Abstract
Background:
Hepatitis C (HCV) is the most common infectious disease among people who inject drugs (PWID). Engaging PWID in harm reduction services, such as syringe service programs (SSPs), is critical to reduce HCV and HIV transmission. Additionally, testing for HIV and HCV among PWID is important to improve diagnosis and linkage to care. On March 1, 2018, Florida’s only legal SSP implemented bundled opt-out HIV/HCV testing at enrollment. We aimed to examine the differences in HIV/HCV testing uptake before and after the implementation of the opt-out testing policy.
Methods:
Multivariable logistic regression was used to assess predictors of accepting HIV/HCV tests, controlling for opt-in and opt-out policy. Monthly estimates of the percent of participants accepting an HIV test, HCV test, or both were generated. Interrupted Time Series (ITS) analysis evaluated the immediate policy impact on level of uptake and trend in uptake over time for bundled HIV/HCV testing before and after the opt-out testing policy.
Results:
The total study period was 37 months between December 2016–January 2020 with 512 SSP participants 15 months prior and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing. Significant predictors of accepting both HIV/HCV tests were cocaine injection (aOR = 2.36), self-reported HIV positive status (aOR = 0.39) and self-reported HCV positive status (aOR = 0.27). Based on the ITS results, there was a significant increase in uptake of HIV/HCV testing by 42.4% (95% CI: 26.2%–58.5%, p < 0.001) immediately after the policy change to opt-out testing.
Conclusion:
Bundled opt-out HIV/HCV testing substantially increased the percentage of SSP clients who received HIV and HCV rapid tests at enrollment into the program, and the effect remained stable across the 22 months post opt-out testing policy. Future investigation must assess PWID-level perspective of testing preferences and examine whether this testing approach improves HIV/HCV detection among PWID previously unaware of their status.
Keywords: Syringe Services Programs, HIV/HCV Testing, Opt-out Testing
Introduction
People who inject drugs (PWID) remain disproportionately affected by blood-borne infections, such as human immunodeficiency virus (HIV) and Hepatitis C (HCV), which contribute to increased morbidity and mortality among this population (Degenhardt et al., 2016). There are an estimated 15 million PWID globally with approximately 17% living with HIV and 52% HCV antibody reactive (Degenhardt et al., 2017). In addition, there are approximately 2.3 million HIV/HCV co-infections worldwide, with the majority (1.3 million) among PWID (Platt et al., 2016). In 2018, PWID accounted for approximately 9% of all new HIV diagnoses in the United States (CDC, 2019), and an increasing number of jurisdictions have reported HIV outbreaks among this population (Alpren et al., 2020; Golden et al., 2019; Peters et al., 2016; Samoff et al., 2020; Tookes et al., 2019). In addition, reported cases of acute HCV infection have increased nationally, with injection drug use as the primary risk factor for disease transmission (CDC Hepatitis Report 2019). Due to the syndemic nature of HIV, HCV, and opioid use disorder (Perlman & Jordan, 2018), PWID are a key population to engage in HIV and HCV prevention, testing, and linkage to treatment services in order to work towards HIV and HCV elimination.
Recent research has demonstrated missed opportunities with HIV and HCV testing among PWID in traditional healthcare settings, with approximately 90% of PWID not receiving an HIV or HCV test upon clinical encounter in the US (Bull-Otterson et al., 2020). Different venues that tailor services towards PWID, like syringe service programs (SSPs), may be more suitable to engage this population in HIV/HCV testing and cost-effective (Manca, Robinson, Dillon, & Boyd, 2020) than other settings. SSPs are community-based programs that provide health-related services to PWID, including access to new syringes and other injection equipment, HIV and HCV testing, and referrals to treatment for substance use disorder. With advancements in point-of-care (POC) rapid antibody testing for HIV and HCV (Khuroo, Khuroo, & Khuroo, 2015; Shivkumar, Peeling, Jafari, Joseph, & Pai, 2012), integrating testing services at SSPs has the potential to increase identification of new infections and facilitate improvements in the HIV and HCV care continuums. In addition, delivery of HCV rapid testing is acceptable to PWID (Latham et al., 2019) and simultaneous, bundled HIV/HCV testing may improve linkage to care outcomes (Bottero et al., 2015).
A recent study assessing the availability of HIV/HCV testing at SSPs determined that the majority of SSP sites sampled in the U.S. offered on-site HIV/HCV testing, but uptake of HIV/HCV testing was low with only 15%–17% of SSP participants accepting testing services on-site (Behrends et al., 2018). Population-level analysis has also demonstrated low HIV testing uptake (Cooley et al., 2016) and potentially a decline in HIV testing among PWID (Burt, Tinsley, & Glick, 2017), suggesting that simply providing access to testing may not translate to widespread testing.
In order to streamline the identification of HIV infection in the U.S., the Centers for Disease Control and Prevention (CDC) has recommended that health care providers adopt an “opt-out” testing approach (Galletly, Pinkerton, & Petroll, 2008). Opt-out, routine HIV screening is highly effective at making new HIV diagnoses (CDC, 2020b) and reduces stigma associated with HIV testing (Young, Monin, & Owens, 2009). With new HCV testing recommendations from the U.S. Preventive Services Task Force (Chou et al., 2020), healthcare providers, such as emergency departments (Grant et al., 2020) and federally qualified health centers (Coyle et al., 2019), have integrated routine opt-out testing for both HIV and HCV. However, current studies examining the effectiveness of opt-out testing have lacked appropriate comparators to estimate intervention effects. In addition, existing interventions to increase uptake of HCV testing among PWID have been limited to on-site testing and dried blood spot (DBS) models, with the majority of studies being conducted within hospital settings (Bajis et al., 2017) and few studies examining opt-out testing, including SSPs (Zhou et al., 2015).
To the best of our knowledge, no studies have examined HIV and HCV testing policies, specifically providing opt-out routine bundled HIV/HCV testing, at SSPs serving PWID. This study aims to assess the predictors of bundled HIV/HCV testing uptake at an SSP and to examine the impact of an opt-out HIV/HCV screening policy on HIV and HCV testing uptake.
Methods
Human subjects
This study was determined not to be human subjects research by the Institutional Review Board of the University of Miami (IRB #20200408) due to the use of anonymous program data as part of routine pilot program evaluation.
Study setting
Programmatic data collected at the IDEA SSP fixed location in Miami, FL was used for this analysis. The IDEA SSP remains the first and only legal SSP in Florida, administered by the University of Miami Miller School of Medicine, and primarily serves residents of Miami-Dade County.
