perfusion in these vulnerable patients. In addition, renal denervation has been shown to improve renal function in patients with chronic kidney diseases [28]. Our analysis suggests that RDN neither harms nor ameliorates renal function in HFrEF patients. Several other included studies reported electrolytes including sodium, potassium and BUN levels; however, whether RDN improved renal function in the setting of HFrEF requires future studies.
The current studies have several limitations. One major limitation of current studies is the small sample sizes of the included studies. In addition, the clinical profiles of the patients from included studies varied. Ages of patients may have effects on outcomes of patients that receive RDN. One study that included relatively young patients (mean age was 47.6) and one study that included old patients (mean age was 65) both showed that heart failure patients benefited from RDN at 12-month follow-up. However, the association between age and other clinical features and outcomes based on the small numbers of trials and heterogeneities among different studies is unclear. Second, the RDN procedure varies considerably due to high intrinsic procedural variability, interventionist and center experience, and catheter technology. In the included studies, details concerning the denervation procedure including the number of ablation points, the sites of denervation, the catheter types, and the operator experience were not extensively described. Liu et al used renal nerve stimulation to identify the nerve-enriched areas during RDN in order to improve the efficacy of the procedure in a dog model of hypertension, indicating that renal nerve stimulation could serve as a useful guide to locate the optimal ablation targets [29]. In addition, a recent study carried out in rabbits and pigs suggests that a transfer function analysis of the coupling between renal blood flow and BP on a beat by beat basis may be used as an endpoint in determining the completeness of RDN during the procedure [30]. Additionally, none of the studies used hard endpoints like mortality, death from cardiovascular causes, or heart failure admissions that drive changes in the standard of care for heart failure patients. Furthermore, it is not known yet how long following diagnosis of CHF that RDN should be initiated. Geng et al. demonstrated that patients in early-stage HF could benefit more from RDN, however, the appropriate time point to initiate RDN treatment in HF patients still requires future investigation. [31],There are limited RCT trials that focused on the effect of renal denervation. Most trials were observational, single-armed studies, or nonrandomized clinical trial. Large, multicenter, sham-controlled randomized trials with gold-standard endpoints are needed to definitively test the therapeutic potential of catheter-based RDN in chronic HF patients.
5. Conclusion
Based on a modest number of human trials, bilateral RDN appears safe and well-tolerated in patients with HFrEF. RDN improved the signs and symptoms of HF and slightly decreased systolic and diastolic BP without affecting renal function in the clinical trials performed to date. More comprehensive studies need to be carried out to evaluate the extent to which this therapy is additive or replaces current medical therapy. Furthermore, studies in patients with HFpEF will need to be evaluated.
Declarations
Author contribution statement
Zhiqiu Xia and Li Han: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Peter R. Pellegrino, Robert L. Lobato and Steven J. Lisco: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Alicia M. Schiller and Logan D. Harrold: Analyzed and interpreted the data.
Irving H. Zucker and Han Jun Wang: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by NIH grants (2R01 HL126796,1R01 HL-152160 and 1R01 HL-121012).