Study population and period
Data were collected at the fixed SSP location between December 2016 and January 2020 as part of routine SSP operations. Clients are assigned a unique identifier upon enrollment into the SSP that prevents duplication of individuals. A detailed description of enrollment data collected at the IDEA SSP is presented in (Bartholomew et al., 2020). Data were stratified into opt-in policy (December 1, 2016-February 28, 2018) and opt-out policy (March 1, 2018-January 31, 2020) periods. Based on the new HIV/HCV opt-out testing implementation date, there were 15 months of the opt-in testing policy and 22 months of the opt-out testing policy.
Data collection
HIV and HCV testing are offered at both enrollment and routinely every three months thereafter; however, only enrollment data were used for this analysis. All assessments were administered in face-to-face interviews in a confidential setting, and no personal identifying information was collected, by state statute. Data were managed by the study team, and assessment data were collected and monitored using REDCap® software (Harris et al., 2009), an online electronic data collection system.
Measures
Socio-demographics
Socio-demographic measures used in this analysis included age cat (categorized to 18–29, 30–49, and older than 50), reported biological sex at birth (male/female), reported race/ethnicity (categorized into Non-Hispanic White, Non-Hispanic Black, and Hispanic), educational attainment (dichotomized to less than or equal to high school education/greater than high school education), annual income (dichotomized to <$14,999/>$15,000 per year), and housing status (currently experiencing homelessness/not homeless).
Drug use/Risk behaviors
Measures used in this analysis were all self-reported for the past 30 days and included drugs injected (heroin, cocaine, methamphetamine, heroin/cocaine), sharing of injection equipment (i.e. syringes, cookers, water, cottons) (any/none), reuse of syringes (any/none), and number of injections per day (dichotomized to <5 injections/≥5 injections, based on even distribution of two categories). Sexual risk measures included condomless sex (yes/no) and men who have sex with men (yes/no).
HIV/HCV testing
During baseline enrollment assessments, participants were asked to self-report their HIV and HCV status based on their most recent test. During the opt-in policy phase, comprehensive syringe services were offered to the participant and s/he could request HIV/HCV testing (i.e. opt-in) in addition to syringe exchange, naloxone, wound care and a variety of other services. Under the new opt-out testing policy, participants were informed that HIV/HCV testing was part of routine care at the SSP; however, they were allowed to decline testing (i.e. opt-out). If clients accepted the testing, both results of each test were recorded (reactive/negative). The point-of-care tests offered were OraQuick Advance® Rapid HIV-1/2 Antibody test or Chembio SURE CHECK® HIV 1/2 Assay for HIV and OraQuick® HCV Rapid Antibody Test for HCV, with both tests using a blood sample collected via fingerstick. Results were reported to the participant immediately with appropriate post-test counseling and education. For those who tested reactive, active linkage to care was offered.
Outcome measure
The measure of interest for this present analysis was individual-level uptake of both HIV/HCV rapid tests and the monthly percentage of participants accepting both HIV/HCV rapid tests. Participants who accepted only HIV tests, only HCV tests, or accepted no tests were grouped together and compared to participants who accepted both tests. Individual-level and monthly aggregate data were divided into opt-in and opt-out periods with an index date of March 1, 2018.
Statistical analysis
Frequency distributions stratified by opt-in and opt-out testing period were calculated to describe the overall sample characteristics. Persons chi-squared tests were used to examine the differences between HIV and HCV testing uptake and test results among those self-reporting HIV positive, HIV negative, HCV positive, and HCV negative. Of note, those reporting HIV negative or unknown status were grouped, and HCV negative or unknown status were grouped, together reflecting those participants at risk for HIV and HCV infection, respectively. Bivariate and multivariable logistic regression models were used to assess the unadjusted and adjusted associations between socio-demographics, drug use, injection risk behaviors, sexual risk behaviors, and self-reported HIV/HCV serostatus and uptake of bundled HIV/HCV tests, at baseline enrollment, controlling for testing opt-in and opt-out testing policy period. Additional sub-analysis was performed examining those accepting an HIV test and HCV test, separately. The distribution and correlations between independent variables were assessed to reduce multicollinearity. All variables were retained in the final model, and significance testing was set at alpha <0.05.
Finally, to estimate the impacts of the opt-out bundled testing policy on HIV/HCV testing uptake, we conducted an interrupted time series analysis (ITS) (Bernal, Cummins, & Gasparrini, 2017). ITS is a type of quasi-experimental study design that enables control of pre-existing trends by comparing the observed outcome post-policy implementation with the expected outcomes if the policy had not been implemented. The percentage of participants accepting both HIV/HCV tests was aggregated by month and stratified by the index date.
A visual inspection of the data (i.e. scatter plot) was used to understand the trend in the data (Jandoc, Burden, Mamdani, Lévesque, & Cadarette, 2015). Next, we used a segmented regression analysis (Bernal et al., 2017; Wagner, Soumerai, Zhang, & Ross-Degnan, 2002) using PROC AUTOREG (Maradiaga, Pujula, & Zapata, 2013) to assess the immediate impact and long-term impacts of the opt-out testing policy on uptake of HIV/HCV testing. The regression model included opt-in testing trend, level change in the outcome variable immediately after implementation of opt-out testing, and the opt-out testing trend. Durbin-Watson tests were used to assess autocorrelation (order 1–4) over time (Ali & Sharma, 1993; Durbin & Watson, 1950), and Engle-Granger cointegration test was used to assess stationarity (Engle & Granger, 1987). Based on the non-significant Durbin-Watson test, we concluded that there was little evidence of the errors in the model being correlated and the Engle-Granger test rejected non-stationarity. All statistical analyses were performed using SAS 9.4 (SAS, Cary, NC) and all significance tests were set at alpha<0.05.
Results
In total, 37 months of data were included from 1,059 individuals; 512 participants were included during 15 months of opt-in testing and 547 participants during 22 months of opt-out testing. Overall, there were an average of 29 observations per month. Socio-demographic, risk behaviors and uptake of testing of the study population during opt-in and opt-out testing are presented in Table 1. The median age of the overall sample was 37 years (IQR = 30–45). The majority of the sample were male (75.1%) and reported injection of heroin (74.1%) in the previous 30 days. The proportion of participants accepting both HIV/HCV tests was 33.1% (IQR = 20.0%–42.4%) during opt-in testing and 91.3% (IQR = 87.2%–100%) during opt-out testing.
Table 1.
Descriptive Statistics of Sample during opt-in and opt-out testing policy (N1,059)
| Characteristic | Total Sample (N = 1,059) | Opt-In HIV/ HCV Testing (N = 512) | Opt-Out HIV/ HCV Testing (N = 547) |
|---|---|---|---|
| Age (n, %) | |||
| 18–29 | 271 (25.6) | 124 (24.2) | 147 (26.9) |
| 30–49 | 626 (59.1) | 302 (59.0) | 324 (59.2) |
| 50 and older | 162 (15.3) | 86 (16.8) | 76 (13.9) |
| Sex (n, %) | |||
| Male | 787 (75.1) | 391 (76.4) | 396 (73.9) |
| Female | 261 (24.9) | 121 (23.6) | 140 (26.1) |
| Race/Ethnicity (n, %) | |||
| Non-Hispanic White | 561 (55.0) | 283 (57.5) | 278 (52.6) |
| Non-Hispanic Black | 54 (5.3) | 23 (4.7) | 31 (5.9) |
| Hispanic | 406 (39.8) | 186 (37.8) | 220 (41.6) |
| Educational attainment (n, %) | |||
| ≤High School | 509 (48.9) | 257 (50.7) | 252 (47.1) |
| >High School | 533 (51.2) | 250 (49.3) | 283 (52.9) |
| Annual income (n, %) | |||
| ≤$14,999 | 510 (52.7) | 226 (49.2) | 277 (54.5) |
| >$15,000 | 457 (47.3) | 233 (50.8) | 231 (45.5) |
| Currently homeless (n, %) | |||
| Yes | 367 (36.6) | 188 (40.6) | 179 (33.2) |
| No | 636 (63.4) | 275 (59.4) | 361 (66.8) |
| Drugs injected in the previous 30 days (n, %) | |||
| Heroin | 785 (74.1) | 426 (83.2) | 359 (65.6) |
| Cocaine | 290 (27.4) | 123 (24.0) | 167 (30.5) |
| Methamphetamine | 176 (16.6) | 60 (11.7) | 116 (21.2) |
| Speedball | 189 (17.9) | 99 (19.3) | 90 (16.5) |
| Share injection equipment in the previous 30 days (n, %) | |||
| Any | 325 (30.7) | 182 (35.6) | 143 (26.1) |
| None | 734 (69.3) | 330 (64.4) | 404 (73.9) |
| Reuse syringes in the previous 30 days (n, %) | |||
| Any | 671 (68.3) | 342 (75.3) | 329 (62.2) |
| None of the time | 312 (31.7) | 112 (24.7) | 200 (37.8) |
|
Number of injections per day in previous
30 days (n, %) | |||
| <5 | 587 (60.1) | 284 (57.5) | 303 (62.9) |
| ≥5 | 389 (39.9) | 210 (42.5) | 179 (37.1) |
| Male who has sex with men (MSM) (n, %) | |||
| Yes | 117 (11.1) | 48 (9.4) | 69 (12.6) |
| No | 942 (88.9) | 464 (90.6) | 478 (87.4) |
| Condomless sex in previous 30 days (n, %) | |||
| Yes | 449 (45.2) | 192 (38.1) | 257 (52.5) |
| No | 545 (54.8) | 312 (61.9) | 233 (47.5) |
| Uptake of testing (n, %) | |||
| No test accepted | 296 (28.0) | 257 (50.2) | 39 (7.1) |
| HIV test only | 79 (7.5) | 71 (13.9) | 8 (1.5) |
| HCV test only | 18 (1.70) | 17 (3.3) | 1 (0.2) |
| HIV/HCV accepted | 666 (62.9) | 167 (32.6) | 499 (91.2) |
Table 2 provides descriptive statistics of clients who self-reported HIV or HCV positive/negative and accepted HIV/HCV tests, with the results of those tests if accepted. Overall, 55.3% of self-reported HIV positive participants and 49.4% of self-reported HCV positive participants accepted bundled HIV/HCV tests. However, only 7% of self-reported HIV positive clients accepted testing in the opt-in testing period, and 93.1% accepted testing in the opt-out testing period (P < 0.001). Similar results were seen for self-reported HCV positive participants; 13.0% during opt-in testing and 91.3% in the opt-out testing policy period (P < 0.001). There were 7 (0.79%) newly identified HIV positive participants and 76 (13.4%) newly identified HCV positive participants discovered in the overall sample (those who self-reported negative or unknown status and tested positive). The majority of those self-reporting HIV or HCV negative or unknown status who tested positive were identified in the opt-out testing period; however, there were no statistically significant differences between testing policy periods. In addition, there were 8 (7.8%) who self-reported HIV positive but tested HIV non-reactive on the rapid point-of-care test and 22 (6.4%) participants who self-reported HCV positive but tested HCV non-reactive on the point-of-care test.
Table 2.
Self-report HIV and HCV status, HIV and HCV testing uptake, and HIV and HCV test results stratified by opt-in and opt-out testing policy period.
| Characteristic | Total Sample (N = 1,059) | Opt-In HIV/HCV Testing (N = 512) | Opt-Out HIV/HCV Testing (N = 547) | P-value |
|---|---|---|---|---|
| Self-report (SR) HIV positive | 103 (10.4) | 45 (9.4) | 58 (11.4) | 0.29 |
| (N = 103) | (N = 45) | (N = 58) | ||
| Accepted HIV test | 57 (55.3) | 3 (6.7) | 54 (93.1) | <0.001 |
| Tested HIV positive | 49 (47.6) | 2 (4.4) | 47 (81.0) | 0.32 |
| Tested HIV negative | 8 (7.8) | 1 (2.2) | 7 (12.1) | — |
| Self-report (SR) HIV negative or unknown | 885 (89.6) | 435 (90.6) | 450 (88.6) | 0.29 |
| (N = 885) | (N = 435) | (N = 450) | ||
| Accepted HIV test | 633 (71.5) | 213 (49.0) | 420 (93.3) | <0.001 |
| Tested HIV positive | 7 (0.79) | 1 (0.23) | 6 (1.3) | 0.28 |
| Tested HIV negative | 620 (70.1) | 208 (47.8) | 412 (91.6) | — |
| Self-report (SR) HCV positive | 346 (37.9) | 185 (42.1) | 161 (34.1) | 0.01 |
| (N = 346) | (N = 185) | (N = 166) | ||
| Accepted HCV test | 171 (49.4) | 24 (13.0) | 147 (91.3) | <0.001 |
| Tested HCV positive | 144 (41.6) | 21 (11.4) | 123 (76.4) | 0.49 |
| Tested HCV negative | 22 (6.4) | 2 (1.1) | 20 (12.4) | — |
| Self-report (SR) HCV negative or unknown | 566 (62.1) | 255 (57.9) | 311 (65.9) | 0.01 |
| (N = 566) | (N = 255) | (N = 311) | ||
| Accepted HCV test | 401 (70.9) | 111 (43.5) | 290 (93.3) | <0.001 |
| Tested HCV positive | 76 (13.4) | 27 (10.6) | 49 (15.8) | 0.07 |
| Tested HCV negative | 325 (57.4) | 84 (32.9) | 241 (77.5) | — |
Note. Boldedp-value represent significance at alpha <0.05
Predictors of bundled HIV/HCV testing uptake
Results of the multivariable regression model for bundled testing uptake are presented in Table 3. Based on the final regression model, controlling for testing policy period, self-reported cocaine injection in the previous 30 days (aOR = 2.38, 95% CI: [1.41–4.01]) was significantly associated with higher odds of bundled HIV/HCV testing uptake. In addition, self-reported HIV positive status (aOR = 0.33, 95% CI: [0.13–0.88]) and self-reported HCV positive status (aOR = 0.28, 95% CI: [0.17–0.46]) were significantly associated with lower odds of bundled HIV/HCV testing uptake.