Hanjun Wang was supported by the Margaret R. Larson Professorship in Anesthesiology. Irving H. Zucker was supported by the Theodore F. Hubbard Professorship for Cardiovascular Research.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., Johnson M.R., Kasper E.K., Levy W.C., Masoudi F.A., McBride P.E., McMurray J.J., Mitchell J.E., Peterson P.N., Riegel B., Sam F., Stevenson L.W., Tang W.H., Tsai E.J., Wilkoff B.L. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Krum H., Schlaich M., Whitbourn R., Sobotka P.A., Sadowski J., Bartus K., Kapelak B., Walton A., Sievert H., Thambar S., Abraham W.T., Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 3.Schirmer S.H., Sayed M.M., Reil J.C., Ukena C., Linz D., Kindermann M., Laufs U., Mahfoud F., Bohm M. Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J. Am. Coll. Cardiol. 2014;63:1916–1923. doi: 10.1016/j.jacc.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 4.Chen W., Ling Z., Xu Y., Liu Z., Su L., Du H., Xiao P., Lan X., Shan Q., Yin Y. Preliminary effects of renal denervation with saline irrigated catheter on cardiac systolic function in patients with heart failure: a Prospective, Randomized, Controlled, Pilot Study. Cathet. Cardiovasc. Interv. 2017;89:E153–e161. doi: 10.1002/ccd.26475. [DOI] [PubMed] [Google Scholar]
- 5.Schiller A.M., Haack K.K., Pellegrino P.R., Curry P.L., Zucker I.H. Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R886–892. doi: 10.1152/ajpregu.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller A.M., Pellegrino P.R., Zucker I.H. The renal nerves in chronic heart failure: efferent and afferent mechanisms. Front. Physiol. 2015;6:224. doi: 10.3389/fphys.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J.Q., Yang W., Liu Z.J. Percutaneous renal artery denervation in patients with chronic systolic heart failure: a randomized controlled trial. Cardiol. J. 2019;26:503–510. doi: 10.5603/CJ.a2018.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w264. [DOI] [PubMed] [Google Scholar]
- 9.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Deeks JJe. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Higgins J.P.T., Green S., editors. The Cochrane Collaboration; 2011. Chapter 7: selecting studies and collecting data.www.handbook.cochrane.org (updated March 2011) Available from. [Google Scholar]
- 11.Wells G., Shea B., O'Connell D., Peterson j, Welch V., Losos M., Tugwell P. 2000. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-randomized Studies in Meta-Analysis. [Google Scholar]
- 12.Hopper I., Gronda E., Hoppe U.C., Rundqvist B., Marwick T.H., Shetty S., Hayward C., Lambert T., Hering D., Esler M., Schlaich M., Walton A., Airoldi F., Brandt M.C., Cohen S.A., Reiters P., Krum H. Sympathetic response and outcomes following renal denervation in patients with chronic heart failure: 12-month outcomes from the symplicity HF feasibility study. J. Card. Fail. 2017;23:702–707. doi: 10.1016/j.cardfail.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Drozdz T., Jastrzebski M., Moskal P., Kusiak A., Bednarek A., Styczkiewicz K., Jankowski P., Czarnecka D. Renal denervation in patients with symptomatic chronic heart failure despite resynchronization therapy - a pilot study. Adv. Interventional Cardiol. 2019;15:240–246. doi: 10.5114/aic.2019.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies J.E., Manisty C.H., Petraco R., Barron A.J., Unsworth B., Mayet J., Hamady M., Hughes A.D., Sever P.S., Sobotka P.A., Francis D.P. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int. J. Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Gao J.Q., Xie Y., Yang W., Zheng J.P., Liu Z.J. Effects of percutaneous renal sympathetic denervation on cardiac function and exercise tolerance in patients with chronic heart failure. Rev. Port. Cardiol. 2017;36:45–51. doi: 10.1016/j.repc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Dai Q., Lu J., Wang B., Ma G. Effect of percutaneous renal sympathetic nerve radiofrequency ablation in patients with severe heart failure. Int. J. Clin. Exp. Med. 2015;8:9779–9785. [PMC free article] [PubMed] [Google Scholar]