Table 3.
Bivariate and multivariable logistic regression results for accepting both HIV/HCV tests, controlling for opt-in and opt-out testing policy period
| Characteristic | Uptake of HIV/HCV Testing (N = 666) | ||
|---|---|---|---|
| n, %a | OR, 95% CI | aOR, 95% CI | |
| Age | |||
| 18–29 | 153 (66.5) | 1.67 (1.12, 2.53) | 1.38 (0.64, 2.98) |
| 30–49 | 425 (63.7) | 1.48 (1.04, 2.09) | 1.70 (0.90, 3.22) |
| 50 and older | 88 (54.3) | Ref | Ref |
| Sex | |||
| Male | 486 (61.8) | 0.88 (0.66, 1.18) | 0.95 (0.56, 1.61) |
| Female | 169 (64.8) | Ref | Ref |
| Race/Ethnicity | |||
| Hispanic | 262 (64.5) | 1.14 (0.87, 1.49) | 1.26 (0.78, 2.01) |
| Non-Hispanic Black | 35 (64.8) | 1.15 (0.64, 2.07) | 1.37 (0.49, 3.86) |
| Non-Hispanic White | 345 (61.5) | Ref | Ref |
| Educational Attainment | |||
| ≤High School | 313 (61.5) | 0.89 (0.69, 1.14) | 0.86 (0.55, 1.35) |
| >High School | 343 (64.4) | Ref | Ref |
| Annual Income | |||
| ≤$14,999 | 336 (65.9) | 1.20 (0.92, 1.56) | 1.28 (0.80–2.06) |
| >$15,000 | 282 (61.7) | Ref | Ref |
| Currently Homeless | |||
| Yes | 228 (62.1) | 0.84 (0.65, 1.10) | 1.14 (0.69–1.88) |
| No | 420 (66.0) | Ref | Ref |
| Drugs injected in the previous 30 days | |||
| Heroin | 473 (60.3) | 0.64 (0.47, 0.86) | 1.41 (0.75–2.65) |
| Cocaine | 210 (72.4) | 1.80 (1.34, 2.42) | 2.38 (1.41–4.01) |
| Methamphetamine | 126 (71.6) | 1.60 (1.12, 2.28) | 1.11 (0.52–2.38) |
| Speedball | 108 (57.1) | 0.75 (0.54, 1.03) | 0.57 (0.30–1.06) |
| Share works in the previous 30 days | |||
| Any | 196 (60.3) | 0.85 (0.65, 1.12) | 1.22 (0.75–1.98) |
| None of the time | 470 (64.0) | Ref | Ref |
| Reuse syringes in the previous 30 days | |||
| Any | 419 (62.4) | 0.71 (0.53, 0.94) | 0.99 (0.59–1.64) |
| None of the time | 219 (70.2) | Ref | Ref |
| Amount inject per day in previous 30 days | |||
| ≥5 | 231 (59.4) | 0.78 (0.60, 1.01) | 0.75 (0.47, 1.20) |
| <5 | 383 (65.3) | Ref | Ref |
| Male who has sex with men (MSM) | |||
| Yes | 79 (67.5) | 1.26 (0.84, 1.89) | 1.53 (0.59, 3.95) |
| No | 587 (62.3) | Ref | Ref |
| Self-report HIV positive | 56 (54.4) | 0.70 (0.46, 1.05) | 0.33 (0.13–0.88) |
| Self-report HCV positive | 169 (48.8) | 0.42 (0.32, 0.56) | 0.28 (0.17–0.46) |
Note. Bolded unadjusted (OR) and adjusted odds ratios (aOR) represent significance at P < 0.05
Represents the proportion of each characteristic accepting HIV/HCV testing
Based on the sub-analysis examining the correlates of HIV testing uptake and HCV testing uptake separately, self-reported HIV positive status was significantly associated with a lower odds of HIV testing uptake (aOR = 0.28, 95% CI: [0.12–0.69]), and self-reported HCV positive status was significantly associated with a lower odds of HCV testing uptake (aOR = 0.27, 95% CI: [0.17–0.44]). Self-reported HIV positive status was not significantly associated with HCV testing uptake (aOR = 0.58, 95% CI: [0.25–1.26]) (Supplemental Table 2).
Impact of Opt-out bundled HIV/HCV testing on uptake of HIV/HCV tests
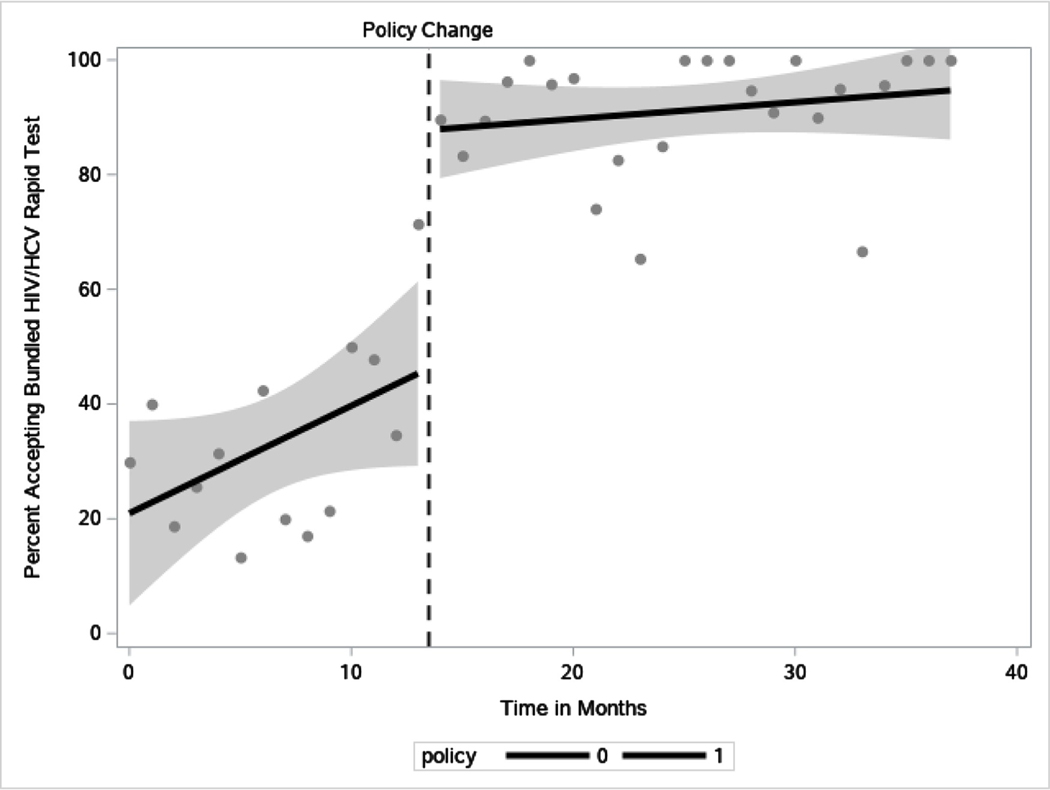
Based on the segmented regression results (Table 4, Fig. 1), the trend line during opt-in testing for uptake of bundled testing increased by 1.87% per month (95% CI: 0.25%–3.5%, P0.03). The trend line for uptake of bundled testing during the opt-out testing period and the change in the slope of the trend line from opt-in to opt-out were non-significant. However, there was a significant increase in uptake of HIV/HCV testing by 42.4% (95% CI: 26.2%−58.5%, P<0.001) immediately after the implementation of opt-out testing.
Table 4.
Results of Segmented Regression Analysis on Uptake of HIV/HCV tests
| Durbin-Watson Tests | Order | DW | Pr<DW | Pr>DW |
|---|---|---|---|---|
| 1 | 1.772 | 0.108 | 0.892 | |
| 2 | 1.883 | 0.261 | 0.739 | |
| 3 | 2.068 | 0.568 | 0.432 | |
| 4 | 2.331 | 0.889 | 0.111 | |
| Engle-Granger Test | Type | Lags | Tau | Pr<Tau |
| Single mean | 0 | −5.437 | 0.001 | |
| Regression | Coefficients | 95% CI | t-value | p-value |
| Intercept | 20.96 | 8.55, | 3.43 | 0.002 |
| 33.38 | ||||
| Opt-in testing policy trend | 1.87 | 0.25, 3.50 | 2.34 | 0.030 |
| Immediate effect of opt-out | 42.35 | 26.21, | 5.33 | <0.001 |
| testing policy | 58.49 | |||
| Change in trend during opt-out | −1.58 | −3.35, 0.20 | −1.81 | 0.080 |
| testing |
Fig. 1.
Percent uptake of bundled HIV/HCV testing by policy period.
Discussion
In this study of PWID participants at an urban U.S. SSP, we found that uptake of baseline HIV and HCV screening increased slowly over time with opt-in testing policies in place, but that bundled HIV/HCV testing drastically increased testing uptake by 42% after implementation of an opt-out testing policy. Internationally, there is rich literature on interventions to enhance the HCV care continuum among people who inject drugs in diverse settings (Cullen et al., 2006; Evon et al., 2011; Hagedorn et al., 2007; Hickman et al., 2008; Ho et al., 2015; Lubega, Agbim, Surjadi, Mahoney, & Khalili, 2013; Merchant et al., 2014; Rosenberg et al., 2010), however, there are no studies of which we are aware investigating the impact of an opt-out testing strategy to enhance HIV/HCV screening specifically at SSPs. While previous studies have been conducted in emergency departments, substance use disorder clinics, prison, and general practice, the literature on HIV/HCV screening at low barrier venues such as SSPs is lacking (Behrends et al., 2018; Burt et al., 2017; Cooley et al., 2016). Improving HIV and HCV testing among PWID is necessary to reach the World Health Organization’s targets for HIV and HCV elimination (WHO, 2020). Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population. Worldwide, increased HIV screening among PWID is necessary to achieve UNAIDS 90–90-90 targets (UNAIDS, 2020). Likewise, the first pillar of Ending the HIV Epidemic: A Plan for America is to diagnose all people living with HIV as early as possible (Fauci, Redfield, Sigounas, Weahkee, & Giroir, 2019). Increasing screening in high incidence communities such as PWID should be implemented with a low stigma approach in trusted settings such as SSPs. This study supports that opt-out testing can improve uptake within a harm reduction framework.
The jurisdictions impacted by the Ending the HIV Epidemic plan include geographic hotspots where greater than 50% of new HIV infections occur in the U.S. Targets largely include southern states where SSPs have long been prohibited due to a carceral approach to drug policy. There appears to be a shift in these anti-public health policies with Florida, Georgia, North Carolina, and Louisiana, among others, recently passing legislation to explicitly authorize SSPs (LawAtlas, 2019). SSPs are touted as one of the pillars of President’s plan to end HIV in the U.S. by preventing infection (Fauci et al., 2019), so there is potential over the next decade to further drive policy change in states late to adopt SSPs by highlighting the crucial role SSPs play in providing not only access to HIV/HCV testing, but an acceptable venue for PWID (Barocas, Linas, Kim, Fangman, & Westergaard, 2016). Additionally, given the potential effect of SSPs in preventing HCV acquisition (Platt et al., 2018), access to needles and syringes will be critical to reduce the risk of HCV reinfection following HCV treatment and to achieve HCV elimination in the U.S. (Fraser et al., 2019). Southern states in the U.S. also have the most restrictive HCV treatment policies, many with “sobriety” requirements for the treatment of HCV (CHLPI, 2020). The evolving approach to injection drug use and its infectious sequelae in the U.S. should promote SSPs as an important venue not only for the prevention of HIV and HCV acquisition (Aspinall et al., 2014), but also for diagnosis and treatment.
Miami, Florida has the highest HIV incidence of any municipality in the U.S. (CDC, 2019), so improving testing uptake among this high risk population is especially crucial for improving HIV and HCV detection and linkage to care along the care continuum. Implementation of this opt-out HIV/HCV testing policy has already directly facilitated the detection of an HIV transmission network among our participants, and the subsequent public health investigation and response that followed (Tookes et al., 2019). Our work shows that a small unobtrusive change in SSP policy can lead to major downstream effects on community health of PWID.
In our cohort, participants who injected cocaine in the previous 30 days, compared to other drugs, had higher odds of accepting HIV/HCV testing. In general, people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs compared to people who use non-stimulant drugs (Allen et al., 2020; Bobashev, Zule, Osilla, Kline, & Wechsberg, 2009; El-Bassel, Wechsberg, & Shaw, 2012). These behaviors, which are associated with increased HIV and other STI incidence, may lead people who use stimulants to be more accepting of HIV screening. In contrast, the most direct surrogate of HIV/HCV risk—shared equipment—was not associated with increased testing uptake. Further qualitative work will be important to understand how PWID’s understanding of risk and overall access to testing outside of the SSP influences their uptake of HIV/HCV screening.