- 17.Esler M.D., Krum H., Sobotka P.A., Schlaich M.P., Schmieder R.E., Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt D.L., Kandzari D.E., O'Neill W.W., D'Agostino R., Flack J.M., Katzen B.T., Leon M.B., Liu M., Mauri L., Negoita M., Cohen S.A., Oparil S., Rocha-Singh K., Townsend R.R., Bakris G.L. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 19.Azizi M., Sapoval M., Gosse P., Monge M., Bobrie G., Delsart P., Midulla M., Mounier-Véhier C., Courand P.Y., Lantelme P., Denolle T., Dourmap-Collas C., Trillaud H., Pereira H., Plouin P.F., Chatellier G. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 20.Townsend R.R., Mahfoud F., Kandzari D.E., Kario K., Pocock S., Weber M.A., Ewen S., Tsioufis K., Tousoulis D., Sharp A.S.P., Watkinson A.F., Schmieder R.E., Schmid A., Choi J.W., East C., Walton A., Hopper I., Cohen D.L., Wilensky R., Lee D.P., Ma A., Devireddy C.M., Lea J.P., Lurz P.C., Fengler K., Davies J., Chapman N., Cohen S.A., DeBruin V., Fahy M., Jones D.E., Rothman M., Böhm M. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 21.Azizi M., Schmieder R.E., Mahfoud F., Weber M.A., Daemen J., Davies J., Basile J., Kirtane A.J., Wang Y., Lobo M.D., Saxena M., Feyz L., Rader F., Lurz P., Sayer J., Sapoval M., Levy T., Sanghvi K., Abraham J., Sharp A.S.P., Fisher N.D.L., Bloch M.J., Reeve-Stoffer H., Coleman L., Mullin C., Mauri L. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 22.Kandzari D.E., Böhm M., Mahfoud F., Townsend R.R., Weber M.A., Pocock S., Tsioufis K., Tousoulis D., Choi J.W., East C., Brar S., Cohen S.A., Fahy M., Pilcher G., Kario K. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad Y., Francis D.P., Bhatt D.L., Howard J.P. Renal denervation for hypertension: a systematic review and meta-analysis of randomized, blinded, placebo-controlled trials. JACC Cardiovasc. Interv. 2021 doi: 10.1016/j.jcin.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Gulati R., Raphael C.E., Negoita M., Pocock S.J., Gersh B.J. The rise, fall, and possible resurrection of renal denervation. Nat. Rev. Cardiol. 2016;13:238–244. doi: 10.1038/nrcardio.2016.1. [DOI] [PubMed] [Google Scholar]
- 25.Kiuchi M.G., Esler M.D., Fink G.D., Osborn J.W., Banek C.T., Böhm M., Denton K.M., DiBona G.F., Everett THt, Grassi G., Katholi R.E., Knuepfer M.M., Kopp U.C., Lefer D.J., Lohmeier T.E., May C.N., Mahfoud F., Paton J.F.R., Schmieder R.E., Pellegrino P.R., Sharabi Y., Schlaich M.P. Renal denervation update from the international sympathetic nervous system summit: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73:3006–3017. doi: 10.1016/j.jacc.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp T.E., 3rd, Polhemus D.J., Li Z., Spaletra P., Jenkins J.S., Reilly J.P., White C.J., Kapusta D.R., Lefer D.J., Goodchild T.T. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J. Am. Coll. Cardiol. 2018;72:2609–2621. doi: 10.1016/j.jacc.2018.08.2186. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H., Iwanaga Y., Miyaji Y., Yamamoto H., Miyazaki S. Renal denervation mitigates cardiac remodeling and renal damage in Dahl rats: a comparison with β-receptor blockade. Hypertens. Res. 2016;39:217–226. doi: 10.1038/hr.2015.133. [DOI] [PubMed] [Google Scholar]
- 28.Ott C., Mahfoud F., Schmid A., Toennes S.W., Ewen S., Ditting T., Veelken R., Ukena C., Uder M., Böhm M., Schmieder R.E. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J. Hypertens. 2015;33:1261–1266. doi: 10.1097/HJH.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Chen W., Lai Y., Du H., Wang Z., Xu Y., Ling Z., Fan J., Xiao P., Zhang B., Wang J., Gyawali L., Zrenner B., Woo K., Yin Y. Selective renal denervation guided by renal nerve stimulation in canine. Hypertension (Dallas, Tex : 1979) 2019;74:536–545. doi: 10.1161/HYPERTENSIONAHA.119.12680. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrino P.R., Zucker I.H., Chatzizisis Y.S., Wang H.J., Schiller A.M. Quantification of renal sympathetic vasomotion as a novel end point for renal denervation. Hypertension (Dallas, Tex : 1979) 2020;76:1247–1255. doi: 10.1161/HYPERTENSIONAHA.120.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng J., Chen C., Zhou X., Qian W., Shan Q. Influence of renal sympathetic denervation in patients with early-stage heart failure versus late-stage heart failure. Int. Heart J. 2018;59:99–104. doi: 10.1536/ihj.16-413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.