While routine opt-out HIV/HCV testing at the IDEA SSP has been successful in increasing the number of tests performed and identifying new HIV and HCV diagnoses, we remain vigilant in trying to balance participant autonomy—avoiding coercive policies—with improving community health. As noted in our results, participants did continue to decline testing during the opt-out period, but at a lower rate than previously, indicating that many continued to feel comfortable with this opt-out approach. Furthermore, we showed that the trend line for uptake of HIV/HCV testing (Fig. 1) was steadily and consistently increasing naturally even before the policy change, indicating a growing community understanding and uptake for testing. Nonetheless, further research is warranted to identify how opt-out testing policies are perceived by participants and whether it has any negative effect on their trust and engagement with the SSP.
Also notable in these data were the high prevalence of undiagnosed HIV and HCV infection. Knowledge of HIV/HCV status has been shown to decrease syringe sharing among PWID (Bryant, 2014; Spelman et al., 2015). Screening for HIV/HCV is indicated under the current U.S. Preventative Services Task Force recommendations, and repeat screening is “reasonable” in patients with additional sexual or injection drug use related risk, in addition to patients in a high prevalence setting (USPSTF, 2020). Efforts to increase testing in communities at risk are especially justifiable in Miami given the high rates of HIV infection. Recent research has shown that in urban areas such as Miami, SSPs and opt-out testing strategies are indeed cost-effective HIV prevention strategies, whereas other more well-resourced urban locations may have different priorities (Krebs et al., 2020). Interestingly, there were people who self-reported HIV/HCV positive but tested negative, illustrating the importance of routine screening in a high incidence population, coupled with post-test counseling and education on the meaning of the test results and how to prevent infection in the future.
In areas where SSPs have been absent, like much of the southern U.S., the center of the US HIV epidemic (Reif, Safley, McAllaster, Wilson, & Whetten, 2017), a more aggressive testing strategy has the advantage of identifying people living with HIV and HCV who need access to care and have traditionally been disenfranchised. Injection drug use poses additional challenges for HCV infection. For PWID to receive life-saving HCV treatment, many state Medicaid programs, including Florida, require negative drug screens and drug abstinence agreements in order to gain access to antiviral therapies (CHLPI, 2020). These policies are misguided and contrast with extensive clinical data showing successful HCV treatment outcomes among PWID, even among those who continue to use drugs (Aspinall et al., 2013; Grebely et al., 2018; Hajarizadeh et al., 2018). Despite challenges in the treatment of HCV, identification of HCV could lead to decrease in high risk injection practices (Bryant, 2014; Spelman et al., 2015) and thereby mitigate transmission in the community.
In the U.S., many jurisdictions late to adopt SSPs have onerous restrictions on funding SSPs, such as Florida, which by statute, prohibits the use of state or local funds (FloridaLegislature, 2020). Additionally, accessing federal funding requires the Department of Health to apply to the CDC for a Determination of Need, making funding of SSPs extraordinarily challenging in some locations. In Florida, the 2016 Infectious Disease Elimination Act authorized the pilot SSP in Miami effective July 1, 2016. Due to funding restrictions and requirements, the program did not open until December 1, 2016. In addition, the state of Florida did not obtain a Determination of Need from the CDC until February 1, 2020 (CDC, 2020a). Longitudinal investment from programs like Gilead FOCUS, a program that seeks to increase testing for HIV/HCV and linkage to care (Gilead, 2020), can be essential to setting up and hiring vital linkage to care staff at SSP sites to address the second pillar of the Ending the HIV Epidemic strategy: treat (Fauci et al., 2019). In Florida, statute requires anonymous HIV/HCV testing at SSPs; however, the Department of Health will only support and fund confidential testing. While controversial, implementation of routine opt-out HIV/HCV testing through foundations and corporate philanthropy can be necessary for SSPs to provide critical medical services, like testing, in additional to the distribution of injection equipment.
Limitations
There are several limitations to this study. First, the data on HIV and HCV testing history relies on self-report, which can be subject to recall and social desirability bias. However, interviews were conducted by SSP staff in confidential settings. For the purpose of this analysis, unknown and negative status for HIV and HCV infection were grouped together, in order to reflect testing in participants at risk for infection. Second, the data was limited to the fixed SSP site, but over 200 participants enrolled into the IDEA SSP have done so via the mobile unit or street level outreach. Due to the lack of confidential settings, less testing occurs among these participants; however, innovative testing strategies must be implemented to engage harder-to-reach populations in testing (Iyengar, Kravietz, Bartholomew, Forrest, & Tookes, 2019). As a real-world before/after study, we cannot prove with certainty that the testing policy change fully accounts for the increase in testing in the opt-out period. However, interrupted time series analysis is a robust method to analyze this policy change. Lastly, the number of observations per monthly aggregated data point were relatively small. This may have led to high variability in the data, resulting in reduced power to detect changes in the outcome.
Conclusion
Implementation of bundled, routine opt-out HIV/HCV testing substantially increased the percentage of SSP participants who received HIV and HCV screening at enrollment into the program, and the effect remained stable across the 22 months of opt-out testing policy. More work needs to be done to assess PWID-level perspective of testing policy preferences and examine whether this testing approach improves HIV/HCV detection among PWID who did not previously know their status.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health, National Institutes on Drug Abuse (R01DA045713), and the National Institute on Mental Health (P30MH116867) under Award Number P30CA240139, and Frontlines of Communities in the United States, a program of Gilead Sciences, Inc. The FOCUS Program is a public health initiative that enables partners to develop and share best practices in routine blood-borne virus (HIV, HCV, HBV) screening, diagnosis, and linkage to care in accordance with screening guidelines promulgated by the U.S. Centers for Disease Control and Prevention (CDC), the U.S. Preventive Services Task Force (USPSTF), and state and local public health departments. FOCUS funding supports HIV, HCV, and HBV screening and linkage to the first medical appointment after diagnosis. FOCUS partners do not use FOCUS awards for activities beyond linkage to the first medical appointment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Gilead Sciences, Inc.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.drugpo.2020.102875.
References
- Ali MM, & Sharma SC (1993). Robustness to nonnormality of the Durbin-Watson test for autocorrelation. Journal of Econometrics, 57(1–3), 117–136. [Google Scholar]
- Allen ST, White RH, O’Rourke A, Ahmad NJ, Hazelett T, Kilkenny ME, et al. (2020). Correlates of transactional sex among a rural population of people who inject drugs. AIDS and Behavior, 24(3), 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpren C, Dawson EL, John B, Cranston K, Panneer N, Fukuda HD, et al. (2020).Opioid use fueling HIV transmission in an urban setting: an outbreak of HIV infection among people who inject drugs—massachusetts, 2015–2018. American Journal of Public Health, e1–e8 (0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al.(2013). Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clinical Infectious Diseases, 57(suppl_2), S80–S89. [DOI] [PubMed] [Google Scholar]
- Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, et al. (2014). Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: A systematic review and meta-analysis. International Journal of Epidemiology, 43(1), 235–248. [DOI] [PubMed] [Google Scholar]
- Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, & Grebely J. (2017). Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. International Journal of Drug Policy, 47, 34–46. [DOI] [PubMed] [Google Scholar]
- Barocas JA, Linas BP, Kim AY, Fangman J, & Westergaard RP (2016). Acceptability of rapid point-of-care hepatitis C tests among people who inject drugs and utilize syringe-exchange programs. Paper presented at the Open forum infectious diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew TS, Tookes HE, Bullock C, Onugha J, Forrest DW, & Feaster DJ (2020). Examining risk behavior and syringe coverage among people who inject drugs accessing a syringe services program: A latent class analysis. International Journal of Drug Policy, 78, Article 102716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends CN, Nugent AV, Des Jarlais DC, Frimpong JA, Perlman DC, & Schackman BR (2018). Availability of HIV and HCV on-site testing and treatmentat syringe service programs in the United States. Journal of Acquired Immune Deficiency Syndromes (1999), 79(2), e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal JL, Cummins S, & Gasparrini A. (2017). Interrupted time series regression for the evaluation of public health interventions: a tutorial. International Journal of Epidemiology, 46(1), 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobashev GV, Zule WA, Osilla KC, Kline TL, & Wechsberg WM (2009).Transactional sex among men and women in the south at high risk for HIV and other STIs. Journal of Urban Health, 86(1), 32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero J, Boyd A, Gozlan J, Carrat F, Nau J, Pauti M-D, et al. (2015). Simultaneous human immunodeficiency virus-hepatitis B-hepatitis C point-of-care tests improve outcomes in linkage-to-care: results of a randomized control trial in persons without healthcare coverage. Paper presented at the Open forum infectious diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J. (2014). A study of young people who inject drugs: An opportunity to decrease high risk injecting by improving knowledge about hepatitis C prevention. Vulnerable Children and Youth Studies, 9(2), 104–113. [Google Scholar]
- Bull-Otterson L, Huang Y, Zhu W, King H, Edlin BR, & Hoover KW (2020). HIV and Hepatitis C virus infection testing among commercially insured persons who inject drugs, United States,. The Journal of Infectious Diseases, 2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RD, Tinsley J, & Glick SN (2017). A decline in HIV testing among persons who inject drugs in the Seattle area, 2004–2015. JAIDS Journal of Acquired Immune Deficiency Syndromes, 75, S346–S351. [DOI] [PubMed] [Google Scholar]
- CDC. (2019). HIV Surveillance Report, 2018. [Google Scholar]
- CDC. (2020). Determination of Need for Syringe Services Programs. Retrieved from https://www.cdc.gov/ssp/determination-of-need-for-ssp.html.
- CDC. (2020). Opt-Out Screening. [Google Scholar]
- CHLPI. (2020). Hepatitis C: The State of Medicaid Access. Retrieved from https://www.chlpi.org/wp-content/uploads/2013/12/HCV-State-of-Medicaid-Access-Update-11-8-18.pdf.
- Chou R, Dana T, Fu R, Zakher B, Wagner J, Ramirez S, et al. (2020). Screening for hepatitis C virus infection in adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. [DOI] [PubMed] [Google Scholar]
- Cooley LA, Wejnert C, Spiller MW, Broz D, Paz-Bailey G, & Group NS (2016). Low HIV testing among persons who inject drugs—National HIV Behavioral Surveillance, 20 US cities, 2012. Drug and Alcohol Dependence, 165, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C, Moorman AC, Bartholomew T, Klein G, Kwakwa H, Mehta SH, & Holtzman D. (2019). The Hepatitis C virus care continuum: linkage to Hepatitis Cvirus care and treatment among patients at an urban health network, Philadelphia, PA. Hepatology, 70(2), 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen W, Stanley J, Langton D, Kelly Y, Staines A, & Bury G. (2006). Hepatitis C infection among injecting drug users in general practice: a cluster randomised controlled trial of clinical guidelines’ implementation. British Journal of General Practice, 56(532), 848–856. [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Stanaway J, Larney S, Alexander LT, Hickman M, et al. (2016). Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: Findings from the Global Burden of Disease Study 2013. The Lancet Infectious Diseases, 16(12), 1385–1398. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al.(2017). Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global Health, 5(12), e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin J, & Watson GS (1950). Testing for serial correlation in least squares regression: I. Biometrika, 37(3/4), 409–428. [PubMed] [Google Scholar]
- El-Bassel N, Wechsberg WM, & Shaw SA (2012). Dual HIV risk and vulnerabilities among women who use or inject drugs: no single prevention strategy is the answer. Current Opinion in HIV and AIDS, 7(4), 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RF, & Granger CW (1987). Co-integration and error correction: representation, estimation, and testing. Econometrica: journal of the Econometric Society, 251–276. [Google Scholar]
- Evon DM, Simpson K, Kixmiller S, Galanko J, Dougherty K, Golin C, & Fried MW (2011). A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. The American Journal of Gastroenterology, 106(10), 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Redfield RR, Sigounas G, Weahkee MD, & Giroir BP (2019). Ending the HIV epidemic: a plan for the United States. JAMA, 321(9), 844–845. [DOI] [PubMed] [Google Scholar]
- FloridaLegislature. (2020). The 2019 Florida Statutes. Retrieved from http://www.leg.state.fl.us/statutes/index.cfm?mode=View%20Statutes&SubMenu=1&App_mode= Display_Statute&Search_String=syringe+exchange&URL=0300-0399/0381/Sections/0381.0038.html.
- Fraser H, Vellozzi C, Hoerger TJ, Evans JL, Kral AH, Havens J, & Hariri S.(2019). Scaling up hepatitis C prevention and treatment interventions for achieving elimination in the United States: a rural and urban comparison. American Journal of Epidemiology, 188(8), 1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly CL, Pinkerton SD, & Petroll AE (2008). Commentary: CDC recommendations for opt-out testing and reactions to unanticipated HIV diagnoses. AIDS Patient Care and STDs, 22(3), 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead. (2020). HCV Elimination. Retrieved from https://www.gilead.com/purpose/advancing-global-health/hcv-elimination.
- Golden MR, Lechtenberg R, Glick SN, Dombrowski J, Duchin J, Reuer JR, et al.(2019). Outbreak of human immunodeficiency virus infection among heterosexual persons who are living homeless and inject drugs—Seattle, Washington, 2018. Morbidity and Mortality Weekly Report, 68(15), 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C, O’Connell S, Lillis D, Moriarty A, Fitzgerald I, Dalby L, et al. (2020). Opt-out screening for HIV, hepatitis B and hepatitis C: observational study of screening acceptance, yield and treatment outcomes. Emergency Medicine Journal, 37(2), 102–105. [DOI] [PubMed] [Google Scholar]
- Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, et al. (2018). Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. The Lancet Gastroenterology & Hepatology, 3(3), 153–161. [DOI] [PubMed] [Google Scholar]
- Hagedorn H, Dieperink E, Dingmann D, Durfee J, Ho SB, Isenhart C, et al. (2007). Integrating hepatitis prevention services into a substance use disorder clinic. Journal of Substance Abuse Treatment, 32(4), 391–398. [DOI] [PubMed] [Google Scholar]
- Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, & Grebely J. (2018). Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology, 3(11), 754–767. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009).Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman M, McDonald T, Judd A, Nichols T, Hope V, Skidmore S, & Parry J. (2008). Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial. Journal of Viral Hepatitis, 15(4), 250–254. [DOI] [PubMed] [Google Scholar]
- Ho SB, Bräu N, Cheung R, Liu L, Sanchez C, Sklar M, et al. (2015). Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clinical Gastroenterology and Hepatology, 13(11), e2003 2005–2014. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Kravietz A, Bartholomew TS, Forrest D, & Tookes HE (2019). Baseline differences in characteristics and risk behaviors among people who inject drugs by syringe exchange program modality: an analysis of the Miami IDEA syringe exchange. Harm Reduction Journal, 16(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandoc R, Burden AM, Mamdani M, Lévesque LE, & Cadarette SM (2015). Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. Journal of Clinical Epidemiology, 68(8), 950–956. [DOI] [PubMed] [Google Scholar]
- Khuroo MS, Khuroo NS, & Khuroo MS (2015). Diagnostic accuracy of point-of-care tests for hepatitis C virus infection: a systematic review and meta-analysis. PloS ONE, 10(3), Article e0121450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E, Zang X, Enns B, Min JE, Behrends CN, Del Rio C, et al. (2020). The impact of localized implementation: determining the cost-effectiveness of HIV prevention and care interventions across six United States cities. Aids, 34(3), 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham NH, Pedrana A, Doyle JS, Howell J, Williams B, Higgs P, et al. (2019). Community-based, point-of-care hepatitis C testing: perspectives and preferences of people who inject drugs. Journal of Viral Hepatitis, 26(7), 919–922. [DOI] [PubMed] [Google Scholar]
- LawAtlas. (2019). Syringe Service Program Laws. Retrieved from https://lawatlas.org/datasets/syringe-services-programs-laws.
- Lubega S, Agbim U, Surjadi M, Mahoney M, & Khalili M. (2013). Formal hepatitis C education enhances HCV care coordination, expedites HCV treatment and improves antiviral response. Liver International, 33(7), 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca F, Robinson E, Dillon JF, & Boyd KA (2020). Eradicating Hepatitis C: are novel screening strategies for people who inject drugs cost-effective? International Journal of Drug Policy. [DOI] [PubMed] [Google Scholar]
- Maradiaga D, Pujula A, & Zapata H. (2013). Exploring Time Series Data Properties in SAS®. Paper presented at the SAS Global Forum 2013, Paper 456–2013, Statistics and Data Analysis. [Google Scholar]
- Merchant RC, Baird JR, Liu T, Taylor LE, Montague BT, & Nirenberg TD (2014). Brief intervention to increase emergency department uptake of combined rapid human immunodeficiency virus and hepatitis C screening among a drug misusing population. Academic Emergency Medicine, 21(7), 752–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman DC, & Jordan AE (2018). The syndemic of opioid misuse, overdose, HCV, and HIV: structural-level causes and interventions. Current HIV/AIDS Reports, 15(2),96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, et al. (2016). HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. New England Journal of Medicine, 375(3), 229–239. [DOI] [PubMed] [Google Scholar]
- Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. (2016). Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. The Lancet Infectious Diseases, 16(7), 797–808. [DOI] [PubMed] [Google Scholar]
- Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. (2018). Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction, 113(3), 545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif S, Safley D, McAllaster C, Wilson E, & Whetten K. (2017). State of HIV in the US Deep South. Journal of Community Health, 42(5), 844–853. [DOI] [PubMed] [Google Scholar]
- Rosenberg SD, Goldberg RW, Dixon LB, Wolford GL, Slade EP, Himelhoch S, et al. (2010). Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatric Services, 61(9), 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoff E, Mobley V, Hudgins M, Cope AB, Adams ND, Caputo CR, et al. (2020). HIV outbreak control with effective access to care and harm reduction in North Carolina, 2017–2018. American Journal of Public Health, 110(3), 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivkumar S, Peeling R, Jafari Y, Joseph L, & Pai NP (2012). Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Annals of Internal Medicine, 157(8), 558–566. [DOI] [PubMed] [Google Scholar]
- Spelman T, Morris M, Zang G, Rice T, Page K, Maher L, et al. (2015). A longitudinal study of hepatitis C virus testing and infection status notification on behaviour change in people who inject drugs. J Epidemiol Community Health, 69(8), 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tookes H, Bartholomew TS, Geary S, Matthias J, Poschman K, Blackmore C, et al.(2019). Rapid Identification and Investigation of an HIV Risk Network Among People Who Inject Drugs–Miami, FL, 2018. AIDS and behavior, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2020). 90–90-90: Treatment for all. Retrieved from https://www.unaids.org/en/resources/909090.
- USPSTF. (2020). Hepatitis C Virus Infection in Adolescents and Adults: Screening. Retrieved from https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening.
- Wagner AK, Soumerai SB, Zhang F, & Ross-Degnan D. (2002). Segmented regression analysis of interrupted time series studies in medication use research. Journal of Clinical Pharmacy and Therapeutics, 27(4), 299–309. [DOI] [PubMed] [Google Scholar]
- WHO. (2020). Welcome to 2020-the decade for disease elimination. Retrieved from https://www.who.int/news-room/feature-stories/detail/welcome-to-2020-the-decade-for-disease-elimination.
- Young SD, Monin B, & Owens D. (2009). Opt-out testing for stigmatized diseases: a social psychological approach to understanding the potential effect of recommendations for routine HIV testing. Health Psychology, 28(6), 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang X, Zhou S, Xie N, Liu P, Luo L, et al. (2015). Hepatitis C seroconversion in methadone maintenance treatment programs in Wuhan, China. Addiction, 110(5), 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.